This national Department of Veterans Affairs study of hospitalizations for pneumonia found a dramatic increase in broad-spectrum antibiotic use from 2006 to 2010, without an increase in nosocomial pathogens or improvement in the match between coverage and pathogen.
Keywords: pneumonia, antibiotic use, HCAP, nosocomial pathogens, drug-resistant pneumonia
Abstract
Background. In 2005, pneumonia practice guidelines recommended broad-spectrum antibiotics for patients with risk factors for nosocomial pathogens. The impact of these recommendations on the ability of providers to match treatment with nosocomial pathogens is unknown.
Methods. Among hospitalizations with a principal diagnosis of pneumonia at 128 Department of Veterans Affairs medical centers from 2006 through 2010, we measured annual trends in antibiotic selection; initial blood or respiratory cultures positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and Acinetobacter species; and alignment between antibiotic coverage and culture results for MRSA and P. aeruginosa, calculating sensitivity, specificity, and diagnostic odds ratio using a 2 × 2 contingency table.
Results. In 95 511 hospitalizations for pneumonia, initial use of vancomycin increased from 16% in 2006 to 31% in 2010, and piperacillin-tazobactam increased from 16% to 27%, and there was a decrease in both ceftriaxone (from 39% to 33%) and azithromycin (change from 39% to 36%) (P < .001 for all). The proportion of hospitalizations with cultures positive for MRSA decreased (from 2.5% to 2.0%; P < .001); no change was seen for P. aeruginosa (1.9% to 2.0%; P = .14) or Acinetobacter spp. (0.2% to 0.2%; P = .17). For both MRSA and P. aeruginosa, sensitivity increased (from 46% to 65% and 54% to 63%, respectively; P < .001) and specificity decreased (from 85% to 69% and 76% to 68%; P < .001), with no significant changes in diagnostic odds ratio (decreases from 4.6 to 4.1 [P = .57] and 3.7 to 3.2 [P = .95], respectively).
Conclusions. Between 2006 and 2010, we found a substantial increase in the use of broad-spectrum antibiotics for pneumonia despite no increase in nosocomial pathogens. The ability of providers to accurately match antibiotic coverage to nosocomial pathogens remains low.
(See the Editorial Commentary by Mortensen on pages 1411–2.)
Pneumonia is the leading infectious cause of death in the United States, with 1.1 million hospitalizations annually [1, 2]. Optimal treatment involves selecting an empiric antibiotic regimen (before obtaining results of microbiologic tests) that targets pathogens while avoiding drugs that extend the spectrum of coverage to organisms not causing infection. In 2005, the Infectious Disease Society of America and the American Thoracic Society introduced the classification of healthcare-associated pneumonia (HCAP), recommending empiric coverage of methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa in patients meeting criteria for recent prior healthcare exposure [3]. The 2007 update of the community-acquired pneumonia guidelines further emphasized that patients with risk factors for HCAP should be treated with broad-spectrum antibiotics [4]. Efforts to improve pneumonia outcomes have reinforced this recommendation through dissemination of guidelines, performance measures by Medicare, and public health initiatives [5, 6].
Since the introduction of the HCAP classification, concerns have been raised that the risk factors for HCAP are too broad, driving excessive antibiotic use rather than improving providers' ability to identify patients truly at risk for nosocomial pathogens [7]. It is unknown how these recommendations have impacted providers' ability to match empiric broad-spectrum antibiotic treatment to patients at risk for nosocomial pathogens.
The US Department of Veterans Affairs (VA) health system uses a universal electronic health record, with standardized medication and culture data. This allows us to examine trends in initial antibiotic selection and microbiology at the patient level across a large health system. Associations between MRSA cultures and anti-MRSA treatment in infection-related hospitalizations were measured previously [8, 9]. Using the same data set in the current study, we aimed to evaluate annual trends in initial antibiotic selection and nosocomial pathogen identification across the entire VA medical system and to examine trends in the match between initial antibiotics and culture results. We tested the hypothesis that the match between broad-spectrum antibiotic use and culture results would increase in the years after the guidelines were disseminated.
METHODS
Setting
This study was performed with data collected from VA medical centers from 1 January 2006 through 31 December 2010 [10]. We included all facilities with ≥10 operational acute care beds and complete electronic medication administration records. Data were accessed and analyzed using the VA Informatics and Computing Infrastructure [11].
