Abstract
Background
Coronary artery disease (CAD) outcomes consistently improve when they are routinely measured and provided back to physicians and hospitals. However, few centers around the world systematically track outcomes, and no global standards exist. Furthermore, patient-centered outcomes and longitudinal outcomes are under-represented in current assessments.
Methods and Results
The nonprofit International Consortium for Health Outcomes Measurement (ICHOM) convened an international Working Group to define a consensus standard set of outcome measures and risk factors for tracking, comparing, and improving the outcomes of CAD care. Members were drawn from 4 continents and 6 countries. Using a modified Delphi method, the ICHOM Working Group defined who should be tracked, what should be measured, and when such measurements should be performed. The ICHOM CAD consensus measures were designed to be relevant for all patients diagnosed with CAD, including those with acute myocardial infarction, angina, and asymptomatic CAD. Thirteen specific outcomes were chosen, including acute complications occurring within 30 days of acute myocardial infarction, coronary artery bypass grafting surgery, or percutaneous coronary intervention; and longitudinal outcomes for up to 5 years for patient-reported health status (Seattle Angina Questionnaire [SAQ-7], elements of Rose Dyspnea Score, and Patient Health Questionnaire [PHQ-2]), cardiovascular hospital admissions, cardiovascular procedures, renal failure, and mortality. Baseline demographic, cardiovascular disease, and comorbidity information is included to improve the interpretability of comparisons.
Conclusions
ICHOM recommends that this set of outcomes and other patient information be measured for all patients with CAD.
Keywords: coronary artery disease, outcomes, patient-centered
Cardiovascular disease represents the single greatest global disease burden, both in mortality and morbidity.1 Recent alarming increases in incidence noted in low-income and middle-income countries raise concern for future generations.2 However, increasing cardiovascular disease burden is not inevitable. High-income nations have invested heavily in addressing this problem. Mortality from cardiovascular disease, especially coronary artery disease (CAD), has dramatically decreased in the past few decades.3,4 While public health initiatives aimed at primary prevention have certainly led to some of these gains, advances in treatment for patients with CAD have accounted for a significant portion.5,6
Despite overall improvement in high-income countries, significant variation in outcomes for patients with CAD still exists. Significant differences in 30-day mortality following acute myocardial infarction (AMI) have been found between the United Kingdom and Sweden.7 Even among elderly patients admitted for AMI within the United States, a greater than twofold difference in 30-day risk standardized mortality was found, depending on which hospital provided the care.8 These findings suggest an opportunity to identify the best management practices that lead to optimal outcomes and then to implement them across broad populations, lessening the global burden of cardiovascular disease.
Cardiovascular registries have been operating worldwide for several decades in large part to accomplish these goals,9,10 and impressive gains in quality of care and outcomes have been made.11–13 However, the full potential impact of cardiovascular registries is currently constrained by the lack of 2 key factors: international standard definitions and longer-term patient-centered outcomes. With the notable exception of collaborations in the United State between the American College of Cardiology and the Society of Thoracic Surgeons registries, most cardiovascular registries have developed in isolation, and they rarely cross national borders. This approach has led to registries tracking different outcomes and/or using different definitions for equivalent outcomes. This lack of standard outcomes and definitions has limited the validity of international comparisons across providers of different health systems. Educational and quality improvement activities have remained primarily local or regional, and the wider variation in care and outcomes globally has remained unaddressed. In addition, cardiovascular registries have focused primarily on improving quality of care by reporting process measures and short-term outcomes, most commonly tracking in-hospital or 30-day mortality and complications. Longer-term outcomes (for example, after 1 and 5 years), and patient-centered outcomes (for example, angina burden, functional status, and health-related quality of life), more closely reflect the ultimate benefit of care but are rarely tracked in real-world settings. Hence, the true value of individual interventions and of whole systems of health care is never fully understood.
To align outcome measurement efforts globally and to promote more comprehensive measurement of outcomes, the nonprofit International Consortium for Health Outcomes Measurement (ICHOM)14 formed a CAD working group. ICHOM was founded in 2012 by Harvard Business School, The Boston Consulting Group, and The Karolinska Institutet and has so far completed 11 other standard sets of outcomes. It is funded through support from the founders and from a wide range of international sponsoring partners. In accordance with the goals of ICHOM, the CAD Working Group aimed to define a parsimonious, consensus, standard set of outcomes that are meaningful to patients with CAD and are able to be tracked for an appropriate length of time across diverse health systems.
