Abstract
Background
In patients with chronic heart failure, abnormal ventilation at cardiopulmonary testing (expressed by minute ventilation-to-carbon dioxide production, or VE/VCO2 slope, and resting end-tidal CO2 pressure) may derive either from abnormal autonomic or chemoreflex regulation or from lung dysfunction induced by pulmonary congestion. The latter hypothesis is supported by measurement of pulmonary capillary wedge pressure, which cannot be obtained routinely but may be estimated noninvasively by measuring transthoracic conductance (thoracic fluid content 1/kΩ) with impedance cardiography.
Methods and Results
Preliminarily, in 9 patients undergoing invasive hemodynamics during cardiopulmonary testing, we demonstrated a significant relationship between VE/VCO2 slope and resting end-tidal CO2 pressure with baseline and peak pulmonary capillary wedge pressure. Later, noninvasive hemodynamic evaluation by impedance cardiography was performed before cardiopulmonary testing in 190 patients with chronic systolic heart failure and normal lung function (aged 67±3 years, 71% with ischemia, ejection fraction 32±7%, 69% with implantable cardioverter-defibrillator or cardiac resynchronization therapy). In this group, we determined the relationship between abnormal ventilation (VE/VCO2 slope and resting end-tidal CO2 pressure) and transthoracic conductance. In the whole population, thoracic fluid content values were significantly related to VE/VCO2 slope (R=0.63, P<0.0001) and to resting end-tidal CO2 pressure (R=−0.44, P<0.001).
Conclusions
In patients with chronic heart failure, abnormal ventilation during exercise may be related in part to pulmonary congestion, as detected by resting baseline impedance cardiography.
Keywords: abnormal ventilation, cardiopulmonary test, chronic heart failure, impedance cardiography, pulmonary congestion
The cause of limited exercise capacity and dyspnea in patients with heart failure is still a matter of debate.1,2 Although left ventricular ejection fraction is a poor predictor of maximal exercise capacity, left ventricular diastolic function correlates better with functional status.3–5 In contrast, in the genesis of exertional dyspnea, many data support the importance of abnormal ventilation, which is easily assessed during cardiopulmonary tests.6–12 In several pathophysiological studies, the abnormal ventilatory pattern that characterizes advanced heart failure has been associated with pulmonary congestion due to impaired hemodynamics, or with abnormal chemoreflex and autonomic responses.13–18 However, precise characterization of the mechanisms of functional capacity and exertional dyspnea in the clinical setting and, more specifically, in the single patient is challenging. Invasive hemodynamic assessment at rest or during exercise cannot become a routine procedure, and chemoreflex testing remains primarily a research tool.
Several methods for noninvasively determining the hemodynamic status of patients have been developed recently, and studies using these techniques are appearing, most aiming at cardiac output evaluation19–21 with a few also examining pulmonary congestion.22,23 The present study analyzed the relationship between resting noninvasive hemodynamic evaluation and cardiopulmonary testing.
Methods
Patients
As a preliminary phase of the study, we analyzed the data of 9 patients with chronic advanced heart failure in whom we performed invasive right hemodynamics during cardiopulmonary testing. The remaining part of the study was carried out in 190 patients with chronic systolic heart failure and without pulmonary disease (as estimated by history, routine lung function tests, and chest x-ray) who, from January 2012 to December 2014, performed a cardiopulmonary test before enrollment in our rehabilitation program. Patient characteristics are presented in Table 1. In both groups, therapy was optimized and stable for at least 3 months before stress testing. For both groups of patients, baseline echocardiographic data and brain natriuretic peptide values are presented but we did not discuss them further in this study. Patients gave written informed consent to participate in the studies, which were approved by the institutional ethics committee (Istituto Auxologico Italiano IRCCS) and conformed to the principles of the Declaration of Helsinki.
Table 1.
Clinical Data of Patients Undergoing Both Studies
| Invasive Study (n=9) | Noninvasive Study (n=190) | |
|---|---|---|
| Age | 66±7 | 67± 3 |
| Sex, male/female | 8/1 | 151/39 |
| Ischemic/nonischemic | 6/3 | 135/55 |
| NYHA I | – | 18 |
| NYHA II | 2 | 81 |
| NYHA III | 7 | 91 |
| NYHA IV | – | – |
| SAP, mm Hg | 112±16 | 118±26 |
| DAP, mm Hg | 67±10 | 71±8 |
| HR, bpm | 66±12 | 68±10 |
| EF, % | 30±5 | 32±7 |
| PAP, mm Hg | 47±14 | 48±11 |
| Moderate to severe mitral regurgitation | 4 (48%) | 24 (46%) |
| BNP, pg/mL | 475.0±49.3 | 403.5±69.7 |
| Therapy (% of patients) | ||
| ACE inhibitors and/or ATII inhibitors | 100% | 85% |
| β-blockers | 72% | 84% |
| Amiodarone | 54% | 60% |
| Diuretics | 100% | 90% |
| Others (digoxin, ivabradine) | 2% | 6% |
| ICD and/or ICD+CRT | 72% | 69% |
Data are expressed as mean±1 SD except as noted. ACE indicates angiotensin-converting enzyme; ATII, angiotensin II; BNP indicates brain natriuretic peptide; CRT, cardiac resynchronization therapy; DAP, diastolic arterial pressure; EF, ejection fraction; HR, heart rate; ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association classification; PAP, estimated pulmonary arterial pressure; SAP, systolic arterial pressure.