Subject Selection
During the study period, we selected all hospitalizations of patients ≥18 years of age in acute medical, surgical, or neurological wards and intensive care units (ICUs). We then identified hospitalizations with a principal International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) [12] code consistent with pneumonia (codes 481–486), similar to other studies [13, 14], excluding those with ICD-9-CM codes for viral pneumonia and influenza (480.0–480.9 and 487.0–488.19). To evaluate whether diagnostic coding for pneumonia was consistent during the study period relative to other diseases, we also identified all hospitalizations with principal ICD-9-CM codes consistent with sepsis (038.x, 995.91, 995.92, and 785.52), skin and soft-tissue infections (680–686), and genitourinary infections (590, 595.0, 597, 598.0, 599.0, 614.0, 614.1–5, 616.0, 616.1, 616.3, and 616.4).
Measurements: Antibiotic Use
We measured antibiotic use using bar code medication administration, which records all medications administered to hospitalized patients [15]. Because we were interested in the use of antibiotics before culture results were available, initial antibiotic therapy was defined as the systemic administration of at least 1 dose of an antibiotic within the first 2 calendar days of hospitalization. We calculated the proportion of hospitalized patients receiving antibiotics for pneumonia. We combined respiratory fluoroquinolones (moxifloxacin, levofloxacin, and gatifloxacin), macrolides (azithromycin, clarithromycin, and erithromycin), and tetracyclines (doxycycline and tetracycline) into single categories.
Because multiple antibiotics may be administered concurrently and we wanted to detect breadth of coverage for pneumonia pathogens, we then classified antibiotics with activity against the following pathogen types: atypical organisms (respiratory fluoroquinolone, macrolide, or tetracycline), standard organisms (guideline-concordant coverage with either an antipneumococcal or nonantipseudomonal β-lactam [listed in the “Results” section] plus a macrolide or a respiratory fluoroquinolone), MRSA (vancomycin or linezolid), and single coverage for P. aeruginosa (piperacillin-tazobactam, ticarcillin-clavulanate, ceftazidime, cefepime, meropenem, doripenem, imipenem, or aztreonam). Because the guidelines from the Infectious Disease Society of America and the American Thoracic Society call for combination therapy to ensure coverage of P. aeruginosa in selected patients, an additional category was created requiring the combination of single coverage with an antipseudomonal fluoroquinolone (ciprofloxacin or levofloxacin) or aminoglycoside, consistent with guideline recommendations [4].
Measurements: Microbiologic Data
We accessed microbiologic data on all cultures drawn at any time during each hospitalization, which were standardized into Systemized Nomenclature of Medicine (SNOMED-CT) format [16]. Organism data were included if susceptibility testing was performed. Initial positive cultures were identified for 3 common nosocomial pathogens associated with pneumonia—MRSA, P. aeruginosa, and Acinetobacter spp. We classified culture source into blood, respiratory (sputum, endotracheal aspirate, bronchiolar lavage, wash, or biopsy, or pleural fluid), or “other,” which included wound and urine cultures. Because we were interested in identifying pathogens that were present at hospital admission rather than acquired during a hospitalization, we defined a positive initial pneumonia-related culture as any positive result that had been obtained during the first 2 calendar days of the hospitalization, from either blood or respiratory sources.
Statistical Analysis
To assess providers' ability to accurately differentiate patients at risk for nosocomial pathogens, we examined trends in the alignment between the selection of initial antibiotics and recovery of organisms from microbiologic cultures. Our assessment is thus similar to the evaluation of accuracy of a diagnostic test (Table 1). A “true-positive” was defined as the match between initial treatment and positive growth of the targeted organism in culture. A “true-negative” was defined as the absence of coverage for a pathogen and lack of recovery of the corresponding organism. For example, a MRSA true-positive hospitalization was one during which initial anti-MRSA therapy was administered and a pneumonia-related culture was positive for MRSA, and a MRSA true-negative hospitalization one during which no anti-MRSA therapy was administered and no MRSA-positive cultures were identified. Sensitivity was defined as the proportion of admitted patients with MRSA-positive cultures who received anti-MRSA therapy, and specificity the proportion without positive MRSA cultures who did not receive such therapy.
Table 1.
