Abstract
Background
It is unclear whether obesity unaccompanied by metabolic abnormalities is associated with increased cardiovascular disease risk across racial and ethnic subgroups.
Methods and Results
We identified 14 364 postmenopausal women from the Women's Health Initiative who had data on fasting serum lipids and serum glucose and no history of cardiovascular disease or diabetes at baseline. We categorized women by body mass index (in kg/m2) as normal weight (body mass index 18.5 to <25), overweight (body mass index 25 to <30), or obese (body mass index ≥30) and by metabolic health, defined first as the metabolic syndrome (metabolically unhealthy: ≥3 metabolic abnormalities) and second as the number of metabolic abnormalities. We used Cox proportional hazards regression to assess associations between baseline characteristics and cardiovascular risk. Over 13 years of follow-up, 1101 women had a first cardiovascular disease event (coronary heart disease or ischemic stroke). Among black women without metabolic syndrome, overweight women had higher adjusted cardiovascular risk than normal weight women (hazard ratio [HR] 1.49), whereas among white women without metabolic syndrome, overweight women had similar risk to normal weight women (HR 0.92, interaction P=0.05). Obese black women without metabolic syndrome had higher adjusted risk (HR 1.95) than obese white women (HR 1.07; interaction P=0.02). Among women with only 2 metabolic abnormalities, cardiovascular risk was increased in black women who were overweight (HR 1.77) or obese (HR 2.17) but not in white women who were overweight (HR 0.98) or obese (HR 1.06). Overweight and obese women with ≤1 metabolic abnormality did not have increased cardiovascular risk, regardless of race or ethnicity.
Conclusions
Metabolic abnormalities appeared to convey more cardiovascular risk among black women.
Keywords: cardiovascular disease, metabolism (metabolic syndrome), obesity, race/ethnicity, women
Obesity has become a worldwide epidemic,1 so methods to identify those at risk of obesity-related cardiovascular disease (CVD) morbidity and mortality are warranted. The excess cardiovascular risk of obese persons may be attributable primarily to metabolic mediators rather than to obesity per se,2,3 and insulin resistance has been suggested as a key pathophysiological link between obesity and cardiovascular risk.4 Some persons appear to be “metabolically healthy obese,”5,6 in that they are not insulin resistant and lack most of the metabolic abnormalities composing the metabolic syndrome.7 In white populations, the cardiovascular risk of the metabolically healthy obese group appears to be no higher than that of metabolically healthy persons of normal weight.5,8,9 In other racial and ethnic groups, however, the cardiovascular risk among metabolically healthy obese persons is largely unexplored, and may differ from that among white persons; not only does the prevalence of cardiovascular risk factors differ across race and ethnicity, but some risk factors, such as hypertension, appear to be associated with a higher cardiovascular risk in black persons.10,11
We aimed to characterize cardiovascular risk according to obesity level and metabolic health status across racial and ethnic subgroups. In identification of persons with elevated cardiovascular risk in relation to obesity, we also sought to investigate whether the standard definition of metabolically healthy obese applied to the various racial and ethnic subgroups by quantifying the number and the type of metabolic syndrome components.
Methods
Study Population
The Women's Health Initiative (WHI) recruited a large multiethnic cohort of postmenopausal women aged 50 to 79 years at baseline throughout the United States during 1993–1998. The WHI consisted of an observational study cohort and 3 clinical trial cohorts (a diet modification trial, a menopausal hormone therapy trial, and/or a calcium/vitamin D trial) that defined and measured clinical variables uniformly across all studies. The scientific rationale, study design, eligibility criteria, and baseline characteristics of these studies have been reported.12–15 The WHI studies were approved by institutional review committees at each participating center, and all participants gave written informed consent. The present study included women from 2 biomarker subcohorts of the WHI and its Extension Study (2005–2010): the SNP Health Association Resource cohort (n=11 967), which comprised black and Hispanic participants from either the observational trial or the clinical trial cohorts, and the European American Hormone Trial subcohort (n=10 161), which primarily comprised white women from the menopausal hormone therapy trials.
The WHI excluded women with predicted survival of <3 years and women with alcohol or drug dependency or mental illness; the clinical trials also excluded women with any invasive cancer in the previous 10 years, hepatitis or cirrhosis, or blood pressure ≥200/105 mm Hg; and the menopausal hormone therapy trials also excluded women with a history of pulmonary embolus, deep venous thromboembolism, or hypertriglyceridemia. For the present study, we excluded women with prior CVD, defined as a previous history of myocardial infarction, stroke, cardiac catheterization, percutaneous coronary intervention, coronary artery bypass surgery, heart failure, atrial fibrillation, or aortic aneurysm at entry, or with prior diabetes, defined as a self-reported medical history of diabetes, treatment with glucose-lowering agents, or a fasting serum glucose level ≥126 mg/dL (≥7.0 mmol/L). Because of insufficient data for analysis, we also excluded women with missing data on pertinent variables (height, weight, waist circumference, age, smoking, systolic or diastolic blood pressure, treatment for hypertension, triglycerides, high-density lipoprotein cholesterol [HDL-C], fasting serum glucose, baseline hormone use, or any of the exclusion variables), women who provided data only at baseline, and women who were underweight (body mass index [BMI; in kg/m2] <18.5). Race or ethnicity was categorized as non-Hispanic white women (hereafter referred to as white women), non-Hispanic black women (hereafter referred to as black women), and Hispanic women.
Exposure Definitions
During the baseline clinic visit, each study participant completed self-administered questionnaires on medical history, smoking, diet, physical activity, and other lifestyle-related factors and had blood pressure, weight, and height measured. Waist circumference was measured to nearest 0.5 cm at the narrowest part of the torso at the end of a normal expiration. Women in the biomarker studies had blood samples drawn after a 12-hour fast. Glucose was analyzed using the Gluco-quant glucose/hexokinase reagent (Roche), triglycerides were analyzed using the Triglyceride GB reagent (Roche), and HDL-C was analyzed using the HDL-C Plus third-generation direct method (Roche), all on a Roche Modular P chemistry analyzer. All assays for the biomarker studies were done on serum by the University of Minnesota Medical Center laboratory, and average assay correlation variances were 2% for glucose, 2% for triglycerides, and 3% for HDL-C.
