Abstract
Background
Clinical studies suggest that acute inflammation in patients with elevated heart rate (HR) increases morbidity and mortality. The SJL/J (SJL) inbred mouse strain is a unique genetic model that has higher HR and systemic and vascular inflammation compared with C3HeB/FeJ (C3HeB) mice. The goal of this study was to investigate the role of stress on cardiac and vascular complications between 2 strains.
Methods and Results
Radiotelemetry was used for continuous recordings of HR and blood pressure in mice. Hemodynamic differences between mouse strains were very small without stress; however, tail-cuff training generated mild stress and significantly increased HR (≈2-fold) in SJL compared with C3HeB mice. Circulating proinflammatory monocytes (CD11b+Ly6CHi) significantly increased in SJL mice but not in C3HeB mice after stress. Presence of Ly6C+ cells in injured carotids was elevated only in SJL mice after stress; however, a transfer of bone marrow cells from SJL/C3HeB to C3HeB/SJL chimeras had no effect on HR or vascular inflammation following stress. Arterial inflammation (VCAM-1+) was greater in SJL inbred mice or SJL recipient chimeras, even without stress or injury. HR variability was reduced in SJL mice compared with C3HeB mice.
Conclusions
We found that impaired parasympathetic activity is central for stress-induced elevation of HR and systemic and vascular inflammation; however, immune cells from stress-susceptible mice had no effect on HR or vascular inflammation in stress-protected mice.
Keywords: carotid arteries, heart rate variability, inflammation, mouse
Clinical studies have established a strong link between elevated heart rate (HR) and risks of cardiovascular events including coronary artery disease, myocardial ischemic injury, and arrhythmias.1,2 Individuals with elevated HR are at risk for subclinical inflammation with higher levels of circulating immune cells and future cardiovascular events.3,4 Importantly, vascular complications such as nephropathy and retinopathy are more prevalent in type 2 diabetes patients with elevated HR.5 Three pathogenic mechanisms for the role of elevated HR have been proposed: (1) autonomic neuropathy via increased sympathetic nervous system activity, (2) strong association with proinflammatory factors (eg, obesity, high blood pressure [BP], increased proatherogenic lipid profile), and (3) direct detrimental (mechanical) effects on endothelial functions. The exact mechanisms responsible for dysregulation of HR and vascular complications, however, are not well understood.
Animal and clinical studies suggested that impaired parasympathetic activity is responsible for lower HR variability.6–8 An inbred mouse strain, SJL/J (SJL), recapitulates clinical symptoms of patients with elevated HR and vascular complications. We found that SJL mice had higher HR and carotid injury, resulting in robust remodeling and inflammation, compared with C3HeB/FeJ (C3HeB) mice.9–11 In addition, SJL mice have higher levels of circulating white blood cells compared with C3HeB.12 Our recent study in congenic mice suggests that greater expression of aortic vascular cell adhesion molecule 1 (VCAM-1) determines vascular inflammation in SJL mice13; however, others14 have shown that HR values were lower in SJL mice when measured by direct radiotelemetry methods. This could be due to differences in behavioral responses in SJL mice.15 A goal of the present study was to investigate the role of stress on cardiovascular and proinflammatory responses between SJL and C3HeB inbred mouse strains.
Materials and Methods
Animals
Experiments were performed in male C3HeB and SJL mice aged 6 to 8 weeks. All animals were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed individually under 12-hour light/dark cycles (lights on from 6 am to 6 pm) with free access to chow and water. The University of Rochester animal care committee approved all procedures on animals, which were conducted according to the guidelines of the National Institutes of Health and the American Heart Association for the use of laboratory animals.
Experimental Design
Animals were randomly divided into experimental groups. For direct measurements of hemodynamic parameters, mice were surgically implanted with telemetry transmitters and allowed 10 days for recovery. For indirect measurement of hemodynamic parameters, mice experienced 5 days of tail-cuff plethysmography. Blood and tissues were collected on the last day of training (at the same time as animals without tail cuff) to assess systemic inflammation in inbred mice. In separate experiments, mice with or without tail-cuff training for 5 days were immediately subjected to carotid ligations. After 7 days after the ligation surgery blood was collected for corticosterone levels, and mice were perfusion fixed for histological evaluation. The SJL/C3H chimeric mice were allowed for immune cell repopulation for 6 weeks, which was immediately followed by 5 days of tail-cuff training and subsequent carotid ligation with perfusion fixation after 7 days following surgery.