MRSA Culture-Coverage Matching Analysis Characterized in a 2 × 2 Tablea
| Culture MRSA Positive | MRSA Coverage Given |
|
|---|---|---|
| No | Yes | |
| Yes | FN decision | TP decision |
| No | TN decision | FP decision |
Abbreviations: FN, false-negative; FP, false-positive; MRSA, methicillin-resistant Staphylococcus aureus; TN, true-negative; TP, true-positive.
a Measures of performance were defined as follows:
Sensitivity (Se) = TP/(TP + FN); Specificity (Sp) = TN/(TN + FP); Positive likelihood ratio (PLR) = Se/(1 − Sp); Negative likelihood ratio (NLR) = 1 − Se/(Sp); and Diagnostic odds ratio = (TP · TN)/(FN · FP), or PLR/NLR.
The diagnostic odds ratio (DOR) was calculated as a composite measure of performance to reflect both sensitivity and specificity [17]. The DOR equals the positive likelihood ratio divided by the negative likelihood ratio, or (Sensitivity · Specificity)/([1 − Sensitivity] · [1 − Specificity]) (Table 1). The greater the DOR, the greater the overall sensitivity and specificity, and overall matching between culture and coverage, that occurred. For example, sensitivity and specificity of 0.50 would result in a DOR of 1; sensitivity and specificity of 0.75, a DOR of 9. To measure trends in the coverage-culture concordance for MRSA or P. aeruginosa, we calculated annual sensitivity, specificity, and the DOR for each year, and the difference in the proportion of true-positive cases and false-positive cases from 2006 to 2010.
We analyzed temporal trends in antibiotic use, nosocomial pathogens, and alignment between coverage and culture results using logistic generalized estimating equation models, which took facility-level correlations into consideration. Calendar year was added as the single independent variable. All statistical analyses were performed using Stata (version 12.0; StataCorp) and R (http://cran.r-project.org) software. The study was conducted with approval from the University of Utah Institutional Review Board and the Salt Lake City VA Human Research Protection Program.
RESULTS
Temporal Trends: Diagnosis and Demographics
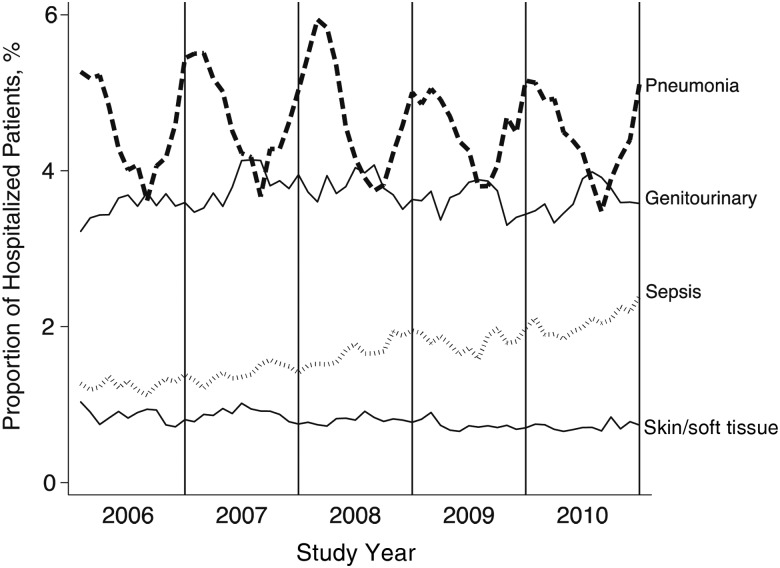
Of a total of 2.4 million hospitalizations at 128 facilities meeting our study criteria, 95 511 (3.9%) had a principal diagnosis of pneumonia during the study period. The proportion of hospitalizations for pneumonia remained stable over time, in contrast to the increase seen in sepsis diagnoses (Figure 1). The median age of patients hospitalized for pneumonia was 71 years (mean, 70 years; interquartile range, 61–81 years), and their median length of stay was 4 days (mean, 6 days; interquartile range, 3–7 days). Of these patients, 12% were initially admitted to the ICU.
Figure 1.
Proportion of hospitalized veterans with principal infectious diagnoses, 2006–2010 (total number hospitalized, 2.4 million).
