Virological suppression is the cornerstone of long-term human immunodeficiency virus treatment success. In low- and middle-income countries, virological suppression rates after 5 years of first-line antiretroviral treatment were favorable (>80%) and stable but declined over time after correction for attrition.
Keywords: antiretroviral therapy, virological monitoring, HIV-1, low- and middle-income countries
Abstract
Background. More than 11.7 million people are currently receiving antiretroviral therapy (ART) in low- and middle-income countries (LMICs), and focused efforts are needed to ensure high levels of adherence and to minimize treatment failure. Recently, international targets have emphasized the importance of long-term virological suppression as a key measure of program performance.
Methods. We systematically reviewed publications and conference abstracts published between January 2006 and May 2013 that reported virological outcomes among human immunodeficiency virus type 1 (HIV-1)–infected adults receiving first-line ART for up to 5 years in LMICs. Summary estimates of virological suppression after 6, 12, 24, 36, 48, and 60 months of ART were analyzed using random-effects meta-analysis. Intention-to-treat (ITT) analysis assumed all participants who were lost to follow-up, died, or stopped ART as having virological failure.
Results. Summary estimates of virological suppression remained >80% for up to 60 months of ART for all 184 included cohorts. ITT analysis yielded 74.7% (95% confidence interval [CI], 72.2–77.2) suppression after 6 months and 61.8% (95% CI, 44.0–79.7) suppression after 48 months on ART. Switches to second-line ART were reported scarcely.
Conclusions. Among individuals retained on ART, virological suppression rates during the first 5 years of ART were high (>80%) and stable. Suppression rates in ITT analysis declined during 4 years.
The World Health Organization (WHO) estimated that nearly 11.7 million people living with human immunodeficiency virus (HIV) in low- and middle-income countries (LMICs) received antiretroviral therapy (ART) in 2013 [1]. Global ART scale-up has been made possible by the use of standardized and simplified treatment protocols and decentralized service delivery, with limited reliance on laboratory monitoring [2, 3]. In order to enhance treatment monitoring, WHO recommended in 2013 that viral load measurements among people receiving ART be performed 6 months after initiating ART and every 12 months thereafter [4, 5].
As the availability of viral load testing grows in LMICs, the percentage of patients with virological suppression can be an important measure of overall ART clinic and program success. The Joint United Nations Programme on HIV/AIDS 90-90-90 by 2020 initiative was established with the goal of achieving virological suppression in 90% of all people receiving ART by the year 2020 [6], emphasizing the need for robust data on short- and long-term programmatic levels of virological suppression against which program performance can be assessed.
In this context, population-level summary estimates of virological suppression measured at different time points are needed to guide ART program managers on the normative levels of population-level virological suppression. The objective of this systematic review was to determine summary estimates of virological suppression among HIV-infected people receiving first-line ART for up to 5 years in LMICs.
METHODS
Search Strategy
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement and the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [7, 8]. We searched the electronic databases Ovid Medline (through PubMed), Embase, and the Cochrane Central Register of Controlled Trials for research papers published in English between 1 January 2006 and 14 May 2013 (Supplementary Material). The start date of 2006 was chosen to search for published virological outcomes from ART programs that followed the WHO public health approach [3]. We defined search terms pertaining to HIV/AIDS or ART combined with LMICs. Subsequently, studies were restricted to those matching search terms for HIV viral load and/or HIV drug resistance. We combined this with search terms specifying time factors in order to select viral load measurements obtained from individuals on ART for 6 months and up to 5 years. Finally, we excluded studies of children (aged <13 years) from the search. Additionally, we manually searched conference abstracts of the International AIDS Society in 2011 and 2012 and the Conference on Retroviruses and Opportunistic Infections in 2012 and 2013. Finally, unpublished cohort data from the Pan-African Studies to Evaluate Resistance Monitoring (PASER-M) program and TREAT Asia (TAHOD [TREAT Asia HIV Observational Database] and TASER [TREAT Asia Studies to Evaluate Resistance]) programs were added as additional sources of data [9].
Study Selection
We included original research papers and conference abstracts that reported on virological outcomes among HIV type 1 (HIV-1)–infected adults on first-line combination ART for up to 5 years in LMICs. Studies reporting on both adults and adolescents (aged >13 years) were included if the majority of the population (>90%) was aged >18 years. Cross-sectional studies, cohort studies (prospective and retrospective), and clinical trials were eligible if they reported virological outcomes. Included studies had to report data on the proportion of participants below (or above) a threshold of HIV RNA in copies per milliliter at a specified duration of ART. LMICs were categorized by gross national income according to the World Bank [10]. Previous use of antiretroviral drugs for (pre- or post-exposure) prophylaxis, prevention of mother-to-child transmission, or treatment of HIV infection did not preclude inclusion.