We defined the metabolic abnormalities composing the metabolic syndrome according to the 2009 joint interim statement of the International Diabetes Federation and the American Heart Association/National Heart, Lung, and Blood Institute,7 based on increased waist circumference ≥80 cm, increased level of triglycerides ≥150 mg/dL (≥1.7 mmol/L), decreased level of HDL-C <50 mg/dL (<1.3 mmol/L), increased blood pressure with either systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or treatment with antihypertensive drugs, and impaired fasting serum glucose ≥100 mg/dL (5.6 mmol/L). Consistent with other studies, we defined metabolically unhealthy as the presence of ≥3 metabolic abnormalities (metabolic syndrome) and metabolically healthy as ≤2 metabolic abnormalities.16
Primary End Point
The primary outcome was time to first CVD event defined as nonfatal and fatal coronary heart disease or ischemic stroke, identified using annual mailed questionnaires (semiannual for clinical trials, response rates >95%) and adjudicated locally and centrally by physicians blinded to exposure data by use of medical records and standard criteria.17 Standardized criteria for electrocardiographic changes, elevated cardiac enzymes, or both were used to define nonfatal myocardial infarction. A diagnosis of ischemic stroke required a hospital admission with rapid onset of neurological deficit persisting >24 hours, attributed to occlusion of cerebral or extracranial arteries with infarction and without evidence of other cause. Fatal events were confirmed by documentation in hospital, by autopsy reports, or both. The participants were followed until December 31, 2010, or until first CVD event, loss to follow-up, death from any cause, or last visit prior to the end of Extension Study I at December 31, 2010, whichever came first.
Statistical Methods
We categorized the study population by metabolic health status within each BMI category: normal weight (18.5 to <25.0), overweight (25.0 to <30.0), or obese (BMI ≥30.0).
We assessed the associations between obesity and metabolic subtypes and the risk of CVD by use of multivariable Cox proportional hazards regression. Because the aim of the study was to examine the race-specific cardiovascular risk according to obesity and metabolic health subtypes, analyses were performed separately by race or ethnicity (white, black, Hispanic). Normal weight women with <3 (0, 1, or 2) metabolic abnormalities were used as a reference category in all analyses unless otherwise stated. Metabolic abnormalities were categorized using 3 different approaches. First, metabolic abnormalities were categorized according to the binary metabolic syndrome (≥3 metabolic abnormalities, yes or no) to allow for direct comparisons between prior studies and our findings for white women and, afterward, to compare our results across races and ethnicities. Second, metabolic abnormalities were categorized as accumulated numbers of metabolic abnormalities to further explore our findings. Given that almost all overweight (79%) and obese (98%) women had a waist circumference ≥80 cm, groups with 0 or 1 metabolic abnormality were combined for overweight and obese women (leaving 4 categories: 0 to 1, 2, 3, or ≥4 metabolic abnormalities). To enable comparison with primary analyses, the reference category comprised normal weight women with ≤2 metabolic abnormalities (without the metabolic syndrome). Third, we assessed the associations between the most frequent combinations of the metabolic syndrome components and cardiovascular risk (no metabolic abnormalities as the reference category) to explore whether specific combinations of abnormalities drove our results. Due to few events in Hispanic women, we conducted analyses according to combinations of metabolic abnormalities only in white and black women. The first 2 approaches were categorized based on metabolic abnormalities and BMI category. The categories in the third approach were independent of BMI category but adjusted for BMI and waist circumference. All categories were nominal; no ordering was assumed.
In each approach, we adjusted for age, smoking, and hormone use at baseline in primary analyses. All models were stratified by hormone therapy trial study arm and hysterectomy status because stratification allowed us to control for potential confounding. In separate models, we also adjusted for physical activity, education, parity (number of term pregnancies), and low-density lipoprotein cholesterol (LDL-C) and treatment with lipid-lowering drugs. Overall incidence rates per 1000 person-years for each race or ethnicity were age standardized to 1998 US population data using the Surveillance, Epidemiology, and End Results (SEER) program. The proportional hazards assumption was assessed by visual examination of Schoenfeld residual plots, which appeared to be satisfied unless otherwise indicated. To explore whether associations between obesity and metabolic health subtypes and cardiovascular risk differed according to race or ethnicity, we tested for effect modification through the use of statistical interaction terms in the metabolic syndrome model, but not in the models with metabolic health defined by number of metabolic abnormalities or combination of metabolic syndrome components due to lack of statistical power. Furthermore, we included only white and black women because of the small number of Hispanic women; we tested whether the associations between metabolic health status and cardiovascular risk within each BMI category differed between white and black women by use of pair-wise comparison tests.
In sensitivity analyses we included women with diabetes at baseline. In additional sensitivity analyses, we used the higher threshold for impaired fasting glucose of 110 mg/dL (6.1 mmol/L) used by European guidelines18 and the World Health Organization because several studies have found no incremental risk with a threshold of 100 mg/dL.19,20 We also conducted a sensitivity analysis in which all women receiving hormone therapy at baseline or in the active intervention arm of the hormone therapy trials were excluded. Moreover, we evaluated a different definition of metabolically unhealthy obese, that is, by being obese, defined as a BMI ≥30 or waist circumference ≥80 cm, in addition to at least 2 other metabolic syndrome components.9 In the latter analyses, normal weight and overweight women with a waist circumference <80 cm but ≥2 metabolic abnormalities were considered metabolically unhealthy, albeit not obese, and categorized separately. Finally, we explored the associations between presence of the metabolic syndrome and CVD risk according to BMI as a continuous variable using normal weight, metabolically healthy women as reference.
Analyses were performed with SAS software, version 9.3 (SAS Institute, Inc), and R 3.0 (R Foundation for Statistical Computing).
Results
Of 22 128 women with biomarker data, 7438 met exclusion criteria and 326 had missing data on covariates, yielding a final analytic cohort of 14 364 women (Figure 1). The median age of the study population was 64 years (interquartile range 57 to 69 years), and 47% were white, 36% were black, and 18% were Hispanic. As BMI increased, all components of the metabolic syndrome became more common, as did clustering of metabolic abnormalities (Table 1). Elevated blood pressure was more common in black women, but untreated hypertension was uncommon in all race and ethnicity categories (Table 2). High triglycerides and low HDL-C were more common in white and Hispanic women than in black women (Table 2). Over a median follow-up of 13 years (interquartile range 12 to 14 years), 1101 women (7.7%) had a first cardiovascular event (718 coronary heart disease, 411 ischemic stroke, 48 both events), for an incidence rate of 6.4 per 1000 person-years. Race- or ethnicity-specific incidence rates of CVD were 8.8 per 1000 person-years (95% confidence interval [CI] 8.1 to 9.6 per 1000 person-years) for white women, 4.8 per 1000 person-years (CI 4.2 to 5.5 per 1000 person-years) for black women, and 3.2 per 1000 person-years (CI 2.5 to 4.2 per 1000 person-years) for Hispanic women.
Figure 1.
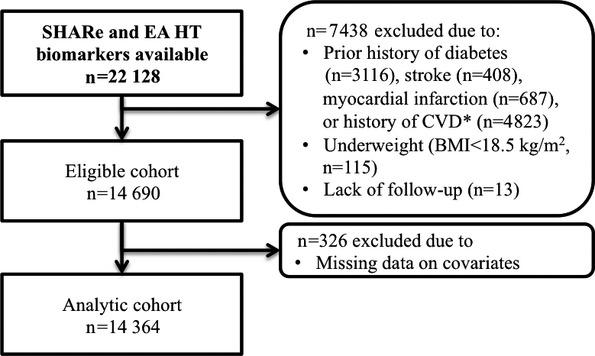
Flowchart of study. *Cardiac arrest, heart failure, cardiac catheterization, atrial fibrillation, aortic aneurism, angioplasty of coronary arteries, coronary bypass surgery, or other heart problems. BMI indicates body mass index; EA HT, European American Hormone Trial subcohort; SHARe, SNP Health Association Resource cohort.
