In this retrospective study of patients evaluated at a North Carolina medical center <20% of patients with positive Lyme disease test result had illnesses compatible with the infection. False-positive results greatly exceed true-positive in this geographic setting.
Keywords: Borrelia burgdorferi, Lyme, serology, positive predictive value, diagnostic testing
Abstract
Background. Lyme disease is diagnosed by 2-tiered serologic testing in patients with a compatible clinical illness, but the significance of positive test results in low-prevalence regions has not been investigated.
Methods. We reviewed the medical records of patients who tested positive for Lyme disease with standardized 2-tiered serologic testing between 2005 and 2010 at a single hospital system in a region with little endemic Lyme disease. Based on clinical findings, we calculated the positive predictive value of Lyme disease serology. Next, we reviewed the outcome of serologic testing in patients with select clinical syndromes compatible with disseminated Lyme disease (arthritis, cranial neuropathy, or meningitis).
Results. During the 6-year study period 4723 patients were tested for Lyme disease, but only 76 (1.6%) had positive results by established laboratory criteria. Among 70 seropositive patients whose medical records were available for review, 12 (17%; 95% confidence interval, 9%–28%) were found to have Lyme disease (6 with documented travel to endemic regions). During the same time period, 297 patients with a clinical illness compatible with disseminated Lyme disease underwent 2-tiered serologic testing. Six of them (2%; 95% confidence interval, 0.7%–4.3%) were seropositive, 3 with documented travel and 1 who had an alternative diagnosis that explained the clinical findings.
Conclusions. In this low-prevalence cohort, fewer than 20% of positive Lyme disease tests are obtained from patients with clinically likely Lyme disease. Positive Lyme disease test results may have little diagnostic value in this setting.
Lyme disease is a tick-borne zoonotic bacterial infection caused by Borrelia burgdorferi sensu lato. It is the most common vector-borne infectious disease in the temperate northern hemisphere, reported in tens of thousands of residents of the United States each year [1]. Lyme disease most commonly presents with a distinctive erythema migrans (EM) skin lesion, but if untreated the disease can disseminate to other organ systems, causing arthritis, meningitis, cranial and peripheral neuropathy, and cardiac conduction abnormalities. These syndromes are not unique to Lyme disease, and in the absence of the characteristic EM rash, serologic testing is necessary to differentiate Lyme disease from other conditions [2].
Lyme disease transmission is geographically heterogeneous, however, and for any given clinical presentation the likelihood of Lyme disease will be influenced by regional disease prevalence. This is primarily a function of tick populations, particularly the density of host-seeking nymphal black-legged ticks infected with B. burgdorferi [3, 4]. States and regions where infected ticks are uncommon have low transmission rates of Lyme disease. North Carolina, for example, has a low annual incidence of Lyme disease (<0.5 cases per 100 000 population), and entomologic data suggest there is a very low risk of human Lyme disease there [3, 5, 6]. In endemic regions, such as the northeastern and upper Midwestern United States, Lyme disease is responsible for an appreciable burden of meningitis, arthritis, and cranial neuropathy. By contrast B. burgdorferi infection will be responsible for a much smaller proportion of these syndromes in areas with little or no Lyme disease transmission. Considering that the pretest probability of a disease strongly influences interpretation of any diagnostic test result, we hypothesized that positive Lyme disease test results will be less meaningful in regions with low disease prevalence. To this end, we performed a large cross-sectional retrospective study of patients undergoing evaluation for Lyme disease presenting to clinics and hospitals located in an area with little Lyme disease transmission.
METHODS
Study Design
We performed a retrospective study of electronic medical records for adults and children evaluated at both inpatient and outpatient sites in the Duke University Health System between 1 January 2005 and 31 December 2010. The institutional review board approved the study protocol with a waiver of informed consent.