Antibiotic Use
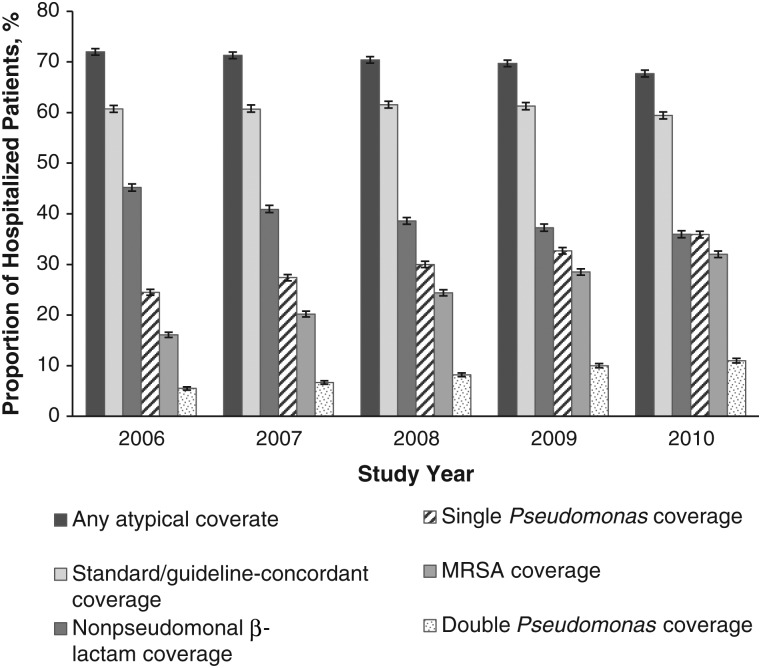
Antibiotic use for veterans hospitalized with pneumonia shifted significantly during the 5-year period (Figure 2). Initial use of vancomycin increased from 16% to 31% (P < .001), use of piperacillin-tazobactam increased from 16% to 27% (P < .001), and use of ceftriaxone and azithromycin decreased (P < .001). Use of macrolides (azithromycin in 99.5%) decreased from 39.5% to 36.0% (P < .001). Overall, antipseudomonal coverage and MRSA therapy increased, and a significant decline in initial therapy with standard and atypical coverage was observed (Figure 3; P < .001 for all). The proportion of hospitalizations with initial double antipseudomonal coverage, though low, also increased significantly during the study period (from 5% to 11%; P < .001). Overall 8.1% of hospitalized patients did not receive any guideline-concordant antibiotics. The most common antibiotics used in these hospitalizations were ciprofloxacin (single therapy), clindamycin, and trimethoprim-sulfamethoxazole. We found a small, nonsignificant increase in hospitalizations without guideline-concordant therapy during the study period (from 7.7% in 2006 to 8.3% in 2010; P = .055).
Figure 2.
Five-year trends in initial antibiotic use for hospitalized patients with pneumonia, 2006–2010. Error bars represent 95% confidence intervals.
Figure 3.
Trends in antimicrobial coverage for hospitalized patients with pneumonia in 2006–2010. Error bands represent 95% confidence intervals, For coverage, any atypical coverage includes respiratory fluoroquinolones, macrolide, and tetracycline; standard or guideline-concordant coverage includes nonpseudomonal β-lactam plus a macrolide and respiratory fluoroquinolone; nonpseudomonal β-lactam coverage includes ampicillin, amoxicillin, ampicillin-sulbactam, amoxicillin-clavulanate, cefuroxime, cefotaxime, ceftriaxone, ceftizoxime, cefixime, cefpodoxime, ceftibuten, cefdinir, and ertapenem; single Pseudomonas coverage includes piperacillin-tazobactam, ticarcillin-clavulanate, ceftazidime, cefepime, meropenem, doripenem, imipenem, aztreonam, and aminoglycoside; methicillin-resistant Staphylococcus aureus (MRSA) coverage includes vancomycin and linezolid; and double Pseudomonas coverage includes any 2 of the above or 1 of the above plus ciprofloxacin or levofloxacin.
Microbiology and Culture-Coverage Concordance
Overall, 84.5% of pneumonia hospitalizations had documentation of ≥1 culture obtained from a blood or respiratory specimen within 2 days of admission. Respiratory cultures were less frequently collected (34.0% of hospitalization) than blood cultures (81.7% of hospitalizations). We found a small increase in blood culture collection over time (79.7% in 2006 to 82.1% in 2010, P < .001) and no significant change in proportion of hospitalizations with respiratory cultures obtained.
