Abstract
Background
Elevated serum phosphorus might aggravate the effect of hypertension on mortality. The objective of this study was to examine the joint effect of hypertension and serum phosphorus on the risk of mortality.
Methods and Results
A large prospective (n=15 833), population-based cohort of participants from the National Health and Nutritional Examination Survey III was examined to test potential synergism between hypertension, elevated serum phosphorus, and the risk of mortality. Interaction on additive scale and multiplicative scale was estimated. After a median follow-up of 14.3 years, 1691 cases of cardiovascular mortality and 3875 cases of all-cause mortality were identified. Interaction was observed between hypertension and elevated serum phosphorus on the additive scale for cardiovascular mortality (relative excess risk due to interaction, 0.99, 95% CI: 0.06; 1.92, adjusted for age, gender, race, and estimated glomerular filtration rate). No statistically significant interaction was found between hypertension and serum phosphorus for all-cause mortality on the additive scale. No significant interaction was detected on the multiplicative scale. In sensitivity analysis, excluding participants who died in first 2 years and adjustment for additional confounders resulted in essentially similar findings.
Conclusions
The joint effect of hypertension and elevated serum phosphorus was larger than the sum of the independent effects on cardiovascular mortality but not on all-cause mortality. Future studies should investigate whether controlling elevated serum phosphorus in hypertensive individuals helps in prevention of extra risk of cardiovascular mortality.
Keywords: hypertension, interaction, mortality, RERI, serum phosphorus
Hypertension is a global public health problem. It is one of the major causes of premature deaths worldwide, killing nearly 8 million people every year, and 92 million disabled years are attributed to hypertension.1 Lowering blood pressure reduces risk of cardiovascular morbidity and mortality, and all-cause mortality.2 Besides controlling hypertension itself, understanding other modifiable risk factors, which might enhance complications in hypertensive individuals, is important.
Recently, a number of observational studies have shown an independent relationship between elevated serum phosphorus and risks of cardiovascular as well as all-cause mortality in the general population.3,4 Impaired intestinal phosphate absorption, renal phosphate reabsorption, and phosphate metabolism might elevate serum phosphorus level.5 Elevated serum phosphorus increases mortality risk through vascular injury and calcification.6,7 Among hypertensive individuals, it is well established that the left ventricular wall thickens in response to elevated blood pressure as a compensatory mechanism to minimize wall stress.8 Subsequently, after a series of poorly characterized events (“transition to failure”), the left ventricular ejection fraction declines and the risk of mortality increases. It is possible that, in individuals with both elevated serum phosphorus and hypertension, impaired vessel walls (resulting from elevated serum phosphorus) together with high blood pressure might aggravate the response of the left ventricular wall and ultimately the risk of mortality.9 Therefore, the joint effect of hypertension and elevated serum phosphorus on mortality might be larger than the sum of their independent effects, particularly on cardiovascular mortality.
This hypothesis was tested in a large, population-based, prospective cohort of adult general population by examining the joint effect of hypertension and elevated serum phosphorus on risk of cardiovascular and all-cause mortality.
Methods
Study Design and Population
Data of subjects participating in the Third National Health and Nutritional Examination Survey 1988–1994 (NHANES III), a nationally representative sample of civilian noninstitutionalized US population was used. Briefly, the NHANES surveys are cross-sectional, multistage, stratified, clustered probability samples of the noninstitutionalized US civilian population. The details of the study can be found at http://www.cdc.gov/nchs/nhanes.htm. For the current study, data of participants older than 18 years with complete data on blood pressure, serum phosphorus and mortality, and other relevant covariates (n=15 833) were examined. The National Centres for Health Statistics Ethics Review Board approved the study protocol, and each participant provided written informed consent.
Measurements
Blood pressure and hypertension
Blood pressure was measured by a trained research assistant 3 times during the in-home visits and 3 additional times by a trained clinician during the visit to the mobile examination clinic. In both settings, blood pressures were measured with the participant in the sitting position after 5 minutes of rest. For systolic and diastolic blood pressure, separately, the second and third measurements from each visit were averaged. Hypertension was defined as self-reported history of hypertension, measured systolic blood pressure ≥140 mm Hg, measured diastolic blood pressure ≥90 mm Hg, or self-reported use of blood pressure medications.10
Measurement of serum phosphorus
Serum phosphorus was measured using a Hitachi model 737 multichannel analyzer (Boehringer Mannheim Diagnostics) by reacting inorganic phosphorus with ammonium molybdate in an acidic solution to form ammonium phosphomolybdate, which was quantified in the UV range (340 nm) through the use of a sample-blanked end-point method.11 Elevated serum phosphorus was defined as serum phosphorus concentration ≥1.36 mmol/L (4.2 mg/dL).