Patient Identification and Data Abstraction
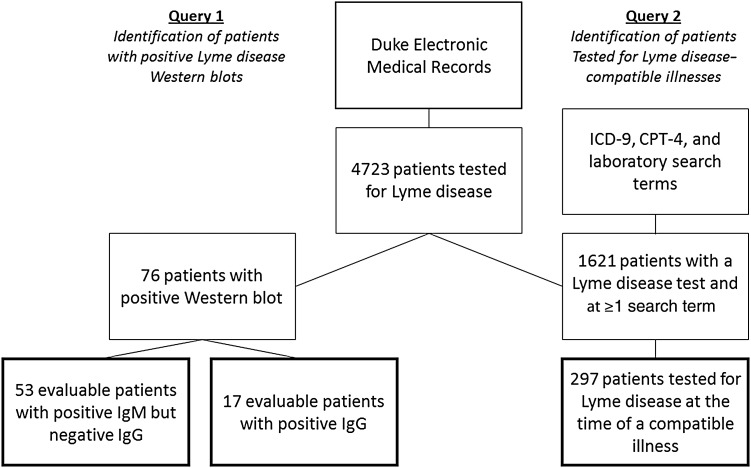
We queried the electronic medical records to identify 2 cohorts of patients: those with a positive 2-tiered Lyme disease serologic test result and those tested for Lyme disease during a compatible illness (Figure 1). For the first cohort, we reviewed the electronic medical records to determine whether each patient had a clinical presentation compatible with active Lyme disease documented within 1 month of when the diagnostic test was obtained. We abstracted testing results, information about their clinical presentation, documentation of an alternative diagnosis, and documentation of tick exposure in a Lyme disease endemic state.
Figure 1.
Workflow used to conduct electronic medical record queries. Abbreviations: CPT-4, Current Procedure Terminology 4; ICD-9, International Classification of Diseases, Ninth Revision; IgG, immunoglobulin G; IgM, immunoglobulin M.
For the second cohort, we focused on patients with oligoarticular arthritis of large joints, meningitis, and cranial nerve palsy. Although these syndromes do not encompass the full clinical spectrum of disseminated Lyme disease, we selected the conditions that are most frequently attributable to Lyme disease in endemic areas [7–16]. We searched for patients who had been tested for Lyme disease and whose record contained International Classification of Diseases, Ninth Revision diagnostic codes, Current Procedure Terminology 4 procedure codes, or laboratory codes compatible with arthritis, meningitis, or cranial neuropathy (Supplementary Table 1). We then reviewed the medical records to confirm documentation of arthritis, meningitis, or cranial neuropathy at the time of Lyme disease testing.
To identify each cohort we performed queries of the Duke electronic medical records. Our search terms identified inpatients and outpatients of all ages tested at Duke-affiliated laboratories. We excluded patients from both cohorts without available electronic medical records to review. All patients were tested using the Meridian Premier Lyme EIA kit (catalog Nos. 696016 and 696032). Specimens reactive by this kit were then tested by Western blot using the Trinity Biotech B. burgdorferi IgG and IgM MarBlot Strip Test systems (catalog Nos. 40–2075 G and 40–2075 M).
Outcome Measure
We defined a case of Lyme disease as the coexistence of a positive 2-tiered Lyme disease serologic test and a compatible clinical illness. This is in accordance with recommended clinical and diagnostic practices, definitions accepted for Lyme disease clinical trials, as well as the Centers for Disease Control and Prevention surveillance definition of Lyme disease [17–19]. A positive 2-tiered test is conventionally defined as positive or equivocal results of an enzyme-linked immunosorbent assay (ELISA) using a B. burgdorferi whole-cell sonicate, followed by a positive immunoglobulin (Ig) M or IgG Western immunoblot, defined as ≥2 of 3 reactive IgM bands or ≥5 of 10 reactive IgG bands [20]. The Duke University Health System clinical laboratories perform ELISA followed by automatic Western blot analysis in the event of positive or equivocal ELISA results. Individual band results are not reported to clinicians. We classified patients who were seropositive by IgM criteria as “false-positive” if they did not have a positive IgG Western blot within 2 months of symptom onset [19, 20]. Although conventionally a 1-month cutoff is recommended, beyond which the IgM results should no longer be considered [3], we chose 2 months given the difficulty of precisely dating symptom onset in a retrospective study.
We classified seropositive patients as “true-positive” if they had chart documentation of any of the following clinical presentations: EM-like skin lesions, large-joint arthritis (including clinical or radiographic documentation of a joint effusion or inflammatory synovial fluid), meningitis (documented by elevated lymphocyte counts in the cerebrospinal fluid [CSF]), motor cranial neuropathy, radiculopathy or peripheral neuropathy, or atrioventricular block (documented by electrocardiography). Patients with an alternative diagnosis that explained their syndrome were reclassified as false-positives.