The overall proportions of hospitalizations with positive initial cultures were 2.2% for MRSA, 2.0% for P. aeruginosa, and 0.2% for Acinetobacter spp. A significant decline in MRSA was observed, whereas P. aeruginosa and Acinetobacter spp. remained stable (Figure 4). Of the positive cultures, 88% positive for MRSA, 96% for P. aeruginosa, and 76% for Acinetobacter spp. were from a respiratory source.
Figure 4.
Proportion of hospitalized patients with pneumonia with cultures positive for Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), or Acinetobacter spp. within 2 days of hospitalization.
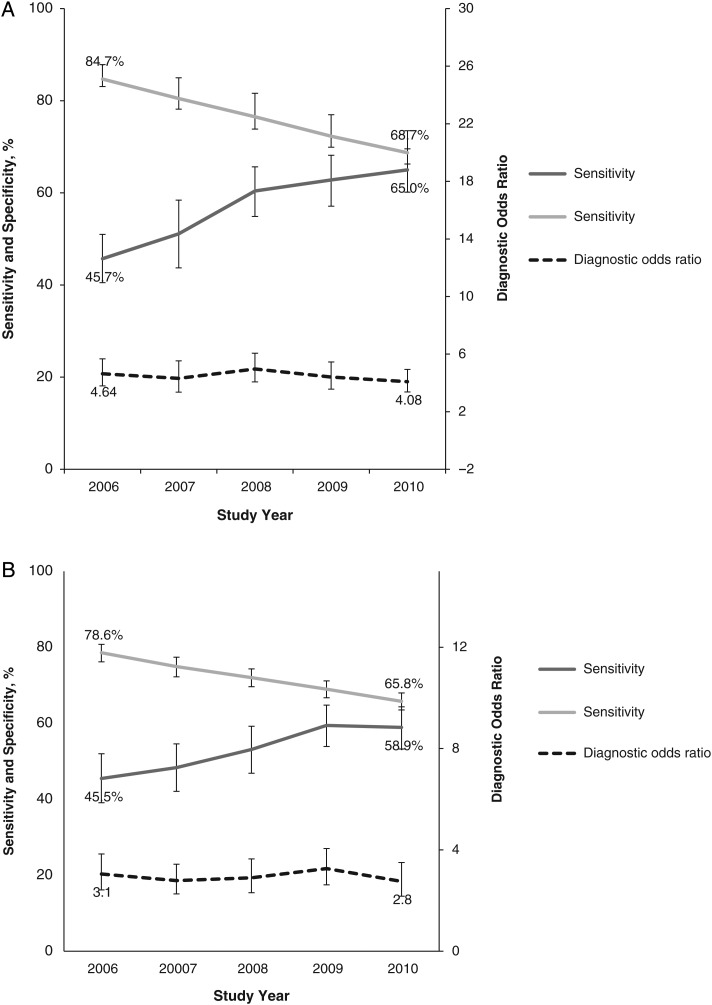
Figure 5 displays annual trends in sensitivity, specificity, and DORs for the alignment between antibiotic coverage and culture results for MRSA and P. aeruginosa. For both pathogens, sensitivity increased and specificity decreased substantially. The resulting annual DORs for both pathogens demonstrated no significant change from 2006 to 2010 (MRSA, from 4.6 to 4.1 [P = .57]; P. aeruginosa, 3.1 to 2.8 [P = .95]). The proportion of true-positive cases increased slightly for both pathogens (from 1.1% to 1.3% for MRSA and from 0.9% to 1.2 for P. aeruginosa), but the proportion of false-positive cases increased significantly (from 15.0% to 30.6% for MRSA and from 21.0% to 33.6% for P. aeruginosa; P < .001 for both).
Figure 5.
Trends in match between coverage and culture for methicillin-resistant Staphylococcus aureus (MRSA) (A) and Pseudomonas aeruginosa (B). Error bands represent 95% confidence intervals. Sensitivity was defined as the proportion of patients with MRSA- or Pseudomonas-positive cultures who received anti-MRSA or antipseudomonal therapy; specificity, the proportion without positive cultures who did not receive such therapy. The diagnostic odds ratio is a composite measure of performance reflecting both sensitivity and specificity (see “Statistical Analysis” section and Table 1).