We excluded studies in which participants were HIV-1/HIV-2 coinfected, were receiving mono- or duotherapy, had started second-line ART, were selected based on treatment failure, lived in high-income countries, or were children (aged <13 years or >10% adolescents aged 13–18 years). Studies reporting a median follow-up of <4 months or >5 years on ART only were not eligible for inclusion. Because we wanted to extract and compare summary estimates of virological suppression after 6, 12, 24, 36, 48, and 60 months of ART initiation, studies were excluded if the reported interquartile range (IQR) of follow-up duration extended beyond 2 time points. For example, a study reporting on persons with a median ART duration of 43 months (IQR 28–61) was excluded. When more than 1 study reported on the same cohort of patients, we included the publication that contained the most complete information. Two reviewers independently selected eligible papers and abstracts for inclusion in the analysis and removed duplicates. If the description of the study was unclear with respect to eligibility criteria, we contacted the authors for further information. Any disputes about inclusion were resolved by discussion between the 2 reviewers with help of a third reviewer.
Data Extraction
The following information was extracted from the included articles: location(s) and time frame of the study, study design, number of participants, participants’ characteristics at time of ART initiation, ART regimens (nonnucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor with nucleoside reverse transcriptase inhibitor (NRTI) backbone or triple NRTI regimen), time on first-line ART, number of switches to second-line ART, viral load threshold used to define virological suppression, number of participants with and without viral load results, number of participants with virological suppression, and categories of patient attrition (ie, lost to follow-up [LTFU], death, stop ART) as reported in the study. When 1 study reported virological outcomes at multiple time points and/or reported multiple thresholds to define virological suppression, all outcomes were extracted. When multiple definitions of virological suppression were provided, the study definition closest or equal to 1000 copies per milliliter was included in the analysis to fit the current WHO definition of virological failure and ART program benchmarks [4, 11, 12]. Studies reporting patient outcomes separately, for example, clinical trial arms, were considered to consist of different cohorts. Therefore, the number of cohorts reported in this review exceeds the number of studies included. We assessed quality of reporting using the STROBE checklist (Supplementary Material) [8].
Statistical Analyses
The proportion of study participants meeting the definition of virological suppression was determined for each cohort and for each duration of ART (ie, 6, 12, 24, 36, 48, or 60 months). The primary analysis for each time point was the on-treatment (OT) analysis, that is, the number of participants with virological suppression divided by the number of participants retained in care and with viral load results available at a given time point. The secondary analysis included only studies reporting attrition over time. This intention-to-treat (ITT) analysis included the number of people who were deceased, LTFU, and/or who stopped ART in the denominator of estimates of virological suppression. Switches to second-line ART were not considered virological failures in the OT and ITT analyses. Switch rates were calculated separately by dividing the number of switches to second-line ART by the number of participants at baseline.
Descriptive statistics were used to assess the proportion of virological suppression, followed by random-effects meta-analysis; we determined the summary estimates of people with virological suppression at each time point. The variance of the raw proportions was stabilized using a Freeman–Tukey arcsine square root transformation and subsequently back transformed to the original scale. Between-study heterogeneity was reported by means of the τ2 of the meta-analysis of the transformed proportions [13]. Pooled proportions and 95% confidence intervals (CIs) were displayed using forest plots.
Three sensitivity and subgroup analyses were carried out to explore reasons for heterogeneity observed in the analyses. First, we explored the role of study design on the reported outcomes. We excluded trials and analyzed data from programmatic settings only in order to explore the effect of a controlled trial setting. Second, we only included studies in which at least 90% of the participants received NNRTI-based first-line ART in order to look at the effect of WHO-recommended first-line regimens [4]. Third, we explored the role of geographic location by analyzing regions separately. Subgroup differences were tested through χ2 test for heterogeneity. Statistical analyses were performed using Stata 12 [14].