Hemodynamic Protocol
A 7-Fr Swan-Ganz thermodilution catheter (model 131HF7; Baxter Healthcare Corporation) was advanced via the right jugular vein into the pulmonary capillary wedge position. The optimal balloon position was verified by the presence of characteristic wedge pressure waveforms. Pulmonary capillary wedge pressure (PCWP) was measured with the zero level at the midaxillary line. Cardiac output was measured using the thermodilution method; derived hemodynamic variables were calculated by standard formulas. PCWP was averaged over pressure waveform data obtained during a 10-seond interval and expressed as a mean. After a stabilization period of 30 minutes, measurements of all variables were obtained in the resting supine position before cardiopulmonary testing; only PCWP was also calculated at peak exercise.
Noninvasive Hemodynamic Monitoring by Thoracic Bioimpedance
Impedance cardiography was performed using commercial equipment (BioZed; Niccomo).23 Two dual sensors were placed at the base of the neck under each ear, and 2 were placed on either side of the chest, on the midaxillary line at the level of the xyphoid. A cable with 8 impedance cardiography lead wires was attached to the sensor sites. The cuff of an integrated validated oscillometric blood pressure measuring device cuff was connected to the patient's arm. Recording was performed for 15 minutes; an average report was stored for analysis. For this study, we took into account transthoracic conductance (TFC; thoracic fluid content[TT]=1/Z0[TT]=1/kΩ) and stroke volume (in milliliters).
Exercise Test Protocol
Exercise was performed on an electrical bicycle using a cardiopulmonary exercise system (V2900; SensorMedics) for breath-by-breath measurements of minute ventilation (VE), oxygen consumption per unit time (VO2), and carbon dioxide production (VCO2).10 Calibration was performed before each test. Derived entities such as VE for O2 and CO2 (VE/VO2, VE/VCO2), respiratory quotient VCO2/VO2, and respiratory rate were viewed on the monitor online. A 12-lead ECG was monitored, and blood pressure and heart rate were measured every 2 minutes. Tests began with 2 minutes of supine rest and 2 minutes of resting on the bicycle, followed by 2 minutes of freewheeling warm-up; then, a ramp test started, with an increase of workload by 10 W/min. Patients exercised until either dyspnea or fatigue appeared. Respiratory quotient, VO2, VE/VO2, and VE/VCO2 were averaged during the last 30 seconds of exercise. Only tests with a respiratory quotient ≥1.05 were considered.7,11 Anaerobic threshold was calculated by the V-slope method. The VE/VCO2 slope, relating the rate of increase in ventilation per unit increase in CO2 production, was calculated by linear regression from averaged VE and VCO2 data collected until anaerobic compensation. Other variables taken into account were O2 pulse (mL/beat), the relationship of which with stroke volume has been demonstrated,24 and resting end-tidal CO2 pressure (PETCO2), also related to poor ventilatory performance and prognosis.25 According to test results, patients were classified according to both the Weber-Janicki classes26 and the ventilatory classes described by Arena et al.27
Statistical Analysis
We used commercial software for analysis (OriginPro 7.0; Microcal). Data are expressed as mean±1 SD. Linear regression with a least squares fitting routine was used for related continuous response with explanatory variables; for each regression, the correlation coefficient R was considered. Differences in the continuous variables between rest and peak exercise were evaluated by paired t test. ANOVA was used to compare variables among groups of patients; when allowed by the F value for multiple comparisons, Bonferroni correction was used to compare pairs of observations. A P value <0.05 was considered significant.