DISCUSSION
The selection of empiric antibiotics for pneumonia is challenging, with consequences to both individual patients and the public health that are difficult to weigh. Because current microbiologic tests are imperfect, whether or not a patient is given broad-spectrum antibiotics depends on a provider's estimation of the probability of resistant pathogens as well as his/her threshold for treatment. When a causative pathogen is uncertain, as is the case for most patients with pneumonia, providers must perform the difficult task of weighing the risks and benefits of overtreatment versus undertreatment.
Our 5-year study of over 95 000 hospitalizations for pneumonia across the VA system is, to our knowledge, the first to examine trends in antibiotic use and culture results together on a large scale. We found a substantial shift toward broad-spectrum agents. During the same time period, we found no increase in initial cultures positive for the 3 most common nosocomial pathogens.
The shift in antibiotic use to broad-spectrum agents for pneumonia is not unique to the VA system. Berger et al [18] reviewed nationwide antibiotic practices in the United States and found similar trends for hospitalized non-ICU patients with pneumonia . The decrease seen in MRSA pneumonia has also been suggested in other studies during the same time period [19–22], in contrast to the period of 2000–2005, when there was a rise in diagnostic coding for MRSA [23]. The prevalence of P. aeruginosa among hospitalized patients with pneumonia has also been reported to be stable [20, 22].
By combining antibiotic administration and culture data, we examined whether the observed trends in antibiotic use observed resulted in a change in clinicians' ability to match broad-spectrum antibiotic coverage to cultures for nosocomial pathogens. The increase in broad-spectrum antibiotics was comparable in patients with and without positive cultures, and the match between treatment and pathogen remained unchanged.
These results suggest that the shift toward broad-spectrum antibiotics reflects a change in the threshold for treatment rather than a response to increased prevalence or enhanced ability to identify patients at risk for resistant organisms. Increased recognition of HCAP risk factors, driven largely by dissemination of guidelines, probably modified clinicians' perception of risk. This change was not accompanied by an improved ability to determine which patients actually have MRSA or P. aeruginosa infection. Although the increase in broad-spectrum antibiotics did result in an increase in coverage of more patients with nosocomial pathogens, this came at the cost of treating many patients unnecessarily with broad-spectrum antibiotics, and more than a third of patients with positive cultures still failed to receive appropriate initial coverage.
Our study was not designed to determine whether the shift in antibiotic use had a positive or negative impact on clinical outcomes. Because patients meeting HCAP criteria tend to have higher mortality risk and risk of nosocomial pathogens, it was thought that the observed increase in mortality rates may be due to inadequate therapy [24]. However, since the adoption of the HCAP definition, studies examining clinical outcomes have found either no improvement or even an increase in mortality rates associated with the use of broad-spectrum antibiotics in this group [25–29]. A single study suggested a possible benefit to broader-spectrum therapy when applying a more refined risk assessment [30]. In our study, as the proportion of patients receiving broad-spectrum antibiotics increased, the proportion receiving standard therapies, including coverage for atypical pneumonia pathogens, decreased. This raises concern that excessive use of broad-spectrum antibiotics could promote underuse of more standard therapies and failure to cover more common pneumonia pathogens.
Currently, clinicians have few ways to improve their ability to match antibiotics with likely pathogens for pneumonia. Refining and validating the clinical risk factors for nosocomial pathogens has been a major recent focus of research [31, 32], although accuracy in predicting resistant pathogens remains low [32–35]. The development of new accurate rapid diagnostic tests as well as more consistent use of tests currently available may increase our yield of pathogens and include initial respiratory cultures (documented in only 32% of our patients, although the majority of positive cultures were identified from a respiratory source) and MRSA surveillance (the VA's system-wide program has demonstrated high negative predictive value compared with cultures for patients with pneumonia) [9] In lieu of rapid identification and reliable clinical risk assessment, comparative effectiveness research evaluating the consequences of conservative versus aggressive antibiotic coverage strategies (ie, withholding broad-spectrum therapy until cultures result vs tailoring antibiotics after cultures) will also improve our ability to base our empiric antibiotic selection on actual risks.
We recognize several limitations to our study. We used administrative data that relied on principal diagnostic codes to identify cases of pneumonia, and we did not identify patient-level clinical factors. Our results therefore may have been affected by unmeasured changes in clinical population or diagnostic coding practices. Defining the study population using principal diagnoses for pneumonia plus sepsis and respiratory failure with pneumonia as secondary may have provided more cases of severe pneumonia in the later years and identified more hospitalizations with broad-spectrum antibiotic use and positive cultures. However, the profound rate of increase in both piperacillin-tazobactam and vancomycin use suggests that providers have decreased their threshold for usage in similar patients. We did not report trends in more typical pneumonia pathogens or less common resistant pathogens, such as those carrying extended-spectrum β-lactamases, for which broad-spectrum antibiotics are indicated. At the individual level, it is impossible to determine the appropriateness of broad-spectrum antibiotic use for any given patient from our study. Rather, we aimed to develop a population metric that is informative about thresholds for decision making.
