Abstract
Background
In adults, exercise blood pressure seems to be more closely related to cardiovascular risk than resting blood pressure; however, few data are available on the effects of familial risk factors, including smoking habits, on exercise blood pressure in adolescents.
Methods and Results
Blood pressure at rest and during exercise, parental smoking, and other familial risk factors were investigated in 532 adolescents aged 12 to 17 years (14.6±1.5 years) in the Kiel EX.PRESS. (EXercise PRESSure) Study. Exercise blood pressure was determined at 1.5 W/kg body weight using a standardized submaximal cycle ergometer test. Mean resting blood pressure was 113.1±12.8/57.2±7.1 mm Hg, and exercise blood pressure was 149.9±19.8/54.2±8.6 mm Hg. Parental smoking increased exercise systolic blood pressure (+4.0 mm Hg, 3.1 to 4.9; P=0.03) but not resting blood pressure of the subjects (adjusted for age, sex, height, body mass index percentile, fitness). Parental overweight and familial hypertension were related to both higher resting and exercise systolic blood pressure values, whereas associations with an inactive lifestyle and a low educational level of the parents were found only with adolescents’ blood pressure during exercise. The cumulative effect of familial risk factors on exercise systolic blood pressure was more pronounced than on blood pressure at rest.
Conclusions
Parental smoking might be a novel risk factor for higher blood pressure, especially during exercise. In addition, systolic blood pressure during a submaximal exercise test was more closely associated with familial risk factors than was resting blood pressure, even in adolescents.
Keywords: adolescents, blood pressure measurement, exercise testing, risk factors, smoking
Arterial hypertension is among the most important cardiovascular risk factors in adults.1 In addition, it has been suggested that an excessive rise of blood pressure (BP) during exercise testing is more closely associated with cardiovascular prognosis and is a better predictor for future hypertension, even independent of BP at rest.2–7 The higher susceptibility of office resting BP to situational influences such as psychological factors could explain its lower prognostic significance compared with BP during acute exercise.8 Consequently, exercise BP might be a more reliable parameter than resting BP,8,9 especially in youth, because it is less affected by psychological components.10
Although higher resting BP values seem to continue from adolescence into adulthood (tracking phenomenon),11,12 little is known about the significance of exercise BP in adolescents. This could be of particular interest in younger subjects because it has been assumed that an excessive BP response during exercise precedes the onset of hypertension13–15 and permits an early diagnosis.16–18
For this purpose, we studied resting and exercise BP in a large sample of adolescents (aged 12 to 17 years) in the Kiel EX.PRESS. (EXercise PRESSure) Study. Elevated BP in children and adolescents is caused by a complex interaction of various biological and behavioral but also familial risk factors, with a high probability of resulting in an increasing number of future young hypertensive individuals.19,20 Because no data are available linking passive tobacco exposure to exercise BP in young persons, the influence of parental smoking was determined in the current substudy. There are many associations between smoking and BP regulation in adults.21,22 Even parental smoking seems to influence children’s resting BP.23
In the present investigation, particular emphasis was placed on the effect of parental smoking on systolic BP during exercise and at rest. In a few studies, an association between exercise BP and individual cardiovascular risk factors was demonstrated in health participants for adiposity,18,24 insulin resistance,18 and left ventricular hypertrophy25 but not for familial risk factors except family history of hypertension.13 Consequently, in our study, exercise and resting systolic BP were investigated in relation to parental smoking and several known familial risk factors.
In addition, information on the relevance of exercise BP compared with resting BP with respect to cardiovascular risk in adolescents might be obtained by differentiating the associations of known familial risk factors with both parameters.
Methods
Subjects
The present cross-sectional data are based on a substudy of our teenage sample from the Kiel EX.PRESS. Study, for which participants were recruited from 6 public schools in Kiel, northern Germany. The schools participated in our study after a written request to the school administration. We initially invited 645 young subjects, and a total of 532 healthy adolescents aged 12 to 17 years were enrolled (271 boys and 261 girls; 82.5% of the invited participants due to lack of willingness) from March 2011 to October 2012. BP measurements at rest and during exercise, additional anthropometric assessments, and a submaximal aerobic fitness test were added to a detailed interview of the parents about family history of diseases and social status. The study fulfilled the criteria of the Declaration of Helsinki and was approved by the local scientific ethics committee of the Medical Faculty at Kiel University. Children with hypertension or other cardiovascular diseases were excluded from the study. Parents and children were informed about the study through oral and written information and gave their signed consent.
BP and Clinical Measurements
Body weight of the subjects (without shoes) to the nearest of 0.1 kg and height to the nearest of 0.1 cm were determined by standard anthropometric methods with the use of electronic scales and a fixed stadiometer in an upright position. After weighing, 500 g were subtracted for the light garments that were left on. The body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). BMI percentiles were derived from German reference values defined as BMI ≥90th percentile for overweight and ≥97th percentile for obesity.26 BMI percentiles were chosen over waist circumference for 2 reasons. First, BMI percentiles are more accurate because age and sex are considered. Second, even though waist circumference is also an important prognostic parameter, more studies have been conducted using BMI in adolescents.