Results
Invasive Hemodynamic Evaluation
As shown in Table 2, at the baseline hemodynamic evaluation, patients showed moderate pulmonary hypertension (39.5±10.1 mm Hg) and low resting stroke volume (53.6±7.1 mL), the latter not related to peak VO2 (R=0.11, P=0.73). PCWP was slightly increased at baseline (17.2±6.7 mm Hg) and was significantly related to VE/VCO2 slope (R=0.80, P<0.01) (Figure 1A) and resting PETCO2 (R=−0.71, P<0.05) (Figure 2A). At peak exercise, PCWP was increased (32.7± 11.7 mm Hg, P<0.02 compared with baseline) and maintained a significant relationship with both VE/VCO2 slope (R=0.80, P<0.01) (Figure 1B) and resting PETCO2 (R=−0.71, P<0.05) (Figure 2B). In contrast, VE/VCO2 slope and resting PETCO2 were unrelated to peakVO2 (R=−0.29 for VE/VCO2 slope and R=0.31 for PETCO2; P=0.35 and P=0.26, respectively). Exercise capacity of this group of patients was reduced, with manifest abnormal ventilation, as shown by the classifications of Weber-Janicki and Arena et al26 (Table 2).
Table 2.
Cardiopulmonary Test and Invasive and Noninvasive Hemodynamics
| Invasive Study (n=9) | Noninvasive Study (n=190) | |
|---|---|---|
| Hemodynamic during cardiopulmonary test | ||
| CVP, cm H2O | 13.5±2.1 | NA |
| SVRI resting, dyn/s/cm−5/m2 | 2968.7±662.8 | NA |
| PAPs resting, mm Hg | 39.5±10.1 | NA |
| PCWP resting, mm Hg | 17.2±6.7 | NA |
| PCWP peak, mm Hg | 32.7±11.7* | NA |
| Stroke volume resting, mL | 53.6±7.1 | NA |
| Resting impedance cardiograhy | ||
| SVRI rest, dyn/s/cm−5/m2 | NA | 2062.1±874.7 |
| TFC, 1/kΩ | NA | 37.3±6.8 |
| Stroke volume resting, mL | NA | 70.1±21.7 |
| Cardiopulmonary test results | ||
| Peak VO2, mL/kg/min | 16.5±2.4 | 14.4±3.7 |
| Oxygen pulse, mL/beat | 10.3±1.9 | 10.5±3.2 |
| Resting PETCO2, mm Hg | 30.1±4.1 | 32.8±4.7 |
| VE/VCO2 slope | 37.4±5.2 | 36.5±5.8 |
| Functional classification, n (%) | ||
| Weber-Janicki class A | 0 (0) | 10 (5) |
| Weber-Janicki class B | 4 (44) | 54 (28) |
| Weber-Janicki class C | 5 (56) | 98 (52) |
| Weber-Janicki class D | 0 (0) | 28 (15) |
| Ventilatory classification, n (%) | ||
| Arena et al class 1 | 0 (0) | 15 (8) |
| Arena et al class 2 | 4 (44) | 84 (44) |
| Arena et al class 3 | 4 (44) | 80 (42) |
| Arena et al class 4 | 1 (12) | 11 (6) |
Data are expressed as mean±1 SD except as noted. CVP indicates central venous pressure; NA, not available; PAPs, pulmonary artery pressure systolic; PCWP, pulmonary capillary wedge pressure; PETCO2, end-tidal CO2 pressure; SVRI, systemic vascular resistances, indexed; TFC, transthoracic conductance; VE/VCO2, minute ventilation-to-carbon dioxide production; VO2, oxygen consumption per unit time.
P<0.02 vs resting.
Figure 1.

Relationship between VE/VCO2 slope and resting (A) and peak (B) pulmonary capillary wedge pressure (PCWP) during cardiopulmonary test in 9 patients undergoing hemodynamic study. VE/VCO2 indicates minute ventilation-to-carbon dioxide production.
Figure 2.
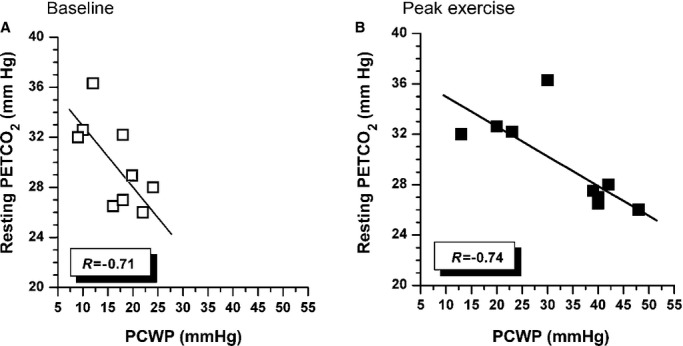
Relationship between resting PETCO2 and resting (A) and peak exercise (B) pulmonary capillary wedge pressure (PCWP) during cardiopulmonary test in 9 patients undergoing hemodynamic study before the test. PETCO2 indicates end-tidal CO2 pressure.
