Abstract
Background
Animal study results point to oxidative stress as a key mechanism triggering postoperative atrial fibrillation (PoAF), yet the extent to which specific biomarkers of oxidative stress might relate to PoAF risk in humans remains speculative.
Methods and Results
We assessed the association of validated, fatty acid–derived oxidative stress biomarkers (F2-isoprostanes, isofurans, and F3-isoprostanes) in plasma and urine, with incident PoAF among 551 cardiac surgery patients. Biomarkers were measured at enrollment, the end of surgery, and postoperative day 2. PoAF lasting ≥30 seconds was confirmed with rhythm strip or electrocardiography and centrally adjudicated. Outcomes were assessed until hospital discharge or postoperative day 10, whichever occurred first. Urine level of each oxidative stress biomarker rose at the end of surgery (2- to 3-fold over baseline, P<0.001) and subsequently declined to concentrations comparable to baseline by postoperative day 2. In contrast, plasma concentrations remained relatively stable throughout the perioperative course. Urine F2-isoprostanes and isofurans at the end of surgery were 20% and 50% higher in subjects who developed PoAF (P≤0.009). While baseline biomarker levels did not associate significantly with PoAF, end of surgery and postoperative day 2 isoprostanes and isofurans demonstrated relatively linear associations with PoAF. For example, the end of surgery extreme quartile multivariate adjusted OR (95% CI) for urine isofurans and F3-isoprostanes were 1.95 (1.05 to 3.62; P for trend=0.01) and 2.10 (1.04 to 2.25, P for trend=0.04), respectively. The associations of biomarkers with PoAF varied little by demographics, surgery type, and medication use (P≥0.29 for each).
Conclusions
These novel results add to accumulating evidence supporting the likely key pathogenic role of elevated oxidative stress in PoAF.
Clinical Trial Registration
URL: Clinicaltrials.gov Unique identifier: NCT00970489.
Keywords: atrial fibrillation, cardiac surgery, isofurans, isoprostanes, oxidative stress
Postoperative atrial fibrillation (PoAF) commonly complicates cardiac surgery. PoAF increases the risk of thromboembolic events, prolongs hospital care, increases healthcare costs, and elevates long-term mortality.1 Current frontline therapy such as β-blockers and amiodarone only partly prevent PoAF, likely reflecting the complex, multifactorial pathogenesis of PoAF.2 Improved understanding of the precise molecular and physiologic mechanisms contributing to PoAF could promote the development of novel targeted interventions.
Experimental evidence suggests oxidative stress can contribute to the pathogenesis of PoAF.2 Oxidative stress occurs when excess production of reactive oxygen species (ROS) overwhelms endogenous antioxidant defenses, resulting in tissue injury. In animal studies, oxidative stress contributed to cardiac myocyte dysfunction, electrical and structural remodeling, and the development of AF.3–5 Furthermore, oxidative stress could potentiate PoAF via activation of stress signaling pathways and inflammatory responses, which in turn amplify tissue injury.6,7 Evidence supporting the relationship between oxidative stress and PoAF in humans, however, remains limited. Studies based on analyses of human atrial tissues collected during cardiac surgery suggest a pathogenic role of oxidative stress and, in particular, identified elevated atrial nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity as associated with PoAF.8,9 Conversely, measurements of circulating oxidative stress biomarkers have yielded mixed findings,9–11 although such studies typically assessed oxidative stress by using biomarkers with low sensitivity and specificity, which could obscure potential associations.12 Furthermore, although levels of oxidative stress vary throughout the perioperative time course,13 there is a lack of comprehensive evaluation of the relationship of oxidative stress with PoAF at different cardiac surgery time points.
To investigate further the role of oxidative stress in PoAF, we tested the hypothesis that plasma and urine levels of F2-isoprostanes (F2-isoP), isofurans (IsoF), and F3-isoprostanes (F3-IsoP) associate with incident PoAF in a subset of 551 subjects in the Omega-3 Fatty Acids for Prevention of Post-Operative Atrial Fibrillation (OPERA) trial.14 F2-isoP, IsoF, and F3-IsoP are highly sensitive and robust lipid oxidation biomarkers derived from arachidonic acid (F2-isoP and IsoF) and eicosapentaenoic acid (F3-IsoP).15,16 Concentrations of these biomarkers were measured at baseline (ie, at study recruitment, representing baseline ambulatory levels of oxidative stress), at the end of cardiac surgery (reflecting acute changes in oxidative damage following ischemia–reperfusion), and again at postoperative day 2 (to capture trends in persistently elevated or oxidative stress or, conversely, return to homeostasis).