Methods
Objectives
The primary goal of this initiative was to identify a parsimonious, consensus, set of outcomes with standard definitions for patients with CAD that could be tracked by health systems and clinical registries around the world. In particular, this standard set would encompass a range of outcomes relating to mortality, morbidity, and patient health status (ie, symptoms, functional status, and health-related quality of life). The use of a standard set of outcome measurements would not preclude any system or registry collecting and reporting additional measures as desired. A secondary goal was to identify a standard set of variables to be systematically collected to enable case-mix adjustment, which would support comparison of CAD outcomes among providers and health systems with different case mixes of patients.
Composition of Working Group
ICHOM brought together an internationally recognized group of clinician and nonclinician leaders in the field of CAD with expertise in (1) clinical trials and registries, (2) public and private health system management, (3) patient-centered outcomes research, (4) outcomes measurement, (5) quality improvement, and (6) patient advocacy. There were a total of 17 members from 4 continents and 6 countries. ICHOM also formed a project team, which consisted of a project leader (C.J.S., then T.A.K.), who coordinated the process and a research fellow (E.S.S.), a cardiologist who provided subject-specific expertise.
Process
A modified Delphi technique was used to develop consensus around all major decision areas, including the scope of the population to be covered, the minimum outcome set, and the risk factors required for case-mix adjustment. Consistent with this method, a combination of teleconferences and surveys was used to forge consensus. Between December 2012 and November 2013, the Working Group participated in 11 conference calls, 10 of which were followed by surveys on key decision points. Prior to each teleconference, the ICHOM project team developed an agenda, listed key proposals, and summarized relevant evidence from the literature. The Working Group reviewed these documents in advance and discussed them during each teleconference.
The Working Group selected outcomes based on 4 criteria: (1) the frequency of the outcome; (2) its impact on the patient; (3) the potential to modify the outcome; and (4) the feasibility of “capturing” the outcome in clinical practice. Additional criteria for patient-reported outcome measures (PROMs) included (1) the domain coverage; (2) the psychometric properties; (3) the feasibility to implement; and (4) the clinical interpretability. Next, time points for data collection were selected for each outcome. Risk-adjustment variables were selected based on 3 criteria: (1) the relevance (strength of the causal linkage between the risk factor and the outcome), (2) the risk factor independence, and (3) feasibility of measurement.
Following each call, the ICHOM team circulated detailed minutes and an electronic survey on each key decision point. Decisions were finalized when more than two thirds of the Working Group members concurred. In cases where consensus was not achieved, further discussion ensued during subsequent teleconferences, which was followed by a second survey. The final standard set was approved unanimously by all members of the Working Group.
Results
Cohort Definition
As defined by the CAD Working Group, the target population is all patients with CAD, including patients presenting with any of the following qualifying diagnoses, test findings, or interventions: angina, acute coronary syndrome, AMI, CAD noted on angiography or other coronary imaging modalities (eg, computed tomography; magnetic resonance imaging), stress testing suggestive of CAD, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG). Informing the Working Group's approach to cohort selection was the recognition that the same set of longitudinal outcomes (eg, survival, symptoms, and quality of life) was relevant to all patients with CAD, regardless of disease state or treatment received, while additional treatment and event-specific outcomes could be described for patients experiencing AMI or undergoing PCI or CABG. In addition, the Working Group decided that the outcomes should be assessed at particular times after initial diagnosis and from each major event (AMI, PCI, and CABG).
Outcomes
The CAD Working Group decided to focus on both short-term (hospitalization, 30 days posthospitalization) and long-term (1 year and 5 years from first enrollment) outcomes (Figure 1). To inform its work, the Working Group reviewed the outcome domains, relevant definitions, and methods of ascertainment used in 13 established registries (Table 1). However, as these registries mainly report outcomes occurring during index hospitalizations and do not include patient-reported outcomes, the CAD Working Group proposed additional timelines for outcome measurement as well as collection of a set of PROMs.
Figure 1.