Noninvasive Hemodynamic Evaluation
The results from impedance cardiography and cardiopulmonary tests in the whole population of noninvasively studied patients are illustrated in Table 2. In parallel to what was observed in patients studied invasively, peak VO2 (expression of global exercise tolerance) was unrelated to baseline TFC or stroke volume at impedance cardiography (R=0.11 and R=0.16, P=0.33 and P=0.22, respectively). VE/VCO2 slope showed a significant relationship with baseline TFC (R=0.63, P<0.0001) (Figure 3). A significant relationship with TFC was also present for resting PETCO2 (R=−0.44, P<0.001) (Figure 4). In Table 2 we also show cardiopulmonary and noninvasive hemodynamic data grouped according to the classifications of Weber-Janicki and Arena et al.26 Regarding Weber-Janicki classes, comparing only patients in class D with those in class A, we observed a higher VE/VCO2 slope, lower resting PETCO2 and peak O2 pulse, and higher TFC. In contrast, a progressive reduction in peak VO2, peak O2 pulse, and resting PETCO2 and an increase in TFC values were evident across the whole spectrum of the classification suggested by Arena et al28 (Table 3).
Figure 3.
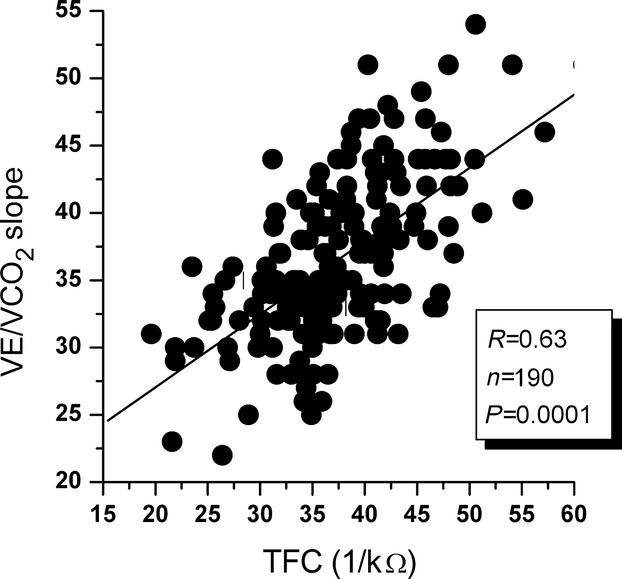
Relationship between baseline transthoracic conductance (TFC) and VE/VCO2 slope at cardiopulmonary testing in 190 patients undergoing noninvasive hemodynamic evaluation before the test. VE/VCO2 indicates minute ventilation-to-carbon dioxide production.
Figure 4.

Relationship between baseline transthoracic conductance (TFC) and resting PETCO2 at cardiopulmonary testing in 190 patients undergoing noninvasive hemodynamic evaluation before the test. PETCO2 indicates end-tidal CO2 pressure.
Table 3.
Cardiopulmonary Test and Noninvasive Hemodynamics According to Functional and Ventilatory Classes
| Weber-Janicki classes | A (n=10) | B (n=54) | C (n=98) | D (n=28) |
|---|---|---|---|---|
| Peak VO2, mL/kg/min | 22.5±0.9 | 17.8±1.2 | 13.4±1.5 | 9.3±1.3 |
| Oxygen pulse, mL/beat | 14.7±3.3 | 12.5±3.1 | 10.0±2.7 | 8.2±2.7* |
| Resting PETCO2, mm Hg | 36.1±4.7 | 33.1±4.0 | 32.9±4.9 | 31.7±5.5* |
| VE/VCO2 slope | 31.8±3.9 | 35.4±4.2 | 36.9±3.2 | 38.9±6.9* |
| TFC, 1/KΩ | 36.0±2.9 | 36.7±5.2 | 37.8±3.1 | 39.3±9.2* |
| Stroke volume resting, mL | 85.5±17.8 | 68.0±3.6 | 70.6±23.3 | 70.4±16.9 |
| Arena et al classes | 1 (n=15) | 2 (n=84) | 3 (n=80) | 4 (n=11) |
|---|---|---|---|---|
| Peak VO2, mL/kg/min | 17.0±4.5 | 14.9±3.7 | 14.3±3.1 | 11.7±3.3† |
| Oxygen pulse, mL/beat | 12.2±2.7 | 11.4±3.3 | 10.1±3.0 | 9.5±2.2† |
| Resting PETCO2, mm Hg | 37.3±2.8 | 35.1±3.2 | 30.8±3.8 | 27.8±3.1‡ |
| VE/VCO2 slope | 26.8±2.1 | 33.0±1.6 | 39.8±2.9 | 48.2±2.2‡ |
| TFC, 1/KΩ | 31.3±4.1 | 34.6±5.3 | 39.7±5.7 | 45.9±6.1‡ |
| Stroke volume resting, mL | 78.1±19.5 | 77.9±24.2 | 68.1±19.5 | 50.1±13.6† |
Data are expressed as mean±1 SD. PETCO2, end-tidal CO2 pressure; TFC indicates transthoracic conductance;VE/VCO2, minute ventilation-to-carbon dioxide production; VO2, oxygen consumption per unit time.