Methods
Design and Population
This investigation was designed as an ancillary study of the OPERA trial, which was a randomized, placebo-controlled study that found perioperative fish oil supplementation did not reduce PoAF.14 Design of the OPERA study has been reported in detail previously.17,18 In brief, the study enrolled 1516 patients from 28 medical centers undergoing cardiac surgery in 3 countries (United States, Italy, and Argentina). The inclusion criteria were broad: age ≥18 years, presence of sinus rhythm on the screening electrocardiogram (ECG), and being scheduled for cardiac surgery on the following day or later. Exclusions were absence of sinus rhythm at screening, regular use of fish oil, known intolerance or allergy to fish oil or olive oil (placebo), unable or unwilling to provide informed consent, being currently pregnant, or having an existing or a planned cardiac transplant or use of ventricular assist device. All participants provided written informed consent, and the study was approved by the human subjects committees of all participating institutions. Seventeen study centers participated in this ancillary study.
Oxidative Stress Biomarkers
EDTA-anticoagulated fasting blood and spot urine samples were collected from subjects with the use of standardized kits and techniques at baseline, the end of cardiac surgery, and postoperative day 2. Biologic samples were stored at −70°C at each study center and subsequently transferred on dry ice to a central sample bank for long-term storage at −80°C. After completion of the OPERA study, oxidative stress biomarkers were measured at the Eicosanoid Core Laboratory at Vanderbilt University with the use of highly robust and specific gas chromatography–mass spectrometry.19 Limits of detection were 1, 5, and 10 pg/mL for F2-IsoP, F3-Iso, and IsoF, respectively. Interassay coefficient of variations were ≤15%. Levels of oxidative stress biomarkers in plasma are reported in picograms per milliliter, whereas their levels in urine are normalized to creatinine clearance and reported as nanograms per milligrams of creatinine. We were unable to quantify oxidative stress biomarkers in a small portion of samples (≤2% for F2-IsoP and IsoF, ≤12% for F3-IsoP) due to coeluting contaminants, and these samples were thus excluded from the analyses. If the level of an oxidative stress biomarker in a sample was less than the assay detection limit, its value was imputed as half of the detection limit.
Covariates
Other potential risk factors for PoAF were assessed by using standardized methods including demographics, lifestyle behaviors, cardiovascular risk factors, anthropometric measurements, medical and surgical history, comorbidities, medication use (outpatient and inpatient), echocardiographic evaluation, and laboratory measures. Additional data were collected regarding surgical procedure and daily follow-up and discharge information.
Outcomes
A centralized events committee of cardiac electrophysiologists reviewed and adjudicated all suspected episodes of PoAF, based on documentations including duration, clinical information, and confirmatory rhythm strip or 12-lead ECG. The primary end point for this analysis is incident PoAF of ≥30 seconds’ duration, with outcomes assessed until hospital discharge or postoperative day 10, whichever occurred first.
Statistical Analysis
The interrelationships between individual oxidative stress biomarkers were assessed at each time point by using Spearman correlation coefficients. Changes in biomarker level between study time points were assessed with paired t test. Oxidative stress biomarker concentrations were evaluated in quartiles as indicator variables, and their associations with incident PoAF were investigated by using multivariable-adjusted logistic regression. Biomarkers were also examined as continuous variables after log transformation because these variables were right-skewed. Tests of linear trend were conducted by assigning to participants the median value in each quartile and assessing this as a single continuous variable. We also examined possible nonlinear relationships between circulating and urine biomarkers and risk of PoAF nonparametrically by using restricted cubic splines, after excluding participants with extreme values (<1st or >99th percentile) to minimize the effects of outliers. To minimize confounding, we adjusted for age, sex, country, body mass index, prevalent hypertension, prevalent diabetes, prevalent coronary heart disease, prevalent chronic renal failure, prevalent heart failure, smoking, dyslipidemia, statin medication use, left ventricular ejection fraction, and logistic Euroscore.20 Adjustment for treatment did not make a difference and was not included in the final model. Missing covariates (<3%) were singly imputed by best-subset-regression with demographic/risk variables. Results were similar excluding those with missing covariate values. In sensitivity analyses, we also assessed the association between changes in oxidative stress biomarker concentrations from baseline to end of surgery and PoAF.
Effect modification were evaluated in stratified analyses for several subgroups including age (<median, ≥median), sex, country, body mass index (<median, ≥median), type of surgery (valve surgery versus nonvalve surgery), smoking (current versus ex-smokers and nonsmokers), aspirin use, and statin use. For each subgroup, oxidative stress biomarkers were assessed in stratum-specific quartiles as indicator variables. The significance of potential effect modification was tested by using the Wald test for a multiplicative interaction term between the biomarker quartiles (evaluated as ordinal variables) and the stratification variable, with Bonferroni-corrected α level to account for multiple comparisons. All P values were 2-tailed (α=0.05), and analyses were performed with Stata 13.1 (Stata Corp).
Results
The mean age of the participants was 63 years, and the majority (72%) were men (Table1). Subjects were enrolled in the United States (57%), Italy (32%), and Argentina (11%). As expected, there was a high prevalence of cardiometabolic risk factors, including hypertension and dyslipidemia, and nearly 50% of the subjects have a history of coronary heart disease.
Table 1.