Clinicians continue to be challenged by the dilemma of whether to use broad-spectrum antibiotics in the treatment of pneumonia. Unnecessary use of broad-spectrum antibiotics carries substantial risk, both to individual patients and to public health. So does undertreatment of resistant pathogens. Given the raised awareness of antimicrobial resistance prompted by practice guidelines, it is not surprising that providers at the VA, as in other systems, have lowered their threshold for using broad-spectrum antibiotics since the guidelines were disseminated. Our study suggests, however, that even with a higher-caliber shotgun, we are still missing the mark. Our study supports an urgent need for research on the impact of this change in threshold on outcomes and better strategies to identify patients who would truly benefit from broad-spectrum antibiotics. Without better evidence that helps providers weigh the risks and benefits of broad-spectrum antibiotic use for their patients with pneumonia, we are likely to see continued excessive use of this important resource.
CONCLUSIONS
We found a substantial increase in the use of both vancomycin and piperacillin-tazobactam from 2006 to 2010 in a national population of 95 511 hospitalized veterans with pneumonia, with no increase in initial positive cultures for MRSA, P. aeruginosa, or Acinetobacter spp. Although providers' thresholds for broad-spectrum antibiotic use have decreased, the ability to accurately match antibiotic coverage to culture results for MRSA and P. aeruginosa has not improved.
Notes
Acknowledgments. We thank Pat Nechodom, PhD, and Saundra Duffy-Hawkins for administrative support and Kevin Nechodom, PhD, for guidance with data management.
Author contributions. B. E. Jones had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Other contributions included data collection/management: M. J., B. H., and M. R.; study design: G. S., T. G., K. M. K., and M. S.; analysis: G. S., K. A. B., and V. W.; and manuscript preparation: M. J., B. H., G. S., T. G., B. S., K. M. K., M. R., M. B. G., and M. S.
Disclaimers. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Financial support. This work was supported by the National Institutes of Health (training grant to B. E. J.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Pneumonia. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm Accessed 15 September 2014. [Google Scholar]
- 2.US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013; 310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society/Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416. [DOI] [PubMed] [Google Scholar]
- 4.Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society, Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Facts about ORYX for hospitals (national hospital quality measures). The Joint Commission. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/HospitalOverviewOfSpecs200512.pdf. Accessed 11 August 2015.
- 6.Centers for Medicare & Medicaid Services. Premier Hospital Quality Incentive Demonstration. Available at: https://www.cms.gov/hospitalqualityinits/35_hospitalpremier.asp Accessed 15 September 2015.
- 7.Wunderink RG. Community-acquired pneumonia versus healthcare-associated pneumonia. The returning pendulum. Am J Respir Crit Care Med 2013; 188:896–8. [DOI] [PubMed] [Google Scholar]
- 8.Huttner B, Jones M, Huttner A, Rubin M, Samore MH. Antibiotic prescription practices for pneumonia, skin and soft tissue infections and urinary tract infections throughout the US Veterans Affairs system. J Antimicrob Chemother 2013; 68: 2393–9. [DOI] [PubMed] [Google Scholar]
- 9.Jones M, Huttner B, Leecaster M et al. Does universal active MRSA surveillance influence anti-MRSA antibiotic use? a retrospective analysis of the treatment of patients admitted with suspicion of infection at Veterans Affairs Medical Centers between 2005 and 2010. J Antimicrob Chemother 2014; 69:3401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Department of Veterans Affairs, Veterans Health Administration. 2010 VHA facility quality and safety report. Available at: http://www.va.gov/health/docs/HospitalReportCard2010.pdf Accessed 6 August 2013.
- 11.US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI). Available at: www.hsrd.research.va.gov/for_researchers/vinci Accessed 19 February 2014.