Mortality follow-up
Adult NHANES III participants were followed for mortality through December 31, 2006. Probabilistic matching was used to link NHANES III participants with the National Death Index to ascertain vital status. Matching was based on 12 identifiers for each participant (eg, Social Security number, gender, and date of birth), leading to correct matching in 96.1% of deceased participants and 99.4% of living participants. Cardiovascular mortality was defined by International Classification of Diseases, Tenth Edition, Clinical Modification codes 100 to 178 derived from death certificate data.
Covariates
Information on age, gender, race, cigarette smoking, history of cardiovascular disease, and family income was self-reported by the participants and was obtained via interview during in-home visits. Socioeconomic status was assessed by the poverty income ratio, which is the ratio of family income to the federal poverty threshold (specific to the year of the interview).
Height and weight were measured using standardized methods, and body mass index was calculated as weight in kilograms divided by height in meters squared. Serum creatinine, cholesterol, and glucose were measured as previously described.12 Kidney function was assessed from estimated glomerular filtration rate (eGFR). Glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation after calibrating serum creatinine values to Cleveland Clinic reference values.13 Diabetes was defined in accordance with the criteria of the American Diabetes Association (ie, fasting plasma glucose ≥7 mmol/L [126 mg/dL] and/or current use of insulin or oral hypoglycemic agents).14
Statistical Analysis
A Cox proportional hazards model was used to estimate hazard ratios of cardiovascular and all-cause mortality with 95% CI. Because eGFR is related to serum phosphorus and hypertension, relationship between hypertension, elevated serum phosphorus, and mortality was examined in a multivariate analysis adjusted for eGFR along with age, gender, and race. Proportional hazards assumption was evaluated by Schönfeld's residuals and by inspection of log-log plots.
Given that the nature of a joint effect can be additive or multiplicative,15,16 the joint effect of hypertension and elevated serum phosphorus on risk of mortality was investigated by testing interaction between hypertension and elevated serum phosphorus on an additive and multiplicative scale. Additive interaction indicates that an effect is more than additive when the risk ratios found are greater than the sum of individual risk ratios. Interaction on the additive scale was assessed by calculating the relative excess risk caused by interaction (RERI), using the algorithm of Andersson et al.17 RERI was calculated as (HR11−HR10−HR01)+1, where HR11 is the hazard ratio for both risk factors present, HR10 is the hazard ratio for hypertension but no elevated serum phosphorus, and HR01 is the hazard ratio for no hypertension but elevated serum phosphorus. RERI effects were considered significant when 95% CI of RERI did not contain zero. Interaction on the multiplicative scale was assessed by comparing multiplicative models with and without an interaction term using the log-likelihood ratio test.
Sensitivity analyses were performed to test the robustness of our findings. First, to account for possible reverse causation, we performed analyses after excluding participants who died in the first 2 years of follow-up. Second, because use of diuretic antihypertensive medication can affect blood pressure, risk of mortality, as well as affect serum phosphorus levels, we performed analyses after excluding participants who were on diuretic medication. Third, we additionally adjusted for dietary phosphorus intake. Finally, interactions were tested after adjustment for additional covariates in association between hypertension, elevated serum phosphorus, and mortality. Models were additionally adjusted for socioeconomic status, body mass index, smoking, cardiovascular disease history, diabetes, serum cholesterol, and vitamin D. In accordance with NHANES analytic guidelines, NHANES III specific sampling weights were incorporated to account for its complex survey design (http://www.cdc.gov/nchs/tutorials/nhanes/stata_tips.htm). All analyses were performed using Stata Statistical Software (version 13.0; Stata Corp).