Baseline Characteristics of Subjects With Oxidative Stress Biomarker Measures in the OPERA Trial (N=551)
| Characteristics | Summary Statistics* |
|---|---|
| Country, % | |
| USA | 57.0 |
| Italy | 32.1 |
| Argentina | 10.9 |
| Age, y | 62.7±12.7 |
| Sex, % male | 72.2 |
| Planned valve surgery, % | 55.0 |
| Planned CABG, % | 48.3 |
| Current smoking, % | 13.3 |
| Diabetes mellitus, % | 29.8 |
| Body mass index, kg/m2 | 29.0±6.1 |
| Hypertension, % | 77.1 |
| COPD, % | 14.3 |
| Dyslipidemia, % | 64.1 |
| Chronic renal failure, % | 6.5 |
| Coronary heart disease, % | 44.5 |
| Prior myocardial infarction, % | 22.9 |
| Prior PCI, % | 14.5 |
| Prior arrhythmias, % | 13.8 |
| Prior atrial fibrillation, % | 8.0 |
| Congestive heart failure, % | 20.0 |
| Ejection fraction, % | 56.2±11.2 |
| β-Blocker, % | 54.1 |
| Statins, % | 56.6 |
| ACE inhibitor, % | 35.6 |
| Aspirin, % | 61.9 |
| Antiarrhythmics, % | 4.4 |
| Logistic Euroscore, units | 6.2±7.5 |
CABG indicates coronary artery bypass graft surgery; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; ACE, angiotensin-converting enzyme.
Values are mean±SD for continuous variables, and percent for categorical variables.
The levels of individual oxidative stress biomarkers and their correlations are shown in the online supplemental data (Table S1). While the concentrations of F2-IsoP and IsoF were similar, they were an order of magnitude larger than F3-Iso in both plasma and urine. Biomarkers measured in the same compartment (plasma or urine) showed moderate to high correlations, with the strongest associations observed for F2-IsoP and IsoF. For example, Spearman correlation coefficients at the end of cardiac surgery were between 0.43 and 0.72 among urine oxidative stress biomarkers (P<0.001 for each); conversely, plasma concentrations of each biomarker correlated either not at all or only weakly with their respective urine levels. For example, plasma and urine isofurans did not correlate significantly at any of the time points assessed (r = −0.05 to 0.05, P≥0.32 for each).
There were significant changes in the concentrations of oxidative stress biomarkers aftert cardiac surgery, with distinct patterns for plasma and urine measurements, as well as for subjects who did or did not develop PoAF (Figures1 and 2). One of the most pronounced change was the 2- to 3-fold increase over baseline in urine level of all 3 oxidative stress biomarkers following cardiac surgery (P<0.001 for each). Furthermore, at the end of surgery, F2-isoP and IsoF levels were ≈20% and ≈50% higher in subjects who subsequently developed PoAF (P≤0.009, adjusted for enrollment biomarker concentrations). At postoperative day 2, urine levels of all 3 biomarkers fell significantly (paired t test relative to end of surgery, P≤0.03 for each), to concentrations comparable to baseline. Plasma levels of oxidative stress biomarkers did not show such marked variation as urine concentrations throughout the study. At postoperative day 2, levels of F2-IsoP and IsoF were slightly lower relative to the end of surgery (≈10%, P≤0.04 for each), and F2-IsoP levels were ≈20% higher in subjects who developed PoAF than in those who did not (P=0.05). Plasma F3-Iso was higher at the end of cardiac surgery (≈40%, P<0.001) and remained similar at postoperative day 2.
Figure 1.
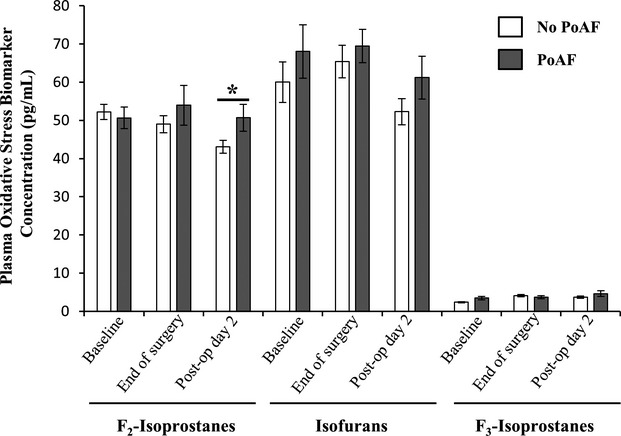
Plasma oxidative stress biomarkers concentration (pg/mL) at baseline (recruitment), end of surgery (at time of closure), and postoperative day 2. Data shown are mean±SE. The white and gray bars represent subjects without and with incident postoperative atrial fibrillation (PoAF), respectively. *Mean plasma level of F2-isoprostanes were ≈20% higher in patients who developed PoAF than in those who did not (P=0.05, adjusted for end of surgery F2-isoprostane concentrations).
Figure 2.
