Abstract
Tumefactive multiple sclerosis (MS) is an aggressive form of MS that can be difficult to treat with standard therapies. In severe MS relapses, plasma exchange (PLEX) has shown some benefit, but reports of its use in patients with tumefactive MS are limited. This article describes the successful use of PLEX in a patient with tumefactive MS. A 46-year-old right-handed woman with a recent diagnosis of MS presented with drowsiness, dysarthria, horizontal nystagmus, and quadriparesis. Her brain magnetic resonance images demonstrated multiple tumefactive demyelinating lesions in the medulla, bilateral periventricular white matter, and corona radiata white matter. She was initially treated with a 10-day course of intravenous methylprednisolone without benefit; therefore, PLEX was initiated. After the second exchange, the patient started to improve and was discharged initially to rehabilitation and then home. She was started on disease-modifying therapy with natalizumab and did not experience further relapses but had slow clinical decline during the next year, which led to discontinuation of natalizumab treatment. PLEX may be used as second-line treatment in corticosteroid-resistant MS relapses, but there are limited reports of its use in patients with tumefactive MS. This patient presented with aggressive disease with multiple tumefactive lesions and did not respond to standard treatment with corticosteroids. PLEX was successful in improving her symptoms, allowing her to return home, although the disease progressed during the next year.
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating disease that typically has a relapsing and remitting course at onset. Disability can accumulate quickly if relapses are severe and recovery is incomplete, but any relapse may result in accumulation of disability.1 Tumefactive MS is a particularly aggressive form, characterized by large (>2 cm), tumor-like demyelinating lesions seen on magnetic resonance imaging (MRI).2 These lesions may occur as solitary or multiple lesions.2
Standard treatment for MS relapses consists of high-dose corticosteroids; however, this treatment may not be effective for these severe relapses, and additional treatment options in these situations should be explored. Plasma exchange (PLEX) is beneficial in severe, corticosteroid-resistant relapses of MS and neuromyelitis optica (NMO); however, there are few reports of its use in tumefactive MS.2–4 We describe a fulminant course of MS with multiple tumefactive lesions treated with PLEX.
Case Report
A 46-year-old right-handed woman was referred to our tertiary-care center for continued management of a severe MS relapse. She had recently been diagnosed as having relapsing-remitting MS (RRMS) based on two relapses in the preceding 10 months, as well as MRI findings typical of demyelinating disease meeting McDonald criteria for dissemination in time and space.5,6 Her initial attack consisted of lip paresthesia and gait difficulties that resolved spontaneously, and her second relapse occurred 4 months before this presentation and consisted of right hemiparesis, speech difficulties, and bilateral leg paresthesia treated with a 10-day course of intravenous methylprednisolone. Her Expanded Disability Status Scale (EDSS) score7 during this relapse was 6.5. Disease-modifying therapy had been discussed and agreed on but not yet initiated.
She presented to her local hospital with gait and swallowing difficulties. On examination, she was drowsy and severely dysarthric with marked horizontal nystagmus, hypotonia in all four limbs, left hemiplegia, and mild right hemiparesis. Her reflexes were normal in the upper extremities and pathologically brisk at the knees and ankles, with clonus at the ankles. Her plantar responses were extensor bilaterally. She was initially treated for a suspected pneumonia, but she continued to decline and was intubated for hypercarbic respiratory failure. She was treated with a 10-day course of intravenous methylprednisolone, followed by a prednisone taper, but she did not improve.
She was transferred to our tertiary-care center for further management. At this time, her EDSS score was 9.5. Findings from extensive blood work performed to rule out other causes of inflammatory lesions in the brain were all negative and included antinuclear antibodies, extractable nuclear antigens, antineutrophil cytoplasmic antibodies, antithyroid antibodies, arbovirus, Lyme, human immunodeficiency virus, hepatitis, toxoplasmosis, cryptococcal antigen, tissue transglutaminase, B12, and serum angiotensin-converting enzyme. Her NMO antibodies were negative. Her cerebrospinal fluid showed slightly elevated protein levels at 466 mg/L (reference range, 200–400 mg/L) and 6 × 106/L nucleated cells. Cerebrospinal fluid cytology, herpes simplex virus polymerase chain reaction, and oligoclonal bands were negative. This lumbar puncture was performed after the long course of corticosteroids. Her MRIs showed multiple tumefactive lesions in the medulla, bilateral periventricular white matter, and white matter of the corona radiata (Figure 1). An expert neuroradiologist (DHL) reviewed previous MRIs and concluded that the findings were consistent with her previous MS diagnosis and with a new tumefactive presentation. Because this was deemed a corticosteroid-resistant relapse, PLEX was initiated 19 days after symptom onset for a total of five exchanges of 3 L each, performed every other day.
