Abstract
Stress and hormones released in response to stress influence the effects of nicotine and the severity of nicotine withdrawal. Here, we systematically examine the contribution of a stress response gene, FKBP5, to the acute and chronic behavioral effects of nicotine in smokers. Subjects were European- and African-American (EA and AA) heavy smokers who participated in an intravenous (IV) nicotine administration study (total n=169). FKBP5 rs3800373 genotype was analyzed for association to several outcomes, including nicotine withdrawal and the acute subjective, heart rate (HR), blood pressure and plasma cortisol responses to IV nicotine. Nicotine withdrawal was also examined in relation to rs3800373 allele frequencies in an independent cohort of EA and AA current smokers (n=3821). For a subset of laboratory subjects FKBP5 mRNA (n=48) expression was explored for an association to the same outcomes. The rs3800373 minor allele was associated with less severe nicotine withdrawal in laboratory subjects and the independent cohort of smokers. The rs3800373 minor allele was also associated with lower subjective ratings of negative drug effects in response to IV nicotine. Low FKBP5 mRNA expression was associated lower cortisol levels, lower subjective ratings of negative drug effects and a blunted HR response to nicotine. Stress hormone regulation via FKBP5 warrants further investigation as a potential contributor to the effects of nicotine withdrawal, which occurs commonly and has an important role in the maintenance of smoking behavior and relapse following a quit attempt.
Keywords: stress, smoking, PTSD, withdrawal, genetics, FKBP5
Introduction
Exposure and the differential response to stressful stimuli affect the development and maintenance of dependence on a number of drugs, including nicotine.1 The important role of stress with respect to risk of nicotine dependence (ND) is evident when one considers the high prevalence of ND in stress-related disorders such as post-traumatic stress disorder (PTSD). Individuals with PTSD are about twice as likely to have ND as those without PTSD.2 Further, smokers with PTSD smoke more cigarettes per day, report experiencing more severe withdrawal symptoms, and have lower quit rates than smokers without PTSD.3, 4 Although the underlying mechanisms that link stress-related disorders to ND have not been fully elucidated, disturbances in stress response and hypothalamic-pituitary-adrenal (HPA) axis activation are the focus of intense research. HPA-axis abnormalities are a hallmark of stress-related disorders such as PTSD,5 and subjects with PTSD responded differently to stressful stimuli compared to subjects unaffected by PTSD.6
There are consistent findings across basic neurobiological and genetic studies that the gene encoding FK506 binding protein 5 (FKBP5) is among those that modulate the stress response and HPA-axis activity in humans. FKBP5 has been linked to stress hormone dysregulation,7-9 risk of PTSD, anxiety disorders, and depressive disorder,10-14 and recently, alcohol withdrawal.15 FKBP5 functions in a feedback loop that regulates HPA-axis activity. FKBP5 expression is induced by glucocorticoids16 and the encoded protein, FK506 binding protein 51, represses glucocorticoid receptor (GR) signaling.17 The HPA-axis and the nicotinic cholinergic system are closely linked. However, the function of this critical regulatory gene in the context of smoking-related behaviors and the effects of nicotine has not been thoroughly investigated. Nicotine activates the HPA-axis in a dose-dependent fashion, resulting in the release of adrenocorticotropic hormone (ACTH) and cortisol.18, 19 Glucocorticoids have been shown to inhibit or desensitize nicotinic acetylcholine receptors20, 21 and may enhance nicotine self-administration in rats possibly by increasing the dose needed to produce a particular level of reinforcement.22 Stress hormones also influence the severity of nicotine withdrawal in rats23 and potentially, relapse to smoking in humans.24, 25 Thus, FKBP5, a stress-response gene, may function in pathways that modulate sensitivity to nicotine and the severity of nicotine withdrawal. To our knowledge, no previous studies have examined the modulation of nicotine responses and withdrawal severity by FKBP5.
The goal of this study was to examine systematically the contribution of FKBP5 to the acute and chronic behavioral effects of nicotine in smokers, starting with a laboratory paradigm. We included European American (EA) and African American (AA) heavy smokers. The behavioral outcomes studied were nicotine withdrawal severity and the acute subjective, heart rate, blood pressure and plasma cortisol responses to IV nicotine. In addition, the effect of FKBP5 variation on nicotine withdrawal severity was examined separately in a large cohort of EA and AA current smokers who participated in genetic studies of substance dependence.