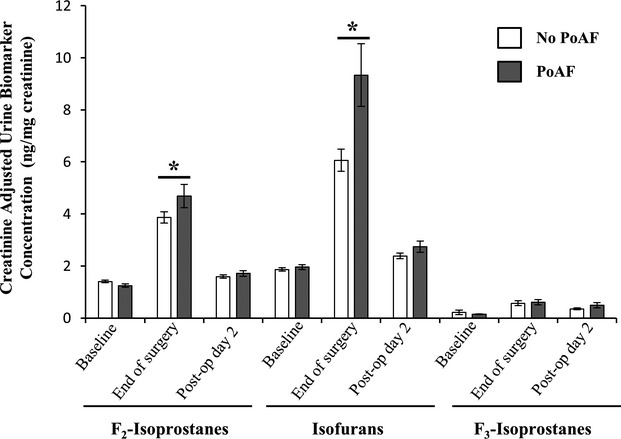
Urine creatinine-adjusted concentration of oxidative stress biomarkers (ng/mg) at baseline (recruitment), end of surgery (at time of closure), and postoperative day 2. Data shown are mean±SE. The white and gray bars represent subjects without and with incident postoperative atrial fibrillation (PoAF), respectively. *Mean urine F2-isoprostane and isofuran levels were ≈20% and ≈50% higher in subjects who subsequently developed PoAF (P≤0.009, adjusted for baseline concentrations).
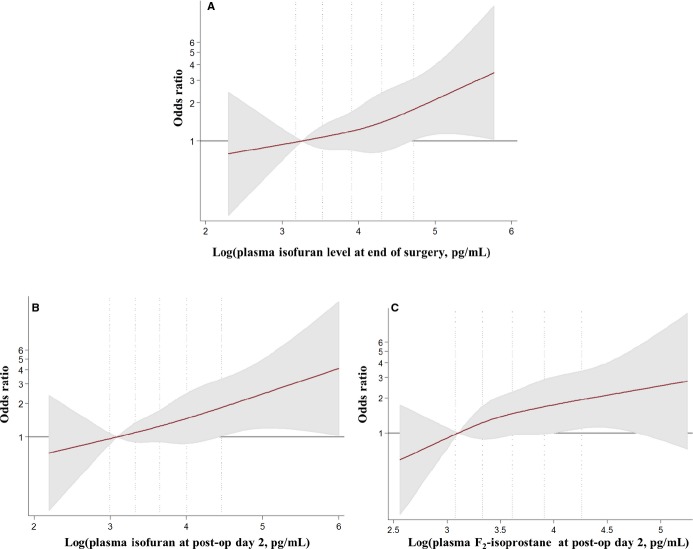
Among the 551 subjects in the study, 171 developed PoAF during follow-up. Oxidative stress biomarkers in plasma or urine at baseline did not associate significantly with PoAF (online supplemental data, Tables S2 and S3). By the end of surgery, however, the concentrations of IsoF in urine and plasma associated with elevated risk of PoAF. For example, the OR (95% CI) for participants in the highest compared with the lowest quartile of urine IsoF was 1.95 (1.05 to 3.62, P for trend=0.01). Urine, but not plasma F3-IsoP, concentration also associated with higher PoAF risk, with extreme quartile OR (95% CI) of 2.10 (1.04 to 2.25, P for trend=0.04). F2-IsoP at the end of surgery did not associate with PoAF. At postoperative day 2, plasma F2-IsoP and IsoF both associated with higher PoAF risk, with extreme quartile OR (95% CI) of 2.53 (1.14 to 5.58) and 2.98 (1.22 to 7.25), respectively. Urine concentrations of these biomarkers did not significantly associate with PoAF. Similarly, levels of F3-IsoP at postoperative day 2 in urine and plasma were not associated with subsequent PoAF. Semiparametric analyses with restricted cubic splines suggested relatively linear (monotonic) associations of oxidative stress biomarkers at the end of surgery and postoperative day 2 with incident PoAF (Figures3 and 4).
Figure 3.
Multivariable-adjusted association of plasma (A) isofuran at end of surgery (B) isofuran at postoperative day 2 and (C) F2-isoprostanes at postoperative day 2 with postoperative atrial fibrillation (PoAF), evaluated by restricted cubic splines and logistic regression. Biomarkers underwent log-transformation before analyses as they were right-skewed, and analyses adjusted for age (years), sex (male/female), country (US, Italy, Argentina), body mass index (kg/m2), prevalent hypertension (yes/no), prevalent diabetes (yes/no), prevalent coronary heart disease (yes/no), prevalent chronic renal failure (yes/no), prevalent heart failure (yes/no), smoking (never or former/current), dyslipidemia (yes/no), statin medication use (yes/no), ejection fraction (%), and logistic Euroscore (continuous). The solid red line and shaded areas represent the OR and 95% CIs, respectively, in comparison to the reference level representing the median value of the lowest quartile (12.5th percentile). Dotted vertical lines correspond to the 10th, 25th, 50th, 75th, and 90th percentiles of the log biomarker concentrations. There was overall linear association for each biomarkers with PoAF (P≤0.02) but little evidence of nonlinearity (P≥0.83).