Table 1.
Baseline Characteristics of the Analytic Cohort of Postmenopausal Women According to BMI Categories (n=14 364)
| Normal Weight (BMI 18.5 to <25) | Overweight (BMI 25 to <30) | Obese (BMI ≥30) | |
|---|---|---|---|
| n=3625 | n=5335 | n=5404 | |
| Age, y | |||
| 50 to 59 | 28 | 31 | 37 |
| 60 to 69 | 42 | 45 | 45 |
| 70 to 79 | 30 | 24 | 18 |
| Race/ethnicity | |||
| Non-Hispanic white | 56 | 46 | 42 |
| Black or African American | 25 | 35 | 43 |
| Hispanic/Latino | 19 | 19 | 15 |
| Waist circumference | |||
| ≥80 cm | 23 | 79 | 98 |
| ≥88 cm | 4 | 36 | 88 |
| High glucose (≥100 mg/dL) | 14 | 22 | 34 |
| Glucose (mg/dL), mean (SD) | 90.3 (9.4) | 92.9 (9.9) | 96.3 (10.9) |
| High measured blood pressure* (SBP ≥130 and/or DBP ≥85) | 41 | 49 | 56 |
| Hypertension* | |||
| Never hypertensive | 77 | 66 | 55 |
| Untreated hypertensive | 6 | 9 | 9 |
| Treated hypertensive | 17 | 25 | 35 |
| High triglycerides (≥150 mg/dL) | 18 | 31 | 34 |
| Low HDL-C (≤50 mg/dL) | 23 | 38 | 48 |
| Insulin resistance† | 25 | 42 | 49 |
| LDL-C‡ (mg/dL), median (IQR) | 142 (120 to 168) | 149 (125 to 174) | 148 (125 to 174) |
| Lipid-lowering drugs‡ | 10 | 14 | 12 |
| Smoking | |||
| Never | 52 | 53 | 53 |
| Former | 35 | 38 | 40 |
| Current | 13 | 9 | 7 |
| Total minutes of physical activity, hours/week | |||
| <1 | 24 | 30 | 43 |
| 1 to 2 | 15 | 17 | 17 |
| 2 to 3.5 | 19 | 20 | 17 |
| ≥3.5 | 41 | 33 | 23 |
| Hormone use | |||
| Never | 58 | 58 | 62 |
| Past | 21 | 20 | 19 |
| Current | 21 | 21 | 19 |
| HRT arm and hysterectomy status | |||
| Not randomized to HRT plus no hysterectomy | 19 | 20 | 20 |
| Not randomized to HRT plus hysterectomy | 17 | 21 | 23 |
| E-alone intervention | 10 | 11 | 13 |
| E-alone control | 9 | 12 | 13 |
| E+P intervention | 22 | 18 | 16 |
| E+P control | 22 | 18 | 15 |
| US region | |||
| Northeast | 22 | 20 | 20 |
| South | 31 | 34 | 36 |
| Midwest | 21 | 21 | 24 |
| West | 26 | 25 | 21 |
| Education‡ | |||
| Did not go to school | 0 | 0 | 0 |
| Grade school (1 to 4 years) | 1 | 1 | 1 |
| Grade school (5 to 8 years) | 1 | 2 | 2 |
| Some high school (9 to 11 years) | 4 | 5 | 6 |
| High school diploma or GED | 17 | 17 | 19 |
| Vocational or training school | 11 | 12 | 13 |
| Some college or associate degree | 26 | 27 | 28 |
| College degree or bachelor degree | 10 | 9 | 8 |
| Some postgraduate or professional | 10 | 9 | 8 |
| Master degree | 16 | 14 | 12 |
| Doctoral degree | 2 | 2 | 2 |
| Number of term pregnancies‡ | |||
| Never pregnant | 9 | 7 | 7 |
| Never had term pregnancy | 4 | 3 | 4 |
| 1 | 11 | 10 | 10 |
| 2 | 23 | 22 | 21 |
| 3 | 22 | 23 | 21 |
| 4 | 16 | 16 | 16 |
| ≥5 | 14 | 18 | 21 |
Data are shown as percentages, except as noted. BMI indicates body mass index; DBP, diastolic blood pressure; E+P, estrogen plus progestin; E-alone, estrogen alone; GED, General Educational Development; HDL-C, high-density lipoprotein cholesterol; HRT, hormone replacement therapy; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
In the models, high blood pressure comprised high measured blood pressure and treated hypertension.
Triglycerides/HDL-C >2.47 (75th percentile among healthy-weight women).
Variables with missing data: LDL-C (n=118), lipid-lowering drugs (n=5), education (n=122), and number of term pregnancies (n=92).
Table 2.