RESULTS
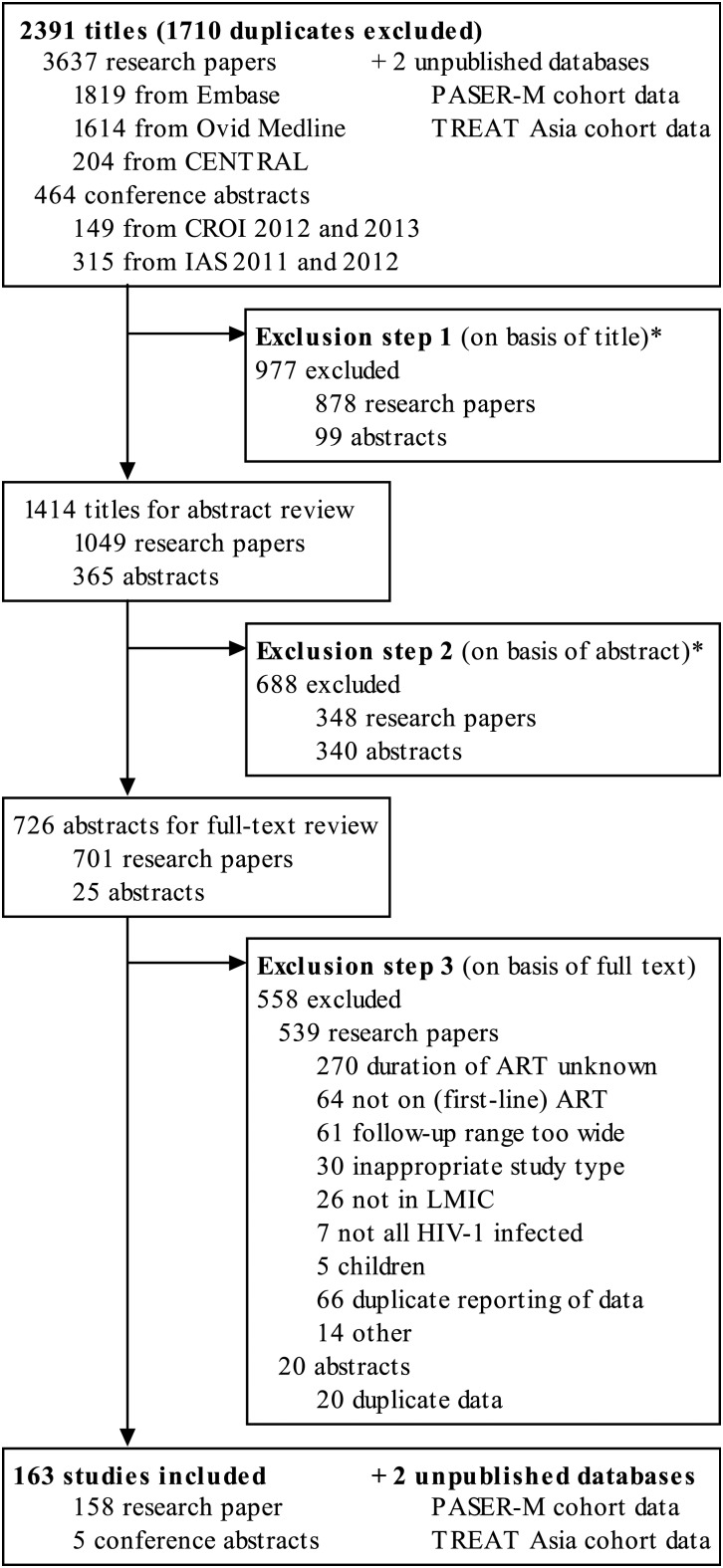
A total of 2391 research papers and conference abstracts were deemed potentially eligible after duplicate papers were removed. On the basis of title and abstract, 1665 reports were excluded (Figure 1). Of the remaining 726 studies, 163 met the inclusion criteria, including 158 full-text research papers and 5 conference abstracts. In addition, we included unpublished outcome data from the PASER-M cohort (24 and 36 months of follow-up) and TREAT Asia cohort (6, 12, 24, 36, 48, and 60 months of follow-up).
Figure 1.
Flow chart of study selection. An asterisk (*) indicates studies without original or virological data, studies with a design not meeting inclusion criteria or without specific data on duration of antiretroviral treatment (ART), studies outside low- and middle-income countries, and studies with only pediatric participants. Abbreviations: CENTRAL, Cochrane Central Register of Controlled Trials; CROI, Conference on Retroviruses and Opportunistic Infections; HIV, human immunodeficiency virus; IAS, International AIDS Society; LMIC, low- and middle-income countries; PASER, Pan-African Studies to Evaluate Resistance; TREAT Asia, Therapeutics Research, Education, and AIDS Training in Asia.
In total, 184 cohorts from 35 countries were retrieved from 163 studies (Figure 2); 69.0% of the cohorts originated from sub-Saharan Africa. Almost half (48.4%) of the studies were prospective cohorts, 20.1% were trials, and the remaining were either cross-sectional or retrospective cohort designs. Almost three-quarters (73.4%) of the studies reported at least 90% of participants initiating NNRTI-based first-line ART. Fifty-four percent adhered to all STROBE guidelines. Study characteristics are summarized in Table 1, and a full list and characteristics of all included studies are provided in Supplementary Table. Sample sizes ranged from 8 to 27 516 study participants, and most studies report outcomes up to 2 years after ART initiation (Table 2). All outcomes beyond 2 years from ART initiation originated from Asia and sub-Saharan Africa, and 5-year outcomes were only available for OT outcomes.
Figure 2.
World map indicating 35 countries reporting data included.
Table 1.