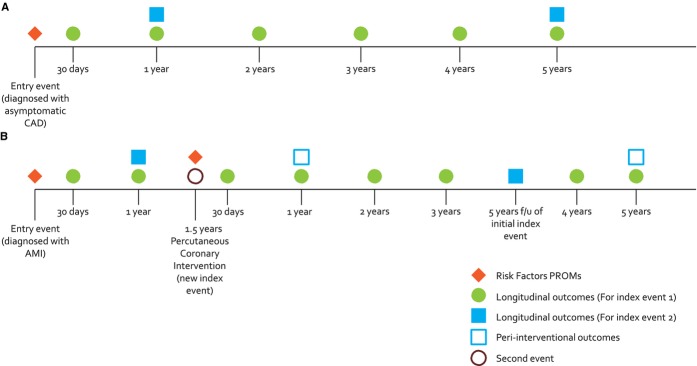
A, Example timeline for a patient diagnosed with asymptomatic CAD without any subsequent events. B, Example timeline for a patient initially diagnosed at time of an AMI and who subsequently undergoes a PCI one and a half years later. A new revascularization procedure or a new diagnosis of AMI constitutes a new index event, and tracking of PROMs should reset from this point, tracking again at 30 days, and then annually for 5 years. Given that longitudinal outcomes are obtained from administrative data, they are collected for both the entry event (eg, AMI) and the new event (eg, PCI). AMI indicates acute myocardial infarction; CAD, coronary artery disease; PCI, percutaneous coronary intervention; PROMs, patient-reported outcome measures.
Table 1.
Registries Reviewed to Inform Outcome and Risk Factor Domains and Definitions
| Country | Registry Name | Reference |
|---|---|---|
| Australia | Coronary Angiogram Database of South Australia (CADOSA) | 15 |
| Singapore | Singapore Myocardial Infarct Registry | 16 |
| Spain (Catalonia) | Codi Infarct Registry | 17 |
| Sweden | Swedeheart: Heart Surgery Registry | 18 |
| Sweden | Swedeheart: Swedish Coronary Angiography and Angioplasty Registry (SCAAR) | 18 |
| Sweden | Swedeheart: Swedish Register of Cardiac Intensive Care (RIKS-HIA) | 18 |
| Sweden | Swedeheart: Swedish Secondary Prevention Registry (SEPHIA) | 18 |
| United Kingdom | Myocardial Ischemia National Audit Project (MINAP) | 19 |
| United Kingdom | British Cardiovascular Intervention Society National PCI Audit | 20 |
| United Kingdom | Society for Cardiothoracic National Adult Cardiac Surgery Audit | 21 |
| United States | NCDR ACTION Registry—GWTG | 22,23 |
| United States | NCDR CathPCI Registry | 22,23 |
| United States | Society of Thoracic Surgeons National Database: Adult Cardiac | 24 |
GWTG indicates Get With The Guidelines; NCDR, National Cardiovascular Data Registry.
Survival
The Working Group unanimously agreed to assess survival at 30 days postdischarge, and at 1 and 5 years following cohort entry. While longer follow-up is clearly important to patients, it may be less feasible for health systems to capture. Nonetheless, for the 3 selected time periods, the CAD Working Group decided to assess all-cause mortality using death registers. Limitations of such death registries were discussed, including the expense and time lag involved in obtaining these data. Additionally, in some countries, access to death registers is limited; in such circumstances, ICHOM will advocate that governments make these data more available. Disease-specific mortality was not selected as it is less meaningful to patients than all-cause mortality; additionally, the validity of the data may be limited if cause of death is not clinically adjudicated.
Longitudinal Outcomes
The CAD Working Group focused on longitudinal outcomes of CAD that are frequent, that are associated with a high morbidity (affecting patients’ quality of life and functional status), and that are costly. It elected to measure outcomes at 1 and 5 years from cohort entry. Patients who experience a cardiovascular event (eg, AMI) or undergo a cardiac procedure (PCI or CABG) during the follow-up period will not be censored; rather, they will continue to be followed for the entire 5-year period from the time they entered the cohort. However, they will also be included in a second cohort with an additional 5 years of follow-up from the new index event or procedure. The CAD Working Group elected to include the following outcomes, representing CAD progression and common, high-impact health outcomes related to cardiovascular disease: all-cause mortality (from death registers); AMI, revascularization procedures (PCI or CABG); new hospitalizations for acute coronary syndrome, heart failure, hemorrhagic and/or ischemic stroke; and advanced renal failure (detected as a new requirement for dialysis). It was decided not to include peripheral artery disease as an outcome as there is substantial international variation in detection of peripheral artery disease, depending on the intensity of diagnostic practices and therapeutic capacities.