Methods
Nicotine laboratory study: subjects, procedure and assessments
Non-treatment-seeking smokers (n=169) were recruited from the New Haven, Connecticut area for a laboratory study examining factors that moderate nicotine withdrawal and reward. Subjects from this population have been included in previous studies, including genetic studies.26, 27 All participants smoked at least 10 cigarettes/day and were otherwise medically healthy. Subjects were evaluated with the Structured Clinical Interview for DSM-IV28 and subjects that were dependent on alcohol or drugs (other than nicotine) or affected with any major psychiatric disorder were excluded from the study. A urine drug screen verified lack of drug use other than nicotine. Demographic information and smoking-related characteristics for the laboratory subjects are shown in Supplemental Information (Table S1).
The study procedure has been described in detail elsewhere.26, 27 Briefly, experimental sessions were conducted the morning (0800) after an overnight abstinence from smoking that was confirmed biochemically. All laboratory subjects had expired carbon monoxide levels less than 10 parts per million and plasma nicotine levels less than 4 ng/ml, indicating abstinence from smoking. After intravenous (IV) lines were established, at baseline, subjects completed the Minnesota Nicotine Withdrawal Scale (MNWS),29 the Positive and Negative Affect Schedule (PANAS),30 and the Brief Questionnaire of Smoking Urges (BQSU).31 They then were administered an IV dose of saline, followed by two doses of IV nicotine per 70 kg of body weight: a moderate dose (0.5 mg) and a high dose (1 mg) in uniform order, separated by 30 minutes. During the experimental session, blood pressure and heart rate were monitored and the effects of nicotine were assessed with the Drug Effects Questionnaire (DEQ). Approximately 20 minutes after the final dose of nicotine, subjects repeated the MNWS, PANAS and BQSU assessments. Institutional review boards at Yale University and VA Connecticut Healthcare System (West Haven, CT) approved the study, written consent was provided before participating in the study, and subjects were paid for their participation.
DSM-IV-based withdrawal symptoms in current smokers
Subjects were recruited at multiple Eastern US sites for genetic studies of drug and alcohol dependence, including genomewide association studies of alcohol,32 cocaine,33 opioid,34 and nicotine dependence.35 The institutional review board at each participating site approved the studies and all subjects provided written informed consent to participate. The National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism issued Certificates of Confidentiality to protect study participants. The sample's demographic details, smoking-related information, and major psychiatric diagnoses are shown in Supplemental Information (Table S2). The subjects were interviewed using the polydiagnostic Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA) to obtain DSM-IV diagnoses for all major psychiatric disorders and the additional variables used in the analysis.36 Subjects included in this analysis were current cigarette smokers, i.e., they had smoked within the last week. A smoking-withdrawal symptom score was calculated by summing the reports of DSM-IV smoking withdrawal symptoms. Specifically, subjects were asked about problems that they might have had when they stopped smoking or smoked less tobacco than usual. They answered “yes” or “no” to each of nine questions: 1) Were you irritable, angry, or frustrated? 2) Were you nervous or anxious? 3) Were you restless? 4) Did you have trouble concentrating? 5) Did your heart slow down? 6) Did you feel down or depressed? 7) Did you have such a strong desire for cigarettes that you couldn't think of anything else? 8) Did your appetite increase or did you gain weight? 9) Did you have trouble sleeping? In a separate stage of this assessment, subjects were asked whether symptoms 1-7 lasted for at least 24 hours. For each assessment, only subjects that reported at least one withdrawal symptom were included in the analysis.
Genotyping and Genetic Analysis
Laboratory participants were genotyped for rs3800373 with a 2μl TaqMan allelic discrimination assay (Applied Biosystems, Foster City, CA, USA). This SNP tags a previously studied common FKBP5 haplotype.11-14, 37 Laboratory subjects were assigned to EA or AA subject groups based on a previously described genetic marker method26, 38 or based on self-report. Rs3800373 was directly genotyped for subjects with DSM-IV-based withdrawal symptoms by either the HumanOmni1-Quad v1.0 or the Infinium® HumanCore Exome BeadChip (Illumina, San Diego, CA, USA). One subject was retained from each pair determined to be related (identity-by-descent>0.25), and subjects with sex-mismatches where excluded. The overall genotype-missing rate was <2%. The array-genotyped subjects were clustered as EA or AA based on the first two principal components, and subjects with clustering that was discordant from their self-reported race were excluded.