P<0.05 Weber-Janicki class D vs Weber-Janicki class A (ANOVA with Bonferroni correction).
P<0.05 Arena et al class 4 vs either Arena et al class 1 or class 2 (ANOVA with Bonferroni correction).
P<0.05 each of the Arena et al classes compared with the others (ANOVA with Bonferroni correction).
In this group of relatively stable patients undergoing cardiac rehabilitation, only 21 (11%) presented with exercise periodic breathing. This incidence is low compared with other studies,29 which showed poorer exercise tolerance and more abnormal ventilation (lower peak VO2 and resting PETCO2, higher VE/VCO2 slope); in fact, their small number prevented any statistical analysis.
Discussion
In humans, VE is finely regulated to tightly match VCO2; the relationship between VE and VCO2 is linear and is relatively constant from rest to moderate exercise unless significant acidosis occurs.28 Nevertheless, several pathological settings may alter such a relationship. In chronic heart failure, reduced ventilatory efficiency reflects an increased ventilatory demand for every unit of VCO2 and indicates more severe disease, independently predicting prognosis in addition to or even better than peak VO29,11,25; however, the pathophysiological significance of abnormal ventilation has not been completely clarified.12,27–30 Moreover, because pathophysiology and prognosis are likely to be interrelated, the question arises of whether targeting ventilation and ventilatory control in chronic heart failure could modify its prognosis.31
The regulation of CO2 by VE is described by a hyperbolic relationship:
| 1 |
Consequently, in patients with chronic heart failure, VE/VCO2 slope has been primarily related to increased dead space32 and to augmented chemoreflex sensitivity.33 In addition, the vascular tone of pulmonary vessels and right ventricular function have been related to the abnormal ventilatory response to exercise in patients.10 Finally, invasively measured pulmonary hemodynamics showed a clear relationship of enhanced left ventricular filling pressures with VE/VCO2 slope 15,33 and with PETCO2.34 Although obtained in a small subset of patients, our invasive results are in keeping with these observations.
In the individual patient, any of the mechanisms mentioned may induce an increased ventilatory response to exercise. We observed a strong correlation between VE/VCO2 slope and PCWP at rest and at peak exercise, confirming that ventilatory inefficiency during effort combines hemodynamic maladaptation to exercise with the hemodynamic derangement already present at rest.35 The tight connection between VE/VCO2 slope and PCWP is in keeping with the results of Lewis et al,10 who studied the effects of sildenafil on hemodynamics and ventilatory efficiency in chronic heart failure and showed a relationship between the reduction of PCWP and of VE/VCO2 after treatment. This finding suggests that a higher starting point for PCWP at rest influenced pulmonary vascular hemodynamics and ventilatory efficiency during exercise.
Thoracic Impedance and Pulmonary Congestion
In a nonselected population of ambulatory heart failure patients, we showed a direct correlation between ventilatory inefficiency during exercise and thoracic fluid content at rest. The latter index was obtained with a simple noninvasive evaluation that, in our previous studies, was strongly related to PCWP 23 and that was performed similarly to a comprehensive Doppler echocardiogram for detecting high filling pressures and diastolic dysfunction.36
The correlation of TFC with VE/VCO2 slope was weaker than that observed between VE/VCO2 slope and PCWP. It has to be kept in mind that thoracic conductance reflects a balance between intra- and extravascular (ie, interstitial) fluid: Only the former represents the source of PCWP, whereas the remaining fluid depends on lung and interstitial conditions. In this study, we purposely excluded patients with abnormal pulmonary function and restrictive, potentially “wet” lungs, which could have accounted for exercise hyperpnea37,38; however, we cannot rule out the possibility that part of the conductance we measured depended on extravascular fluid accumulation. The possibility of simultaneously assessing invasive and noninvasive hemodynamics, looking for an increase of transthoracic impedance during exercise similar to that observed with PCWP, would have been useful regarding this issue to further investigate the relationship between PCWP and TFC.23 Unfortunately, our noninvasive equipment was not suitable for continuous monitoring during exercise, and invasive and noninvasive measurements were obtained at the same time in resting conditions in only 2 patients.