At each examination, measurements of height, weight, and BP were obtained by trained examiners according to a standardized protocol. All BP measurements were performed by the same trained investigator using a standard stethoscope and sphygmomanometer. Resting BP was measured in a seated position by auscultation using the Riva-Rocci-Korotkoff method after 5 minutes of rest. Systolic and diastolic BP were determined by the onset and disappearance of the Korotkoff sounds (K1 and K5), respectively. Appropriate cuff sizes were selected according to the child’s upper right arm circumference by referring to standardized procedural guidelines.27 Three BP readings at rest were taken at 2-minute intervals. The lowest value of those measurements was used in the analysis. Elevated resting systolic BP was defined as ≥95th percentile, dependent on age, height, and sex, corresponding to German reference data.28 Systolic BP values between the 90th and 95th percentiles indicated a high normal BP.
Exercise BP was studied during a graded cycle ergometer submaximal exercise test measured at 1.5 W/kg body weight, which corresponds approximately to the recommendation for adults.8 In addition, it has also been reported that the effect of psychological factors on BP is negligible at a workload above 1.0 W/kg body weight.10 Aerobic fitness was determined as the physical working capacity at a heart rate of 170 beats per minute (PWC 170 test), which has been shown to be a reliable and largely valid parameter of submaximal physical fitness.29 Heart rate was recorded continously during the test using a heart rate monitor (Polar X30; Polar Electro). After an initial workload of 1.0 W/kg body weight, the workload was gradually increased every 3 minutes by 0.5 W/kg body weight until a heart rate ≥170 beats per minute (PWC 170 test) was expressed as watts relative to body weight in kilograms. Because physical performance is not proportional to body weight, the weight-related test protocol was modified in overweight and obese adolescents, using their age- and sex-specific healthy weight in accordance with the 50th percentile26 to determine the initial and incremental workload. BP was taken at the end of each 3-minute workload while subjects were still exercising.
Because exercise diastolic BP cannot be measured reliably,9,30 only systolic resting and exercise BP were taken for statistical comparison in this study.
Familial Risk Factors
Parents filled out a questionnaire to provide information about the mother’s and father’s ages, weight, height, educational levels, current smoking habits, physical activity, and family history of cardiovascular diseases (eg, arterial hypertension, overweight or obesity). BMI was classified based on the World Health Organization definitions of underweight or lean (BMI 18.5 kg/m2), normal weight (BMI ≥18.5 and >25 kg/m2), overweight (BMI 25 to 30 kg/m2), and obese (BMI >30 kg/m2). Because passive smoking at a younger age is usually due to living with parents who smoke,31 self-reported smoking status was used as an indirect indicator of passive exposure to tobacco smoke of the study participants, a method previously used by Simonetti et al.23 With regard to family history, the interviewed parent was asked to state whether such diseases were once diagnosed by a physician. Mothers, fathers, and grandparents were classified as hypertensive if hypertension had been diagnosed, if drug therapy had already been initiated, or both. The parents’ educational levels were used as an indicator of social status in this study. The educational level of the family was classified as high if at least 1 parent had completed a college degree. Finally, parents were asked about amount and type of physical activity.
Statistical Analysis
For descriptive purposes, data are expressed as the mean±one standard deviation (SD) unless stated otherwise. BP values are given as mean resting and exercise systolic BP for the group. Data were analyzed by bivariate partial correlation analysis using the Pearson coefficient to evaluate simple associations between individual parameters. Dichotomous variables were compared with the chi-square test. Independent Student’s t tests assessed sex differences for all measured continuous variables. To consider characteristic BP changes during maturation, ANCOVA was performed for group comparisons of examined risk factors with systolic BP as outcome and age, height, and sex as covariates. The same analysis was repeated with additional correction for BMI percentile and aerobic fitness (W/kg body weight) as potential confounders or mediators of associations. Available-case analysis was used for handling missing data to limit the reduction in sample size. All statistical calculations were performed with SPSS software version 20.0 (IBM Corp), with P<0.05 considered significant.