Summary of Cohort Characteristics, by Analysis Type
| Characteristic | On-Treatment Analysis |
Intention-to-Treat Analysisa |
||
|---|---|---|---|---|
| N | % | N | % | |
| Region | ||||
| Sub-Saharan Africa | 127 | 69.0 | 61 | 65.6 |
| Asia | 38 | 20.7 | 23 | 24.7 |
| Latin America and the Caribbean | 14 | 7.6 | 7 | 7.5 |
| Eastern Europe | 1 | 0.5 | 0 | 0.0 |
| Multiple regions | 4 | 2.2 | 2 | 2.2 |
| Study design | ||||
| Prospective cohort | 89 | 48.4 | 48 | 51.6 |
| Retrospective cohort | 39 | 21.2 | 21 | 22.6 |
| Trial (any) | 37 | 20.1 | 23 | 24.7 |
| Cross-sectional | 19 | 10.3 | 1 | 1.1 |
| Year of antiretroviral therapy initiation | ||||
| Before 2006 | 81 | 50.9 | 34 | 43.6 |
| After 2006 | 78 | 49.1 | 44 | 56.4 |
| Not reported | 25 | 13.6 | 15 | 16.1 |
| Antiretroviral therapy regimens | ||||
| Nonnucleoside reverse transcriptase inhibitor–basedb | 135 | 73.4 | 70 | 75.3 |
| Protease inhibitor–basedb | 7 | 3.8 | 6 | 6.5 |
| Other or unknownc | 42 | 22.8 | 17 | 18.3 |
| Total | 184 | 100.0 | 93 | 50.5 |
For a full list and characteristics of study cohorts included in the systematic review, see Supplementary Table.
a Studies reporting numbers of deaths and persons lost to follow-up were included in the intention-to-treat analysis.
b Nonnucleoside reverse transcriptase inhibitor/protease inhibitor (NNRTI/PI)–based regimen defined as >90% of participants receiving an NNRTI/PI as part of first-line antiretroviral therapy.
c Including triple nucleoside reverse transcriptase inhibitor regimens.
Table 2.
Virological Suppression after 6 to 60 Months of First-Line Antiretroviral Therapy
| Months on Antiretroviral Therapy | Cohorts, N |
Participants, N |
Descriptive Statistics |
Random-Effects Meta-analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Total | Median | Interquartile Range |
Median | Interquartile Range |
Summary Estimate | 95% Confidence Interval |
τ2a | ||||
| Q1 | Q3 | Q1 | Q3 | Low | High | |||||||
| On-treatment analysis | ||||||||||||
| 6 | 100 | 116 051 | 173 | 84 | 453 | 87.2 | 78.7 | 91.3 | 84.9 | 83.5 | 86.3 | 0.03 |
| 12 | 117 | 103 632 | 174 | 78 | 427 | 87.7 | 80.8 | 91.9 | 85.6 | 84.4 | 86.9 | 0.04 |
| 24 | 45 | 39 694 | 203 | 78 | 475 | 86.0 | 80.5 | 91.9 | 84.4 | 82.0 | 86.9 | 0.05 |
| 36 | 21 | 18 729 | 373 | 163 | 980 | 90.1 | 82.5 | 93.6 | 88.5 | 85.5 | 91.4 | 0.06 |
| 48 | 13 | 4673 | 201 | 104 | 384 | 90.9 | 85.1 | 94.6 | 88.6 | 84.2 | 93.0 | 0.09 |
| 60 | 6 | 1433 | 117 | 60 | 505 | 86.6 | 78.6 | 94.1 | 85.2 | 76.6 | 93.9 | 0.09 |
| Intention-to-treat analysis | ||||||||||||
| 6 | 40 | 47 838 | 106 | 40 | 371 | 72.9 | 63.8 | 82.3 | 74.7 | 72.2 | 77.2 | 0.02 |
| 12 | 67 | 40 938 | 153 | 66 | 351 | 71.9 | 59.6 | 77.6 | 67.3 | 63.6 | 71.0 | 0.10 |
| 24 | 23 | 6332 | 142 | 66 | 321 | 65.0 | 52.4 | 72.2 | 64.6 | 60.8 | 68.4 | 0.03 |
| 36 | 4 | 1411 | 309 | 160 | 546 | 63.0 | 61.3 | 75.1 | 68.1 | 58.0 | 78.2 | 0.03 |
| 48 | 4 | 504 | 130 | 66 | 187 | 59.0 | 51.4 | 72.4 | 61.8 | 44.0 | 79.7 | 0.13 |
a τ2 is a measure of between-study heterogeneity that is less affected by the number of studies than other common measures.