Figure 1.
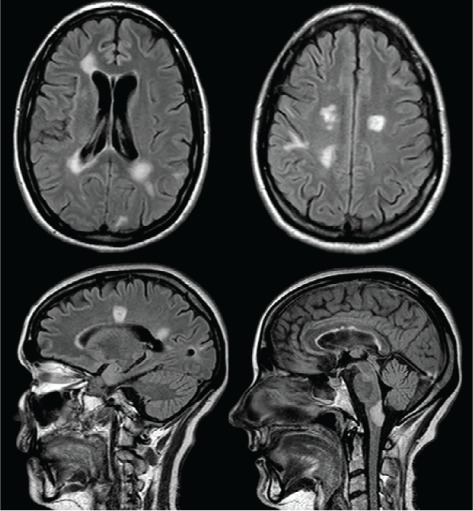
Axial and sagittal fluid-attenuated inversion recovery images showing multiple tumefactive lesions in the periventricular white matter, white matter of the corona radiata, and medulla
Improvements were noted after the first PLEX treatment. When transferred back to her peripheral hospital, she was following one-step commands, speaking with a spastic dysarthria and echolalia, and had at least antigravity power in both legs and in the right arm.
Two months after the completion of PLEX, the patient was ambulating with assistance, and her EDSS score was 6.5. She was started on disease-modifying therapy with natalizumab given the aggressive nature of her disease and approval through her private drug benefits. However, during the next year she demonstrated slow progressive neurologic decline consistent with a secondary progressive MS diagnosis; her MRIs did not show any new lesions or recurrence of her tumefactive lesions (Figure 2). Thus, 1 year later, natalizumab was stopped owing to clinical progression and an EDSS score of 7.5 to 8.0.
Figure 2.
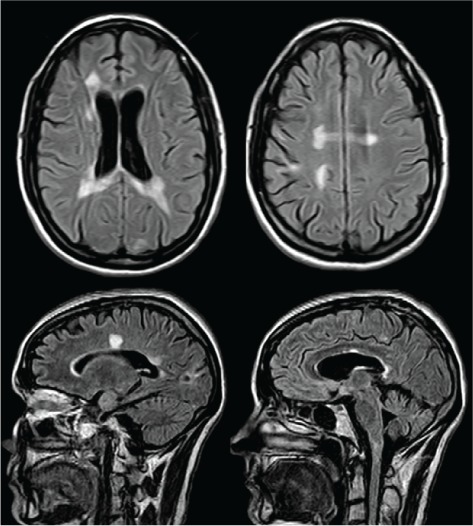
Axial and sagittal fluid-attenuated inversion recovery images at the same levels as in Figure 1, 2 years later, showing resolution of the medullary lesion, coalescence of the periventricular lesions, and interval development of diffuse brain atrophy
Discussion
Tumefactive MS is characterized by large (>2 cm) T2 or fluid-attenuated inversion recovery lesions seen on MRI.2,8 These lesions are also more likely to cause edema and mass effect, and as they are often singular, they may be mistaken for a neoplastic etiology.8 Presentations of MS with an aggressive course or tumefactive lesions continue to present a management challenge for clinicians. First-line treatment with corticosteroids may not always be effective, and evidence supporting the use of other therapies, such as PLEX, is limited.
PLEX has been studied in patients with any inflammatory demyelinating disease whose presentations are resistant to corticosteroids, including several randomized controlled trials,9–12 although these studies were all performed more than 25 years ago. In addition, there are multiple reports of its use in fulminant attacks of MS without tumefactive lesions.13 Reports of its use in tumefactive MS, however, are few,3,4 and some cases are reported with the first presentation of a tumefactive demyelinating lesion,14 leaving the diagnosis of MS in question.