Results
Subject Characteristics
A total of 532 adolescents underwent anthropometric and BP measurements. Participant characteristics are shown in Table1. Distribution by sex was 50.9% male and 49.1% female. The mean age was 14.6±1.5 years (range 12 to 17 years). The mean BMI was 21.0±3.4 kg/m2 (range 13.2 to 35.5 kg/m2). Overweight (BMI between the 90th and 97th percentiles) was found in 42 participants (7.9%; 24 boys and 18 girls), and obesity (≥97th percentile) was observed in 29 (5.5%) of the examined adolescents (14 boys and 15 girls). As expected, male participants were taller and heavier, but BMI was similar for male and female participants. In addition, female participants had a lower mean resting systolic BP but higher diastolic BP. The full sample had an average resting BP of 113.1±12.8/57.2±7.1 mm Hg (range 82.0 to 158.0/36.0 to 86.0 mm Hg). The prevalence of a resting systolic BP >95th percentile was 7.9% (n=42).
Table 1.
Basic Characteristics of 532 Adolescents
| Male (n=271) | Female (n=261) | P Value | |
|---|---|---|---|
| Age, y | 14.6±1.5 | 14.6±1.5 | 0.827 |
| Height, cm | 172.9±9.8 | 165.1±6.8 | <0.001 |
| Weight, kg | 60.5±12.6 | 58.0±10.6 | <0.001 |
| BMI, kg/m² | 21.0±3.4 | 21.2±3.4 | 0.295 |
| BMI percentile | 58.4±27.4 | 59.5±27.1 | 0.375 |
| Resting systolic BP, mm Hg | 115.5±13.2 | 110.6±11.9 | <0.001 |
| Resting diastolic BP, mm Hg | 56.4±8.1 | 58.1±7.1 | <0.001 |
| Exercise systolic BP*, mm Hg | 153.6±20.6 | 146.1±18.2 | <0.001 |
| Exercise diastolic BP*, mm Hg | 54.1±8.5 | 54.3±8.7 | 0.784 |
Values are mean±SD. BMI indicates body mass index; BP, blood pressure.
n=492 (boys n=252, girls n=240).
Of the 271 boys and 261 girls who participated in the Kiel EX.PRESS. Study, 252 boys and 240 girls had valid measured BP values during exercise; 7.5% did not perform the ergometer test, mostly because of illness or reduced performance resulting from injuries. The mean exercise BP was 149.9±19.8/54.2±8.6 mm Hg (range 108.0 to 240.0/30.0 to 74.0 mm Hg).
Parent Characteristics
Data describing the parental characteristics are presented in Table2. Fathers were more likely to be overweight than mothers in this study. Of the 374 families, at least 1 parent was overweight in 60.2% of families and both parents were overweight in 18.2%. Moreover, 22% of the mothers and 24% of the fathers were smokers. In 35% of the families investigated, at least 1 parent smoked, and in 9%, both mothers and fathers were currently smoking. A higher educational level was found in two-thirds (66.8%) of the families. A large fraction of the interviewed mothers (66.1%) and fathers (60.5%) engaged in sports during their leisure time; only a small number of the families were totally inactive (25.0%). The prevalence of parental hypertension was 29.0%; however, more than half of the participants (61.8%) had a positive family history (parents and grandparents) of arterial hypertension, and 3.5% of the examined adolescents had both hypertensive mothers and fathers. There was no difference in the prevalence of being overweight between smoking and nonsmoking parents (35.2% versus 34.6%, P=0.908). A higher proportion of smokers was found among parents with a low educational level (54.8% versus 24.8%, P<0.001). This subgroup of smokers was less likely to be normal weight than overweight (20.8% versus 40.7%, P<0.001). Moreover, smokers were more likely to be inactive (52.1% versus 29.0%, P<0.001), but an association with the presence of hypertension did not occur in this subgroup (35.5% versus 35.5%, P=0.988).
Table 2.
Basic Characteristics of Parents
| Mother (n=389) | Father (n=359) | P Value | |
|---|---|---|---|
| Age, y | 45.4±4.6 | 48.2±6.0 | <0.001 |
| Height, cm | 168.1±6.4 | 181.7±7.0 | <0.001 |
| Weight, kg | 67.1±11.8 | 86.2±11.0 | <0.001 |
| BMI, kg/m² | 23.7±3.8 | 26.1±3.2 | <0.001 |
| Overweight (≥25 kg/m²), % | 26.9 | 58.4 | 0.001 |
| Arterial hypertension, % | 14.5 | 25.2 | 0.001 |
| Smoker, % | 21.9 | 24.0 | 0.493 |
| Educational level, % | |||
| Low | 51.3 | 41.8 | <0.001 |
| High | 48.7 | 58.2 | |
| Physical activity, % | |||
| Inactive (both) | 33.9 | 39.5 | <0.001 |
| Active | 66.1 | 60.5 | |
Values are mean±SD or percentage. BMI indicates body mass index.
Resting and Exercise Systolic BP
Moderate correlations were observed between resting and exercise systolic BP (r=0.564, P<0.001) after adjusting for age, sex, and height. Participants with resting systolic BP values classified as high-normal or hypertensive (12.4%) showed 13.8mm Hg higher exercise systolic BP during cycle ergometer testing, even after adjustment for age, sex, height, BMI percentile, and physical fitness. The partial correlation coefficient for both resting and exercise systolic BP indicated a positive significant association with BMI percentile (r=0.289 versus r=0.312, P<0.001).