The CAD Working Group decided to restrict longitudinal outcomes to those that could be captured via administrative data, as most current registries and electronic health record databases are not designed to capture events occurring outside of the acute care episode. It was recognized that for many countries without a single-payer healthcare system or an all-payer claims database, long-term outcomes can be difficult to ascertain. Therefore, linking of clinical data with administrative data was felt to be the most feasible and least resource-intense approach to collecting long-term outcomes.
Patient-Reported Health Status
PROMs are increasingly recognized as providing valuable information about health-related quality of life and are guiding more informed discussions about care management. A review of existing PROMs used in describing cardiovascular disease revealed 3 main instruments, specifically, the Seattle Angina Questionnaire25 (SAQ-7), Rose Dyspnea Score,26 Patient Health Questionnaire27 (PHQ-2), Quality of Life Index28 (QLI) Cardiac Version IV, and Quality of Life after Myocardial Infarction (QLMI-2/MacNew).29 These instruments measure cardiac-related symptoms (eg, shortness of breath, chest pain), functional status, and quality of life. The Working Group considered the above measurement tools based on the following qualities: domain coverage (symptom burden, functional status, and quality of life), psychometric properties (validity, sensitivity, and quality of life), feasibility to implement (length of questionnaire, language availability, and cost to implement), and clinical interpretability (knowledge of how to interpret scores in a clinically meaningful way). The SAQ-7, which is short, widely translated into many languages, and has a high degree of clinically interpretability most closely aligned with these qualities and was recommended in the standard set. The Working Group desired additional questions to assess patients’ level of dyspnea and depressive symptoms. The PHQ-2, a widely used 2-item questionnaire assessing signs of depression, and 2 items from the Rose Dyspnea Score were included to cover these domains. The Working Group also desired but did not reach a consensus on including time to return to normal activities, return to work, sexual function, or medication side effects within the Standard Set, primarily due to a lack of standardized assessments. The set will evolve over time, and we anticipate these factors may be included in the future.
Complications
In addition to survival, longitudinal outcomes, and patient-reported health status, the Working Group voted to measure complications following PCI and CABG procedures that can have a significant impact on patients’ quality of life and health outcomes, and that can enable comparison of providers’ and institutions’ technical quality of care. Based on registries from the Society for Thoracic Surgeons and the National Cardiovascular Data Registry, the Working Group selected several periprocedural outcomes for inclusion in the standard set. These outcomes include stroke, renal failure, and length of stay (for patients undergoing PCI and CABG); prolonged ventilation, sternal wound infections, and reoperations (for patients undergoing CABG only); and coronary dissection/perforation, emergent CABG, and vascular complications (for patients undergoing PCI only) (Table 2). The Working Group excluded conditions that were considered to be particularly rare, variably detected depending on intensity of care, and difficult to diagnose, (eg, periprocedural MI [variable detection; unclear if clinically meaningful], cardiac tamponade [rare]; restenosis rate [variable detection]; pneumonia among patients undergoing CABG [often not distinguishable from atelectasis]; and deep venous thrombosis among patients with CABG [rare]).
Table 2.
Summary of Standard Set of Outcomes for Patients With Coronary Artery Disease
| Category (Cohorts) | Measure | Details | Timing | Data Source |
|---|---|---|---|---|
| Longitudinal outcomes (All) | All-cause mortality | Date of death | Tracked for 5 years after index event—reported at 1 and 5 years | Administrative |
| Admissions (for AMI, hemorrhagic stroke, ischemic stroke or heart failure) | Date of each admission & discharge | |||
| Procedural interventions | Date of PCI and/or CABG | |||
| Acute renal failure | New requirement for dialysis | |||
| Patient- reported health status (All) | Angina, dyspnea, depression, functional status, health-related quality of life | SAQ-7, PHQ-2, Rose Dyspnea | 30 days+then annually to 5 years after index event | Patient reported |
| Acute complications of treatment (PCI & CABG) | Mortality post procedure | Date of death | Within index hospitalization+within 30 days of procedure | Clinical or administrative |
| Place of death | Options: Home; acute care hospital or rehab; nursing home or hospice | |||
| Stroke and stroke type | Ischemic; hemorrhagic; unknown | |||
| Acute renal failure | New requirement for dialysis | |||
| Total length of stay | Date at arrival and discharge | Within index hospitalization | ||
| Post-procedure length of stay | Date of intervention and discharge | |||
| Major surgery complications (CABG only) | Prolonged ventilation | Mechanical ventilation >24 h post-surgery | Within index hospitalization | Clinical |
| Deep sternal wound infection | Requires operative intervention, positive culture & antibiotics | Within index hospitalization+within 30 days of procedure | ||
| Reoperation required | Return to operating theatre (for other than wound) | |||
| Interventional cardiology complications (PCI only) | Significant dissection | Type C to F dissections | Within index hospitalization | Clinical |
| Perforation | Angiographic or clinical evidence of perforation | |||
| Emergent CABG for failed PCI | Emergency cardiothoracic surgery | |||
| Vascular complications requiring intervention | At percutaneous entry site | Within index hospitalization+within 30 days of procedure | ||
| Bleeding event within 72 h | Within 72 h of PCI | Within index hospitalization+within 72 h of procedure |
The full list of definitions is available on the website (http://www.ichom.org/project/coronary-artery-disease/). AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; PHQ-2, Patient Health Questionnaire; SAQ-7, Seattle Angina Questionnaire.