Data Analysis
Laboratory data were analyzed using mixed models with the following independent variables: age, sex, race (EA or AA), FKBP5 rs3800373 (coded as minor allele carrier versus TT to increase power), ordered experimental dose (saline, 0.5 mg nicotine and 1 mg nicotine per 70 kg body weight) and the interaction of experimental dose with FKBP5 genotype (as fixed effects), and subject (as a random effect). Rs3800373 distributions are show in Table S1 and S2. Based on prior work suggesting that the DEQ items may not represent independent constructs,39 we factor analyzed the DEQ prior to conducting statistical analyses and found evidence for the following three domains: 1) “stimulatory” effects comprising “feel stimulated”, “feel effects” and “feel high”; 2) “feel good” effects comprising “like”, “feel good” and “want more”; and 3) “negative” effects comprising “feel anxious”, “feel down” and “feel bad”. Each response was rated on a visual 100 mm scale that was then converted to a rating from 1-10. Blood cortisol levels were collected at five time points described in Figure 3. Log-transformed cortisol levels were analyzed in mixed models that included the interaction of FKBP5 genotype with time point rather than experimental dose. MNWS, PANAS and BQSU data were analyzed at the pre- and post-nicotine conditions separately with age, sex, and race as covariates. DSM-IV nicotine withdrawal symptoms were summed, log-transformed and analyzed in regression models that used age, sex, anxiety or depression diagnosis (coded dichotomously), the first three PCs (determined for the EA and AA populations separately), and rs3800373 (coded as minor allele carrier versus TT) as predictors. The results for EAs and AAs were combined by meta-analysis. Analyses and quality control procedures were performed using PLINK40 and JMP® Pro 10.0.
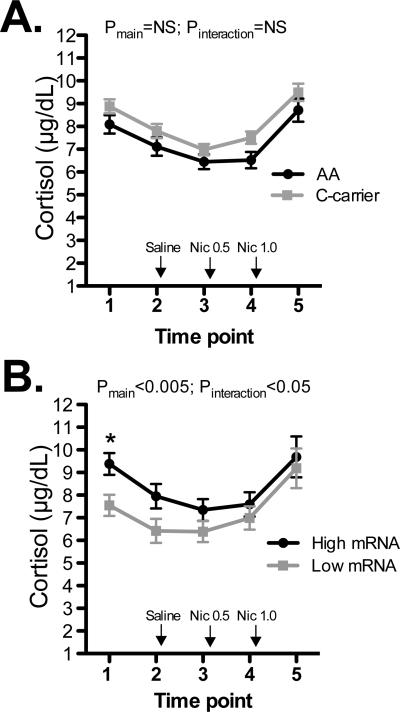
Figure 3.
FK506 binding protein 5 (FKBP5) mRNA expression is positively correlated with cortisol levels. Effects were tested for (A) rs3800373 genotype (AA versus C-carrier) and (B) FKBP5 mRNA (graphed as high or low based on a median split). Time points are, 1. Post-IV line insertion; 2. Pre-saline injection; 3. Pre-nicotine (0.5 mg/70 kg) injection; 4. Pre-nicotine (1.0 mg/70 kg) injection and 5. Post-infusions. FKBP5 mRNA levels were obtained prior to time point 2. The mean value is presented (±s.e.m.) and main effects of FKBP5 mRNA or genotype and interactive effects with time point are shown. *Pairwise p <0.005. Nic=nicotine.
RNA expression analysis
For a subset of laboratory subjects (N=48) whole blood was collected into PAXgene Blood RNA tubes (PreAnalytiX, Hombrechtikon, Switzerland) for RNA analysis. All experimental sessions began at 0800 and blood for RNA analysis was collected immediately prior to saline infusion for all subjects. RNA was extracted with the PaxGene Blood miRNA Kit (Qiagen, Hilden, Germany). The RNA quality was checked with an Agilent Bioanalyzer and the average RNA Integrity Number was 8.7 (range=6.2-9.9). An additional purification step was performed with the RNeasy MinElute Cleanup kit (Qiagen) to reduce salt concentrations. RNA was prepared for hybridization to HumanHT-12 v4 arrays at the Yale Center for Genome Analysis (YCGA) using the Illumina TotalPrep RNA Amplification kit (Life Technologies/Ambion, Foster City, CA). After hybridization, the arrays were scanned with a Bead Array Reader (Illumina, San Diego, CA, USA). Gene-level expression values were cubic-spline normalized using BeadStudio software (Illumina, San Diego, CA, USA). All subjects had FKBP5 expression values above a threshold detection level of p<0.00001. RNA expression was analyzed using mixed models as described above for genotype. To validate the array-based FKBP5 expression values we used RT-PCR to confirm the association with cortisol levels. RNA from 24 subjects (from 4 different arrays) was reverse transcribed using the ABI High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's specifications. One-fortieth of each cDNA reaction was amplified in triplicate in a 10 μl PCR reaction containing 500 nM forward and 500 nM reverse primer, 0.1 × Sybr Green I (Molecular Probes, Eugene, OR, USA), and 1x TaqMan Genotyping Master Mix (Applied Biosystems, Foster City, CA, USA) using an ABI Prism 7900HT Sequence Detection System. The expression of a housekeeping gene, HPRT, was used to normalize expression values between subjects. Primer sequences were, HPRT Fwd TGAGGATTTGGAAAGGGTGT, HPRT Rev CCTCCCATCTCCTTCATCAC, FKBP5 Fwd AAGTTTGCAGAGCAGGATGC, FKBP5 Rev GGCCCTCAGGTTTCTCTTCT. The relative level of gene expression was determined with the ΔΔCt method using the mean value for two experiments. Consistent with array-based results, the mean cycle threshold was associated with cortisol levels at time point 1 (Figure 1) in a regression model adjusted for age, sex and race (p<0.05, Figure S1).