Associations Between Systolic BP and Familial Risk Factors
All parental risk factors were associated with higher levels of adolescents systolic BP at rest and during exercise. Compared with exercise systolic BP, there was a weaker influence on resting BP. After full adjustment for age, sex, height, BMI percentile, and fitness, children with a positive family history of hypertension showed higher systolic BP values at rest (+4.4 mm Hg, P<0.001) and during submaximal exercise (+4.7 mm Hg, P=0.007) compared with nonpredisposed adolescents. Parental obesity indirectly affected only children’s exercise systolic BP (+5.2 mm Hg, P=0.005) because no effects were observed after controlling for their own BMI percentile und fitness. With respect to resting systolic BP, even this association could not be verified. Furthermore, a low educational level was associated with higher systolic BP values only during exercise (+5.2 mm Hg, P=0.006), even after adjusting for all covariates. In addition, children from families with at least 1 active parent exhibited lower exercise systolic BP values (+4.9 mm Hg, P=0.013) than children with inactive parents, whereas resting systolic BP level did not differ between these groups.
Parental Smoking
There was no influence of parental smoking on resting systolic BP. In contrast, exercise systolic BP was significantly higher (+6.3 mm Hg, P=0.001) in subjects exposed to parental smoking than in unexposed children. Even after further adjustment for BMI percentile and fitness, the influence of parental smoking remained largely unchanged (+4.0 mm Hg, P=0.03). The effect on exercise systolic BP increased in children for which both mother and father were smokers in comparison to children of nonsmoking parents (+6.2 mm Hg, P=0.044). No significant difference was found when systolic BP values were additionally adjusted according to the parents’ educational levels.
Mean systolic BP differences at rest and during exercise are summarized in Table3.
Table 3.
The Effect of Familial Risk Factors on Resting and Exercise Systolic Blood Pressure (n=374)
| Difference, mm Hg | |||||||
|---|---|---|---|---|---|---|---|
| Resting Systolic BP | Exercise Systolic BP | ||||||
| Prevalence, %* | MD | 95% CI | P Value | MD | 95% CI | P Value | |
| Parental overweight | 60.2 | +1.0 | 0.7 to 1.4 | 0.397 | +3.4 | 2.8 to 3.8 | 0.061 |
| Parental smoking | 35.0 | −0.1 | −0.7 to 0.5 | 0.945 | +4.0 | 3.1 to 4.9 | 0.030 |
| Parental hypertension | 29.0 | +4.1 | 3.6 to 4.7 | 0.001 | +3.9 | 3.1 to 4.8 | 0.029 |
| Familial hypertension† | 61.8 | +4.4 | 4.0 to 4.6 | <0.001 | +4.7 | 4.2 to 5.3 | 0.007 |
| Inactive parents (both) | 25.0 | +1.0 | 0.1 to 1.9 | 0.467 | +4.9 | 3.5 to 6.4 | 0.013 |
| Low educational level | 33.2 | +1.3 | 0.6 to 1.9 | 0.315 | +5.2 | 4.3 to 6.2 | 0.006 |
ANOVA analyses were adjusted for age, sex, height, BMI percentile, and physical fitness of adolescents. BMI indicates body mass index; BP, blood pressure; MD, mean difference.
At least 1 parent.
Mother, father, and grandparents.
Cumulative Effect of Risk Factors
To investigate the cumulative effect of risk factors on resting versus exercise systolic BP, subgroups with different individual familial risk profiles were compared using the nonrisk group as a reference. The figure illustrates the progressive increase in systolic BP at rest and during exercise depending on the number of familial risk factors present (parental overweight, parental smoking, low educational level, positive family history of hypertension) after adjustment for age, sex, and height, respectively. Both mean resting and exercise systolic BP values were lower in the group without any risk factors, but exercise systolic BP was more strongly influenced by the number of accompanying risk factors.
Figure 1.
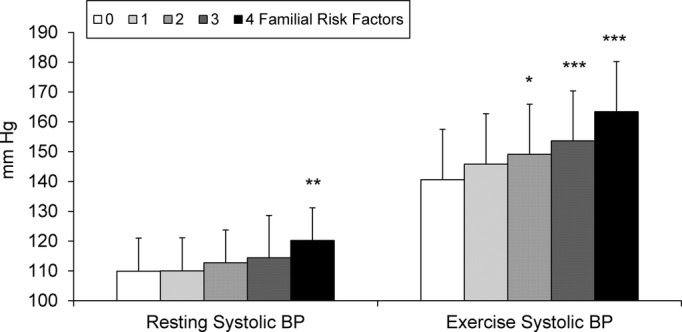
Progressive increase of resting and exercise systolic blood pressure (BP) with cumulative number of familial risk factors (parental overweight, parental smoking, low educational level, positive family history of hypertension) in adolescents. Mean ± SD. Adjusted for age, sex, and height. *P<0.05, **P<0.01, ***P<0.001 compared with participants without any risk factor.