Case-Mix Adjustment
The Working Group was tasked with defining a minimum set of risk factors that would qualify as candidate variables for case-mix adjustment of both clinical and patient-reported outcomes. Risk factors common to all CAD patients were defined as well as add-on variables for patients with AMI, PCI, and CABG (Table 3). The intent was to highlight factors that every health system should be collecting using a standard definition to enable comparisons across health systems. Informing the selection of risk factors was a review of existing risk models commonly used to assess severity of illness and prognosis (eg, the GRACE model30; TIMI risk score31) as well as assessing the impact that a specific risk factor has on the outcomes in our set, based on the literature and expertise of the Working Group. Of note, socioeconomic status and psychosocial factors were not included due to the difficulty of standardizing these variables in the international setting and the lack of consensus regarding adjusting for these variables in risk models. The set of covariates is not intended to be exhaustive but will serve as a basis of developing international risk models for adjusting outcome performance across institutions.
Table 3.
Summary of Standard Set of Risk Factors for Patients With Coronary Artery Disease
| Timing for Collection | Measure | Details | Data Source |
|---|---|---|---|
| First contact with hospital services (eg, outpatient clinic or emergency department) | Age | Date of birth | Administrative, patient reported, or clinical |
| Sex | Sex at birth | ||
| Height | Documented | Clinical | |
| Weight | Documented | ||
| Previous AMI | Documented in history | ||
| Heart Failure | Documented in history | ||
| Hypertension | Documented in history | ||
| Stroke | Documented in history | ||
| Diabetes | Documented in history | ||
| Insulin dependence | Documented in history | ||
| Peripheral arterial disease | Documented in history | ||
| Chronic lung disease | Documented in history | ||
| Liver cirrhosis | Documented in history | ||
| Dementia | Documented in history | ||
| Dialysis dependent | Documented in history | ||
| Baseline creatinine | Documented in history | ||
| At time of presentation with AMI or at time of PCI or CABG | Cardiogenic shock | At first medical contact | |
| Cardiac arrest | Prehospital or in ED | ||
| Creatinine | First measurement for AMI or last measurement prior to PCI or CABG | ||
| At time of presentation with AMI | Troponin | Troponin T or I, lab's upper limit of normal | |
| Heart rate | First measurement for episode of care | ||
| Systolic blood pressure | First measurement for episode of care | ||
| Type of AMI | NSTEMI or STEMI | ||
| At time of PCI or CABG | Procedure Status | Including elective, urgent, emergent, emergent salvage | |
| Left main disease | ≥50% compromise of vessel diameter or 30% –50% with an FFR <0.75 or a minimum lumen area <6 mm2 or a minimum lumen diameter <2.8 mm | ||
| Number of diseased vessels | ≥50% narrowing of any vessel preoperatively, left main disease counted as 2 vessels | ||
| Previous CABG/PCI | Including date of procedure |
The full list of definitions is available in a Reference Guide on the International Consortium for Health Outcomes Measurement web site (http://www.ichom.org/project/coronary-artery-disease/). AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; ED, emergency department; FFR, fractional flow reserve; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data Collection
A very important long-term goal of this effort is to produce data that can be easily compared between providers, centers, and countries. To achieve this, we recommend processes to reduce variability including the use of similar data sources, recognizing that the specific details of data collection will necessarily differ by center. As outlined in Tables 2 and 3, the potential sources include administrative data and death registries, patient-reported sources, and clinical abstraction or physician-reported sources, and we recommend that the source of data as well as the response rate (if patient reported) be tracked for every measure. A data collection manual that further describes each measure, its definition, inclusion and exclusion criteria, and potential data sources is available on the ICHOM web site (http://www.ichom.org/project/coronary-artery-disease/).