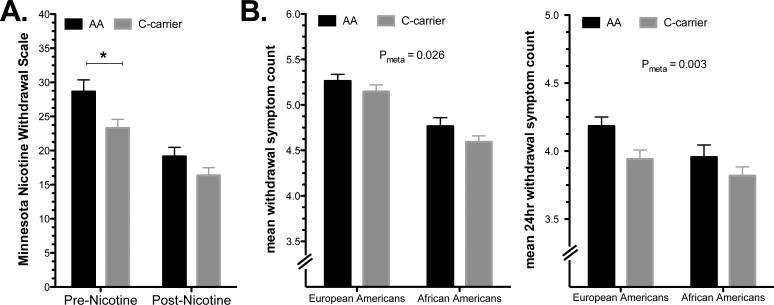
Figure 1.
FK506 binding protein 5 (FKBP5) rs3800373 is associated with nicotine withdrawal. A. Mean Minnesota Nicotine Withdrawal Scale scores for laboratory participants at the pre- and post-nicotine conditions and B. Mean DSM-IV symptom count for any withdrawal symptom (left) or symptom lasting 24 hours (right) in an independent cohort of current smokers. Rs3800373*C carriers reported lower MNWS scores at the pre-nicotine condition (*P=0.007) and fewer DSM-IV withdrawal symptoms. Error bars are ± s.e.m.
Results
FKBP5 variation and nicotine withdrawal severity
Smokers with the rs3800373*C allele, compared to those homozygous for the A allele, reported lower MNWS scores at baseline (main effect for group: F(1,164)=7.37; P<0.010), but not after nicotine (Figure 1A). FKBP5 mRNA was not associated with MNWS, nor was FKBP5 genotype or mRNA associated with QSU or PANAS scores.
To extend our observation on the nicotine-related behavioral effects of FKBP5 variation, we examined the association of rs3800373 to nicotine withdrawal symptoms in a population of current EA and AA smokers (EA and AA; total n=3821) that were recruited for genetic studies of substance dependence. The demographic characteristics and major psychiatric comorbidities are shown in Table S2 (Supplemental Information). We examined subjects’ reports of any DSM-IV nicotine withdrawal symptom and those lasting for 24 hours. Subjects with at least one rs3800373*C allele reported fewer nicotine withdrawal symptoms than those homozygous for rs3800373*A (Figure 1B) (main effect for group: Pmeta < 0.05) and fewer nicotine withdrawal symptoms that lasted for 24 hours (main effect for group: Pmeta < 0.005). Mean withdrawal counts for EA and AA genotype groups from each array are shown in Table S3 (Supplemental Information). In this analysis, comorbid anxiety or depression was used as a covariate in the regression models to control for the effects of anxiety and depression on the severity of withdrawal symptoms.
FKBP5 variation and the acute response to nicotine
For the subjective responses, both 0.5 and 1.0 mg nicotine/70 kg, compared to saline, increased the ratings of the “stimulatory” (main effect for dose: F(2,2643)=123.5; P<0.0001) and “feel good” (main effect for dose: F(2,2644)=124.6; P<0.0001) domains but not the “negative” domain (main effect for dose: F(2,2644)=0.31; P>0.05). The 1.0 mg nicotine/70 kg dose also increased subjective responses relative to the 0.5 mg nicotine/70 kg dose for ratings of “stimulatory” (main effect for dose: F(1,1811)=9.4; P<0.005) and “feel good” (main effect for dose: F(1,1810)=6.6; P<0.05) effects but not “negative” effects. Smokers who were C- carriers reported lower levels of “negative” effects than A-homozygotes in response to saline administration (main effect for group: F(1,192)=5.2; P<0.05 and group-by-dose interaction: F (2, 2640)=8.52; P<0.0005) (Figure 2A). There was a modest genotype-by-dose interactive effect on reports of “feel good” effects (group-by-time interaction; F(1,192)=4.0; P<0.05), with no significant group differences for the saline or nicotine doses (Supplementary Figure S2).