With children without any familial risk factor as a reference, a synergistic effect of the different risk factors on both resting (P<0.001) and exercise (P<0.001) systolic BP was detected. A significantly higher resting systolic BP was found only in the high-risk group with 4 risk factors compared with the reference group (+10.3 mm Hg, P=0.001). During exercise, mean systolic BP increased progressively in proportion to the number of risk factors present; however, associations were already significant with fewer risk factors present compared with resting systolic BP. Mean systolic BP differences during exercise were up to 22.8 mm Hg (P<0.001) among children with all of the respective familial risk factors, up to 13.0 mm Hg (P=0.01) with 3 risk factors, and at 8.5 mm Hg (P=0.03) with 2 cumulative risk factors. If only 1 familial risk factor was identified, exercise-induced systolic BP values no longer differed from participants without a family risk factor.
Discussion
The primary goal of the present cross-sectional study was to investigate the association between systolic BP response during acute exercise and parental smoking in addition to other familial risk factors in a large sample of adolescents.
To the best of our knowledge, no previous study has addressed whether passive tobacco exposure is related to exaggerated exercise BP values in adolescents. The role of active and passive tobacco exposure in various functions of the cardiovascular system is well documented in adults,21,22 including adverse consequences on BP and heart rate as well as exercise tolerance, coronary vasoconstriction, endothelial dysfunction, and increased risk of thrombosis.32,33 Fewer data are available concerning the health consequences of active and passive smoking on the cardiovascular system and arterial BP in children and adolescents. Previously, passive tobacco smoke has been related to altered endothelial function, increased thickness of intima media, and higher resting BP in healthy preschool children.23,34,35 A novelty of our data was the association between parental smoking and exercise systolic BP, whereas resting BP was not affected by passive exposure to tobacco smoke. This relation was independent of BMI percentile and physical fitness of the participants but disappeared after correction for family educational level. Social disparities are believed to be associated with increased cardiovascular risk including unhealthy lifestyle patterns of both parents and children.36 This might have resulted in the higher exercise systolic BP of our participants and would be consistent with other studies.23 Further research is needed to differentiate the impact of parental smoking and educational level on BP because both factors seem to increase children’s exercise systolic BP.
The results indicate that exaggerated systolic BP values during submaximal exercise were associated with classic familial risk factors even at an early age.13,15,24 In the literature, a positive family history of hypertension has been found to be among the strongest predictors of adulthood hypertension.37 Our data provide further evidence that a relationship also exists between familial BP and BP in adolescents, especially during exercise.13
In the current analysis, some familial risk factors were associated with only exercise systolic BP and not resting BP, whereas other risk factors showed a tendency for stronger correlation with exercise systolic BP versus resting BP.
We found exercise systolic BP but not resting BP to be related to low parental educational level, even after adjusting for fitness and BMI percentile, although effects of socioeconomic conditions on BP were shown to be partly mediated via children’s BMI in previous research.38,39 As expected, we observed adolescents’ BMI percentile to be positively correlated to parental overweight,40 which contributes to exaggerated exercise systolic BP values via a mediating effect.24 Surprisingly, parental overweight failed to affect resting systolic BP; however, it can be assumed that parents and children share similar lifestyle-associated risk factors41,42 as a consequence of the parental role model. Beneficial effects of an active parental lifestyle on children’s BMI and activity are well known.38,43 In our study, parental physical activity further influenced adolescents’ systolic BP during exercise, independent of BMI percentile and fitness. Consequently, the impact of similar environmental conditions and behavioral patterns should be considered when assessing the cardiovascular risk of children and adolescents. Our data do not allow for complete differentiation between genetic and lifestyle-related causes for familial risk factors. Although familial hypertension has an important genetic component, other familial risk factors such as obesity, physical inactivity, low social status, and smoking are modifiable lifestyle risk factors investigated in our study.
To date, cumulative effects of familial risk factors have been detected only on resting systolic BP of preschool children.23 This study, however, found that familial risk factors appear to act synergistically on systolic BP not only at rest but also during exercise. In addition, the cumulative influence of these multiple risk factors was more pronounced on exercise systolic BP compared with the effect on resting BP. Differences in systolic BP during exercise became significant even with 2 or more familial risk factors, whereas a significantly higher resting systolic BP was demonstrated only if 4 risk factors were present.