Discussion
The ICHOM CAD Working Group identified a consensus standard set of outcomes for the spectrum of patients with a diagnosis of CAD in order to provide a foundation for making appropriate comparisons among countries and health systems in efforts to improve quality.32 The set incorporates frequently unreported outcomes that are important to patients, such as symptom burden and quality of life, as well as traditional outcomes such as mortality and periprocedural complications. The Working Group recommended not only short-term in-hospital outcomes but longer-term (1- and 5-year) outcomes. As appropriate comparison of outcomes requires robust risk adjustment, this set also includes baseline patient demographic and clinical information. To our knowledge, this is the first coordinated, multinational effort to recommend a standard set of outcomes that all health systems should aspire to collect.
Defining value in health care and sharing data across health systems will require standard definitions of outcomes and patient characteristics.32 Well-designed and implemented standardized registries have attempted to fill this void and have provided the foundation for quality improvement in CAD in many countries.11–13 However, there have been few international comparisons outside of selected populations within clinical trials. Recent investigations into treatment patterns33 and outcomes7 have shed some light but are limited to select countries with well-developed registries. Even within these comparisons, subtle but potentially important differences exist in definitions for patient clinical characteristics and outcomes. Key data elements and definitions for measuring outcomes among patients with CAD have been put forth from the European Union34 and the American Heart Association/American College of Cardiology.35 The current effort supports the principles of these initiatives, taking into consideration the different health systems and different data-collecting capabilities across the globe. In this way, valid comparisons will be feasible across a wider practice variation than found within a single country in the hope of improving quality of care for a broader population. In addition, international registry-based randomized clinical trials will become more feasible.
Notably, the current set emphasizes outcomes that are most important to patients, including PROMs. The “patient voice” has often been neglected in clinical registries and quality improvement efforts,36,37 but awareness of its importance is increasing.38 The American Heart Association recently released a scientific statement advocating for patient-reported health status as a measure of cardiovascular health.39 Understanding that collecting PROMs will place an increased burden of collection in most current practice settings, the working group recommends a parsimonious group of well-validated PROMs to efficiently cover the 3 main components of patient health status—symptom burden, functional status, and health-related quality of life—by using elements of the SAQ-7,25 Rose Dyspnea Score,26 and PHQ-2.27
Current outcome assessments frequently are confined to shorter-term, often in-hospital, outcomes. When collected, longer-term outcomes are usually limited to mortality or a narrow list of events, such as myocardial infarctions, interventions, or surgical procedures. While important and relatively easy to obtain, these outcomes do not represent the full time horizon of health experience important to patients. Significant differences have been found evaluating hospital performance depending on the use of in-hospital mortality or 30-day mortality.40 Longer-term outcomes, including assessments at 1 and 5 years after events or treatment, would provide a clearer picture and a more appropriate basis for comparing different strategies and health systems. However, the implementation of these longer-term outcomes will present challenges that will need to be periodically assessed.
A unique strength of this effort was the diversity of the CAD Working Group members, which included a patient representative as well as physician leaders from around the world, including middle-income countries. All shared in common significant expertise in outcomes measurement, quality improvement, and policy. The Working Group members’ unique, global perspectives were critical to informing the minimum standard outcomes set. While teleconferences were oriented around current literature and practices, the members shared their own country or health system's current efforts and challenges to implementing outcomes measures. Having designed this set, the CAD Working Group has elected a steering committee to oversee its continual iteration to reflect changes in data collection capacity, to clarify outcome and patient characteristic definitions as needed, and to respond to any improvements in outcome assessment. In particular, outcomes such as return to daily activities, productivity, and medication or device safety signals were not included in this set. Although acknowledged as important, these outcomes were not included in the initial minimum standard set due to the need for further investigation into how best to standardize assessment.
We recognize that implementation of a globally standardized set of indicators will not be easy. Key implementation barriers to overcome include (1) infrastructure cost to collect patient-reported outcomes, (2) linkages with longitudinal administrative data sources, (3) streamlining clinician data collection within electronic health records, and (4) aligning existing registries and data-collection efforts to map to the global ICHOM standard. The near-term goal will be to partner with pioneering provider institutions, including selected members of this Working Group, to implement all or part of the set and to use this as a proof of concept towards broader adoption in registries as well as endorsement by payers and governments. In this way, we can move in a step-by-step fashion towards our ultimate goal—internationally comparable data on patient-centered outcomes.