Results
A total of 1691 cases of cardiovascular mortality and 3875 cases of all-cause mortality were identified over a median follow-up of 14.3 years. Baseline characteristics of the study population are shown in Table 1. The HRs of hypertension and elevated serum phosphorus, adjusted for age, gender, race, and eGFR, are shown in Table 2. Hypertension and elevated serum phosphorus separately were significantly associated with the risk of cardiovascular and all-cause mortality. The joint effect of hypertension and elevated serum phosphorus on the risk of mortality is shown in Table 3, and is visualized in Figure 1 for cardiovascular mortality. The joint effect of hypertension and elevated serum phosphorus on the risk of cardiovascular mortality and all-cause mortality was (HR=2.80, 95% CI: 2.09; 3.73) and (HR=1.76, 95% CI: 1.49; 2.07), respectively. For cardiovascular mortality, interaction was observed between hypertension and elevated serum phosphorus on the additive scale (RERI=0.99, 95% CI: 0.06; 1.92, adjusted for age, gender, race, and eGFR), suggesting that the joint effect of hypertension and elevated serum phosphorus is stronger than is the sum of the independent effects of individual risk factor. With respect to all-cause mortality, no clear evidence of interaction was observed between hypertension and elevated serum phosphorus on the additive scale (RERI=0.16, 95% CI: −0.25; 0.57, adjusted for age, gender, race, and eGFR). No substantial interaction was observed on the multiplicative scale between hypertension and elevated serum phosphorus either for cardiovascular mortality (P=0.130, log-likelihood ratio test) or all-cause mortality (P=0.741, log-likelihood ratio test).
Table 1.
Baseline Characteristics of Hypertensive Cases, Elevated Serum Phosphorus Cases, Hypertensive Cases With Elevated Serum Phosphorus, and Entire Cohort
| Characteristics | Hypertension (n=5850) | Elevated Serum Phosphorus (n=1325) | Hypertension and Elevated Serum Phosphorus (n=455) | Entire Cohort (N=15 833) |
|---|---|---|---|---|
| Age, y | 54 (0.5) | 43 (0.6) | 56 (0.9) | 44 (0.4) |
| Gender (male), % | 49 | 37 | 33 | 48 |
| Race, % | ||||
| Whites | 77 | 70 | 72 | 76 |
| African Americans | 13 | 15 | 15 | 11 |
| Mexican Americans | 4 | 7 | 6 | 5 |
| Others | 6 | 8 | 8 | 8 |
| Serum phosphorus, mmol/L | 1.10 (<0.1) | 1.43 (<0.1) | 1.44 (0.1) | 1.11 (<0.1) |
| Poverty (yes), % | 13 | 19 | 19 | 12 |
| Smoking, % | 56 | 54 | 57 | 54 |
| CVD history (yes), % | 13 | 6 | 14 | 6 |
| Body mass index, kg/m2 | 29 (0.2) | 26 (0.3) | 29 (0.3) | 27 (0.1) |
| SBP, mm Hg | 138 (0.6) | 121 (0.9) | 141 (1.0) | 122 (0.4) |
| DBP, mm Hg | 80 (0.2) | 73 (0.4) | 79 (0.6) | 74 (0.2) |
| Diabetes mellitus (yes), % | 13 | 7 | 16 | 7 |
| Serum glucose, mmol/L | 5.83 (0.1) | 5.24 (0.1) | 6.03 (0.1) | 5.36 (<0.1) |
| Serum cholesterol, mmol/L | 5.71 (<0.1) | 5.34 (0.1) | 5.77 (0.1) | 5.29 (<0.1) |
| eGFR, mL/min per 1.73 m2 | 85 (0.6) | 93 (1.2) | 81 (1.4) | 94 (0.5) |
Continuous variables are presented as mean (standard error), categorical variables are presented as percentages. CVD indicates cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Table 2.
Crude Incidence Rate and Adjusted* Hazard Ratios of Cardiovascular and All-Cause Mortality for Hypertension and Elevated Serum Phosphorus
| Risk Factors | Cases (n) | Crude Incidence Rate | Hazard Ratio | P Value |
|---|---|---|---|---|
| Cardiovascular mortality | ||||
| Hypertension | 1234 | 17.7 | 1.86 (1.48 to 2.34) | <0.001 |
| Elevated serum phosphorus | 141 | 7.8 | 1.45 (1.12 to 1.88) | 0.005 |
| All-cause mortality | ||||
| Hypertension | 2551 | 36.6 | 1.45 (1.25 to 1.67) | <0.001 |
| Elevated serum phosphorus | 296 | 16.4 | 1.22 (1.05 to 1.41) | 0.01 |
Adjusted for age, gender, race, and estimated glomerular filtration rate.