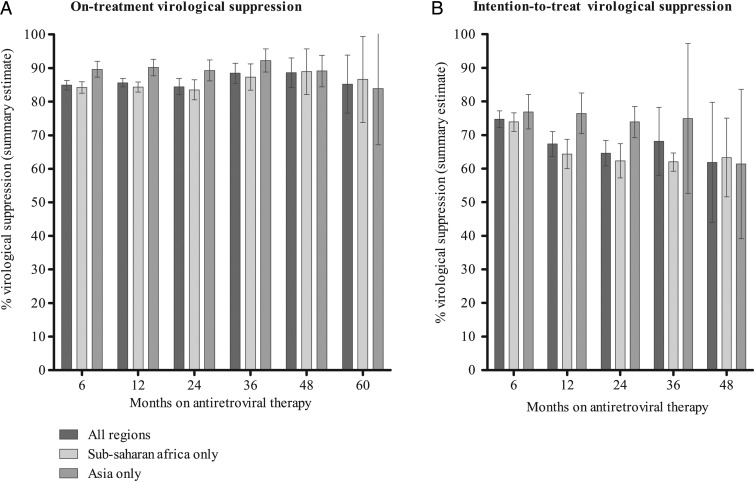
OT summary estimates of virological suppression for all time points assessed were >80%. For cohorts on ART for 6, 12, and 24 months, OT summary estimates were 84.9% (95% CI, 83.5–86.3; N = 116 051), 85.6% (95% CI, 84.4–86.9; N = 103 632), and 84.4% (95% CI, 82.0–86.9; N = 39 694), respectively. After 3, 4, and 5 years of ART, the summary estimates were 88.5% (95% CI, 85.5–91.4; N = 18 729), 88.6% (95% CI, 84.2–93.0; N = 4673), and 85.2% (95% CI, 76.6–93.9; N = 1414), respectively.
The ITT analysis included a subset of 93 (50.5%) cohorts reporting LTFU and mortality outcomes. Summary estimates of the ITT analysis after 6, 12, 24, 36, and 48 months of ART were 74.7% (95% CI, 72.2–77.2; N = 47 838), 67.3% (95% CI, 63.6–71.0; N = 40 938), 64.6% (95% CI, 60.8–68.4; N = 6332), 68.1% (95% CI, 58.0–78.2; N = 1411), and 61.8% (95% CI, 44.0–79.7; N = 504) virological suppression, respectively. Descriptive and summary estimates of OT and ITT virological suppression are presented in Table 2 and Figure 3; forest plots displaying individual and summary estimates can be found in Supplementary Figures.
Figure 3.
Summary estimates of virological suppression outcomes. Note: All summary estimates are informed by a forest plot (see Supplementary Figures). Full tables of the subgroup analysis are provided in Supplementary Tables.
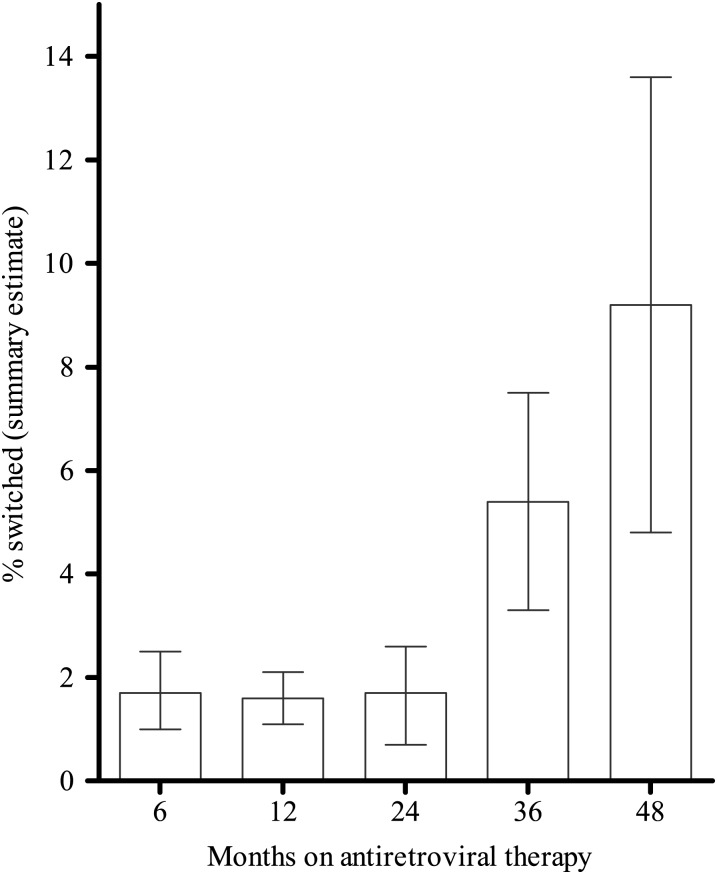
Of 93 cohorts included in the ITT analysis, 46.4% reported the number of switches to second-line ART. Summary estimates of the reported switches were <2% during the first 2 years of ART: 1.7% (95% CI, 1.0–2.5; N = 24 451), 1.6 (95% CI, 1.1–2.1; N = 19 923), and 1.7% (95% CI, .7–2.6; N = 7100) after 6, 12, and 24 months of first-line ART, respectively. After 36 and 48 months, 5.4% (95% CI, 3.3–7.5; N = 2407) and 9.2% (95% CI, 4.8–13.6; N = 595) of participants switched (Table 3, Figure 4).
Table 3.