Across the Weber-Janicki classes, there was not a clear change in ventilation variables and pulmonary congestion; only class D patients showed abnormalities in these variables. In contrast, the gradual worsening of VE/VCO2 described by Arena et al27 was matched by a progressive increase in pulmonary conductance, suggesting that abnormal central hemodynamics may influence ventilation more than exercise capability, which in patients can also depend on physical deconditioning. 1,2,11
Clinical Implications
In our study, impedance cardiography was used as a simple and rapid instrument that was complementary to cardiopulmonary exercise testing in noninvasively assessing the hemodynamic status of patients and evaluating the role of left ventricular filling pressures in exercise pathophysiology. We showed that increased PCWP (measured directly and estimated noninvasively) significantly contributed to abnormal ventilation in chronic advanced heart failure. In other words, rather than low cardiac output or abnormal autonomic balance, congestion may be the crucial component eventually leading to exertional dyspnea. Indeed, hemodynamic derangement and congestion carry significant prognostic information.39 Furthermore, the concept that treating congestion per se may not only relieve dyspnea but also improve the outcome of patients is currently being investigated in studies noninvasively measuring indexes such as intrathoracic impedance40 and pulmonary artery pressure.41,42
In a given patient, the combination of TFC and VE/VCO2 slope might indicate if and how pulmonary congestion determines abnormal exercise physiology. With this in mind, the combination of cardiopulmonary testing and impedance cardiography might be viewed as a tool to recognize pulmonary congestion in otherwise asymptomatic patients and to offer them early treatment.
Acknowledgments
We are grateful to the nurses of the Heart Failure Unit (Ada Spiezia RN and Elena Bressan RN) for their help in performing cardiopulmonary stress tests and impedance cardiography.
Disclosures
None.
References
- Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corrà U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani GQ, Agostoni P Working Group ‘Exercise Physiology, Sport Cardiology and Cardiac Rehabilitation’, Italian Society of Cardiology. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part I. Eur J Cardiovasc Prev Rehabil. 2010;17:637–642. doi: 10.1097/HJR.0b013e3283361dc5. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corrà U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani G, Agostoni P Working Group ‘Exercise Physiology, Sport Cardiology and Cardiac Rehabilitation’, Italian Society of Cardiology. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part II. Eur J Cardiovasc Prev Rehabil. 2010;17:643–648. doi: 10.1097/HJR.0b013e32833f3aa5. [DOI] [PubMed] [Google Scholar]
- Lapu-Bula R, Robert A, De Kock M, D'Hondt AM, Detry JM, Melin JA, Vanoverschelde JL. Relation of exercise capacity to left ventricular systolic function and diastolic filling in idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 1999;83:728–734. doi: 10.1016/s0002-9149(98)00979-5. [DOI] [PubMed] [Google Scholar]
- Skaluba SJ, Litwin SE. Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation. 2004;109:972–977. doi: 10.1161/01.CIR.0000117405.74491.D2. [DOI] [PubMed] [Google Scholar]
- Smart N, Haluska B, Leano R, Case C, Mottram PM, Marwick TH. Determinants of functional capacity in patients with chronic heart failure: role of filling pressure and systolic and diastolic function. Am Heart J. 2005;149:152–158. doi: 10.1016/j.ahj.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Kleber FX, Vietzke G, Wernecke KD, Bauer U, Opitz C, Wensel R, Sperfeld A, Gläser S. Impairment of ventilatory efficiency in heart failure. Prognostic impact. Circulation. 2000;101:2803–2809. doi: 10.1161/01.cir.101.24.2803. [DOI] [PubMed] [Google Scholar]
- Corrà U, Mezzani A, Bosimini E, Scapellato F, Imparato A, Giannuzzi P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am Heart J. 2002;143:418–426. doi: 10.1067/mhj.2002.120772. [DOI] [PubMed] [Google Scholar]
- Arena R, Humphrey R. Comparison of ventilatory expired gas parameters used to predict hospitalization in patients with heart failure. Am Heart J. 2002;143:427–432. doi: 10.1067/mhj.2002.119607. [DOI] [PubMed] [Google Scholar]