Table 3.
Crude Incidence Rate and Adjusted* Hazard Ratios of Cardiovascular and All-Cause Mortality for Joint Effect of Hypertension and Elevated Serum Phosphorus
| Risk Factors | Cases (n) | Crude Incidence Rate | Hazard Ratio | P Value |
|---|---|---|---|---|
| Cardiovascular mortality | ||||
| No hypertension | ||||
| No elevated serum phosphorus | 422 | 3.3 | Reference | |
| Elevated serum phosphorus | 33 | 2.6 | 1.01 (0.65 to 1.56) | 0.97 |
| Hypertension | ||||
| No elevated serum phosphorus | 1124 | 17.5 | 1.80 (1.41 to 2.28) | <0.001 |
| Elevated serum phosphorus | 108 | 19.8 | 2.80 (2.09 to 3.73) | <0.001 |
| All-cause mortality | ||||
| No hypertension | ||||
| No elevated serum phosphorus | 1222 | 9.5 | Reference | |
| Elevated serum phosphorus | 97 | 7.7 | 1.16 (0.89 to 1.51) | 0.26 |
| Hypertension | ||||
| No elevated serum phosphorus | 2340 | 36.4 | 1.44 (1.24 to 1.67) | <0.001 |
| Elevated serum phosphorus | 199 | 36.4 | 1.76 (1.49 to 2.07) | <0.001 |
Adjusted for age, gender, race, and estimate glomerular filtration rate.
Figure 1.
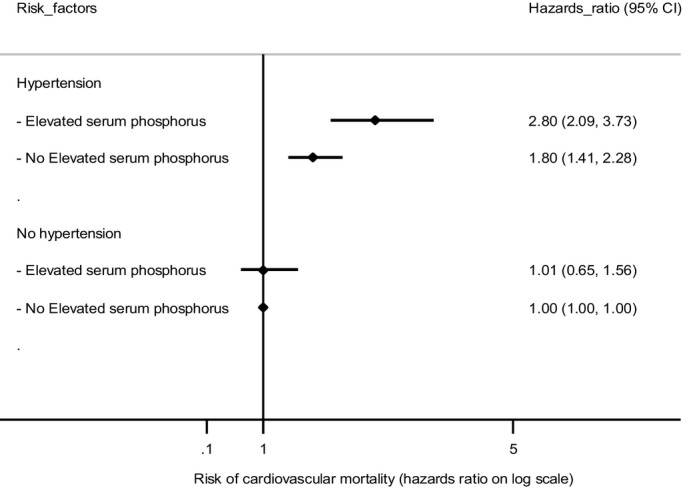
Joint and individual effects of hypertension and elevated serum phosphorus on the risk of cardiovascular mortality.
The joint effect of hypertension and elevated serum phosphorus on the risk of cardiovascular mortality and all-cause mortality after excluding participants who died in the first 2 years of follow-up is shown in Table 4. In this case, RERI for cardiovascular mortality was significant (RERI=1.21, 95% CI: 0.26; 2.17) and not for all-cause mortality (RERI=0.17, 95% CI: −0.27; 0.62, adjusted for age, gender, race, and eGFR). After excluding participants on diuretic medication, RERI for cardiovascular mortality was statistically significant (RERI=1.31, 95% CI: 0.30; 2.33) but not for all-cause mortality (RERI=0.20, 95% CI: −0.22; 0.62). After adjusting for dietary phosphorus intake, RERI for cardiovascular mortality was statistically significant (RERI=1.10, 95% CI: 0.09; 2.10) and not for all-cause mortality (RERI=0.18, 95% CI: −0.22; 0.58). Results after additional adjustment for socioeconomic status, body mass index, smoking, history of cardiovascular disease, diabetes, serum cholesterol, and vitamin D are shown in Table 4. In this case, RERI for cardiovascular mortality, although not statistically significant, was positive and tended to be higher (RERI=0.88, 95% CI: −0.07, 1.83) compared to all-cause mortality (RERI=0.10, 95% CI: −0.35; 0.56). The interactions on the multiplicative scale were not significant in either of the sensitivity analyses.