PLEX was first reported to be effective for the treatment of progressive MS in 1980.15,16 Since then, it has been investigated in chronic progressive MS (CPMS) and acute relapses, with varying effect. A randomized controlled trial of PLEX in addition to immunosuppression with azathioprine in patients with CPMS did not show benefit compared to azathioprine alone.11 Another study showed that PLEX with azathioprine did not reduce MRI lesion load in comparison to placebo.12 Based on this evidence, PLEX is not recommended for treating patients with CPMS.17
For acute relapses, studies of PLEX have reported varying effects, with a response rate varying from 42%10 to 74%.18 However, the clinical presentations and treatment regimens used in these studies vary widely. The populations studied are heterogeneous and include patients with any inflammatory demyelinating disease (IDD), such as MS, acute disseminated encephalomyelitis, neuromyelitis optica, or clinically isolated syndromes.
There has been one randomized controlled trial using PLEX in the treatment of acute, corticosteroid-resistant relapses in patients with an IDD.10 This crossover study enrolled 11 patients in each group and compared PLEX with sham treatment. They found that patients in the treatment group had a moderate-to-marked improvement during treatment compared with the sham treatment group (42.1% vs. 5.9%).
Another randomized study looked at patients with RRMS and CPMS and compared PLEX plus immunosuppression with oral cyclophosphamide with sham PLEX with oral cyclophosphamide.19 The Kurtzke disability survival scale score was 6.1 at entry. There was no statistically significant difference between groups. However, after a logistic regression analysis, they found a significant benefit of PLEX at 2 weeks when they adjusted for attack severity. This study also showed that patients with RRMS receiving PLEX showed improvement at 1 and 12 months. There was no improvement in the CPMS group, even after statistical adjustment.
More recently, a prospective observational trial looked at MRI changes associated with the treatment of corticosteroid-resistant relapses with PLEX.20 Fifteen patients with a severe idiopathic IDD presentation that was corticosteroid resistant were prospectively followed, examining radiologic changes associated with treatment of the relapse with PLEX as well as clinical improvement. The mean EDSS score before PLEX treatment was 4.8 (range, 3.5–9). Fourteen of 15 patients had moderate or marked improvement in symptoms. Radiologic resolution was seen in 60% of patients and partial improvement in 20%; however, 33% had new lesions on follow-up MRI.
Although no randomized trial has been performed specifically with any one population of patients with IDDs, there are numerous reports establishing the efficacy of PLEX in patients with NMO spectrum disorders. It is thought that PLEX is beneficial in NMO by decreasing the amount of circulating antibodies to aquaporin 4, which are felt to be pathogenic.21,22 It has been shown that PLEX decreases the levels of antibodies by 14% after one exchange and by 85% after six exchanges.22 This study did not correlate the reduction of antibody levels with clinical improvements; however, 50% showed improvement immediately, and 78% had improvement at 6 months. In contrast to patients with MS, maintenance therapy with PLEX has also been found to be effective in NMO.21 Khatri et al.21 reviewed seven patients with fulminant NMO unresponsive to corticosteroids and immunomodulating therapies with interferons, glatiramer acetate, or intravenous immunoglobulin who stabilized or clinically improved after acute and maintenance therapy with PLEX. The frequency of exchanges was every 2 to 8 weeks, and many patients also received corticosteroids or cyclophosphamide concurrently.