- Arena R, Myers J, Hsu L, Peberdy MA, Pinkstaff S, Bensimhon D, Chase P, Vicenzi M, Guazzi M. The minute ventilation/carbon dioxide production slope is prognostically superior to the oxygen uptake efficiency slope. J Card Fail. 2007;13:462–469. doi: 10.1016/j.cardfail.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008;1:227–233. doi: 10.1161/CIRCHEARTFAILURE.108.785501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfatto G, Branzi G, Giglio A, Ciambellotti F, Villani A, Parati G, Facchini M. Diastolic dysfunction and abnormal exercise ventilation predict adverse outcome in elderly patients with chronic systolic heart failure. Eur J Prev Cardiol. 2012;19:396–403. doi: 10.1177/1741826711401047. [DOI] [PubMed] [Google Scholar]
- Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Arena R. Maximal dyspnea on exertion during cardiopulmonary exercise testing is related to poor prognosis and echocardiography with tissue Doppler imaging in heart failure. Congest Heart Fail. 2009;15:277–283. doi: 10.1111/j.1751-7133.2009.00107.x. [DOI] [PubMed] [Google Scholar]
- Metra M, Faggiano P, D'Aloia A, Nodari S, Gualeni A, Raccagni D, Dei Cas L. Use of cardiopulmonary exercise testing with hemodynamic monitoring in the prognostic assessment of ambulatory patients with chronic heart failure. J Am Coll Cardiol. 1999;33:943–950. doi: 10.1016/s0735-1097(98)00672-x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe PD, Aaronson KD, Cody RJ, Koelling TM. Impact of serial changes in cardiac hemodynamics on exercise performance in patients with heart failure due to ischemic and nonischemic cardiomyopathy. Am J Cardiol. 2003;91:164–168. doi: 10.1016/s0002-9149(02)03103-x. [DOI] [PubMed] [Google Scholar]
- Arena R, Owens DS, Arevalo J, Smith K, Mohiddin SA, McAreavey D, Ulisney KL, Tripodi D, Fananapazir L, Plehn JF. Ventilatory efficiency and resting hemodynamics in hypertrophic cardiomyopathy. Med Sci Sports Exerc. 2008;40:799–805. doi: 10.1249/MSS.0b013e31816459a1. [DOI] [PubMed] [Google Scholar]
- Chua TP, Ponikowski P, Webb-Peploe K, Harrington D, Anker SD, Piepoli M, Coats AJ. Clinical characteristics of chronic heart failure patients with an augmented peripheral chemoreflex. Eur Heart J. 1997;18:480–486. doi: 10.1093/oxfordjournals.eurheartj.a015269. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Pesek CA, van de Borne PJ, Kato M, Somers VK. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation. 1999;100:262–267. doi: 10.1161/01.cir.100.3.262. [DOI] [PubMed] [Google Scholar]
- Passino C, Poletti R, Bramanti F, Prontera C, Clerico A, Emdin M. Neuro-hormonal activation predicts ventilatory response to exercise and functional capacity in patients with heart failure. Eur J Heart Fail. 2006;8:46–53. doi: 10.1016/j.ejheart.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lang CC, Karlin P, Haythe J, Tsao L, Mancini DM. Ease of noninvasive measurement of cardiac output coupled with peak VO2 determination at rest and during exercise in patients with heart failure. Am J Cardiol. 2007;99:404–405. doi: 10.1016/j.amjcard.2006.08.047. [DOI] [PubMed] [Google Scholar]
- Hummel YM, Bugatti S, Damman K, Willemsen S, Hartog JW, Metra M, Sipkens JS, van Veldhuisen DJ, Voors AA. Functional and hemodynamic cardiac determinants of exercise capacity in patients with systolic heart failure. Am J Cardiol. 2012;110:1336–1341. doi: 10.1016/j.amjcard.2012.06.039. [DOI] [PubMed] [Google Scholar]
- Myers J, Wong M, Adhikarla C, Boga M, Challa S, Abella J, Ashley EA. Cardiopulmonary and noninvasive hemodynamic responses to exercise predict outcomes in heart failure. J Card Fail. 2013;19:101–107. doi: 10.1016/j.cardfail.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Packer M, Abraham WT, Mehra MR, Yancy CW, Lawless CE, Mitchell JE, Smart FW, Bijou R, O'Connor CM, Massie BM, Pina IL, Greenberg BH, Young JB, Fishbein DP, Hauptman PJ, Bourge RC, Strobeck JE, Murali S, Schocken D, Teerlink JR, Levy WC, Trupp RJ, Silver MA for the Prospective Evaluation and Identification of Cardiac Decompensation by ICG Test (PREDICT) Study Investigators and Coordinators. Utility of impedance cardiography for the identification of short term risk of clinical decompensation in stable patients with chronic heart failure. J Am Coll Cardiol. 2006;47:2245–2252. doi: 10.1016/j.jacc.2005.12.071. [DOI] [PubMed] [Google Scholar]