Switches to Second-Line Antiretroviral Therapy after 6 to 48 Months of First-Line Antiretroviral Therapy
| Months on Antiretroviral Therapy | Cohorts, N |
Participants, N (at baseline) |
Descriptive Statistics |
Random-Effects Meta-analysis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | % of Studies from Intention-to-Treat Analysis | Total | Median | Interquartile Range |
Median | Interquartile Range |
Summary Estimate | 95% Confidence Interval |
τ2a | ||||
| Q1 | Q3 | Q1 | Q3 | Low | High | ||||||||
| 6 | 22 | 55.0 | 24 451 | 67 | 27 | 382 | 0.0 | 0.0 | 0.0 | 1.7 | 1.0 | 2.5 | 0.09 |
| 12 | 25 | 37.3 | 19 923 | 230 | 61 | 596 | 0.0 | 0.0 | 1.4 | 1.6 | 1.1 | 2.1 | 0.12 |
| 24 | 12 | 52.2 | 7100 | 283 | 104 | 701 | 0.3 | 0.0 | 3.1 | 1.7 | 0.7 | 2.6 | 0.02 |
| 36 | 3 | 75.0 | 2407 | 1009 | 328 | 1070 | 6.1 | 3.6 | 7.0 | 5.4 | 3.3 | 7.5 | 0.01 |
| 48 | 2 | 50.0 | 595 | 298 | 80 | 515 | 10.1 | 7.8 | 12.5 | 9.2 | 4.8 | 13.6 | 0.01 |
a τ2 is a measure of between-study heterogeneity that is less affected by the number of studies than other common measures.
Figure 4.
Switches to second-line therapy.
Sensitivity analyses excluding clinical trials and analyses excluding studies in which <90% of participants received NNRTI-based ART did not yield different summary estimates in either the OT or ITT analyses (Supplementary Tables). However, regional differences were found in subgroup analyses comparing studies conducted in sub-Saharan Africa to studies from Asia, as shown in Figure 3. At 2 time points, there was a statistically significant difference in OT virological suppression. At 6 months, the OT summary estimate was 89.6% (95% CI, 87.3–92.0; N = 3942) in Asia compared with 84.2% (95% CI, 82.5–85.9; N = 100 613) in sub-Saharan Africa (P = <.01). At 12 months, the OT summary estimate was 90.2% (95% CI, 87.7–92.6; N = 5997) in Asia compared with 84.3% (95% CI, 82.8–85.8; N = 93 415) in sub-Saharan Africa (P = <.01). Although summary estimates from Asia were generally higher compared with those from sub-Saharan Africa, no differences were observed at any of the other time points assessed. Statistically significant regional differences were also observed in the ITT analysis at months 12 and 24; summary estimates were approximately 12% higher in Asia compared with sub-Saharan Africa. At 12 months of ART, we found 76.4% (95% CI, 70.4–82.5; N = 2334) virological suppression in Asia vs 64.3% (95% CI, 60.0–68.7; N = 37 898) in sub-Saharan Africa (P = .01). At 24 months, we found 73.9% (95% CI, 69.2–78.5; N = 1634) virological suppression in Asia vs 62.3% (95% CI, 57.2–67.4; N = 4356) in sub-Saharan Africa (P = .01).
DISCUSSION
This review describes summary estimates of virological suppression for people receiving first-line ART for HIV in 35 LMICs for up to 5 years after treatment initiation. Reported virological outcomes for people who remained in care and on treatment were stable over time, with OT virological suppression rates remaining >80% for up to 5 years after ART initiation. In the ITT analysis, which considered all people who died, stopped ART, or were LTFU as having virological failure, the virological suppression rate declined to 62% after 4 years. The outcomes were consistent irrespective of study design and ART regimen. Early virological suppression rates were found to be significantly higher in Asia compared with sub-Saharan Africa, but differences tended to disappear over time.
Overall, OT estimates of virological suppression up to 36 months on ART were consistently high and informed by a large number of cohorts and study participants. Similar trends have been reported in resource-rich countries, where the proportion of patients with virological suppression has been shown to increase over time in the Netherlands and British Columbia [15, 16]. Stable rates of OT virological suppression may be partially explained by the inherent survivor bias of analyses limited to individuals on ART retained in care [17, 18]. Spontaneous viral resuppression and the impact of adherence interventions may also contribute to the maintenance of high levels of virological suppression among individuals on ART. However, the aggregate proportion of individuals who died, were LTFU, or stopped therapy increased over time, leading to decreasing rates of virological suppression in the ITT analysis. Interestingly, we did not observe an increase in the proportion of individuals with virological failure retained in care. This implies that those failing first-line ART either dropped out of care or were switched to a second-line regimen. Although the proportion of patients switching to second-line care did increase over time in the subset of studies with available data, such numbers were small and consistent with the very low uptake of second-line ART in LMICs in general, suggesting that the majority of individuals failing ART most likely dropped out of care before they could be identified [2].