Baseline Characteristics of the Analytic Cohort of Postmenopausal Women According to Race or Ethnicity (n=14 364)
| White | Black | Hispanic | |
|---|---|---|---|
| n=6741 | n=5102 | n=2521 | |
| Age, y | |||
| 50 to 59 | 15 | 45 | 53 |
| 60 to 69 | 48 | 42 | 39 |
| 70 to 79 | 37 | 13 | 9 |
| BMI category | |||
| Normal weight (18.5 to <25) | 30 | 18 | 27 |
| Overweight (25 to <30) | 37 | 36 | 41 |
| Obese (≥30) | 33 | 46 | 32 |
| Waist circumference | |||
| ≥80 cm | 71 | 77 | 66 |
| ≥88 cm | 48 | 52 | 38 |
| High glucose (≥100 mg/dL) | 27 | 23 | 22 |
| Glucose (mg/dL), mean (SD) | 95 (10) | 93 (11) | 92 (10) |
| High blood pressure (SBP ≥130 and/or DBP ≥85) | 50 | 55 | 38 |
| Hypertension | |||
| Never hypertensive | 70 | 52 | 76 |
| Untreated hypertensive | 8 | 9 | 8 |
| Treated hypertensive | 22 | 38 | 16 |
| High triglycerides (≥150 mg/dL) | 35 | 15 | 40 |
| Low HDL-C (≤50 mg/dL) | 42 | 31 | 43 |
| Insulin resistance* | 47 | 26 | 52 |
| LDL-C† (mg/dL), median (IQR) | 152 (130 to 176) | 143 (119 to 171) | 141 (119 to 165) |
| Lipid-lowering drugs† | 12 | 12 | 12 |
| Smoking | |||
| Never | 51 | 49 | 63 |
| Former | 40 | 39 | 30 |
| Current | 9 | 12 | 6 |
| Total minutes of physical activity, hours/week | |||
| <1 | 30 | 37 | 35 |
| 1 to 2 | 17 | 16 | 16 |
| 2 to 3.5 | 19 | 18 | 18 |
| ≥3.5 | 34 | 28 | 31 |
| Hormone use | |||
| Never | 66 | 57 | 47 |
| Past | 26 | 15 | 14 |
| Current | 8 | 28 | 38 |
| HRT arm and hysterectomy status | |||
| Not randomized to HRT plus no hysterectomy | 0 | 36 | 41 |
| Not randomized to HRT plus hysterectomy | 0 | 42 | 32 |
| E-alone intervention | 18 | 6 | 5 |
| E-alone control | 18 | 7 | 6 |
| E+P intervention | 33 | 5 | 9 |
| E+P control | 31 | 5 | 8 |
| US region | |||
| Northeast | 25 | 17 | 12 |
| South | 20 | 49 | 42 |
| Midwest | 28 | 23 | 4 |
| West | 27 | 11 | 42 |
| Education† | |||
| Did not go to school | 0 | 0 | 0 |
| Grade school (1 to 4 years) | 0 | 0 | 4 |
| Grade school (5 to 8 years) | 1 | 1 | 7 |
| Some high school (9 to 11 years) | 4 | 6 | 8 |
| High school diploma or GED | 22 | 13 | 17 |
| Vocational or training school | 12 | 12 | 12 |
| Some college or associate degree | 29 | 26 | 25 |
| College degree or bachelor degree | 9 | 9 | 7 |
| Some postgraduate or professional | 0 | 9 | 6 |
| Master degree | 11 | 19 | 9 |
| Doctoral degree | 2 | 2 | 2 |
| Number of term pregnancies† | |||
| Never pregnant | 7 | 7 | 9 |
| Never had term pregnancy | 2 | 6 | 3 |
| 1 | 7 | 16 | 9 |
| 2 | 20 | 25 | 22 |
| 3 | 25 | 18 | 21 |
| 4 | 18 | 12 | 16 |
| ≥5 | 20 | 15 | 19 |
Data are shown as percentages, except as noted. BMI indicates body mass index; DBP, diastolic blood pressure; E+P, estrogen plus progestin; E-alone, estrogen alone; HDL-C, high-density lipoprotein cholesterol; HRT, hormone replacement therapy; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
Triglycerides/HDL-C >2.47 (75th percentile among healthy-weight women.
Variables with missing data: LDL-C (n=118), lipid-lowering drugs (n=5), education (n=122), and number of term pregnancies (n=92).
Metabolically Healthy Defined by the Metabolic Syndrome
Women who were metabolically unhealthy (based on the standard binary definition of metabolic syndrome) had increased CVD risk regardless of BMI category in all racial and ethnic groups (Figure 2), although there were too few events among the Hispanic women for precise estimates. White women who were overweight or obese but metabolically healthy had a cardiovascular risk that was not statistically different from normal weight metabolically healthy women (Figure 2A). Metabolically healthy obese black women, however, had a significantly increased cardiovascular risk, and overweight metabolically healthy black women showed a trend toward increased cardiovascular risk compared with normal weight metabolically healthy women (Figure 2B). The associations between metabolic health status and cardiovascular risk differed significantly between white and black women; statistically significant interactions were found between BMI categories and race or ethnicity (white or black) within metabolic health categories (Figure 2). Among the metabolically healthy women, overweight black women had higher cardiovascular risk than overweight white women (interaction P=0.05), and obese black women had higher cardiovascular risk than obese white women (interaction P=0.02). Among the metabolically unhealthy women, normal weight black women had higher cardiovascular risk than normal weight white women (interaction P=0.01), overweight black women had a trend toward higher cardiovascular risk than overweight white women (interaction P=0.18), and obese black women had higher cardiovascular risk than obese white women (interaction P=0.04).
Figure 2.
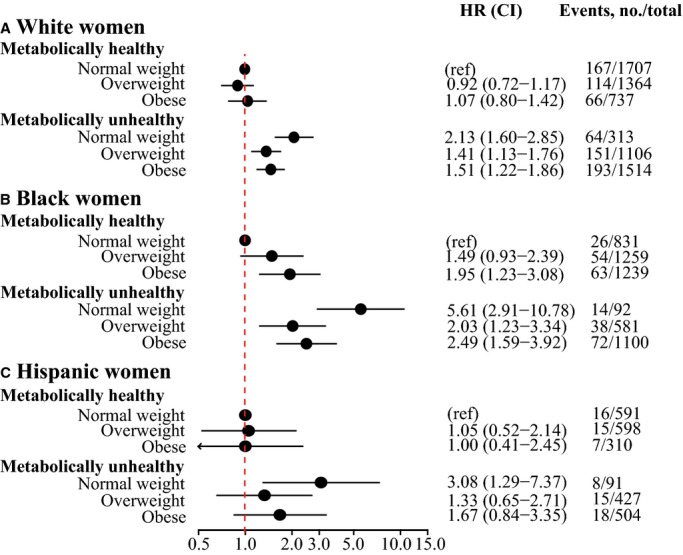
Hazard ratios of the composite outcome of coronary heart disease and ischemic stroke by BMI categories and the presence of the metabolic syndrome in (A) white women, (B) black women, and (C) Hispanic women. Healthy-weight women with <3 (0, 1, or 2) metabolic abnormalities were used as the reference in all models; models were adjusted for age, smoking, and hormone use at baseline and stratified by hormone therapy trial study arm and hysterectomy status. BMI indicates body mass index; HR, hazard ratio; ref, reference.
Metabolically Healthy Defined by Number of Metabolic Disorders
We found a graded association between increasing number of metabolic abnormalities and increased cardiovascular risk in each BMI category in both white and black women (Figure 3A and 3B). Furthermore, black women with just 2 metabolic abnormalities who were overweight or obese had increased risk of CVD (Figure 3B), even though they would be considered metabolically healthy based on the standard binary definition of metabolic syndrome (Figure 1), especially because 1 of these abnormalities was abdominal obesity for 79% of overweight women and 98% of obese women. In contrast, white obese women with 3 metabolic abnormalities did not have a statistically significantly increased cardiovascular risk (Figure 3A), although the standard binary definition of metabolic syndrome would categorize them as metabolically unhealthy. The number of Hispanic women in each category was too small to permit precise risk estimates (Figure 3C).
Figure 3.