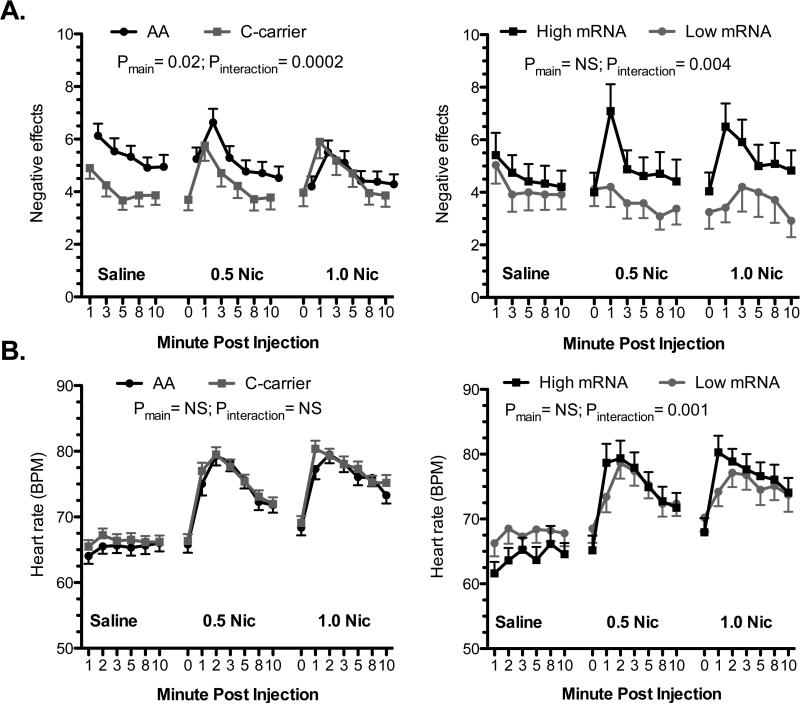
Figure 2.
FK506 binding protein 5 (FKBP5) genotype and mRNA expression are associated with the acute response to IV nicotine. The association of FKBP5 rs3800373 genotype (left panels) and FKBP5 mRNA (right panels) to subjective reports of “negative” drug effects (A) and heart rate (B). For the graph, subjects were grouped as having high (n=24) or low (n=24) FKBP5 expression based on a median split. The mean value for each experimental condition is presented (±s.e.m.) and main effects of FKBP5 mRNA or genotype and interactive effects with dose are shown. Nic = nicotine.
Smokers with low FKBP5 mRNA levels reported lower levels of “negative” effects in response to nicotine than those with high FKBP5 mRNA (group-by-dose interaction: F(2,762)=5.62; P<0.005) (Figure 2A). The association of FKBP5 mRNA-by-dose to “negative” effects was independent of rs3800373 genotype. In a regression model that included genotype and genotype -by-FKBP5 mRNA, there was no significant main (or interactive) effect of genotype, while the FKBP5 mRNA-by-dose term remained significant. Likewise, including baseline cortisol levels, array number (1-4), cigarette pack-years, and RNA Integrity Number as covariates in the model had little effect on the p value for the FKBP5 mRNA-by-dose term.
Smokers with low FKBP5 mRNA had a blunted HR response to nicotine (group-by-dose interaction; F(2,887)=7.8; P<0.0005) (Figure 2B). The HR effect was most evident in the minute following nicotine administration. There was also a modest mRNA-by-dose effect on systolic BP (group-by-dose interaction; F(2,908)=4.7; P<0.01) (Supplementary Figure S3). The HR effects were independent of genotype, baseline cortisol levels, array number, cigarette pack-years, and RNA Integrity Number, which were entered into the models in post-hoc analyses. FKBP5 genotype was not associated to HR or BP outcomes (Supplementary Figure S3).
FKBP5 variation and cortisol regulation in smokers
Smokers with low FKBP5 mRNA expression had lower cortisol levels than those with high FKBP5 expression (main effect for group: F(1,118)=9.46; P<0.005 and group-by-time interaction:F(1,184)=2.9; P<0.05). Consistent with these findings, FKBP5 mRNA expression was associated with cortisol at baseline before the initiation of nicotine administration (r2= 0.20, p =0.001), but not at the end of the study (Figure 3). The cortisol effects were independent of genotype, array number, cigarette pack-years, and RNA Integrity Number, which were entered into the models in post-hoc analyses. FKBP5 genotype was not associated with cortisol levels. The RNA-cortisol relationship was confirmed by RT-PCR (Supplementary Figure S1).