In contrast to previous reviews [19–21], this review included data from all LMICs including Asia and Latin America and the Caribbean for up to 5 years of follow-up. A previously published review of 12-month outcomes across all LMICs reported 84% suppression at 12 months [20]. Two other reviews that reported on virological outcomes on ART in sub-Saharan Africa showed lower OT suppression rates compared with this review: 78%, 76%, 67% vs 75%, 67%, 65% after 6, 12, and 24 months of ART [19, 21]. One of these reviews (concerning sub-Saharan Africa only) also reported ITT outcomes, which were in line with our findings: 78%, 69%, 63% after 6, 12, and 24 months of ART [19]. Early virological suppression rates were found to be significantly higher in Asia compared with sub-Saharan Africa, but differences tended to disappear over time. While this may indicate superior program performance in the early stages of ART in Asia, the paucity of data after 36 months prevents additional analyses.
This review has several limitations. First, it reflects findings from clinics where patients had access to virological monitoring or access to virological testing for research purposes, which likely represent well-resourced ART sites [22]. Therefore, estimates of virological suppression presented in this review may not be representative of actual program conditions that may be prevalent in other clinics and/or settings. ART programs or clinics that evaluated and reported viral loads may have greater resources and better clinical outcomes than those where viral loads were not evaluated and/or where virological outcomes were not reported. Second, in settings where viral loads were evaluated but where poor virological outcomes were observed, researchers may have chosen not to disseminate the results, potentially leading to publication bias. Estimates may therefore overestimate the mean frequency of virological suppression in the broader population receiving ART in LMICs. Third, we searched for studies reporting on virological outcomes of ART and not specifically on attrition. Therefore, the ITT analysis could report biased results as attrition rates could possibly be underreported in the included studies, potentially overestimating program efficacy. Conversely, the assumption that all those who died or were LTFU had detectable viral loads is likely to yield conservative estimates. Fourth, this review included studies reporting OT outcomes from cross-sectional analysis and it did not focus only on studies following cohorts longitudinally. One should take into account that the findings include cross-sectional outcomes from different studies at each time point, which could induce some bias in interpreting differences across time since these differences may be attributable to the studies included in the analysis rather than a true change in virological suppression over time. Fifth, this review might include overlap in data between studies. Because of thorough exclusion, we believe that the presence of duplicate data in our review is minimal.
Strengths of our review include its broad scope, encompassing studies from various ART programs across LMICs and complementary unpublished data from large African and Asian cohort studies. We were able to assemble a large dataset for meta-analysis, especially at the time points up to 3 years after the initiation of ART. Our results were consistent in different sensitivity and subgroup analyses, taking the study design and type of ART into account. This review included both longitudinal and cross-sectional studies from programmatic and clinical trial settings, yet excluding trials from the analysis did not affect summary estimates. Additionally, studies reporting both protease inhibitor- and NNRTI-based regimens were included, but subgroup analyses examining different regimens were comparable to the overall analysis.
In the context of lifelong treatment, data on long-term virological outcomes from LMICs are much needed [23]. This review found high and stable levels of virological suppression (>80%) among individuals retained in care during the first 5 years of first-line ART in LMICs. When accounting for individuals who were LTFU, died, or stopped therapy and if one assumes that all of them had virological failure, available data suggest that suppression rates declined during the first 4 years of ART. This review also highlights the need for more accurate data on retention to inform the development of a comprehensive benchmark framework to assess and monitor program performance. Further research is needed to better describe virological outcomes among individuals who are LFTU and among those who stop therapy, so that they can be taken into account in future estimates of population-level virological suppression.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Annette Sohn and Awachana Jiamsakul for providing further data on behalf of the TAHOD (TREAT Asia HIV Observational Database) (TREAT Asia) (TREAT Asia Studies to Evaluate Resistance [TASER]/TAHOD) program. Also, we thank Ingeborg Nagel for librarian assistance with the search and Caspar Roelofs and Vera van Rijn for their research assistance on literature selection and data extraction.
Author contributions. S. B. conceived the systematic review. T. S. B. did the initial search of published work, checked all full-text articles, extracted data from the full reports and conference abstracts, conceived and coordinated the analyses, and wrote the first draft of the paper. Literature selection and data extraction were performed with the help of 2 research assistants. K. C. E. S. also checked all full-text articles and abstracts, was available to resolve conflicts during data extraction, and supervised the reviewing process. N. F. supported the statistical analyses. J. H. M., M. R. J., N. F., S. K., T. F. R. d. W., J. B., and S. B. participated in discussion of the results. All authors participated in writing of the final paper.
Financial support. This work was supported by the World Health Organization (WHO) with funds provided by the Bill and Melinda Gates Foundation (grant number 38180). In addition, Pan-African Studies to Evaluate Resistance Monitoring data collection was supported by the Ministry of Foreign Affairs of the Netherlands through a partnership with Stichting AidsFonds (grant number 12454), the Embassy of the Kingdom of the Netherlands, Heineken Africa Foundation, Jura Foundation, and the Netherlands Organization for Scientific Research (grant number W 07.10.101). In addition, TASER-TAHOD is supported by the US National Institutes of Health through the International Epidemiologic Databases to Evaluate AIDS consortium (grant number U01AI069907).