- 12.Health information policy council; 1984 revision of the Uniform Hospital Discharge Data Set--HHS. Notice. Fed Regist 1985; 50:31038–40. [PubMed] [Google Scholar]
- 13.Lindenauer PK, Lagu T, Shieh MS et al. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA 2012; 307:1405–13. [DOI] [PubMed] [Google Scholar]
- 14.Rhunke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Marked reduction in 30-day mortality among elderly patients with community-acquired pneumonia. Am J Med 2011; 124:171–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider R, Bagby J, Carlson R. Bar-code medication administration: a systems perspective. Am J Health Syst Pharm 2008; 65:2216, 8–9. [DOI] [PubMed] [Google Scholar]
- 16.International Health Terminology Standards Development Organisation (IHTSDO). SNOMED CT: the global language of healthcare. Available at: http://www.ihtsdo.org/snomed-ct. Accessed 11 August 2015.
- 17.Glas AS, Lijmet JG, Prine MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003; 56:1129–35. [DOI] [PubMed] [Google Scholar]
- 18.Berger A, Edelsberg J, Oster G, Huang X, Weber DJ. Patterns of initial antibiotic therapy for community-acquired pneumonia in US hospitals, 2000 to 2009. Am J Med Sci 2014; 347:347–56. [DOI] [PubMed] [Google Scholar]
- 19.Kallen AJ, Mu Y, Bulens S et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA 2010; 304:641–8. [DOI] [PubMed] [Google Scholar]
- 20.Jain R, Kralovic SM, Evans ME et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011; 364:1419–30. [DOI] [PubMed] [Google Scholar]
- 21.Moran Prevalence of methicillin-resistant Staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis 2012; 54:1126–33. [DOI] [PubMed] [Google Scholar]
- 22.Smith SB, Ruhnke GW, Weiss CH, Waterer GW, Wunderink RG. Trends in pathogens among patients hospitalized for pneumonia from 1993 to 2011. JAMA Intern Med 2014; 174:1837–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zilberberg MD, Shorr AF, Kollef MH et al. Growth and geographic variation in hospitals with resistant infections in the US, 2000–2005. Emerg Infect Dis 2008; 14:1756–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ewig S, Welte T, Torres A. Is healthcare-associated pneumonia a distinct entity needing specific therapy? Curr Opin Infect Dis 2012; 25:166–75. [DOI] [PubMed] [Google Scholar]
- 25.Grenier C, Pépin J, Nault V et al. Impact of guideline-consistent therapy on outcome of patients with healthcare-associated and community-acquired pneumonia. J Antimicrob Chemother 2011; 66:1617–24. [DOI] [PubMed] [Google Scholar]
- 26.Rothberg MB, Zilberberg MD, Pekow PS et al. Association of guideline-based antimicrobial therapy and outcomes in healthcare-associated pneumonia. J Antimicrob Chemother 2015; 70:1573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu JL, Siroka AM, Smith MW et al. One-year outcomes of community-acquired and healthcare-associated pneumonia in the Veterans Affairs Healthcare System. Int J Infect Dis 2011; 15:e382–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attridge RT, Frei CR, Restrepo MI et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J 2011; 38:878–87. [DOI] [PubMed] [Google Scholar]
- 29.Kett DH, Cano E, Quartin AA et al. Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Investigators: implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis 2011; 11:181–9. [DOI] [PubMed] [Google Scholar]
- 30.Madaras-Kelly KJ, Remington RE, Sloan KL, Fan VS. Guideline-based antibiotics and mortality in healthcare-associated pneumonia. J Gen Intern Med 2012; 27:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shindo Y, Ito R, Kobayashi D et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. J Respir Crit Care Med 2013; 188:985–95. [DOI] [PubMed] [Google Scholar]
- 32.Prina E, Ranzani OT, Polverino E et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann the Am Thoracic Soc 2015; 12:153–60. [DOI] [PubMed] [Google Scholar]
- 33.Chalmers JD, Salih W, Eqig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014; 58:330–9. [DOI] [PubMed] [Google Scholar]
- 34.Madaras-Kelly KJ, Remington RE, Fan VS, Sloan KL. Predicting antibiotic resistance to community-acquired pneumonia antibiotics in culture-positive patients with healthcare-associated pneumonia. J Hosp Med 2012; 7:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb BJ, Dascomb K, Stenehjem E, Dean N. Predicting risk of drug-resistant organisms in pneumonia: moving beyond the HCAP model. Respir Med 2015; 109:1–10. [DOI] [PubMed] [Google Scholar]