Statistical Analysis
We calculated the positive predictive value by dividing the true-positives by total positives (true-positives plus false-positives) [21]. We calculated 95% confidence intervals (CIs) around proportions using standard binomial distributions. For all statistical analysis, we used Stata 13.1 statistical software (StataCorp).
RESULTS
During the study period, clinicians ordered 5756 Lyme disease serologic tests for 4723 unique patients; 229 patients were tested ≥2 times. Among the 4723 tested patients, 76 were positive by 2-tiered testing (1.6% of patients; 95% CI, 1.2%–2.0%). Among 70 patients with accessible medical records, 53 were positive by IgM Western blot criteria alone, and 17 patients were positive by IgG Western blot criteria (with or without also meeting IgM criteria).
Among the 17 evaluable subjects who were positive by IgG Western blot criteria (Table 1), 5 were judged to be true-positives by virtue of syndromes characteristic of Lyme disease. One had a peripheral facial nerve palsy, 2 had knee effusions, 1 had a knee effusion as well as arthritis of the temporomandibular joint, and 1 had polyarthritis that included the knee but also (uncharacteristically for Lyme disease) “sausage” digits (however, this patient responded to antibiotic therapy, and no alternative diagnosis was made). Two of the 5 true-positive patients had documented travel to known Lyme disease–endemic regions with potential tick exposure (Connecticut and Maryland). One patient was classified as false-positive based on an alternative medical diagnosis, cranial nerve palsy caused by carcinoma metastatic to the cavernous sinuses. In addition, 1 patient with positive results had isolated uveitis, and another had isolated trigeminal neuralgia. Neither of these conditions is known to be associated with Lyme disease in the absence of other more characteristic manifestations of the infection [22–25]. The remaining 9 patients had syndromes incompatible with Lyme disease and/or an alternative diagnosis (Table 1). Three seropositive patients had histories of Lyme disease, but lacked findings consistent with active infection at the time of the test.
Table 1.
Patients With Positive Lyme Disease Test Results by 2-Tier IgG Criteria
| Patient Sex | Clinical Presentationa | Alternative Diagnosis | Geographic Exposure |
|---|---|---|---|
| Male | Arthralgias | … | … |
| Male | Polyarthralgias | Celiac-associated joint pain | … |
| Male* | Facial nerve palsy | Connecticut | |
| Male* | Knee effusion | Maryland | |
| Male | Arthralgia, history of Lyme disease | Repetitive stress | … |
| Male* | Arthritis | … | |
| Male | Chronic pain | … | … |
| Female | Fever, urticaria, hand swelling | Allergic drug reaction | … |
| Female | Fever, headache, fatigue, negative CSF results | … | … |
| Female | Visual field loss | Retinal lesions | … |
| Male | High fever while in Southeast Asia | … | … |
| Male | Trigeminal neuralgia | … | … |
| Male* | Polyarthritis, sausage digit | … | |
| Male | Skin lesions | Eosinophilic lichenoid dermatitis | … |
| Male | Cranial nerve III palsy | Metastatic cancer to cavernous sinus | … |
| Male | Uveitis | … | … |
| Male* | Knee effusion and TMJ crepitus | … |
Abbreviations: CSF, cerebrospinal fluid; IgG, immunoglobulin G; TMJ, temporomandibular joint.
a Arthralgia was defined as joint pain or stiffness without documentation of joint effusion or inflammation; arthritis, as joint pain or stiffness with such documentation. Cases judged as “true positive” are marked with an asterisk (*).
Among the 53 evaluable subjects who were positive only by IgM criteria, 8 had syndromes compatible with active Lyme disease (15%; 95% CI, 5%–25%). These included 5 individuals with EM-like skin lesions, 1 with a CSF pleocytosis, 1 with facial nerve palsy, and 1 with a knee effusion (Supplementary Table 2). One individual had first-degree atrioventricular block but had presented with a high fever, elevated hepatic transaminases, absolute monocytopenia, and hyponatremia and had a clinical diagnosis of human monocytic ehrlichiosis. Among the remaining subjects who met IgM criteria, 18 had been symptomatic for ≥2 months without positive IgG results, 24 had clinically incompatible illnesses lacking objective findings of Lyme disease, and 3 patients were asymptomatic. Four of the 8 patients with true-positive IgM results had documentation of exposure in highly endemic states: Rhode Island, Connecticut, New York, and New Jersey; for 3 of them, the exposure was clearly recent.