Multiple guidelines on the use of plasmapheresis in neurologic conditions (from the American Academy of Neurology, European Federation of Neurological Societies, and American Society of Apheresis) list acute attacks of IDD, primarily RRMS and NMO, as an indication for PLEX.17,23,24 However, details regarding the number of sessions or when it should be considered once corticosteroids have failed are not delineated. In acute attacks, most treatment regimens use five to seven sessions every other day but vary depending on response to treatment.13,15,20 A response to PLEX is typically seen by the third exchange, or 4 to 5 days after initiation. Treatment early after a failed course of corticosteroids is generally thought to have improved outcomes; however, delayed treatment between 60 and 100 days after symptom onset has been shown to have some benefit.10,18
Attempts have been made to look at characteristics of patients with severe, corticosteroid-resistant relapses to determine features that predict treatment response to PLEX. Clinical features associated with better response include shorter disease duration, RRMS (compared with other forms of IDD), and brisk or normal deep tendon reflexes.18,25 Attack severity did not have an effect on response to treatment,18 although a trend toward better outcome with an EDSS score of less than 8 was seen in one study.25 Patients with ring enhancement of the largest lesion with associated mass effect and edema were more likely to respond to PLEX than those without these radiologic features.18 At 6 months, features associated with a favorable response included a shorter time from symptom onset to initiation of PLEX and improvement at discharge.26 In patients with NMO, factors found to be associated with better recovery include non-optic neuritis attack, preserved reflexes, lower baseline EDSS scores, and fewer previous relapses.27
The present patient had many favorable features predicting a beneficial response to PLEX, including a relatively short disease course of approximately 10 months, relapsing-remitting disease, and brisk reflexes. Her EDSS score improved to her previous baseline score after PLEX, and she had disease stabilization.
Conclusion
Management of tumefactive MS can be a challenge, especially when corticosteroids fail, because standardized guidelines do not exist for these situations. PLEX can be used as a second-line agent for acute MS or NMO relapses that do not respond to corticosteroids. It also seems to be effective for patients with tumefactive lesions, as we have also shown with this case. In addition, earlier treatment with PLEX in corticosteroid-resistant relapses is associated with better outcomes and should be considered early when there is no response to corticosteroids.
PracticePoints.
Tumefactive MS is characterized by large T2/fluid-attenuated inversion recovery hyperintense lesions measuring more than 2 cm. This clinical presentation may be more dramatic than a typical MS relapse and can include cognitive impairment, seizures, decreased level of consciousness, visual field deficits, and hemiparesis. These lesions may be singular or multiple, mimicking a neoplastic cause, thus requiring biopsy.
Plasma exchange (PLEX) may be used as a second-line agent for severe acute MS relapses that do not respond to corticosteroids. Earlier treatment with PLEX in corticosteroid-resistant relapses may be associated with better outcomes.
Acknowledgments
Written informed consent was obtained from the patient's substitute decision maker for publication of this case report and accompanying images.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61:1528–1532. doi: 10.1212/01.wnl.0000096175.39831.21. [DOI] [PubMed] [Google Scholar]
- 2.Hardy TA, Chataway J. Tumefactive demyelination: an approach to diagnosis and management. J Neurol Neurosurg Psychiatry. 2013;84:1047–1053. doi: 10.1136/jnnp-2012-304498. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe SL, Minagar A. Demyelinating pseudotumor. Arch Neurol. 2005;62:1466–1467. doi: 10.1001/archneur.62.9.1466. [DOI] [PubMed] [Google Scholar]
- 4.Seifert CL, Wegner C, Sprenger T et al. Favorable response to plasma exchange in tumefactive CNS demyelination with delayed B-cell response. Mult Scler. 2012;18:1045–1049. doi: 10.1177/1352458511429012. [DOI] [PubMed] [Google Scholar]