Figure 4.
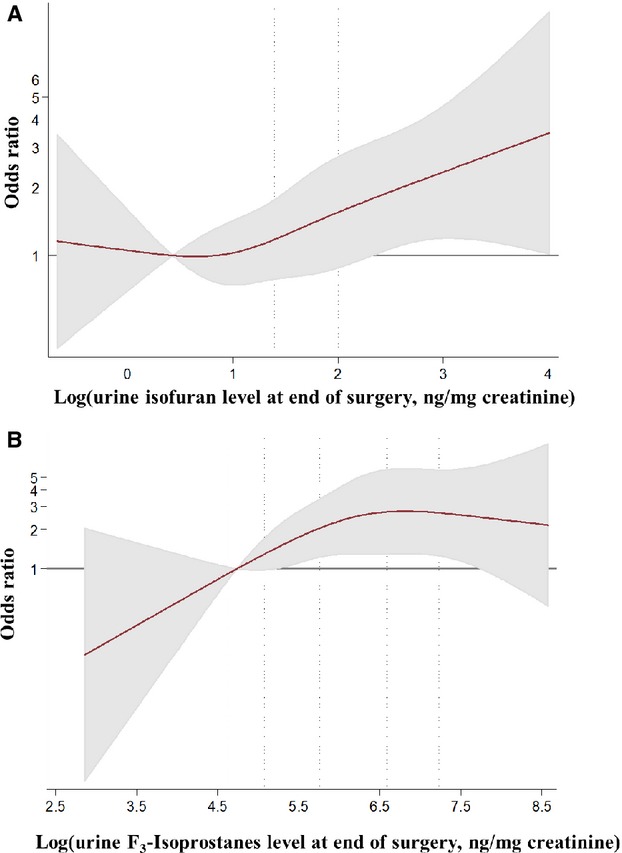
Multivariable-adjusted association of end of surgery (A) urine isofurans and (B) urine F3-isoprostanes with postoperative atrial fibrillation (PoAF), evaluated by restricted cubic splines and logistic regression. Biomarkers underwent log-transformation before analyses as they were right-skewed, and analyses adjusted for age (years), sex (male/female), country (US, Italy, Argentina), body mass index (kg/m2), prevalent hypertension (yes/no), prevalent diabetes (yes/no), prevalent coronary heart disease (yes/no), prevalent chronic renal failure (yes/no), prevalent heart failure (yes/no), smoking (never or former/current), dyslipidemia (yes/no), statin medication use (yes/no), ejection fraction (%), and logistic Euroscore (continuous). The solid red line and shaded areas represent the OR and 95% CIs, respectively, in comparison to the reference level representing the median value of the lowest quartile (12.5th percentile). Dotted vertical lines correspond to the 10th, 25th, 50th, 75th, and 90th percentiles of the log biomarker concentrations. Both biomarkers associated linearly overall with PoAF (P=0.02 for each) but showed little evidence of nonlinearity (P≥0.26 for each).
Results were similar when the biomarkers were assessed continuously. For example, each doubling of urine IsoF at the end of surgery was associated with an OR (95% CI) of 1.20 (1.03 to 1.41, P=0.02). In sensitivity analyses, changes in plasma and urine oxidative stress biomarker concentration from baseline to the end of surgery showed similar direction and magnitude of association with PoAF as end of surgery levels alone (not shown). Further adjustment for study treatment, valve surgery, time on pump, prevalent obstructive pulmonary disease, antiarrhythmic medication, changes in white blood cell count, and aspirin use did not appreciably alter the results (not shown). There was little evidence that country, age, sex, body mass index, type of surgery, smoking, and aspirin and statin use significantly modified the association of oxidative stress biomarkers and PoAF (Bonferroni corrected, P for interaction ≥0.29 for each).
Discussion
In this prospective cohort study of cardiac surgery patients, higher end of surgery and postoperative oxidative stress assessed by F2-IsoP, IsoF, and F3-IsoP associated independently with elevated risk of PoAF. The magnitude of associations was substantial, and subjects in the top quartile of lipid peroxidation biomarker had up to 3-fold higher odds of PoAF. In contrast, baseline IsoP and IsoF did not associate with PoAF.