A strength of our study is the standardized weight-related exercise protocol being adapted in overweight and obese participants by determining the relative capacity based on their age- and sex-specific healthy weight. We examined the exercise systolic BP response by using information at a single exercise intensity (1.5 W/kg body weight). This is opposed to the method used in the European Youth Heart Study,18 which measured BP values over a full range of workloads. Other studies took peak exercise as a reference.17 The full physical capacity with BP measurements at a certain percentage of the maximum power of a person would certainly be of interest. Nevertheless, the feasibility of tests with increasing intensity until exhaustion might be difficult to use in clinical practice. Despite this, our research aimed to identify exaggerated systolic BP response to activity intensity levels equal to everyday stresses, such as climbing stairs.8 Moreover, findings in adults indicate a higher prognostic significance of exercise systolic BP even at a fixed (independent of body weight) definition of workload (eg, 100 W) compared with resting BP values.2,3 Consequently, the procedure used in the present study is even more adapted to the individual than the common clinical practice in adults. In addition, the fact that Mocellin et al10 showed that BP values above a workload of 1.0 W/kg body weight were no longer affected by psychological components justified our approach to determine exercise BP using submaximal workload. Finally, the present investigation is in accordance with data of the European Youth Heart Study showing that the relationship between risk factors and systolic BP response during exercise was stable across all workloads.18
Although our study is unique in that a relationship between parental smoking and exercise systolic BP was found, the results admittedly allowed no accurate estimation of the actual exposure to passive smoke because only the parents were asked. Moreover, we did not correct for active tobacco smoking, but the prevalence of active smokers in our young study group was very low (7%), which is similar (12%) to the general population in Germany (KiGGS Study).44 Furthermore, the proportion of tobacco-consuming parents was almost the same for smoking and nonsmoking adolescents (33% versus 35%).
Generally, the use of self-reported parent data is a limitation of our study. Nevertheless, we identified significant associations despite possible response biases. Confounding effects of variables, such as sexual maturation, cannot be excluded and should be considered with regard to anthropometric data having a major impact on BP. In fact, other factors such as nutrition or birth weight might also be of interest but were not related to the primary goal of our study. The present study has a 2-fold clinical implication. First, the smoking status of the parents should be part of the risk factor assessment of adolescents. Second, exercise BP might be of clinical value in subjects with high familial risk.
In summary, avoiding or removing modifiable risk factors by promoting lifestyle changes as early as possible is a crucial contribution to minimize future cardiovascular disease risk with respect to all family members because increasing evidence shows that risk factors persist and tend to intensify throughout life.45
Perspectives
The findings of this investigation support the importance of exercise BP for youth and reveal a significant relationship between systolic BP during exercise and classic familial risk factors at an early stage of life. This study is the first to demonstrate an effect of parental smoking on the exercise systolic BP of their children. The determination of BP during exercise in clinical practice might be relevant not only to adults but also to adolescents. Although prognostic data are not available at this time, the correlating factors in our study can be helpful as surrogate parameters to assess the importance of exercise systolic BP. Following the results of the Young Finns Study, the age group examined in our research (participants aged 12 to 17 years) seems to be more significant for assessing health perspective than early childhood.46 We suggest that reports of adolescents’ cardiovascular risk profiles should emphasize BP level during exercise. Taking parental smoking habits into consideration might facilitate this evaluation of health risk.
Acknowledgments
We gratefully acknowledge the helpful support of the study staff that made this investigation possible. Special thanks are due to all participating adolescents and their families and teachers for their involvement.
Sources of Funding
The author was funded by a PhD grant of the FAZIT-STIFTUNG.
Disclosures
None.