Overall, 12 of 70 patients who met 2-tiered testing criteria had an illness compatible with active Lyme disease at the time of the test. Without considering travel history, the positive predictive value of a 2-tiered serologic testing was 17% (95% CI, 9%–28%). At least 6 of 12 patients with true-positive results had most likely acquired their disease during travel to endemic regions. Excluding patients with a history of travel to a Lyme disease endemic area, would leave 6 true-positive results in 59 cases, yielding a positive predictive value of 10% (95% CI, 2%–18%) in the nonendemic region studied.
We then identified patients who had been tested for Lyme disease in the setting of a clinical illness compatible with Lyme disease. Applying the search criteria described in the methods section yielded 2569 medical encounters for 1621 unique patients. Of these, 297 patients (18%) had a Lyme disease serologic test at the time of a clinically compatible illness; 110 patients had arthritis of a large joint, 98 had cranial nerve palsy, 75 had meningitis, 11 had both meningitis and cranial neuropathy, and 1 patient each had arthritis with cranial nerve palsy, atrioventricular block alone, or atrioventricular block with cranial nerve palsy (Table 2).
Table 2.
Patients With Select Lyme Disease–Compatible Presentations Identified Through Electronic Medical Record Queries
| Presentation | Patients (Female/Male), No. | Positive Test Resultsa | Age Mean (Range), y |
|---|---|---|---|
| Arthritis | 110 (59/51) | 3 | 31.1 (2–91) |
| Meningitis | 75 (44/31) | 1 | 43.8 (4–82) |
| CN | 98 (49/49) | 1 | 46.8 (7–84) |
| Meningitis plus CN | 11 (4/7) | 1 | 36.5 (8–71) |
| Otherb | 3 (1/2) | 0 | 48 (38–58) |
Abbreviation: CN, cranial neuropathy.
a Positive tests results were defined according to standard 2-tier interpretive criteria, including a reactive enzyme-linked immunosorbent assay followed by positive immunoglobulin M or G Western immunoblot.
b Atrioventricular block, atrioventricular block plus cranial nerve palsy, and arthritis plus cranial nerve palsy in 1 patient each.
Of these 297 patients, 6 tested positive for Lyme disease by 2-tiered serologic testing, 3 by IgG and 3 solely by IgM criteria. These 6 patients had also been identified in our search of all seropositive patients. Three of the 6 had effusions of large joints at the time of presentation; 1 had CSF pleocytosis, 1 had peripheral facial nerve palsy, and 1 had both facial nerve palsy and CSF pleocytosis. Thus, the prevalence of Lyme disease among patients was (at most) 6 of 297 (2%, 95% CI, .7%–4.3%). Three of these 6 seropositive individuals had documented recent travel to Lyme disease–endemic areas, where the infection was probably acquired (Maryland, Connecticut and Massachusetts). The patient with facial nerve palsy and CSF pleocytosis ultimately received a diagnosis of central nervous system vasculitis associated with anti-neutrophil cytoplasmic antibodies. Thus, if we exclude these 4 patients, only 2 of 297 cases (0.7%; 95% CI, .08%–2.4%) were likely to be cases of locally acquired Lyme disease.
DISCUSSION
In a region where Lyme disease is uncommon, even patients with highly characteristic clinical presentations are rarely found to have Lyme disease, and positive test results are seldom associated with clinically probable infection. Indeed, among 70 patients with positive tests during a 6-year period, only 13 had an illness compatible with Lyme disease. Only a small minority of seropositive patients with clinical presentations compatible with disseminated Lyme disease were likely to have acquired the infection disease locally. Our findings raise the question of whether positive Lyme disease test results have diagnostic value in low-prevalence regions, such as North Carolina. With a high background noise of false-positive test results, coupled with a low signal of true-positive cases, it may be impossible to trust a positive result.