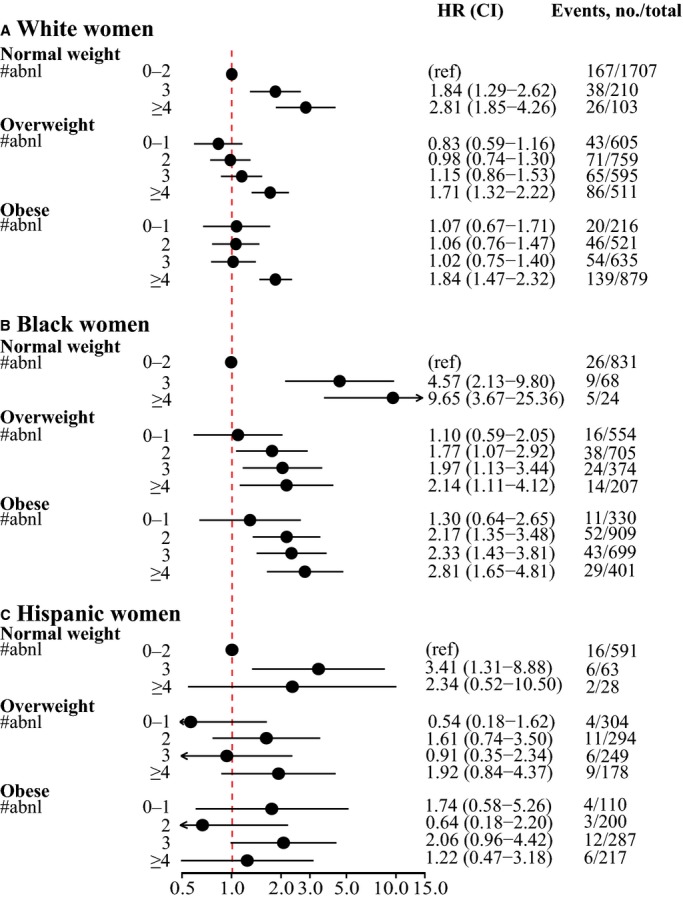
Hazard ratios of the composite outcome of coronary heart disease and ischemic stroke by BMI categories in (A) white women, (B) black women, and (C) Hispanic women, according to incremental number of metabolic abnormalities. Normal weight women with <3 (0, 1, or 2) metabolic abnormalities were used as the reference in all models; models were adjusted for age, smoking, and hormone use at baseline and stratified by hormone therapy trial study arm and hysterectomy status. #abnl indicates number of metabolic abnormalities; BMI, body mass index; HR, hazard ratio; ref, reference.
Cardiovascular Risk According to Combination of Metabolic Abnormalities
We assessed whether specific combinations of risk factors conveyed greater degrees of cardiovascular risk by combining all white women (Figure 4A) and black women (Figure 4B) and applying a model that adjusted for BMI and waist circumference. There was no strong evidence that any particular combination of 2 (or 3) factors conveyed substantially different risk than any other combination (Figure 4). Furthermore, although the sample sizes were small in the racial and ethnic subgroups, elevated blood pressure did not appear to be associated with a different CVD risk for black women compared with for white women (interaction P=0.90).
Figure 4.
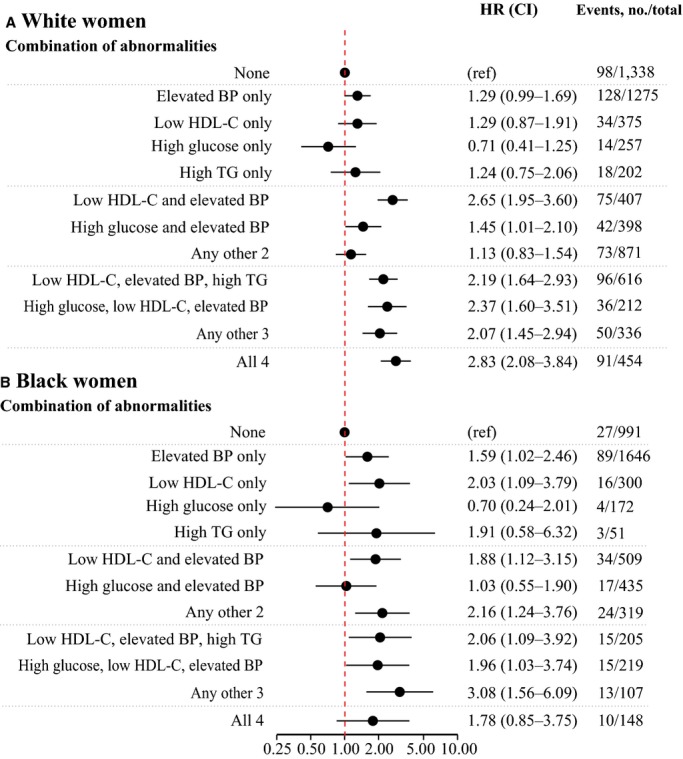
Hazard ratios of the composite outcome of coronary heart disease and ischemic stroke according to BMI categories and specific combinations of metabolic abnormalities in (A) white women and (B) black women. Women with no metabolic abnormalities were used as the reference in all models, and all models were adjusted for age, smoking, BMI, waist circumference, and hormone use at baseline and stratified by hormone therapy trial study arm and hysterectomy status. BMI indicates body mass index; BP, blood pressure; HDL-C, high density lipoprotein-cholesterol; HR, hazard ratio; ref, reference; TG, triglycerides.
Sensitivity Analyses
The main findings of this study were not changed when women with diabetes at baseline were included and categorized as fulfilling the criterion for impaired fasting glucose (ie, glucose ≥100 mg/dL) (Table 3) or when we used the higher threshold for impaired fasting glucose of 110 mg/dL in the primary study cohort (Table 4). Following exclusion of all women receiving hormone therapy at baseline, the population for these sensitivity analyses comprised 3057 white women, 3217 black women, and 1243 Hispanic women. Although limited by few events, particularly among nonwhite participants, the hazard ratios did not change considerably, leaving our main findings unaltered. Furthermore, our results were unchanged when we defined metabolically unhealthy as being obese (waist circumference ≥80 cm or BMI ≥30) in addition to having at least 2 metabolic abnormalities (Tables 5 and 6). Analyses examining associations between the metabolic syndrome and CVD risk, treating BMI as continuous, confirmed our findings that the metabolically healthy obese concept applied to white women but not to all races and ethnicities (Figure 5). Finally, self-reported physical activity, education, parity, and LDL-C and lipid-lowering drugs did not change any of the estimates considerably (data not shown).
Table 3.