Sources of Funding
Costs to run the Working Group were covered by the International Consortium for Health Outcomes Measurement (ICHOM).
Disclosures
Rumsfeld is the Chief Science Officer for the National Cardiovascular Data Registry (NCDR), USA. Kelley and Stowell are employed and paid by ICHOM, USA. Jernberg is the chairman of Swedeheart, National Coronary Artery Disease registry, Sweden. Shahian is chairman of the Society of Thoracic Surgeons Quality Measurement Task Force, USA. Weston is the Clinical Director of the Myocardial Ischaemia National Audit Project (MINAP), UK.
References
- Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 2011. Global status report on noncommunicable diseases. Available at: http://www.who.int/nmh/publications/ncd_report2010/en. Accessed April 14, 2014.
- Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Waganknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125:1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in US deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–1107. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, Jernberg T, Hemingway H. Acute myocardial infarction: a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet. 2014;383:1305–1312. doi: 10.1016/S0140-6736(13)62070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM, Rapp MT, Normand SL, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- Alpert JS. Are data from clinical registries of any value? Eur Heart J. 2000;21:1399–1401. doi: 10.1053/euhj.2000.2265. [DOI] [PubMed] [Google Scholar]
- McNamara RL. Cardiovascular registry research comes of age. Heart. 2010;96:908–910. doi: 10.1136/hrt.2010.198069. [DOI] [PubMed] [Google Scholar]
- Carlhead R, Bojestig M, Peterson A, Aberg C, Garmo H, Lindahl B. Improved clinical outcomes after acute myocardial infarction in hospitals participating in a Swedish quality improvement initiative. Circ Cardiovasc Qual Outcomes. 2009;2:458–464. doi: 10.1161/CIRCOUTCOMES.108.842146. [DOI] [PubMed] [Google Scholar]
- Jernberg T, Johanson P, Held C, Svennblad B, Lindback J, Wallentin L. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA. 2011;305:1677–1684. doi: 10.1001/jama.2011.522. [DOI] [PubMed] [Google Scholar]
- Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, Chen AY, Klein LW, Masoudi FA, McKay C, Hewitt K, Brindis RG, Peterson ED, Rumsfeld JS. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56:254–263. doi: 10.1016/j.jacc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- International Consortium for Health Outcomes Measurement (ICHOM) Available at: www.ichom.org. Accessed April 14, 2014.
- Tavella R, Zeitz C, Arstall M, Chew D, Worthley S, Worthley M, Beltrame J. AMI performance measures in primary PCI in South Australia. Glob Heart. 2014;9:e129. [Google Scholar]
- Mak KH, Kark JD, Chia KS, Tan C, Foong BH, Chew SK. Ethnic differences in utilization of invasive cardiac procedures and in long-term survival following acute myocardial infarction. Clin Cardiol. 2004;27:275–280. doi: 10.1002/clc.4960270507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch X, Curós A, Argimon JM, Faixedes M, Figueras J, Jimenez-Fabrega FX, Masià R, Mauri J, Tresserras R en nombre del comité de creación y los participantes del Codi Infart. Modelo de intervención coronaria percutánea primaria en Cataluña. Rev Esp Cardiol. 2011;suppl 11(C):51–60. [Google Scholar]
- Jernberg T, Attebring MF, Hambraeus K, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallertin L. The Swedish web system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART) Heart. 2010;96:1617–1621. doi: 10.1136/hrt.2010.198804. [DOI] [PubMed] [Google Scholar]
- Herrett E, Smeeth L, Walker L, Weston C. The myocardial ischaemia national audit project (MINAP) Heart. 2010;96:1264–1267. doi: 10.1136/hrt.2009.192328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Cardiovascular Intervention Society. United Kingdom national PCI Audit. Available at: http://www.bcis.org.uk/pages/page_box_contents.asp?pageid=697&navcatid=11. Accessed September 7, 2014.
- Society for Cardiothoracic Surgery in Great Britain & Ireland. National Adult Cardiac Surgery Audit. Available at: www.scts.org. Accessed September 7, 2014.