Serologic testing for Lyme disease is most useful for patients who have an intermediate pretest probability of infection [2]. Patients in endemic areas with characteristic EM-like skin lesion skin findings do not require testing, because they are highly likely to have Lyme disease, and there is significant likelihood of a false-negative test [26]. At the other end of the spectrum, patients with a low pretest probability of Lyme disease are more likely to have a false-positive or nonexplanatory positive test result. These include individuals with no objective manifestations of Lyme disease, including those who have only nonspecific symptoms (eg, fatigue) and those who probably have an alternative diagnosis [27]. They also include patients who live in nonendemic areas and have not traveled to endemic areas, even if their symptoms are compatible with Lyme disease.
Previous studies have shown that patients with objective clinical findings consistent with disseminated Lyme disease have an intermediate likelihood of Lyme disease that will maximize the yield of diagnostic testing. For instance Lyme disease accounts for 22%–34% of facial nerve palsy cases [12, 15], 13%–28% of meningitis cases in children [7, 8, 11, 14], and 31%–67% of monoarthritis cases in children [9, 10, 13, 16]. We must emphasize, however, that these studies were all conducted in regions of the Northeast with exceptionally heavy transmission of Lyme disease. The patients in these studies had both intermediate clinical and epidemiologic risk of B. burgdorferi infection.
We must remember that the coexistence of a positive serologic test and a consistent clinical illness does not absolutely prove that a patient has Lyme disease. Lyme IgM Western blots, in particular, produce many false-positive results [28]. With roughly 3 million Lyme disease tests ordered annually, even a specificity of 99% would yield tens of thousands of false-positive results. A background prevalence of false-positive results can coincidentally overlap with a background prevalence of Lyme disease mimics, resulting in misdiagnosis of Lyme disease in patients with other diagnoses. Arthritis has been diagnosed in >20% of American adults, for example [29]. At the same time, seroreactivity to B. burgdorferi occurs out of proportion to the incidence of clinically apparent Lyme disease, and asymptomatic infection is well documented [30–32]. Awareness of epidemiologic context and the absence of an alternative diagnosis are necessary for a clinician to decide whether a positive test is explanatory or coincidental [33]. On the other hand, the negative predictive value of Lyme disease testing will be very high in a region with low prevalence, and in a region where Lyme disease is emerging, a negative results may provide patients with some reassurance.
Our study has several limitations. First, it was retrospective, and we were limited to the data recorded in the medical record. Travel histories in particular were recorded briefly and seldom described the intensity of exposure to tick habitat during travel; moreover, the absence of travel was almost never specifically documented. Judging the compatibility of each patient's syndrome with Lyme disease depended on a retrospective review of chart documentation, rather than a prospectively defined case definition, and thus individual cases may have been misclassified because of inadequate documentation. To improve the specificity of our study, we selected common manifestations of disseminated Lyme disease that were easily identified by diagnostic and procedural coding, and elected not to perform queries for rarer manifestations, such as carditis. Our aim was not to capture all patients with possible Lyme disease but rather to identify patients with the maximum clinical pretest probability.
We also cannot be sure that our patient cohort is generalizable to all patients in North Carolina, because we conducted our study in a health system that includes a large academic tertiary care center. Physicians in this system may order a large palette of diagnostic tests for patients with rare diseases and unusual presentations. Furthermore, a significant number of physicians received some of their education or training in regions with a higher incidence of Lyme disease. Both factors may inflate the number of low-likelihood Lyme disease tests compared with other types of clinical settings. Importantly, the health system serves rural, suburban, and urban communities including patients presenting to a wide variety of specialty and primary care practices. Finally, our study had a small sample size and was conducted at a single center, making its generalizability uncertain.
The lack of a reliable reference standard test creates a significant challenge in clinical Lyme disease research. True-negative tests are impossible to verify, and true-positives can usually be defined only by the presence of a compatible illness. Although certain tests, such as appropriately performed culture and polymerase chain reaction, may provide more direct evidence of infection, the combination of cost, invasiveness, and lack of sensitivity exclude them from typical clinical practice. In this study, however, for all patients with positive results of 2-tiered Lyme disease serology, we believe that chart documentation sufficed for us to discriminate likely from unlikely Lyme disease.
In summary, we have described a patient cohort in which the positive predictive value of Lyme disease serologic tests is extremely low. In our study population, Lyme disease testing had an 80% rate of false-positives, which puts patients with a positive test result at risk of incorrect Lyme disease diagnoses and adverse drug reactions from inappropriate treatment. In low-transmission settings, a positive Lyme disease test result may be incapable of ruling in Lyme disease with statistical confidence, even when a compatible clinical syndrome is present.
