Abstract
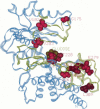
The glycolytic enzyme glucokinase plays an important role in the regulation of insulin secretion and recent studies have shown that mutations in the human glucokinase gene are a common cause of an autosomal dominant form of non-insulin-dependent (type 2) diabetes mellitus (NIDDM) that has an onset often during childhood. The majority of the mutations that have been identified are missense mutations that result in the synthesis of a glucokinase molecule with an altered amino acid sequence. To characterize the effect of these mutations on the catalytic properties of human beta-cell glucokinase, we have expressed native and mutant forms of this protein in Escherichia coli. All of the missense mutations show changes in enzyme activity including a decrease in Vmax and/or increase in Km for glucose. Using a model for the three-dimensional structure of human glucokinase based on the crystal structure of the related enzyme yeast hexokinase B, the mutations map primarily to two regions of the protein. One group of mutations is located in the active site cleft separating the two domains of the enzyme as well as in surface loops leading into this cleft. These mutations usually result in large reductions in enzyme activity. The second group of mutations is located far from the active site in a region that is predicted to undergo a substrate-induced conformational change that results in closure of the active site cleft. These mutations show a small approximately 2-fold reduction in Vmax and a 5- to 10-fold increase in Km for glucose. The characterization of mutations in glucokinase that are associated with a distinct and readily recognizable form of NIDDM has led to the identification of key amino acids involved in glucokinase catalysis and localized functionally important regions of the glucokinase molecule.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., McDonald R. C., Steitz T. A. Sequencing a protein by x-ray crystallography. I. Interpretation of yeast hexokinase B at 2.5 A resolution by model building. J Mol Biol. 1978 Jul 25;123(1):1–13. doi: 10.1016/0022-2836(78)90373-x. [DOI] [PubMed] [Google Scholar]
- Anderson C. M., Zucker F. H., Steitz T. A. Space-filling models of kinase clefts and conformation changes. Science. 1979 Apr 27;204(4391):375–380. doi: 10.1126/science.220706. [DOI] [PubMed] [Google Scholar]
- Andreone T. L., Printz R. L., Pilkis S. J., Magnuson M. A., Granner D. K. The amino acid sequence of rat liver glucokinase deduced from cloned cDNA. J Biol Chem. 1989 Jan 5;264(1):363–369. [PubMed] [Google Scholar]
- Bennett W. S., Jr, Steitz T. A. Structure of a complex between yeast hexokinase A and glucose. II. Detailed comparisons of conformation and active site configuration with the native hexokinase B monomer and dimer. J Mol Biol. 1980 Jun 25;140(2):211–230. doi: 10.1016/0022-2836(80)90103-5. [DOI] [PubMed] [Google Scholar]
- Chien C. T., Tauler A., Lange A. J., Chan K., Printz R. L., el-Maghrabi M. R., Granner D. K., Pilkis S. J. Expression of rat hepatic glucokinase in Escherichia coli. Biochem Biophys Res Commun. 1989 Dec 15;165(2):817–825. doi: 10.1016/s0006-291x(89)80039-7. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Shi J. P., Knill-Jones J., Lowe D. M., Wilkinson A. J., Blow D. M., Brick P., Carter P., Waye M. M., Winter G. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 1985 Mar 21;314(6008):235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Kessler F., Bennardini F., Bachs O., Serratosa J., James P., Caride A. J., Gazzotti P., Penniston J. T., Carafoli E. Partial purification and characterization of the Ca2(+)-pumping ATPase of the liver plasma membrane. J Biol Chem. 1990 Sep 15;265(26):16012–16019. [PubMed] [Google Scholar]
- Lange A. J., Xu L. Z., Van Poelwijk F., Lin K., Granner D. K., Pilkis S. J. Expression and site-directed mutagenesis of hepatic glucokinase. Biochem J. 1991 Jul 1;277(Pt 1):159–163. doi: 10.1042/bj2770159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson M. A. Glucokinase gene structure. Functional implications of molecular genetic studies. Diabetes. 1990 May;39(5):523–527. doi: 10.2337/diab.39.5.523. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990 Jun;39(6):647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. New perspectives on pancreatic islet glucokinase. Am J Physiol. 1984 Jan;246(1 Pt 1):E1–13. doi: 10.1152/ajpendo.1984.246.1.E1. [DOI] [PubMed] [Google Scholar]
- Miwa I., Mitsuyama S., Toyoda Y., Murata T., Okuda J. High-yield purification of glucokinase from rat liver. Prep Biochem. 1990;20(2):163–178. doi: 10.1080/00327489008050187. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Quaade C., Hughes S. D., Milburn J. L. Glucokinase and glucose transporter expression in liver and islets: implications for control of glucose homoeostasis. Biochem Soc Trans. 1990 Oct;18(5):851–853. doi: 10.1042/bst0180851. [DOI] [PubMed] [Google Scholar]
- Nishi S., Stoffel M., Xiang K., Shows T. B., Bell G. I., Takeda J. Human pancreatic beta-cell glucokinase: cDNA sequence and localization of the polymorphic gene to chromosome 7, band p 13. Diabetologia. 1992 Aug;35(8):743–747. doi: 10.1007/BF00429094. [DOI] [PubMed] [Google Scholar]
- Quaade C., Hughes S. D., Coats W. S., Sestak A. L., Iynedjian P. B., Newgard C. B. Analysis of the protein products encoded by variant glucokinase transcripts via expression in bacteria. FEBS Lett. 1991 Mar 11;280(1):47–52. doi: 10.1016/0014-5793(91)80201-d. [DOI] [PubMed] [Google Scholar]
- Stoffel M., Froguel P., Takeda J., Zouali H., Vionnet N., Nishi S., Weber I. T., Harrison R. W., Pilkis S. J., Lesage S. Human glucokinase gene: isolation, characterization, and identification of two missense mutations linked to early-onset non-insulin-dependent (type 2) diabetes mellitus. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7698–7702. doi: 10.1073/pnas.89.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel M., Patel P., Lo Y. M., Hattersley A. T., Lucassen A. M., Page R., Bell J. I., Bell G. I., Turner R. C., Wainscoat J. S. Missense glucokinase mutation in maturity-onset diabetes of the young and mutation screening in late-onset diabetes. Nat Genet. 1992 Oct;2(2):153–156. doi: 10.1038/ng1092-153. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tanizawa Y., Koranyi L. I., Welling C. M., Permutt M. A. Human liver glucokinase gene: cloning and sequence determination of two alternatively spliced cDNAs. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7294–7297. doi: 10.1073/pnas.88.16.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science. 1991 Mar 8;251(4998):1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- Ureta T. The comparative isozymology of vertebrate hexokinases. Comp Biochem Physiol B. 1982;71(4):549–555. doi: 10.1016/0305-0491(82)90461-8. [DOI] [PubMed] [Google Scholar]
- Vionnet N., Stoffel M., Takeda J., Yasuda K., Bell G. I., Zouali H., Lesage S., Velho G., Iris F., Passa P. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992 Apr 23;356(6371):721–722. doi: 10.1038/356721a0. [DOI] [PubMed] [Google Scholar]
- Weinhouse S. Regulation of glucokinase in liver. Curr Top Cell Regul. 1976;11:1–50. [PubMed] [Google Scholar]