References
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular mortality in middle-aged men. Hypertension. 1994;24:56–62. doi: 10.1161/01.hyp.24.1.56. [DOI] [PubMed] [Google Scholar]
- Filipovský J, Ducimetière P, Safar ME. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension. 1992;20:333–339. doi: 10.1161/01.hyp.20.3.333. [DOI] [PubMed] [Google Scholar]
- Singh JP, Larson MG, Manolio TA, O’Donnell CJ, Lauer M, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham Heart Study. Circulation. 1999;99:1831–1836. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- Schultz MG, Otahal P, Cleland VJ, Blizzard L, Marwick TH, Sharman JE. Exercise-induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: a systematic review and meta-analysis. Am J Hypertens. 2013;26:357–366. doi: 10.1093/ajh/hps053. [DOI] [PubMed] [Google Scholar]
- Le V, Mitiku T, Sungar G, Myers J, Froelicher V. The blood pressure response to dynamic exercise testing: a systematic review. Prog Cardiovasc Dis. 2008;51:135–160. doi: 10.1016/j.pcad.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Holmqvist L, Mortensen L, Kanckos C, Ljungman C, Mehlig K, Manhem K. Exercise blood pressure and the risk of future hypertension. J Hum Hypertens. 2012;26:691–695. doi: 10.1038/jhh.2011.99. [DOI] [PubMed] [Google Scholar]
- Franz IW. Ergometry in Hypertensive Patients: Implications for Diagnosis and Treatment. Berlin Heidelberg New York Tokio: Springer; 2012. [Google Scholar]
- Kjeldsen SE, Mundal R, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Supine and exercise systolic blood pressure predict cardiovascular death in middle-aged men. J Hypertens. 2001;19:1343–1348. doi: 10.1097/00004872-200108000-00001. [DOI] [PubMed] [Google Scholar]
- Mocellin R, Rutenfranz J. Methodische Untersuchungen zur Bestimmung der körperlichen Leistungsfähigkeit (W170) im Kindesalter. Z Kinderheilkd. 1970;108:61–80. [PubMed] [Google Scholar]
- Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Dana T, Bougatsos C, Blazina I, Norris SL. Screening for hypertension in children and adolescents to prevent cardiovascular disease. Pediatrics. 2013;131:490–525. doi: 10.1542/peds.2012-3523. [DOI] [PubMed] [Google Scholar]
- Molineux D, Steptoe A. Exaggerated blood pressure responses to submaximal exercise in normotensive adolescents with a family history of hypertension. J Hypertens. 1988;6:361–365. [PubMed] [Google Scholar]
- Grøntved A, Brage S, Møller NC, Kristensen PL, Wedderkopp N, Froberg K, Andersen LB. Hemodynamic variables during exercise in childhood and resting systolic blood pressure levels 6 years later in adolescence: the European Youth Heart Study. J Hum Hypertens. 2011;25:608–614. doi: 10.1038/jhh.2010.103. [DOI] [PubMed] [Google Scholar]
- Lauer RM, Burns TL, Clarke WR, Mahoney LT. Childhood predictors of future blood pressure. Hypertension. 1991;18:I74–I81. doi: 10.1161/01.hyp.18.3_suppl.i74. [DOI] [PubMed] [Google Scholar]
- Fixler DE, Laird WP, Browne R, Fitzgerald V, Wilson S, Vance R. Response of hypertensive adolescents to dynamic and isometric exercise stress. Pediatrics. 1979;64:579–583. [PubMed] [Google Scholar]
- Wanne OP, Haapoja E. Blood pressure during exercise in healthy children. Eur J Appl Physiol Occup Physiol. 1988;58:62–67. doi: 10.1007/BF00636604. [DOI] [PubMed] [Google Scholar]
- Møller NC, Grøntved A, Wedderkopp N, Ried-Larsen M, Kristensen PL, Andersen LB, Froberg K. Cardiovascular disease risk factors and blood pressure response during exercise in healthy children and adolescents: the European Youth Heart Study. J Appl Physiol. 2010;109:1125–1132. doi: 10.1152/japplphysiol.00316.2010. [DOI] [PubMed] [Google Scholar]
- Torrance B, McGuire KA, Lewanczuk R, McGavock J. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139–149. [PMC free article] [PubMed] [Google Scholar]
- Ostchega Y, Carroll M, Prineas RJ, McDowell MA, Louis T, Tilert T. Trends of elevated blood pressure among children and adolescents: data from the National Health and Nutrition Examination Survey 1988–2006. Am J Hypertens. 2009;22:59–67. doi: 10.1038/ajh.2008.312. [DOI] [PubMed] [Google Scholar]
- He Y, Lam TH, Jiang B, Wang J, Sai X, Fan L, Li X, Qin Y, Hu FB. Passive smoking and risk of peripheral arterial disease and ischemic stroke in Chinese women who never smoked. Circulation. 2008;118:1535–1540. doi: 10.1161/CIRCULATIONAHA.108.784801. [DOI] [PubMed] [Google Scholar]
- Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- Simonetti GD, Schwertz R, Klett M, Hoffmann GF, Schaefer F, Wühl E. Determinants of blood pressure in preschool children: the role of parental smoking. Circulation. 2011;123:292–298. doi: 10.1161/CIRCULATIONAHA.110.958769. [DOI] [PubMed] [Google Scholar]
- Dipla K, Zafeiridis A, Koidou I, Geladas N, Vrabas IS. Altered hemodynamic regulation and reflex control during exercise and recovery in obese boys. Am J Physiol Heart Circ Physiol. 2010;299:H2090–H2096. doi: 10.1152/ajpheart.00087.2010. [DOI] [PubMed] [Google Scholar]