- Masoudi FA, Ponirakis A, Yeh RW, Maddox TM, Beachy J, Casale PN, Curtis JP, De Lemos J, Fonarow G, Heidenreich P, Koutras C, Kremers M, Messenger J, Moussa I, Oetgen WJ, Roe MT, Rosenfield K, Shields TS, Spertus JA, Wei J, White C, Young CH, Rumsfeld JS. Cardiovascular care facts: a report from the national cardiovascular data registry: 2011. J Am Coll Cardiol. 2013;62:1931–1947. doi: 10.1016/j.jacc.2013.05.099. [DOI] [PubMed] [Google Scholar]
- Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA. The national cardiovascular data registry (NCDR) data quality brief: the NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Shahian DM, Jacobs JP, Edwards FH, Brennan JM, Dokholyan RS, Prager RL, Wright CD, Peterson ED, McDonald DE, Grover FL. The Society of Thoracic Surgeons’ national database. Heart. 2013;99:1494–1501. doi: 10.1136/heartjnl-2012-303456. [DOI] [PubMed] [Google Scholar]
- Spertus JA, Winder JA, Dewhurst TA, Deyo RA, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- Rose GA, Blackburn H. Cardiovascular Survey Methods. Geneva: World Health Organization; 1968. [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Ferrans CE, Powers MJ. Psychometric assessment of the Quality of Life Index. Res Nurs Health. 1992;15:29–38. doi: 10.1002/nur.4770150106. [DOI] [PubMed] [Google Scholar]
- Höfer S, Saleem A, Stone J, Thomas R, Tulloch H, Oldridge N. The MacNew Heart Disease Health-Related Quality of Life Questionnaire in patients with angina and patients with ischemic heart failure. Value Health. 2012;15:143–150. doi: 10.1016/j.jval.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum Á. Flather MD, Fox KAA for the GRACE Investigators. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. J Am Med Assoc. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- Antman EM, Cohen M, Bernink PM, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non–ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- McNamara RL, Chung S, Jernberg T, Holmes D, Roe M, Timmis A, James S, Deanfield J, Fonorow G, Peterson E, Jeppsson A, Hemmingway H. International comparisons of the management of patients with non-ST segment elevation acute myocardial infarction in the United Kingdom, Sweden, and the United States: the MINAP/NICOR, SWEDEHEART/RIKS-HIA, and ACTION Registry-GWTG/NCDR registries. Int J Cardiol. 2014;175:240–247. doi: 10.1016/j.ijcard.2014.04.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flunn MR, Barret C, Cosı′o FG, Gitt AK, Wallentin L, Kearney P, Lonergan M, Shelley E, Simoons ML. The Cardiology Audit and Registration Data Standards (CARDS), European data standards for clinical cardiology practice. Eur Heart J. 2005;26:308–313. doi: 10.1093/eurheartj/ehi079. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Brindis RG, Chaitman BR, Cohen DJ, Cross JT, Drozda JP, Fesmire FM, Fintel DJ, Fonarow GC, Fox KA, Gray DT, Harrington RA, Hicks KA, Hollander JE, Krumholz H, Labarthe DR, Long JB, Mascette AM, Meyer C, Peterson ED, Radford MJ, Roe MT, Richmann JB, Selker HP, Shahian DM, Shaw RE, Sprenger S, Swor R, Underberg JA, Van de Werf F, Weiner BH, Weintraub WS. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards) J Am Coll Cardiol. 2013;61:992–1025. doi: 10.1016/j.jacc.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167. doi: 10.1136/bmj.f167. [DOI] [PubMed] [Google Scholar]
- Coulter A, Locock L, Ziebland S, Calabrese J. Collecting data on patient experience is not enough: they must be used to improve care. BMJ. 2014;348:g2225. doi: 10.1136/bmj.g2225. [DOI] [PubMed] [Google Scholar]
- Patient-Centered Outcomes Research Institute. Patient-Centered Outcomes Research. Available at: http://www.pcori.org/what-we-do/pcor/. Accessed July 10, 2014.
- Rumsfeld JS, Alexander KP, Goff DC, Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat-Jacobson D, Zerwic JJ. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. doi: 10.1161/CIR.0b013e3182949a2e. [DOI] [PubMed] [Google Scholar]
- Drye EE, Normand SL, Wang Y, Ross JS, Schreiner GC, Han L, Rapp M, Krumholz HM. Comparison of hospital risk-standardized mortality rates calculated by using in-hospital and 30-day models: an observational study with implications for hospital profiling. Ann Intern Med. 2012;6:19–26. doi: 10.1059/0003-4819-156-1-201201030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]