Accumulating experimental evidence supports the biologic plausibility of these findings by indicating a causal role of oxidative stress in pathologic atrial structural remodeling, abnormal ion channel function, and increased vulnerability to AF.21 In animals, risk factors for AF development, such as hypertension and heart failure, link with increased ROS production,22,23 and treatment with antioxidants reduces atrial fibrosis and prevents tissue oxidative damage and occurrence of AF.24–26 Arachidonic acid and eicosapentaenoic acids in cardiac membranes may also influence AF development.27 Structural modification of these polyunsaturated fatty acids by ROS to IsoP and IsoF may disrupt their capacity to regulate protein and ion channel functions.28 Furthermore, not only do IsoP and IsoF serve as sensitive biomarkers of oxidative stress in vivo; they also may contribute to inflammation, such as by enhancing adhesion of monocytes to endothelial cells.29,30
The observed increase in urine F2-IsoP, IsoF, and F3-IsoP between baseline and the end of surgery is consistent with cardiac surgery–induced oxidative stress.31 Our data also indicate the transience of the imbalance in ROS generation and antioxidant defense triggered by cardiac surgery, as levels of urine biomarkers fell to near baseline levels by postoperative day 2. Whereas urine levels of these oxidative stress biomarkers provide a time-integrated measure of subacute systemic oxidative stress, their plasma levels reflect acute systemic oxidative stress, due to their rapid metabolism and renal excretion,32 which may explain the relative stability of plasma levels of these biomarkers observed in our study. Urine IsoF demonstrated a relatively greater increase than IsoP. Although both IsoF and IsoP arise from ROS-mediated peroxidation of polyunsaturated fatty acids, mitochondrial dysfunction and elevated oxygen concentration favor formation of IsoF.16 Our results, therefore, highlight the likely importance of identifying not only endogenous sources of oxidative stress but also factors that expose patients to increased oxygen concentration, such as inspired oxygen supplementation during cardiac surgery.
Our investigation demonstrates that higher oxidative stress at postoperative day 2 associates with subsequent risk of PoAF. These findings highlight the need to identify therapies that could reduce oxidative stress throughout the perioperative time course, specifically targeting oxidants that may cause lipid oxidation. Increasing evidence suggest both statins, which downregulate NADPH oxidase,33 and antioxidant scavengers are of potential interest, and recent meta-analyses of controlled trials found supplementation with statins or putative antioxidants (eg, vitamin C) reduced the risk of PoAF.34,35 However, these findings should be interpreted with caution given the large heterogeneity observed between the results of individual trials and the lack of evidence that the treatments actually reduced oxidative stress in vivo.35 Our findings indicate that future trials could rationally determine the dose and duration of statin or antioxidant therapy for PoAF prevention, based on their ability to reduce plasma or urine IsoP and IsoF. Because oxidative stress may activate inflammation, future studies should also examine inflammatory biomarkers (eg, myeloperoxidase), which could provide additional information.6,36
Our study has important strengths. The use of highly sensitive and specific IsoP and IsoF, assessed across multiple perioperative time points allowed comprehensive evaluation of the relationships of lipid oxidation and PoAF, and inclusion of these complementary biomarkers provide additional insight into potential oxidative stress mechanisms. The study used highly accurate and reproducible assays to measure the biomarkers, limiting the likelihood of misclassification of these exposures. The prospective design minimized selection bias and enabled inference on temporality of associations. Careful collection of information on many potential covariates allowed detailed adjustment to control for confounding. A centralized events committee conducted careful adjudication of PoAF events that lowered the possibility of outcome misclassification. Subjects in the OPERA trial were from different countries with varied demographic and lifestyle characteristics, which enhances generalizability.
Potential limitations also merit consideration. While IsoP and IsoF quantify the extent of lipid peroxidation, they do not provide insight into oxidative damage to other cellular compartments such as proteins and DNA, and these should be assessed in the future by using other validated biomarkers. We adjusted for a range of important demographic and clinical risk factors, but we cannot exclude the possibility of residual confounding due to unmeasured or imprecisely measured factors. Only a subset of OPERA trial subjects participated in this biologic study, which reduced statistical power. Nevertheless, this current investigation represents by far the largest study assessing objective, validated oxidative stress biomarkers in cardiac surgery patients. Although results from this study are relevant to cardiac surgery patients, findings may not be applicable to other populations at risk of AF. Of course, the strong association demonstrated by these data cannot establish causality between oxidative stress and the advent of PoAF. Yet, the grounds for biological plausibility mentioned here do support a causal link between oxidative stress and PoAF. Finally, our findings suggest the need to carefully evaluate the effect of fish oil treatment on oxidative stress levels in future studies.
In summary, cardiac surgery induce acute increase in oxidative stress as assessed with urine levels of IsoP and IsoF. In addition, elevated end of surgery and postoperative levels of these oxidative stress biomarkers relate to higher risk of PoAF. These novel results adds to growing evidence supporting the likely key pathogenic role of elevated oxidative stress in PoAF and suggest that further development of therapies targeting oxidative stress pathways for the prevention of PoAF is warranted.
Sources of Funding
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (1 RC2 HL101816, and R01HL085710); GlaxoSmithKline; Sigma Tau; and Pronova BioPharma. The funding organizations had no role in the design, conduct, or analysis of the OPERA trial or this biologic substudy. The authors thank all OPERA participants.
Disclosures
Dr Mozaffarian reports ad hoc travel reimbursement or honoraria from Bunge, Pollock Institute, Quaker Oats, and Life Sciences Research Organization; ad hoc consulting fees from McKinsey Health Systems Institute, Foodminds, Nutrition Impact, Amarin, Omthera, and Winston and Strawn LLP; membership, Unilever North America Scientific Advisory Board; royalties from UpToDate; and research grants from the National Institutes of Health. The other authors report no conflicts.