Potential conflicts of interest. S. B. and N. F. are staff members of WHO. J. B. is a consultant with the WHO. The authors alone are responsible for the views expressed in this publication, which do not necessarily represent the decisions or stated policies of the WHO. J. H. M. has a dual appointment with the Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, Massachusetts, and the Alfred Hospital, Melbourne, Australia. J. H. M.'s institution (Alfred Hospital) has received payment for consultancy to Gilead and Viiv Healthcare. M. R. J. has an appointment with the Division of Geographic Medicine and Infectious Diseases, Tufts Medical Center, Boston, Massachusetts, and is also a consultant for WHO. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global update on the health sector response to HIV, 2014, 2014. Available at: http://www.who.int/hiv/pub/progressreports/update2014/en/. Accessed 2 April 2015.
- 2.World Health Organization. Access to antiretroviral drugs in low- and middle-income countries: technical report July 2014. Geneva, Switzerland, 2014. Available at: http://www.who.int/hiv/pub/amds/access-arv-2014/en/. Accessed 2 April 2015. [Google Scholar]
- 3.Gilks CF, Crowley S, Ekpini R et al. . The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet 2006; 368:505–10. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection—Recommendations for a public health approach. Geneva, Switzerland, 2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/. Accessed 5 July 2013. [PubMed] [Google Scholar]
- 5.Keebler D, Revill P, Braithwaite S et al. . Cost-effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models. Lancet Glob Heal 2014; 2:e35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS (Joint United Nations Programme on HIV/AIDS). Ambitious treatment targets: writing the final chapter of the AIDS epidemic: writing the final chapter of the AIDS epidemic. Geneva, 2014. [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 82:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamers RL, Oyomopito R, Kityo C et al. . Cohort profile: The PharmAccess African (PASER-M) and the TREAT Asia (TASER-M) monitoring studies to evaluate resistance—HIV drug resistance in sub-Saharan Africa and the Asia-Pacific. Int J Epidemiol 2012; 41:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Bank. World Bank list of economies. Available at: http://data.worldbank.org/about/country-classifications/country-and-lending-groups. Accessed 10 May 2013.
- 11.World Health Organization. HIV drug resistance early warning indicators - June 2010 update. Geneva, Switzerland, 2010. Accessed 20 March 2013. [Google Scholar]
- 12.UNAIDS (Joint United Nations Programme on HIV/AIDS). 90-90-90 - An ambitious treatment target to help end the AIDS epidemic, 2014. Available at: http://www.unaids.org/en/resources/documents/2014/90-90-90. Accessed 20 March 2015.
- 13.Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 2008; 8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP.
- 15.van Sighem A, Gras L, Kesselring A et al. . SHM Monitoring Report 2013 - HIV Infection in the Netherlands. November 2. Amsterdam: Stichting HIV Monitoring, 2013. Available at: http://www.hiv-monitoring.nl/nederlands/onderzoek/monitoring-reports/. Accessed 25 March 2014. [Google Scholar]
- 16.Nosyk B, Montaner JS, Colley G et al. . The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis 2014; 14:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta RK, Goodall RL, Ranopa M et al. . High rate of HIV resuppression after viral failure on first-line antiretroviral therapy in the absence of switch to second-line therapy. Clin Infect Dis 2014; 58:1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okello V, Dlamini S, Jobanputra K, Parker LA, Papo J, Azih C. Routine viral load (VL) monitoring for targeted adherence support among antiretroviral therapy (ART) patients in a resource-limited setting, Swaziland. In: 8th International Conference on HIV Treatment and Prevention 2013. [Google Scholar]
- 19.Barth RE, van der Loeff MFS, Schuurman R, Hoepelman AIM, Wensing AMJ. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis 2010; 10:155–66. [DOI] [PubMed] [Google Scholar]
- 20.McMahon JH, Elliott JH, Bertagnolio S, Jordan MR. Viral suppression after 12 months of antiretroviral therapy in low- and middle-income countries: a systematic review. Bull World Health Organ 2013; 91:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond R, Harry TC. Efficacy of antiretroviral therapy in Africa: effect on immunological and virological outcome measures—a meta-analysis. Int J STD AIDS 2008; 19:291–6. [DOI] [PubMed] [Google Scholar]
- 22.López-Martínez A, O'Brien NM, Caro-Vega Y, Crabtree-Ramírez B, Sierra-Madero J. Different baseline characteristics and different outcomes of HIV-infected patients receiving HAART through clinical trials compared with routine care in Mexico. J Acquir Immune Defic Syndr 2012; 59:155–60. [DOI] [PubMed] [Google Scholar]
- 23.McMahon JH, Medland N. 90-90-90: How Do We Get There? Lancet HIV 2014; 1:e10–1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.