Discussion
We found that variation in FKBP5 was associated with nicotine withdrawal severity, the acute behavioral and physiological effects of nicotine, and cortisol regulation during nicotine withdrawal. FKBP5 genotype was associated with withdrawal severity in subjects both in a laboratory study and in an independent larger cohort of current EA and AA smokers. FKBP5 genotype and RNA expression were associated with differences in the acute negative subjective effects of IV nicotine, while FKBP5 RNA expression was associated with HR response to IV nicotine. FKBP5 mRNA expression was positively correlated with cortisol levels during nicotine withdrawal, but not after IV nicotine administration. Our investigation of effects at the RNA level provided convergent support for some of the genotype effects. For example, FKBP5 mRNA expression and genotype were both associated with the “negative” drug effects domain of the DEQ. Combined, these findings reaffirm the importance of HPA-axis regulation via FKBP5 and show that FKBP5 variation at the DNA and RNA levels contributes to some inter-individual differences in nicotine-related behavior.
The association of FKBP5 genotype with nicotine withdrawal severity in laboratory study participants and the large cohort of current smokers may have clinical relevance, as withdrawal severity is a strong predictor of relapse to smoking.41 Our findings regarding FKBP5 and nicotine withdrawal are consistent with a recent report of an association of FKBP5 to alcohol withdrawal in humans and mice.15 In the Huang et al. study, subjects with the FKBP5 rs3800373 minor allele reported less withdrawal. Together, these findings suggest that some effects of FKBP5 generalize to multiple addictive substances. Indeed, withdrawal symptoms that emerge during abstinence contribute to the maintenance of addiction to nicotine,41 alcohol42 and other drugs.43 These negative effects, as opposed to the positive reinforcing effects, that develop from drug use warrant attention, as they might be mechanistically distinct but independently important for the development of addiction. The pronounced effect of PTSD co-morbidity on nicotine withdrawal suggests that stress reactivity pathways are central to the negative effects that occur during nicotine withdrawal. Stress hormones modulate the activity of neurotransmitter systems in brain regions that are essential for the development and maintenance of addiction to nicotine and other substances. For example, stress hormones alter dopaminergic activity in the ventral tegmentum44 and the nucleus accumbens (NAcc).45 The activity of stress hormones in the NAcc may contribute to the withdrawal effects of nicotine. Marcinkiewcz et al. found that blocking CRF signaling specifically in the NAcc reduced nicotine withdrawal in rats.46
We observed an association of FKBP5 genotype and mRNA expression to subjective reports of “negative” drug effects during the human laboratory study. Genotype group differences were greatest during the saline condition and absent at the high nicotine condition. During the saline condition, the minor allele carriers reported less “negative” drug effects. In contrast, mRNA expression differences were absent during the saline condition and greatest during the high nicotine condition. Subjects with low FKBP5 expression reported less “negative” drug effects in response to nicotine relative to subjects with high FKBP5 expression. We also found that subjects with low FKBP5 mRNA expression had a blunted HR response to nicotine compared to subjects with high FKBP5 mRNA expression, but genotype was not associated with HR response. Stress hormones reduce the sensitivity of nicotinic receptors to nicotine,20, 21, 47 and they moderate the acute behavioral and HR response to nicotine in animal models.48, 49 Our findings regarding FKBP5 variation and the acute subjective and HR response to nicotine may reflect FKBP5-dependent stress hormone modulation of nicotinic receptor sensitivity in humans.
We observed a positive correlation between cortisol levels and FKBP5 mRNA, but not genotype, which is consistent with previous reports.12 The correlation with cortisol levels was strongest when subjects were in nicotine withdrawal and disappeared after IV nicotine administration. Our findings are consistent with previous studies demonstrating the regulatory influence of FKBP5 on glucocorticoid receptors.17, 50 The acute and chronic effects of nicotine exposure on stress hormone levels are relevant to many clinical features of ND. For example, work by Cohen et al. suggests that cortisol level correlates with nicotine withdrawal severity after an acute withdrawal period,51 while others have reported correlations between cortisol and risk of relapse to smoking.24, 25 Additional investigations on the relationship of FKBP5 variation to cortisol levels and the behavioral correlates of FKBP5 mRNA levels in smokers are warranted.
These findings suggest that FKBP5, which has a central function in stress-hormone regulation, could be a useful marker for susceptibility to severe nicotine withdrawal and could help to identify individuals at greatest risk for relapse to smoking following a quit attempt. FKBP5 may also be useful in understanding the link between stress-related disorders, such as PTSD, and the severe nicotine withdrawal that often accompanies it. Treatment of these co-occurring disorders is especially difficult and when they are comorbid, negative health effects may be amplified.3, 4 Risk for ND and PTSD is influenced by genetic variation, with a significant proportion of this risk being common to both disorders.52 FKBP5 could be a common factor involved in facets of each trait. Also, given its role in modulating withdrawal severity, variation in FKBP5 has implications for smoking cessation. This possibility is supported by a study showing that glucocorticoid agonists may reduce cigarette craving and satisfaction in smokers.53 The subjects in the gene expression study (n=48) were also transcriptome-analyzed after the final injection of nicotine (data not shown). Relative to the pre-saline condition, we observed a ~25% reduction in FKBP5 mRNA after the final nicotine injection. This effect was significant based on a transcriptome-wide test correction (Bonferroni corrected significance threshold p< 9.8×10-6), but given the uniform order of administration, saline followed by nicotine, we cannot confidently attribute the gene expression change to the effects of nicotine. This effect, although potentially important, requires additional study.