Our findings have important implications for clinicians and public health workers in North Carolina and epidemiologically similar regions. First, clinicians must critically consider a patient's risk factors, especially recent exposure to Ixodes tick habitats in regions with known Lyme disease transmission, when deciding whether to obtain (and how to interpret) serologic testing for Lyme disease. Second, physicians in nonendemic areas must carefully consider whether a positive Lyme disease test result is authentic, being careful not to miss alternative diagnoses and to counsel the patient accordingly. Finally, Lyme disease surveillance relies on the results of 2-tiered testing, including automated reporting based solely on laboratory results. This is likely to produce a high proportion of false-positives in low-transmission areas, creating further uncertainty as to the burden and distribution of this disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Center for Advancing Translational Sciences of the NIH (grant KL2 TR001115 to P. M. L.), the Ken and Sherrilyn Fisher Center for Environmental Infectious Diseases (P. G. A.), the National Institute for Allergy and Infectious Diseases, NIH (grant K24 AI093969 to F. R. and V. F.), and Boston Children's Hospital (pilot research grant to L. E. N.).
Potential conflicts of interest. J. A. B. has received research grants from DiaSorin, Alere, bioMérieux, Becton Dickinson, and Immunetics. V. F. served as chair of the V710 Scientific Advisory Committee (Merck), has received grant support or has grants pending from Cerexa, Pfizer, Advanced Liquid Logic, MedImmune, and Cubist; has been a paid consultant for Merck, Astellas, Affinium, Theravance, Cubist, Cerexa, Debiopharm, Durata, Pfizer, NovaDigm, Novartis, Medicines Company, Biosynexus, MedImmune, and Inimex, Bayer; and has received honoraria from Merck, Astellas, Cubist, Pfizer, Theravance, and Novartis. P. G. A. has served as an expert witness in malpractice cases related to Lyme disease. All other authors report no conflicts
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hinckley AF, Connally NP, Meek JI et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 2014; 59:676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tugwell P, Dennis DT, Weinstein A et al. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med 1997; 127:1109–23. [DOI] [PubMed] [Google Scholar]
- 3.Pepin KM, Eisen RJ, Mead PS et al. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am J Trop Med Hyg 2012; 86:1062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diuk-Wasser MA, Hoen AG, Cislo P et al. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg 2012; 86:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Reported cases of Lyme disease by state or locality, 2004–2013. Available at: http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html Accessed 12 January 2015.
- 6.Maggi RG, Reichelt S, Toliver M, Engber B. Borrelia species in Ixodes affinis and Ixodes scapularis ticks collected from the coastal plain of North Carolina. Ticks Tick Borne Dis 2010; 1:168–71. [DOI] [PubMed] [Google Scholar]
- 7.Avery RA, Frank G, Glutting JJ, Eppes SC. Prediction of Lyme meningitis in children from a Lyme disease-endemic region: a logistic-regression model using history, physical, and laboratory findings. Pediatrics 2006; 117:e1–7. [DOI] [PubMed] [Google Scholar]
- 8.Cohn KA, Thompson AD, Shah SS et al. Validation of a clinical prediction rule to distinguish Lyme meningitis from aseptic meningitis. Pediatrics 2012; 129:e46–53. [DOI] [PubMed] [Google Scholar]
- 9.Deanehan JK, Kimia AA, Tan Tanny SP et al. Distinguishing Lyme from septic knee monoarthritis in Lyme disease-endemic areas. Pediatrics 2013; 131:e695–701. [DOI] [PubMed] [Google Scholar]
- 10.Deanehan JK, Nigrovic PA, Milewski MD et al. Synovial fluid findings in children with knee monoarthritis in lyme disease endemic areas. Pediatr Emerg Care 2014; 30:16–9. [DOI] [PubMed] [Google Scholar]