Table 4.
Adjusted Hazard Ratios of Cardiovascular and All-Cause Mortality for Joint Effect of Hypertension and Elevated Serum Phosphorus
| Risk Factors | After Exclusion Those Who Died in First 2 Years | After Exclusion Those Who Used Diuretics | Adjusting for Additional Covariates* | Adjusting for Dietary Phosphorus Intake† | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | P Value | Hazard Ratio | P Value | Hazard Ratio | P Value | Hazard Ratio | P Value | |
| Cardiovascular mortality | ||||||||
| No hypertension | ||||||||
| No elevated serum phosphorus | Reference | Reference | Reference | Reference | ||||
| Elevated serum phosphorus | 0.93 (0.56 to 1.54) | 0.78 | 1.08 (0.67 to 1.74) | 0.76 | 0.92 (0.53 to 1.60) | 0.77 | 0.98 (0.58 to 1.66) | 0.94 |
| Hypertension | ||||||||
| No elevated serum phosphorus | 1.77 (1.40 to 2.23) | <0.001 | 1.70 (1.31 to 2.21) | <0.001 | 1.67 (1.31 to 2.15) | <0.001 | 1.87 (1.47 to 2.37) | <0.001 |
| Elevated serum phosphorus | 2.91 (2.13 to 3.98) | <0.001 | 3.10 (2.27 to 4.22) | <0.001 | 2.48 (1.82 to 3.37) | <0.001 | 2.94 (2.24 to 3.87) | <0.001 |
| All-cause mortality | ||||||||
| No hypertension | ||||||||
| No elevated serum phosphorus | Reference | Reference | Reference | Reference | ||||
| Elevated serum phosphorus | 1.18 (0.88 to 1.58) | 0.27 | 1.23 (0.93 to 1.64) | 0.14 | 1.12 (0.83 to 1.51) | 0.46 | 1.14 (0.88 to 1.47) | 0.32 |
| Hypertension | ||||||||
| No elevated serum phosphorus | 1.44 (1.23 to 1.69) | <0.001 | 1.45 (1.24 to 1.71) | <0.001 | 1.36 (1.15 to 1.61) | <0.001 | 1.48 (1.27 to 1.71) | <0.001 |
| Elevated serum phosphorus | 1.79 (1.48 to 2.17) | <0.001 | 1.89 (1.56 to 2.28) | <0.001 | 1.58 (1.27 to 1.97) | <0.001 | 1.80 (1.52 to 2.12) | <0.001 |
Adjusted for age, gender, race, estimated glomerular filtration rate, socioeconomic status, smoking, body mass index, history of cardiovascular disease, diabetes, serum cholesterol, and vitamin D.
Adjusted for age, gender, race, estimated glomerular filtration rate, and dietary phosphorus intake/day.
Discussion
In this prospective study, our findings suggest that regarding cardiovascular mortality the joint effect of hypertension and elevated serum phosphorus is larger than the sum of their individual effects but not regarding all-cause mortality. These findings suggest that controlling serum phosphorus levels in hypertensive individuals may lead to an extra risk reduction regarding cardiovascular mortality.
Interaction in epidemiology tests whether the joint effect of 2 risk factors on a certain outcome differs from the sum or product of their independent effects. Therefore, interaction can be measured on an additive or a multiplicative scale. Interaction on the additive scale indicates the extent to which the observed risk for disease in those with both risk factors is greater (synergism) or lower (antagonism) than the sum of individual risk ratios of each risk factor separately. Interaction on the multiplicative scale concerns whether the risk for disease in those with both risk factors was greater or lower than was the multiplied risk ratios of each risk factor alone.17,18 It is possible that the interaction is present only on 1 scale or both scales. Although there is no consensus on whether the additive or multiplicative scale is the most appropriate, additive interaction may be of more relevance from the viewpoint of translating epidemiological findings into prevention of disease events, as it is readily translated into impact of intervention in terms of absolute number of preventable outcomes.19 The recent STROBE Statement advises to report interaction analyses on an additive and multiplicative scale 20 when evaluating joint effect of exposures. We, therefore, presented interaction results on both an additive and multiplicative scale. Moreover, it allows us to compare our results with existing literature where conventionally interaction is reported on a multiplicative scale.