Our observations on the effects of FKBP5 variation at the DNA and RNA levels are consistent in many respects; however, our study has limitations. To reduce the likelihood that our findings were the result of type-1 error we conducted replication studies in independent samples and additional studies on mRNA expression that provided convergent support, but type-1 error cannot be excluded and replication and extension of these findings will be important. We limited our genotype association analysis to a previously studied SNP to limit the number of statistical tests conducted to preserve power, and to allow for direct comparisons to the two arrays used in our follow-up study on withdrawal effects. Based on prior literature we anticipated that rs3800373 would be highly correlation with rs1360780, an FKBP5 SNP with a putative regulatory function in glucocorticoid receptor binding to DNA.10 Rs3800373, rs1360780, and additional SNPs that form a common FKBP5 haplotype, have been reported to be associated to the presence of a number of different psychiatric traits in EAs and AAs.11-14, 37 We confirmed our expectations regarding linkage disequilibrium with a post-hoc examination of rs1360780 in the laboratory sample; as expected the linkage disequilibrium (determined with Haploview54) for rs3800373 and rs1360780 was high (r2 > 0.9 and D’ > 0.95). Given the LD structure of the gene, a much larger sample will be required to clearly distinguish the independent effects of FKBP5 SNPs (and other variants). The FKBP5 mRNA findings were independent of genotype in our statistical models, but the power to detect interactive or moderating effects of genotype may have been limited by the modest sample size. Alternatively, cell specificity could favor the detection of FKBP5 SNP effects on gene expression in cells from tissues, such as brain, that are more relevant to the behavioral effects of stress, nicotine and nicotine withdrawal. It is interesting that FKBP5 genotype and mRNA are both associated with the “negative” effects domain of the DEQ. Each effect is interactive with nicotine dose, but, as noted above, the effect profiles differ. This may indicate that FKBP5 mRNA expression in blood cells captures some nicotine-related effects not captured by FKBP5 genotype. Unraveling the relationship between these dynamic effects will require additional work.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants, R01 AA011330, R01 AA017535, R01 DA030976, R01 DA12690, R03 DA027474, K24 AA013736, K12 DA00167, T32 MH014276, and T32 AA015496. This work also received support from the U.S. Department of Veterans Affairs (VA) through Cooperative Study #575B, the VISN 1 New England Mental Illness Research, Education and Clinical Center (MIRECC) and VA National Center for PTSD, and a VA VISN 1 CDA and VA MERIT. Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University (contract number N01-HG-65403).
Footnotes
Disclosure: Unrelated to this research, Dr. Kranzler has been a consultant or advisory board member for Alkermes, Lilly, Lundbeck, Otsuka, Pfizer, and Roche. He is also a member of the American Society of Clinical Psychopharmacology's Alcohol Clinical Trials Initiative, which is supported by AbbVie, Ethypharm, Lilly, Lundbeck, and Pfizer and has a U.S. patent pending entitled, “Test for Predicting Response to Topiramate and Use of Topiramate.” Dr. Sofuoglu has served as an expert witness on behalf of Pfizer in lawsuits related to varenicline. All other authors declare no potential conflict of interest.