- 11.Garro AC, Rutman MS, Simonsen K, Jaeger JL, Chapin K, Lockhart G. Prevalence of Lyme meningitis in children with aseptic meningitis in a Lyme disease-endemic region. Pediatr Infect Dis J 2011; 30:990–2. [DOI] [PubMed] [Google Scholar]
- 12.Halperin JJ, Golightly M. Lyme borreliosis in Bell's palsy. Long Island Neuroborreliosis Collaborative Study Group. Neurology 1992; 42:1268–70. [DOI] [PubMed] [Google Scholar]
- 13.Milewski MD, Cruz AI Jr, Miller CP, Peterson AT, Smith BG. Lyme arthritis in children presenting with joint effusions. J Bone Joint Surg Am 2011; 93:252–60. [DOI] [PubMed] [Google Scholar]
- 14.Nigrovic LE, Cohn KA, Lyons TW et al. Enteroviral testing and length of hospital stay for children evaluated for lyme meningitis. J Emerg Med 2013; 44:1196–200. [DOI] [PubMed] [Google Scholar]
- 15.Nigrovic LE, Thompson AD, Fine AM, Kimia A. Clinical predictors of Lyme disease among children with a peripheral facial palsy at an emergency department in a Lyme disease-endemic area. Pediatrics 2008; 122:e1080–5. [DOI] [PubMed] [Google Scholar]
- 16.Thompson A, Mannix R, Bachur R. Acute pediatric monoarticular arthritis: distinguishing lyme arthritis from other etiologies. Pediatrics 2009; 123:959–65. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127:1106–8. [DOI] [PubMed] [Google Scholar]
- 18.Wormser GP, Dattwyler RJ, Shapiro ED et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–134. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Lyme disease (Borrelia burgdorferi): 2011 case definition. Available at: http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=752&DatePub=1/1/2011%2012:00:00%20AM Accessed 12 January 2015.
- 20.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44:590–1. [PubMed] [Google Scholar]
- 21.Kruschke JK. Doing Bayesian data analysis: a tutorial with R and BUGS. Burlington, MA: Academic Press, 2011. [Google Scholar]
- 22.Mikkila H, Seppala I, Leirisalo-Repo M, Karma A. The significance of serum anti-Borrelia antibodies in the diagnostic work-up of uveitis. Eur J Ophthalmol 1997; 7:251–5. [DOI] [PubMed] [Google Scholar]
- 23.Sibony P, Halperin J, Coyle PK, Patel K. Reactive Lyme serology in optic neuritis. J Neuroophthalmol 2005; 25:71–82. [DOI] [PubMed] [Google Scholar]
- 24.Fritz C, Rosler A, Heyden B, Braune HJ. Trigeminal neuralgia as a clinical manifestation of Lyme neuroborreliosis. J Neurol 1996; 243:367–8. [DOI] [PubMed] [Google Scholar]
- 25.Murphy MA, Szabados EM, Mitty JA. Lyme disease associated with postganglionic Horner syndrome and Raeder paratrigeminal neuralgia. J Neuroophthalmol 2007; 27:123–4. [DOI] [PubMed] [Google Scholar]
- 26.Lantos PM, Brinkerhoff RJ, Wormser GP, Clemen R. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis 2013; 13:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakos A, Reiczigel J, Solymosi N. The positive predictive value of Borrelia burgdorferi serology in the light of symptoms of patients sent to an outpatient service for tick-borne diseases. Inflamm Res 2010; 59:959–64. [DOI] [PubMed] [Google Scholar]
- 28.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18:1236–40. [DOI] [PubMed] [Google Scholar]
- 29.Helmick CG, Felson DT, Lawrence RC et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008; 58:15–25. [DOI] [PubMed] [Google Scholar]
- 30.Lakos A, Igari Z, Solymosi N. Recent lesson from a clinical and seroepidemiological survey: low positive predictive value of Borrelia burgdorferi antibody testing in a high risk population. Adv Med Sci 2012; 57:356–63. [DOI] [PubMed] [Google Scholar]
- 31.Fahrer H, van der Linden SM, Sauvain MJ, Gern L, Zhioua E, Aeschlimann A. The prevalence and incidence of clinical and asymptomatic Lyme borreliosis in a population at risk. J Infect Dis 1991; 163:305–10. [DOI] [PubMed] [Google Scholar]
- 32.Steere AC, Sikand VK, Schoen RT, Nowakowski J. Asymptomatic infection with Borrelia burgdorferi. Clin Infect Dis 2003; 37:528–32. [DOI] [PubMed] [Google Scholar]
- 33.Fine AM, Brownstein JS, Nigrovic LE et al. Integrating spatial epidemiology into a decision model for evaluation of facial palsy in children. Arch Pediatr Adolesc Med 2011; 165:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.