- Ketelhut RG, Rode U, Schröter J. Abstract 2113: early end-organ alterations due to high blood pressure in children. Circulation. 2007;116:II_458. [Google Scholar]
- Kromeyer-Hauschild K, Wabitsch M, Kunze D, Geller F, Geiß HC, Hesse V, Hippel A, Jaeger U, Johnsen D, Korte W, Menner K, Müller G, Müller JM, Niemann-Pilatus A, Remer T, Schaefer F, Wittchen H-U, Zabransky S, Zellner K, Ziegler A, Hebebrand J. Percentiles of body mass index in children and adolescents evaluated from different regional German studies. Monatsschr Kinderheilkd. 2001;8:807–818. [Google Scholar]
- National High Blood Pressure Education Program Working Group. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- Neuhauser HK, Thamm M, Ellert U, Hense HW, Rosario AS. Blood pressure percentiles by age and height from nonoverweight children and adolescents in Germany. Pediatrics. 2011;127:e978–e988. doi: 10.1542/peds.2010-1290. [DOI] [PubMed] [Google Scholar]
- Boreham CA, Paliczka VJ, Nichols AK. A comparison of the PWC170 and 20-MST tests of aerobic fitness in adolescent schoolchildren. J Sports Med Phys Fitness. 1990;30:19–23. [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23:3–16. doi: 10.3109/08037051.2014.868629. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Caraballo R, Benowitz N, Repace J. Predictors of cotinine levels in US children: data from the Third National Health and Nutrition Examination Survey. Chest. 2001;120:718–724. doi: 10.1378/chest.120.3.718. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- Sidney S, Sternfeld B, Gidding SS, Jacobs DR, Jr, Bild DE, Oberman A, Haskell WL, Crow RS, Gardin JM. Cigarette smoking and submaximal exercise test duration in a biracial population of young adults: the CARDIA study. Med Sci Sports Exerc. 1993;25:911–916. [PubMed] [Google Scholar]
- Kallio K, Jokinen E, Raitakari OT, Hämäläinen M, Siltala M, Volanen I, Kaitosaari T, Viikari J, Rönnemaa T, Simell O. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115:3205–3212. doi: 10.1161/CIRCULATIONAHA.106.674804. [DOI] [PubMed] [Google Scholar]
- Kallio K, Jokinen E, Saarinen M, Hämäläinen M, Volanen I, Kaitosaari T, Rönnemaa T, Viikari J, Raitakari OT, Simell O. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circ Cardiovasc Qual Outcomes. 2010;3:196–203. doi: 10.1161/CIRCOUTCOMES.109.857771. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Ferrie JE, Singh-Manoux A, Shipley M, Batty GD, Head J, Hamer M, Jokela M, Virtanen M, Brunner E, Marmot MG, Kivimäki M. Socioeconomic differences in cardiometabolic factors: social causation or health-related selection? Evidence from the Whitehall II Cohort Study, 1991–2004. Am J Epidemiol. 2011;174:779–789. doi: 10.1093/aje/kwr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke GL, Savage PJ, Sprafka JM, Selby JV, Jacobs DR, Jr, Perkins LL, Roseman JM, Hughes GH, Fabsitz RR. Relation of risk factor levels in young adulthood to parental history of disease. The CARDIA study. Circulation. 1991;84:1176–1187. doi: 10.1161/01.cir.84.3.1176. [DOI] [PubMed] [Google Scholar]
- Khanolkar AR, Byberg L, Koupil I. Parental influences on cardiovascular risk factors in Swedish children aged 5-14 years. Eur J Public Health. 2012;22:840–847. doi: 10.1093/eurpub/ckr180. [DOI] [PubMed] [Google Scholar]
- Stamatakis E, Wardle J, Cole TJ. Childhood obesity and overweight prevalence trends in England: evidence for growing socioeconomic disparities. Int J Obes (Lond) 2010;34:41–47. doi: 10.1038/ijo.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbilis M, Moschonis G, Mougios V, Manios Y. Obesity in adolescence is associated with perinatal risk factors, parental BMI and sociodemographic characteristics. Eur J Clin Nutr. 2013;67:115–121. doi: 10.1038/ejcn.2012.176. [DOI] [PubMed] [Google Scholar]
- Hanson MD, Chen E. Socioeconomic status and health behaviors in adolescence: a review of the literature. J Behav Med. 2007;30:263–285. doi: 10.1007/s10865-007-9098-3. [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Karppanen A, Ahonen S, Tammelin T, Vanhala M, Korpelainen R. Physical activity and fitness in 8-year-old overweight and normal weight children and their parents. Int J Circumpolar Health. 2012;71:17621. doi: 10.3402/ijch.v71i0.17621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert T, Kuntz B. Tobacco and alcohol consumption among 11- to 17-year-old adolescents: results of the KiGGS study: first follow-up (KiGGS Wave 1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2014;57:830–839. doi: 10.1007/s00103-014-1982-8. [DOI] [PubMed] [Google Scholar]
- Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJ, Cruddas AM, Fall CH. Initiation of hypertension in utero and its amplification throughout life. BMJ. 1993;306:24–27. doi: 10.1136/bmj.306.6869.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N, Järvisalo MJ, Uhari M, Jokinen E, Rönnemaa T, Akerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]