HRs of Cardiovascular Disease According to Body Mass Index and Metabolic Health Including Women With Diabetes*
| White | Black | Hispanic | ||||
|---|---|---|---|---|---|---|
| n=7508 | n=6043 | n=2802 | ||||
| Metabolic Abnormalities | HR | CI | HR | CI | HR | CI |
| Normal weight | ||||||
| 0 to 2 | Ref | — | Ref | — | Ref | — |
| 3 | 1.96 | 1.41 to 2.73 | 3.60 | 1.76 to 7.36 | 2.62 | 1.02 to 6.68 |
| ≥4 | 2.57 | 1.76 to 3.75 | 6.51 | 2.98 to 14.21 | 3.30 | 1.09 to 9.98 |
| Overweight | ||||||
| 0 to 1 | 0.79 | 0.57 to 1.11 | 0.99 | 0.55 to 1.79 | 0.48 | 0.16 to 1.42 |
| 2 | 0.99 | 0.76 to 1.30 | 1.59 | 0.99 to 2.54 | 1.34 | 0.63 to 2.85 |
| 3 | 1.15 | 0.87 to 1.51 | 1.81 | 1.10 to 2.98 | 0.83 | 0.35 to 2.01 |
| ≥4 | 1.89 | 1.49 to 2.37 | 3.29 | 2.03 to 5.31 | 2.30 | 1.15 to 4.58 |
| Obese | ||||||
| 0 to 1 | 1.02 | 0.64 to 1.63 | 1.18 | 0.60 to 2.32 | 1.51 | 0.51 to 4.49 |
| 2 | 1.05 | 0.77 to 1.44 | 1.84 | 1.18 to 2.88 | 0.68 | 0.23 to 2.04 |
| 3 | 1.12 | 0.85 to 1.49 | 2.69 | 1.77 to 4.08 | 2.07 | 1.04 to 4.11 |
| ≥4 | 1.96 | 1.60 to 2.40 | 3.21 | 2.09 to 4.91 | 1.53 | 0.74 to 3.20 |
All models were adjusted for age, smoking, and hormone use at baseline and stratified by hormone therapy trial study arm and hysterectomy status. HR indicates hazard ratio.
Women with diabetes were categorized as having an abnormal glucose metabolism regardless of fasting glucose levels.
Table 4.
HRs of Cardiovascular Disease According to Body Mass Index and Metabolic Health Defining Impaired Glucose Metabolism as Fasting Glucose ≥110 mg/dL in a Cohort of Postmenopausal Women
| White | Black | Hispanic | ||||
|---|---|---|---|---|---|---|
| n=6741 | n=5102 | n=2521 | ||||
| Metabolic Abnormalities | HR | CI | HR | CI | HR | CI |
| Normal weight | ||||||
| 0 to 2 | Ref | — | Ref | — | Ref | — |
| 3 | 1.75 | 1.18 to 2.59 | 2.81 | 1.09 to 7.29 | 3.11 | 1.14 to 8.54 |
| ≥4 | 3.02 | 1.97 to 4.64 | 11.02 | 4.23 to 28.69 | 1.47 | 0.19 to 11.22 |
| Overweight | ||||||
| 0 to 1 | 0.72 | 0.52 to 1.00 | 0.96 | 0.53 to 1.72 | 0.76 | 0.32 to 1.84 |
| 2 | 1.07 | 0.82 to 1.38 | 1.61 | 1.00 to 2.59 | 1.18 | 0.53 to 1.81 |
| 3 | 1.17 | 0.88 to 1.55 | 2.11 | 1.25 to 3.58 | 0.96 | 0.40 to 2.32 |
| ≥4 | 1.76 | 1.33 to 2.33 | 1.29 | 0.57 to 2.95 | 1.81 | 0.75 to 4.37 |
| Obese | ||||||
| 0 to 1 | 0.87 | 0.55 to 1.37 | 1.18 | 0.61 to 2.28 | 2.02 | 0.79 to 5.15 |
| 2 | 1.05 | 0.77 to 1.42 | 1.73 | 1.11 to 2.71 | 0.53 | 0.16 to 1.81 |
| 3 | 1.24 | 0.94 to 1.64 | 2.56 | 1.61 to 4.07 | 1.45 | 0.66 to 3.12 |
| ≥4 | 1.84 | 1.45 to 2.35 | 2.47 | 1.39 to 4.37 | 1.50 | 0.58 to 3.84 |
All models were adjusted for age, smoking, and hormone use at baseline and stratified by hormone therapy trial study arm and hysterectomy status. HR indicates hazard ratio.
Table 5.
HRs of Cardiovascular Disease in a Cohort of Postmenopausal Women According to BMI and Metabolic Health Using a Different Categorization*
| White | Black | Hispanic | ||||
|---|---|---|---|---|---|---|
| n=6741 | n=5102 | n=2521 | ||||
| Binary Model | HR | CI | HR | CI | HR | CI |
| Metabolically healthy* | ||||||
| Normal weight | Ref | — | Ref | — | Ref | — |
| Overweight | 1.05 | 0.81 to 1.37 | 1.54 | 0.95 to 2.52 | 1.30 | 0.60 to 2.78 |
| Obese | 1.25 | 0.92 to 1.69 | 1.85 | 1.15 to 3.00 | 1.13 | 0.44 to 2.91 |
| Metabolically unhealthy† | ||||||
| Normal weight, WC <80 cm | 2.09 | 1.54 to 2.82 | 1.24 | 0.50 to 3.05 | 3.17 | 1.32 to 7.61 |
| Normal weight, WC ≥80 cm‡ | 2.60 | 1.84 to 3.67 | 6.18 | 3.00 to 12.73 | 2.05 | 0.56 to 7.44 |
| Overweight, WC <80 cm | 1.18 | 0.65 to 2.14 | 0.88 | 0.26 to 2.95 | 1.69 | 0.47 to 6.03 |
| Overweight, WC ≥80 cm‡ | 1.66 | 1.31 to 2.12 | 1.88 | 1.11 to 3.19 | 1.32 | 0.59 to 2.96 |
| Obese‡ | 1.74 | 1.38 to 2.20 | 2.46 | 1.53 to 3.94 | 1.88 | 0.89 to 3.98 |
BMI indicates body mass index; HR, hazard ratio; WC, waist circumference.
Metabolically healthy defined as having <2 metabolic abnormalities.
Metabolically unhealthy defined as having ≥2 metabolic abnormalities.
Metabolically unhealthy obese: obese (WC ≥80 cm or BMI ≥30) and ≥2 additional metabolic abnormalities.
Table 6.
HRs of Cardiovascular Disease in a Cohort of Postmenopausal Women According to BMI and Metabolic Health Using a Different Categorization*
| White | Black | Hispanic | ||||
|---|---|---|---|---|---|---|
| n=6741 | n=5102 | n=2521 | ||||
| Numeric Model | HR | CI | HR | CI | HR | CI |
| Normal weight | ||||||
| 0 to 1 | Ref | — | Ref | — | Ref | — |
| 2 | 2.02 | 1.49 to 2.73 | 1.72 | 0.79 to 3.73 | 1.80 | 0.63 to 5.18 |
| ≥3 | 2.79 | 1.98 to 3.95 | 6.09 | 2.71 to 13.69 | 4.72 | 1.81 to 12.28 |
| Overweight | ||||||
| 0 | 0.84 | 0.56 to 1.27 | 1.07 | 0.51 to 2.26 | 0.47 | 0.11 to 2.12 |
| 1 | 1.15 | 0.87 to 1.54 | 1.72 | 1.04 to 2.86 | 1.77 | 0.80 to 3.90 |
| 2 | 1.33 | 0.99 to 1.79 | 1.51 | 0.84 to 2.72 | 0.50 | 0.14 to 1.79 |
| ≥3 | 1.95 | 1.48 to 2.56 | 2.15 | 1.13 to 4.09 | 2.48 | 1.11 to 5.58 |
| Obese | ||||||
| 0 | 1.27 | 0.78 to 2.06 | 1.33 | 0.65 to 2.75 | 2.09 | 0.67 to 6.52 |
| 1 | 1.24 | 0.88 to 1.74 | 2.02 | 1.23 to 3.32 | 0.71 | 0.20 to 2.54 |
| 2 | 1.16 | 0.83 to 1.60 | 2.29 | 1.38 to 3.82 | 2.32 | 1.03 to 5.25 |
| ≥3 | 2.15 | 1.68 to 2.75 | 2.68 | 1.54 to 4.67 | 1.37 | 0.50 to 3.72 |
BMI indicates body mass index; HR, hazard ratio.