The implications of finding interaction on the additive scale between hypertension and elevated serum phosphorus and the risk of cardiovascular mortality may suggest that these risk factors share a common pathway in the pathogenesis of cardiovascular mortality, and that combining serum phosphorus and blood pressure lowering may have an extra risk reduction effect in preventing cardiovascular mortality. Some earlier studies had reported inverse association between high dietary serum phosphorus intake and risk of hypertension.21,22 However, in these studies either the main source of high serum phosphorus was dairy products/leafy vegetables or only high serum phosphorus from dairy products was associated with a reduced risk of hypertension. Dairy products and leafy vegetables are known to be rich in other nutrients such as magnesium, calcium, and potassium and are effective in reducing risk of hypertension,23 which might explain these findings. No previous study has formally examined the joint effect of hypertension and elevated serum phosphorus on the risk of mortality. Only 1 study by Tonelli et al tested interaction between serum phosphorus and hypertension while examining the independent effect of serum phosphorus on mortality in the general population.3 Tonelli et al found no joint effect of hypertension and elevated serum phosphorus on risk of mortality. Interaction, however, was tested on a multiplicative scale only. In our study, interaction of risk factors was tested on both an additive and a multiplicative scale, and it was found that interaction between hypertension and elevated serum phosphorus was present on the additive, but not the multiplicative, scale.
The results obtained in sensitivity analyses corroborate with our main findings. We separately accounted for some of the factors that might affect serum phosphorus levels and risk of mortality (eg, dietary phosphorus intake, hypertensive medication use, and renal function). Results were essentially similar after accounting for these factors. Unfortunately, NHANES-III did not collect information on factors that tightly regulate phosphate metabolism (eg, fibroblast growth factor 23 or intact parathyroid hormone measurement) and, therefore, it was not possible to investigate the effect of phosphate metabolism on our findings. However, it should be noted that the aim of this study was not to investigate the causes of elevated serum phosphorus but to investigate the risk of mortality when individuals have elevated serum phosphorus as well as hypertension. After adjustment for additional covariates, the RERI for cardiovascular mortality was not statistically significant, but it was positive and indicative of joint effect. The low power for RERI after adjustment for additional covariates might be because not all individuals in the study had complete information on all of the covariates. Therefore, adjustment for additional covariates resulted in loss of a substantial number of individuals with elevated serum phosphorus and hypertension (≈13%).
Our study has a number of strengths. The major strength of this analysis is that it was conducted using data from a large, well-characterized cohort of general population. The long-term mortality data are also a significant strength. Potential confounders of the association between hypertension, elevated serum phosphorus, and mortality were carefully considered. The study also has some limitations. Data were only available for onetime assessment of both hypertension and elevated serum phosphorus. Therefore, bias may have been introduced because of risk factor levels that may have changed after baseline measurements. Moreover, data in this study were collected about 20 years ago. Given the changes in health behaviors, disease awareness, and type of antihypertensive medication use in last 20 years, prevalence and incidence of hypertension and elevated serum phosphorus might have changed. However, we believe that the mechanism behind the joint effect of hypertension and serum phosphorus is unlikely to have changed. Like previous studies,24 we also found increased risk of cardiovascular-related mortality from elevated serum phosphorus in men but not women (data not shown). Because the main aim of this study was to investigate the joint effect of elevated serum phosphorus and hypertension, and there were a limited number of participants with both elevated serum phosphorus and hypertension, we could not investigate whether their joint effect differs by sex. It should be investigated in future studies with a larger sample size of participants with both elevated serum phosphorus and hypertension. Finally, as for all observational studies, the present findings do not conclusively demonstrate that the joint effect of hypertension and serum phosphorus for the risk of cardiovascular mortality is causal.
A large body of evidence exists regarding excess risk of mortality risk in kidney disease patients who have high levels of serum phosphorus.25–28 Therefore, clinical management of serum phosphorus is a priority in kidney disease patients. Recently, suggestion has been made to control dietary intake of phosphorus-containing food.6 However, there are no established guidelines for serum phosphorus control in hypertensive individuals. Managing causes of elevated serum phosphorus and thereby controlling an existing elevated serum phosphorus level in hypertensive individuals may be a relevant therapeutic target in preventing excess risk of cardiovascular mortality. Future studies, however, should confirm causality of the relationship between simultaneous existence of serum phosphorus and hypertension and the risk of cardiovascular mortality.