References
- 1.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 2.Hapke U, Schumann A, Rumpf HJ, John U, Konerding U, Meyer C. Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. J Nerv Ment Dis. 2005;193:843–846. doi: 10.1097/01.nmd.0000188964.83476.e0. [DOI] [PubMed] [Google Scholar]
- 3.Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Dedert EA. Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine Tob Res. 2013;15:1122–1129. doi: 10.1093/ntr/nts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- 5.Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- 6.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 7.Menke A, Arloth J, Putz B, Weber P, Klengel T, Mehta D, et al. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology. 2012;37:1455–1464. doi: 10.1038/npp.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menke A, Klengel T, Rubel J, Bruckl T, Pfister H, Lucae S, et al. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes Brain Behav. 2013;12:289–296. doi: 10.1111/gbb.12026. [DOI] [PubMed] [Google Scholar]
- 9.Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 13.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, et al. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang MC, Schwandt ML, Chester JA, Kirchhoff AM, Kao CF, Liang T, et al. FKBP5 Moderates Alcohol Withdrawal Severity: Human Genetic Association and Functional Validation in Knockout Mice. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones. 2004;9:243–252. doi: 10.1379/CSC-32R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J Steroid Biochem Mol Biol. 2006;100:34–41. doi: 10.1016/j.jsbmb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Matta SG, Beyer HS, McAllen KM, Sharp BM. Nicotine elevates rat plasma ACTH by a central mechanism. J Pharmacol Exp Ther. 1987;243:217–226. [PubMed] [Google Scholar]
- 19.Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi LJ, He HY, Liu LA, Wang CA. Rapid nongenomic effect of corticosterone on neuronal nicotinic acetylcholine receptor in PC12 cells. Arch Biochem Biophys. 2001;394:145–150. doi: 10.1006/abbi.2001.2519. [DOI] [PubMed] [Google Scholar]
- 21.Ke L, Lukas RJ. Effects of steroid exposure on ligand binding and functional activities of diverse nicotinic acetylcholine receptor subtypes. J Neurochem. 1996;67:1100–1112. doi: 10.1046/j.1471-4159.1996.67031100.x. [DOI] [PubMed] [Google Scholar]
- 22.Caggiula AR, Donny EC, Epstein LH, Sved AF, Knopf S, Rose C, et al. The role of corticosteroids in nicotine's physiological and behavioral effects. Psychoneuroendocrinology. 1998;23:143–159. doi: 10.1016/s0306-4530(97)00078-4. [DOI] [PubMed] [Google Scholar]
- 23.Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiatry. 1998;43:525–530. doi: 10.1016/S0006-3223(97)00423-X. [DOI] [PubMed] [Google Scholar]
- 26.Herman AI, Jatlow PI, Gelernter J, Listman JB, Sofuoglu M. COMT Val158Met modulates subjective responses to intravenous nicotine and cognitive performance in abstinent smokers. Pharmacogenomics J. 2013 doi: 10.1038/tpj.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37:1509–1516. doi: 10.1038/npp.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for Dsm-Iii-R Personality-Disorders (Scid-Ii) .1. Description. J Pers Disord. 1995;9:83–91. [Google Scholar]
- 29.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 30.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 31.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 32.Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelernter J, Sherva R, Koesterer R, Almasy L, Zhao H, Kranzler HR, et al. Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelernter J, Kranzler HR, Sherva R, Koesterer R, Almasy L, Zhao H, et al. Genome-Wide Association Study of Opioid Dependence: Multiple Associations Mapped to Calcium and Potassium Pathways. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelernter J, Kranzler HR, Sherva R, Almasy L, Herman AI, Koesterer R, et al. Genomewide Association Study of Nicotine Dependence in American Populations: Identification of Novel Risk Loci in Both African- and European-Americans. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, et al. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA). Drug Alcohol Depend. 2005;80:303–312. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, et al. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Arch Gen Psychiatry. 2012;69:62–70. doi: 10.1001/archgenpsychiatry.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang BZ, Zhao H, Kranzler HR, Gelernter J. Practical population group assignment with selected informative markers: characteristics and properties of Bayesian clustering via STRUCTURE. Genet Epidemiol. 2005;28:302–312. doi: 10.1002/gepi.20070. [DOI] [PubMed] [Google Scholar]
- 39.Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O'Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl) 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- 42.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. A J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, et al. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–1752. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pauly JR, Ullman EA, Collins AC. Adrenocortical hormone regulation of nicotine sensitivity in mice. Physiol Behav. 1988;44:109–116. doi: 10.1016/0031-9384(88)90353-8. [DOI] [PubMed] [Google Scholar]
- 48.Robinson SF, Grun EU, Pauly JR, Collins AC. Changes in sensitivity to nicotine and brain nicotinic receptors following chronic nicotine and corticosterone treatments in mice. Pharmacol Biochem Behav. 1996;54:587–593. doi: 10.1016/0091-3057(95)02281-3. [DOI] [PubMed] [Google Scholar]
- 49.Pauly JR, Grun EU, Collins AC. Chronic corticosterone administration modulates nicotine sensitivity and brain nicotinic receptor binding in C3H mice. Psychopharmacology (Berl) 1990;101:310–316. doi: 10.1007/BF02244047. [DOI] [PubMed] [Google Scholar]
- 50.Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- 51.Cohen LM, al'Absi M, Collins FL., Jr. Salivary cortisol concentrations are associated with acute nicotine withdrawal. Addict Behav. 2004;29:1673–1678. doi: 10.1016/j.addbeh.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 52.Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- 53.Reuter M, Netter P, Rogausch A, Sander P, Kaltschmidt M, Dorr A, et al. The role of cortisol suppression on craving for and satisfaction from nicotine in high and low impulsive subjects. Hum Psychopharmacol. 2002;17:213–224. doi: 10.1002/hup.402. [DOI] [PubMed] [Google Scholar]
- 54.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.