Waist circumference not included, and the reference category was defined as <2 metabolic abnormalities to make the models comparable; metabolically healthy obese was defined as being obese (waist circumference ≥80 cm or BMI ≥30) and having <2 additional metabolic abnormalities.
Figure 5.
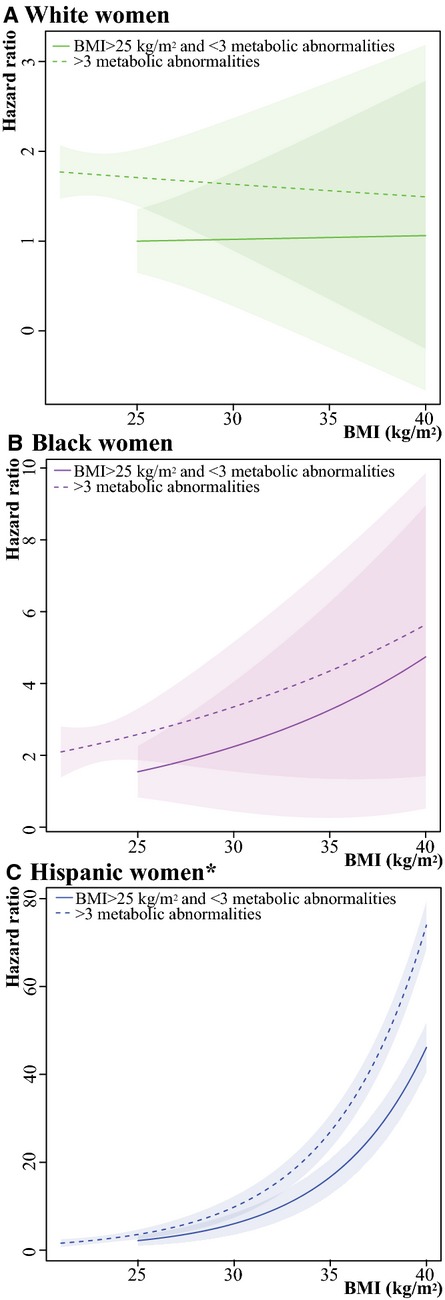
Hazard ratios of the composite outcome of coronary heart disease and ischemic stroke according to presence of the metabolic syndrome by increasing body mass index (BMI) in (A) white women, (B) black women, and (C) Hispanic women. Healthy-weight women with <3 (0, 1, or 2) metabolic abnormalities were used as the reference in all models; models were adjusted for age, smoking, and hormone use at baseline and stratified by hormone therapy trial study arm and hysterectomy status. The shaded regions around the lines gives the 95% confidence band, the standard error, for the mean. *Please note that the scale of the y-axis differs for white (0 to 3), black (0 to 10), and Hispanic (0 to 80) women, so the widths of the CIs are not comparable.
Discussion
In this prospective study of postmenopausal women without diabetes or prior CVD, the risk of developing CVD was more strongly associated with the presence of metabolic abnormalities than with the presence of obesity, regardless of racial or ethnic background. Furthermore, metabolic abnormalities appeared to confer more cardiovascular risk among black women than among white women. Almost all prior studies of obesity, metabolic health, and cardiovascular risk have been limited to white participants and have relied on the presence or absence of metabolic syndrome to define metabolic health.5,9,16 Our finding that obesity in white women was not associated with increased cardiovascular risk unless accompanied by the metabolic syndrome confirms the results of other studies.8,9 Our most novel finding was that black women who were overweight or obese had elevated cardiovascular risk even when they did not have the metabolic syndrome. It appeared that the cardiovascular risk was elevated in black women by the presence of only 2 or 3 metabolic abnormalities to a degree that would require ≥4 metabolic abnormalities among white women (Figure 2). The metabolic syndrome itself has already been evaluated extensively and found to be no greater than the sum of its parts.21,22 This study further suggests that the use of the “metabolic syndrome” to define the metabolically healthy obese group appears to underestimate risk among black women and to overestimate cardiovascular risk in white women. Instead, an individualized approach in which the clinician considers the sum and the type of metabolic syndrome components in light of the patient's overall risk profile should be encouraged, especially when applied to populations other than white.
It has been controversial whether metabolically healthy obesity is a valid concept, and our study suggests that this concept may not apply to black women. A recent meta-analysis and systematic review found that the lower cardiovascular risk in metabolically healthy obese persons was documented only in studies that had limited follow-up (<10 years of follow-up).16 The findings, however, were unadjusted and also might have been limited by ecological bias. Obesity has been found to be associated with long-term cardiovascular risk,23 and long-standing obesity has been somewhat more strongly associated with cardiovascular risk.24 The Norwegian HUNT study found that being obese but metabolically healthy was not associated with increased risk of myocardial infarction but with increased risk of heart failure,9 whereas a study of younger persons found that the composite cardiovascular risk (myocardial infarction, stroke, coronary revascularization) of the metabolically healthy obese group was comparable to normal weight metabolically healthy persons only in analyses adjusted for fitness.5 In our study, adjustment for the level of physical activity did not change the results, perhaps because the WHI population was older and female. Furthermore, in a recent Korean cross-sectional study, being metabolically healthy obese (obese without any metabolic syndrome components and homeostasis model assessment for insulin resistance <2.5) was associated with subclinical coronary atherosclerosis but not after adjustment for lipids (LDL-C, HDL-C, and triglycerides), systolic blood pressure, homeostasis model assessment for insulin resistance, and glucose.25 These findings suggest that obesity is not a harmless condition because it is closely correlated with increased risk of insulin resistance and risk of developing metabolic abnormalities.26 Indeed, a recently published study found that after 5 years of follow-up, 31.8% of metabolically healthy obese individuals (defined as BMI ≥30 and ≤1 metabolic syndrome component, 75% men) had become metabolically unhealthy, rising to 51.5% after 20 years.27 In the absence of metabolic syndrome, obesity might still be associated with increased metabolic risk among black women and thus the need for lifestyle intervention as a “window of opportunity.” Our findings underscore the importance of preventing the development of metabolic disorders, particularly in black overweight and obese women, in whom metabolic disorders were more strongly associated with increased cardiovascular risk than in white women.