In conclusion, our study revealed that the joint effect of hypertension and elevated serum phosphorus is larger than the sum of their individual effects on cardiovascular mortality, but not in case of all-cause mortality. Future studies should investigate whether controlling elevated serum phosphorus in hypertensive individuals helps in prevention of extra risk of cardiovascular mortality.
Acknowledgments
The authors thank the NHANES participants, staff, and investigators.
Disclosures
None.
References
- Lawes CM, Hoorn SV, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2003;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, Jr, Kaplan NM, O'Connor CM, O'Gara PT, Oparil S American Heart Association Council for High Blood Pressure Research; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Hypertension. 2007;50:e28–e55. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Curhan G, Pfeffer M, Sacks F, Thadhani R, Melamed ML, Wiebe N, Muntner P. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation. 2009;120:1784–1792. doi: 10.1161/CIRCULATIONAHA.109.851873. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Sullivan LM, Fox CS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- Lederer E. Regulation of serum phosphate. J Physiol. 2014;592:3985–3995. doi: 10.1113/jphysiol.2014.273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketteler M, Wolf M, Hahn K, Ritz E. Phosphate: a novel cardiovascular risk factor. Eur Heart J. 2013;34:1099–1101. doi: 10.1093/eurheartj/ehs247. [DOI] [PubMed] [Google Scholar]
- Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich ED. Risk mechanisms in hypertensive heart disease. Hypertension. 1999;34:782–789. doi: 10.1161/01.hyp.34.4.782. [DOI] [PubMed] [Google Scholar]
- Beevers GL. The pathophysiology of hypertension. BMJ. 2001;322:912–916. doi: 10.1136/bmj.322.7291.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Chang AR, Grams ME. Serum phosphorus and mortality in the Third National Health and Nutrition Examination Survey (NHANES III): effect modification by fasting. Am J Kidney Dis. 2014;64:567–573. doi: 10.1053/j.ajkd.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94: series 1: programs and collection procedures. Vital Health Stat 1. 1994;32:1–407. [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(suppl 1):s5–s10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Interpretation of interactions: guide for the perplexed. Br J Psychiatry. 2010;197:170–171. doi: 10.1192/bjp.bp.110.081331. [DOI] [PubMed] [Google Scholar]
- Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36:1111–1118. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- Andersson T, Alfredsson L, Köñllberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17:227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112:467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- Alonso A, Nettleton JA, Ix JH, de Boer IH, Folsom AR, Bidulescu A, Kestenbaum BR, Chambless LE, Jacobs DR., Jr Dietary phosphorus, blood pressure, and incidence of hypertension in the Atherosclerosis Risk in Communities Study and the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55:776–784. doi: 10.1161/HYPERTENSIONAHA.109.143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, Brown IJ, Zhao L, Stamler J INTERMAP Cooperative Research Group. Dietary phosphorus and blood pressure: international study of macro- and micro-nutrients and blood pressure. Hypertension. 2008;51:669–675. doi: 10.1161/HYPERTENSIONAHA.107.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer M, Schrezenmeir J. Milk and the metabolic syndrome. Obes Rev. 2007;8:109–118. doi: 10.1111/j.1467-789X.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- Onufrak SJ, Bellasi A, Cardarelli F, Vaccarino V, Muntner P, Shaw LJ, Raggi P. Investigation of gender heterogeneity in the associations of serum phosphorus with incident coronary artery disease and all-cause mortality. Am J Epidemiol. 2009;169:67–77. doi: 10.1093/aje/kwn285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- Bellasi A, Mandreoli M, Baldrati L, Corradini M, Di Nicolò P, Malmusi G, Santoro A. Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol. 2011;6:883–891. doi: 10.2215/CJN.07810910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SC, Hayen A, Macaskill P. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- McGovern AP, de Lusignan S, van Vlymen J, Liyanage H, Tomson CR, Gallagher H, Rafiq M, Jones S. Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: a large community based cohort study. PLoS One. 2013;8:e74996. doi: 10.1371/journal.pone.0074996. [DOI] [PMC free article] [PubMed] [Google Scholar]