Supporting Information
Table S1. Concentrations and Unadjusted Spearman Correlation Coefficients for Plasma and Urine Concentration of Oxidative Stress Biomarkers in the OPERA Trial*
Table S2. Multivariable-Adjusted Risk of Postoperative Atrial Fibrillation According to Plasma Oxidative Stress Biomarkers in the OPERA Trial*
Table S3. Multivariable-Adjusted Risk of Postoperative Atrial Fibrillation According to Creatinine-Adjusted Urine Oxidative Stress Biomarkers in the OPERA Trial*
References
- Mitchell LB. Canadian cardiovascular society atrial fibrillation guidelines 2010: prevention and treatment of atrial fibrillation following cardiac surgery. Can J Cardiol. 2011;27:91–97. doi: 10.1016/j.cjca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. Europace. 2012;14:159–174. doi: 10.1093/europace/eur208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA, Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- Nishijima Y, Sridhar A, Bonilla I, Velayutham M, Khan M, Terentyeva R, Li C, Kuppusamy P, Elton TS, Terentyev D, Gyorke S, Zweier JL, Cardounel AJ, Carnes CA. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure-induced alterations in atrial electrophysiology. Cardiovasc Res. 2011;91:71–79. doi: 10.1093/cvr/cvr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts I, Driesen RB, Hermida N, Holemans P, Heidbuchel H, Janssens S, Balligand JL, Sipido KR, Willems R. Role of nitric oxide and oxidative stress in a sheep model of persistent atrial fibrillation. Europace. 2013;15:754–760. doi: 10.1093/europace/eut012. [DOI] [PubMed] [Google Scholar]
- Pinho-Gomes AC, Reilly S, Brandes RP, Casadei B. Targeting inflammation and oxidative stress in atrial fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibition with statins. Antioxid Redox Signal. 2014;20:1268–1285. doi: 10.1089/ars.2013.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DN, Vowinkel T, Petnehazy T. Modulation of the inflammatory response in cardiovascular disease. Hypertension. 2004;43:924–931. doi: 10.1161/01.HYP.0000123070.31763.55. [DOI] [PubMed] [Google Scholar]
- Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, Marinou K, Nahar K, Jayaram R, Tousoulis D, Bakogiannis C, Sayeed R, Triantafyllou C, Koumallos N, Psarros C, Miliou A, Stefanadis C, Channon KM, Casadei B. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J Am Coll Cardiol. 2012;59:60–70. doi: 10.1016/j.jacc.2011.08.062. [DOI] [PubMed] [Google Scholar]
- Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74. doi: 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- Girerd N, Pibarot P, Fournier D, Daleau P, Voisine P, O’Hara G, Despres JP, Mathieu P. Middle-aged men with increased waist circumference and elevated C-reactive protein level are at higher risk for postoperative atrial fibrillation following coronary artery bypass grafting surgery. Eur Heart J. 2009;30:1270–1278. doi: 10.1093/eurheartj/ehp091. [DOI] [PubMed] [Google Scholar]
- Ramlawi B, Otu H, Mieno S, Boodhwani M, Sodha NR, Clements RT, Bianchi C, Sellke FW. Oxidative stress and atrial fibrillation after cardiac surgery: a case-control study. Ann Thorac Surg. 2007;84:1166–1172. doi: 10.1016/j.athoracsur.2007.04.126. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, II, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCL4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Ulus AT, Aksoyek A, Ozkan M, Katircioglu SF, Basu S. Cardiopulmonary bypass as a cause of free radical-induced oxidative stress and enhanced blood-borne isoprostanes in humans. Free Radic Biol Med. 2003;34:911–917. doi: 10.1016/s0891-5849(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, Latini R, Libby P, Lombardi F, O’Gara PT, Page RL, Tavazzi L, Tognoni G. Fish oil and postoperative atrial fibrillation: the omega-3 fatty acids for prevention of post-operative atrial fibrillation (OPERA) randomized trial. JAMA. 2012;308:2001–2011. doi: 10.1001/jama.2012.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LJ, II, Milne GL. Isoprostanes. J Lipid Res. 2009;50(suppl):S219–S223. doi: 10.1194/jlr.R800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel JP, Jackson Roberts L. Isofurans: novel products of lipid peroxidation that define the occurrence of oxidant injury in settings of elevated oxygen tension. Antioxid Redox Signal. 2005;7:202–209. doi: 10.1089/ars.2005.7.202. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Marchioli R, Gardner T, Ferrazzi P, O’Gara P, Latini R, Libby P, Lombardi F, Macchia A, Page R, Santini M, Tavazzi L, Tognoni G. The omega-3 fatty acids for prevention of post-operative atrial fibrillation trial—rationale and design. Am Heart J. 2011;162:56–63.e53. doi: 10.1016/j.ahj.