- Malfatto G, Blengino S, Perego GB, Branzi G, Villani A, Facchini M, Parati G. Transthoracic impedance accurately estimates pulmonary wedge pressure in patients with decompensated chronic heart failure. Congest Heart Fail. 2012;18:25–31. doi: 10.1111/j.1751-7133.2011.00248.x. [DOI] [PubMed] [Google Scholar]
- Oliveira RB, Myers J, Araújo CG, Abella J, Mandic S, Froelicher V. Maximal exercise oxygen pulse as a predictor of mortality among male veterans referred for exercise testing. Eur J Cardiovasc Prev Rehabil. 2009;16:358–364. doi: 10.1097/HJR.0b013e3283292fe8. [DOI] [PubMed] [Google Scholar]
- Arena R, Peberdy MA, Myers J, Guazzi M, Tevald M. Prognostic value of resting end-tidal carbon dioxide in patients with heart failure. Int J Cardiol. 2006;109:351–358. doi: 10.1016/j.ijcard.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Weber KT, Janicki JS, McElroy PA, Maskin CS. Cardiopulmonary exercise testing in clinical practice. Cardiology. 1987;74:62–70. doi: 10.1159/000174177. [DOI] [PubMed] [Google Scholar]
- Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115:2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest. 2003;124:720–727. doi: 10.1378/chest.124.2.720. [DOI] [PubMed] [Google Scholar]
- Corrà U, Pistono M, Mezzani A, Braghiroli A, Giordano A, Lanfranchi P, Bosimini E, Gnemmi M, Giannuzzi P. Sleep and exertional periodic breathing in chronic heart failure: prognostic importance and interdependence. Circulation. 2006;113:44–50. doi: 10.1161/CIRCULATIONAHA.105.543173. [DOI] [PubMed] [Google Scholar]
- Poon C-S, Tin C. Mechanism of augmented exercise hyperpnea in chronic heart failure and dead space loading. Respir Physiol Neurobiol. 2013;186:114–130. doi: 10.1016/j.resp.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzi M. Abnormalities in cardiopulmonary exercise testing ventilatory parameters in heart failure: pathophysiology and clinical usefulness. Curr Heart Fail Rep. 2014;11:80–87. doi: 10.1007/s11897-013-0183-3. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Zhang YY, Gitt A, Belardinelli R, Koike A, Lubarsky L, Agostoni PG. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96:2221–2227. doi: 10.1161/01.cir.96.7.2221. [DOI] [PubMed] [Google Scholar]
- Chua TP, Ponikowski PP, Harrington D, Chambers J, Coats AJ. Contribution of peripheral chemoreceptors to ventilation and the effects of their suppression on exercise tolerance in chronic heart failure. Heart. 1996;76:483–489. doi: 10.1136/hrt.76.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi-Filho G, Azevedo ER, Parker JD, Bradley TD. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002;19:37–40. doi: 10.1183/09031936.02.00214502. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfatto G, Branzi G, Giglio A, Villani A, Facchini C, Ciambellotti F, Facchini M, Parati G. Transthoracic bioimpedance and brain natriuretic peptide levels accurately indicate additional diastolic dysfunction in patients with chronic advanced systolic heart failure. Eur J Heart Fail. 2010;12:928–935. doi: 10.1093/eurjhf/hfq089. [DOI] [PubMed] [Google Scholar]
- Cundrle I, Jr, Johnson BD, Somers VK, Scott CG, Rea RF, Olson LJ. Effect of cardiac resynchronization therapy on pulmonary function in patients with heart failure. Am J Cardiol. 2013;112:838–842. doi: 10.1016/j.amjcard.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostoni PG, Marenzi GC, Pepi M, Doria E, Salvoni A, Perego G, Lauri G, Giraldi F, Grazi S, Guazzi M. Isolated ultrafiltration in moderate congestive heart failure. J Am Coll Cardiol. 1993;21:424–431. doi: 10.1016/0735-1097(93)90685-t. [DOI] [PubMed] [Google Scholar]
- Lang CC, Agostoni P, Mancini DM. Prognostic significance and measurement of exercise-derived hemodynamic variables in patients with heart failure. J Card Fail. 2007;13:672–679. doi: 10.1016/j.cardfail.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Brachmann J, Böhm M, Rybak K, Klein G, Butter C, Klemm H, Schomburg R, Siebermair J, Israel C, Sinha AM, Drexler H, OptiLink HF Study Executive Board and Investigators. Fluid status monitoring with a wireless network to reduce cardiovascular-related hospitalizations and mortality in heart failure: rationale and design of the OptiLink HF Study (Optimization of Heart Failure Management using OptiVol Fluid Status Monitoring and CareLink) Eur J Heart Fail. 2011;13:796–804. doi: 10.1093/eurjhf/hfr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]