- 5.Polman CH, Reingold SC, Edan G et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, Reingold SC, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 8.Lucchinetti CF, Gavrilova RH, Metz I et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131(pt 7):1759–1775. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner HL, Dau PC, Khatri BO et al. Double-blind study of true vs. sham plasma exchange in patients treated with immunosuppression for acute attacks of multiple sclerosis. Neurology. 1989;39:1143–1149. doi: 10.1212/wnl.39.9.1143. [DOI] [PubMed] [Google Scholar]
- 10.Weinshenker BG, O'Brien PC, Petterson TM et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46:878–886. doi: 10.1002/1531-8249(199912)46:6<878::aid-ana10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Tindall RS, Walker JE, Ehle AL, Near L, Rollins J, Becker D. Plasmapheresis in multiple sclerosis: prospective trial of pheresis and immunosuppression versus immunosuppression alone. Neurology. 1982;32:739–743. doi: 10.1212/wnl.32.7.739. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen PS, Wanscher B, Szpirt W et al. Plasma exchange combined with azathioprine in multiple sclerosis using serial gadolinium-enhanced MRI to monitor disease activity: a randomized single-masked cross-over pilot study. Neurology. 1996;46:1620–1625. doi: 10.1212/wnl.46.6.1620. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez M, Karnes WE, Bartleson JD, Pineda AA. Plasmapheresis in acute episodes of fulminant CNS inflammatory demyelination. Neurology. 1993;43:1100–1104. doi: 10.1212/wnl.43.6.1100. [DOI] [PubMed] [Google Scholar]
- 14.Mao-Draayer Y, Braff S, Pendlebury W, Panitch H. Treatment of steroid-unresponsive tumefactive demyelinating disease with plasma exchange. Neurology. 2002;59:1074–1077. doi: 10.1212/wnl.59.7.1074. [DOI] [PubMed] [Google Scholar]
- 15.Dau PC, Petajan JH, Johnson KP, Panitch HS, Bornstein MB. Plasmapheresis in multiple sclerosis: preliminary findings. Neurology. 1980;30:1023–1028. doi: 10.1212/wnl.30.10.1023. [DOI] [PubMed] [Google Scholar]
- 16.Weiner HL, Dawson DM. Plasmapheresis in multiple sclerosis: preliminary study. Neurology. 1980;30:1029–1033. doi: 10.1212/wnl.30.10.1029. [DOI] [PubMed] [Google Scholar]
- 17.Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76:294–300. doi: 10.1212/WNL.0b013e318207b1f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magana SM, Keegan BM, Weinshenker BG et al. Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol. 2011;68:870–878. doi: 10.1001/archneurol.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner HL, Dau PC, Khatri BO et al. Double-blind study of true vs. sham plasma exchange in patients treated with immunosuppression for acute attacks of multiple sclerosis. Neurology. 1989;39:1143–1149. doi: 10.1212/wnl.39.9.1143. [DOI] [PubMed] [Google Scholar]
- 20.Meca-Lallana JE, Hernandez-Clares R, Leon-Hernandez A, Genoves Aleixandre A, Cacho Perez M, Martin-Fernandez JJ. Plasma exchange for steroid-refractory relapses in multiple sclerosis: an observational, MRI pilot study. Clin Ther. 2013;35:474–485. doi: 10.1016/j.clinthera.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Khatri BO, Kramer J, Dukic M, Palencia M, Verre W. Maintenance plasma exchange therapy for steroid-refractory neuromyelitis optica. J Clin Apher. 2012;27:183–192. doi: 10.1002/jca.21215. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Kim W, Huh SY, Lee KY, Jung IJ, Kim HJ. Clinical efficacy of plasmapheresis in patients with neuromyelitis optica spectrum disorder and effects on circulating anti-aquaporin-4 antibody levels. J Clin Neurol. 2013;9:36–42. doi: 10.3988/jcn.2013.9.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwathmey K, Balogun RA, Burns T. Neurologic indications for therapeutic plasma exchange: 2013 update. J Clin Apher. 2014;29:211–219. doi: 10.1002/jca.21331. [DOI] [PubMed] [Google Scholar]
- 24.Sellebjerg F, Barnes D, Filippini G et al. EFNS guideline on treatment of multiple sclerosis relapses: report of an EFNS task force on treatment of multiple sclerosis relapses. Eur J Neurol. 2005;12:939–946. doi: 10.1111/j.1468-1331.2005.01352.x. [DOI] [PubMed] [Google Scholar]
- 25.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–146. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 26.Llufriu S, Castillo J, Blanco Y et al. Plasma exchange for acute attacks of CNS demyelination: predictors of improvement at 6 months. Neurology. 2009;73:949–953. doi: 10.1212/WNL.0b013e3181b879be. [DOI] [PubMed] [Google Scholar]
- 27.Lim YM, Pyun SY, Kang BH, Kim J, Kim KK. Factors associated with the effectiveness of plasma exchange for the treatment of NMO-IgG-positive neuromyelitis optica spectrum disorders. Mult Scler. 2013;19:1216–1218. doi: 10.1177/1352458512471875. [DOI] [PubMed] [Google Scholar]