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JH, Marchioli R, Silletta MG, Macchia A, Song X, Siscovick DS, Harris WS, Masson S, Latini R, Albert C, Brown NJ, Lamarra M, Favaloro RR, Mozaffarian D. Plasma phospholipid omega-3 fatty acids and incidence of postoperative atrial fibrillation in the OPERA trial. J Am Heart Assoc. 2013;2:e000397. doi: 10.1161/JAHA.113.000397. doi: 10.1161/JAHA.113.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC. Measurement of F2- isoprostanes and isofurans using gas chromatography-mass spectrometry. Free Radic Biol Med. 2013;59:36–44. doi: 10.1016/j.freeradbiomed.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24:881–882. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- Sovari AA, Dudley SC. Antioxidant therapy for atrial fibrillation: lost in translation? Heart. 2012;98:1615–1616. doi: 10.1136/heartjnl-2012-302328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep. 2010;12:135–142. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- Grieve DJ, Shah AM. Oxidative stress in heart failure. More than just damage. Eur Heart J. 2003;24:2161–2163. doi: 10.1016/j.ehj.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Li Y, Sheng L, Li W, Liu W, Gong Y, Xue H, Shan H. Probucol attenuates atrial structural remodeling in prolonged pacing-induced atrial fibrillation in dogs. Biochem Biophys Res Commun. 2009;381:198–203. doi: 10.1016/j.bbrc.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Fukunaga N, Takahashi N, Hagiwara S, Kume O, Fukui A, Teshima Y, Shinohara T, Nawata T, Hara M, Noguchi T, Saikawa T. Establishment of a model of atrial fibrillation associated with chronic kidney disease in rats and the role of oxidative stress. Heart Rhythm. 2012;9:2023–2031. doi: 10.1016/j.hrthm.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Youn JY, Zhang J, Zhang Y, Chen H, Liu D, Ping P, Weiss JN, Cai H. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–79. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JH, Lemaitre RN, King IB, Song X, Sacks FM, Rimm EB, Heckbert SR, Siscovick DS, Mozaffarian D. Association of plasma phospholipid long-chain omega-3 fatty acids with incident atrial fibrillation in older adults: the Cardiovascular Health Study. Circulation. 2012;125:1084–1093. doi: 10.1161/CIRCULATIONAHA.111.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- Leitinger N, Huber J, Rizza C, Mechtcheriakova D, Bochkov V, Koshelnick Y, Berliner JA, Binder BR. The isoprostane 8-iso-PGF(2alpha) stimulates endothelial cells to bind monocytes: differences from thromboxane-mediated endothelial activation. FASEB J. 2001;15:1254–1256. doi: 10.1096/fj.00-0498fje. [DOI] [PubMed] [Google Scholar]
- Huber J, Bochkov VN, Binder BR, Leitinger N. The isoprostane 8-iso-PGE2 stimulates endothelial cells to bind monocytes via cyclic AMP- and p38 MAP kinase-dependent signaling pathways. Antioxid Redox Signal. 2003;5:163–169. doi: 10.1089/152308603764816523. [DOI] [PubMed] [Google Scholar]
- Chambers DJ. Oxidative stress injury during cardiac surgery: how important is it? Cardiovasc Res. 2007;73:626–628. doi: 10.1016/j.cardiores.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Basu S. Metabolism of 8-iso-prostaglandin F2alpha. FEBS Lett. 1998;428:32–36. doi: 10.1016/s0014-5793(98)00481-5. [DOI] [PubMed] [Google Scholar]
- Violi F, Calvieri C, Ferro D, Pignatelli P. Statins as antithrombotic drugs. Circulation. 2013;127:251–257. doi: 10.1161/CIRCULATIONAHA.112.145334. [DOI] [PubMed] [Google Scholar]
- Kuhn EW, Liakopoulos OJ, Stange S, Deppe AC, Slottosch I, Choi YH, Wahlers T. Preoperative statin therapy in cardiac surgery: a meta-analysis of 90,000 patients. Eur J Cardiothorac Surg. 2014;45:17–26. doi: 10.1093/ejcts/ezt181. ; discussion 26. [DOI] [PubMed] [Google Scholar]
- Violi F, Pastori D, Pignatelli P, Loffredo L. Antioxidants for prevention of atrial fibrillation: a potentially useful future therapeutic approach? A review of the literature and meta-analysis. Europace. 2014;16:1107–1116. doi: 10.1093/europace/euu040. [DOI] [PubMed] [Google Scholar]
- Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Concentrations and Unadjusted Spearman Correlation Coefficients for Plasma and Urine Concentration of Oxidative Stress Biomarkers in the OPERA Trial*
Table S2. Multivariable-Adjusted Risk of Postoperative Atrial Fibrillation According to Plasma Oxidative Stress Biomarkers in the OPERA Trial*
Table S3. Multivariable-Adjusted Risk of Postoperative Atrial Fibrillation According to Creatinine-Adjusted Urine Oxidative Stress Biomarkers in the OPERA Trial*