Strengths and Limitations
This large study is unique in having more black participants than other studies and thus could evaluate racial and ethnic differences in the risk of CVD according to obesity and metabolic health status. Its other strengths include the use of prospectively collected data with coronary heart disease and ischemic stroke as adjudicated end points, the long follow-up, and the available fasting measures of glucose and lipid levels for ≈15 000 postmenopausal women without CVD or diabetes.
This study also had several limitations. First, the WHI enrolled only postmenopausal women, so our results may not be generalizable either to younger women or to men. Second, the definition of metabolically healthy obese varies among studies,16 although most use either the ATP III8 or the International Diabetes Federation criteria for the metabolic syndrome.5,9 Without a uniform definition of metabolically healthy obese, it is difficult to compare studies.28 We used the most commonly applied definition, and our findings were unchanged when we applied an alternative, also widely used definition of metabolically healthy,9 namely, ≥2 metabolic abnormalities in addition to obesity (defined as BMI ≥30 or a waist circumference ≥80 cm). Third, menopausal hormone therapy increases HDL-C and triglycerides, which should be taken into account when interpreting the results. Nevertheless, changes induced by hormone therapy were not associated with future risk of coronary heart disease in a WHI study.29 Fourth, the number of Hispanic women was limited, so we could not include an interaction term between race, and the obesity and metabolic abnormality categories in the model with metabolic health defined by number of metabolic abnormalities. Instead, we reported the estimates of risk by race or ethnicity and noted differences that were consistent with our hypothesis and that should be formally tested in future studies designed to have adequate statistical power. The uncertainty surrounding the estimates in the Hispanic women was greater because of the relatively small number of Hispanic women in the cohort. Finally, muscle tissue composes a smaller proportion of overall body mass in postmenopausal women, thus BMI may be lower in these women despite higher fat mass.
In conclusion, in postmenopausal women without diabetes or prior CVD, the presence of even a few metabolic abnormalities was more strongly associated with increased cardiovascular risk in black overweight and black obese women than in white women. Women with ≤1 metabolic abnormality who were overweight or obese did not appear to have increased risk of CVD regardless of race or ethnicity.
Acknowledgments
We would like to acknowledge the Women's Health Initiative investigators and all the participants in the Women's Health Initiative trials. A list of the Women's Health Initiative investigators has been provided in an Appendix S1.
Sources of Funding
The Women's Health Initiative programs is funded by the National Heart, Lung, and Blood Institute, NIH, US Department of Health and Human Services through contracts, HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C. Schmiegelow was supported by a grant from the University of Copenhagen, Denmark. The University of Copenhagen had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
None.
Supporting Information
Appendix S1. Short list of Women's Health Intiative investigators.
References
- WHO. Obesity and overweight, fact sheet no 311. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed May 30, 2013.
- Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Global Burden of Metabolic Risk Factors for Chronic Diseases C. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Endocrinol Metab Clin North Am. 2008;37:581–601. doi: 10.1016/j.ecl.2008.06.005. vii–viii. [DOI] [PubMed] [Google Scholar]
- Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34:389–397. doi: 10.1093/eurheartj/ehs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trondelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–1078. doi: 10.1016/j.jacc.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Howard BV, Criqui MH, Curb JD, Rodabough R, Safford MM, Santoro N, Wilson AC, Wylie-Rosett J. Risk factor clustering in the insulin resistance syndrome and its relationship to cardiovascular disease in postmenopausal white, black, hispanic, and Asian/Pacific Islander women. Metabolism. 2003;52:362–371. doi: 10.1053/meta.2003.50057. [DOI] [PubMed] [Google Scholar]
- Jones DW, Chambless LE, Folsom AR, Heiss G, Hutchinson RG, Sharrett AR, Szklo M, Taylor HA., Jr Risk factors for coronary heart disease in African Americans: the Atherosclerosis Risk in Communities study, 1987–1997. Arch Intern Med. 2002;162:2565–2571. doi: 10.1001/archinte.162.22.2565. [DOI] [PubMed] [Google Scholar]
- Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S78–S86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159:758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jonsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyorala K, Raz I, Schernthaner G, Volpe M, Wood D. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The task force on diabetes and cardiovascular diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chunawala L, Linde R, Reaven GM. Comparison of the 1997 and 2003 American Diabetes Association classification of impaired fasting glucose: impact on prevalence of impaired fasting glucose, coronary heart disease risk factors, and coronary heart disease in a community-based medical practice. J Am Coll Cardiol. 2006;48:293–297. doi: 10.1016/j.jacc.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Levitzky YS, Pencina MJ, D'Agostino RB, Meigs JB, Murabito JM, Vasan RS, Fox CS. Impact of impaired fasting glucose on cardiovascular disease: the Framingham Heart Study. J Am Coll Cardiol. 2008;51:264–270. doi: 10.1016/j.jacc.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Iribarren C, Go AS, Husson G, Sidney S, Fair JM, Quertermous T, Hlatky MA, Fortmann SP. Metabolic syndrome and early-onset coronary artery disease: is the whole greater than its parts? J Am Coll Cardiol. 2006;48:1800–1807. doi: 10.1016/j.jacc.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Simons LA, Simons J, Friedlander Y, McCallum J. Is prediction of cardiovascular disease and all-cause mortality genuinely driven by the metabolic syndrome, and independently from its component variables? The Dubbo study. Heart Lung Circ. 2011;20:214–219. doi: 10.1016/j.hlc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, Derazne E, Tzur D, Shamis A, Vinker S, Rudich A. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364:1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis JP, Loria CM, Lewis CE, Powell-Wiley TM, Wei GS, Carr JJ, Terry JG, Liu K. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–288. doi: 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung HS, Choi Y, Ahn J, Lima JA, Shin H, Guallar E, Ryu S. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–2686. doi: 10.1016/j.jacc.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Schmiegelow MD, Andersson C, Kober L, Andersen SS, Norgaard ML, Jensen TB, Gislason G, Berger SM, Torp-Pedersen C. Associations between body mass index and development of metabolic disorders in fertile women—a nationwide cohort study. J Am Heart Assoc. 2014;3:e000672. doi: 10.1161/JAHA.113.000672. doi: 10.1161/JAHA.113.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JA, Hamer M, Sabia S, Singh-Manoux A, Batty GD, Kivimaki M. The natural course of healthy obesity over 20 years. J Am Coll Cardiol. 2015;65:101–102. doi: 10.1016/j.jacc.2014.09.077. [DOI] [PubMed] [Google Scholar]
- Pataky Z, Bobbioni-Harsch E, Golay A. Open questions about metabolically normal obesity. Int J Obes (Lond) 2010;34(suppl 2):S18–S23. doi: 10.1038/ijo.2010.235. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Cushman M, Greenland P, Lloyd-Jones DM, Bray P, Kooperberg C, Pettinger M, Robinson J, Hendrix S, Hsia J. Inflammatory, lipid, thrombotic, and genetic markers of coronary heart disease risk in the Women's Health Initiative trials of hormone therapy. Arch Intern Med. 2008;168:2245–2253. doi: 10.1001/archinte.168.20.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Short list of Women's Health Intiative investigators.