Radiotelemetry
A telemeter catheter (HD-X11; Data Sciences International) was placed under anesthesia (ketamine 130 mg/kg and xylazine 8.8 mg/kg) into the left carotid artery with its sensing region in the aortic arch in C3HeB and SJL mice. The positive lead of the telemeter was positioned in the left caudal rib region, and the negative lead was situated near the right pectoral muscle. The transmitters were placed in a subcutaneous pocket along the left side of the animals. Mice (n=6 per strain) were allowed to recover for 10 days before experimentation. Analyses of ECG; HR; and systolic, diastolic, and mean BP were performed using Dataquest A.R.T. 4.3 software (Data Sciences International).
Heart Rate Variability
Stable ECG recordings taken at 3 minutes every 2 hours for each animal were exported from Dataquest A.R.T. 4.3. We analyzed HR variability with Kubios HRV 2.2 software.16 Powers (in square milliseconds) for low frequency and high frequency were used for 0.15- to 1.5-Hz bands and 1.5- to 5.0-Hz bands, respectively.17 The low- and high-frequency power and the ratio of low/high-frequency power were calculated for the 12-hour light/dark period for each animal.
Tail-Cuff Plethysmography
Mild stress was induced by tail-cuff plethysmography (Visitech System) for 5 consecutive days of training, as described.11 We had to use PA-C10 transmitters (instead of the HD-X11 model; Data Sciences International) in C3HeB and SJL mice (n=5 to 7) for assessment of hemodynamic responses because they were less sensitive to electromagnetic fields generated by the platform during tail-cuff plethysmography. In each session, we recorded baseline parameters for 15 minutes and accommodated mice for 5 minutes after restraining, followed by 25 minutes of tail-cuff measurements on the platform. Mice were returned to their cages, and telemetry recordings lasted for an additional 60 minutes. HR and systolic BP were averaged every 5 minutes. In addition, we calculated areas under the curve (beats/min×15 minutes or mm Hg×15 minutes) for the 30-minute restraining session to compare hemodynamic reactivity between inbred strains that are not recorded by the tail-cuff software (BP-2000; Visitech Systems).
Corticosterone Levels
Under light anesthesia, blood was collected in EDTA-coated tubes from inbred mice without training or from mice after tail-cuff training (n=5 per group). Plasma was immediately separated and stored at −80°C until use, as described.18 The corticosterone levels in the plasma were quantified using an ELISA kit according to the manufacturer’s instructions (Enzo Life Sciences).
Bone Marrow Transplant
C3HeB mice carry the CD45.2 allele that is common among inbred mouse strains. In contrast, the SJL strain carries a rare CD45.1 allele. We used the differences in CD45 alleles between these inbred strains to create C3HeB/SJL chimeric mice by bone marrow transplant (BMT), as described.19 Briefly, bone marrow (BM) cells were harvested from tibias and femurs of age-matched C3HeB or SJL donor mice. Before BMT, recipient C3HeB mice were lethally irradiated (9.0 Gy) to ablate the host BM by using an RS 2000 irradiator (Rad Source Technologies, Inc). C3HeB recipient mice were injected with 6×106 donor BM cells via tail vein. We split the irradiation into 2 doses on the same day (4.5 and 4.5 Gy) and injected 8×106 donor BM cells because recipient SJL mice were highly sensitive to irradiation. The C3HeB/C3HeB and SJL/C3HeB chimeras exhibited 60% to 70% survival, similar to our previous BMT data in mice on C57BL/6J background19,20; however, SJL/SJL chimeras displayed ≈40% survival, and <30% of C3HeB/SJL chimeras survived (data not shown). Mice that underwent BMT were allowed to recover for 6 weeks before further experimentation.
Flow Cytometry
Peripheral blood from C3HeB/SJL chimeras was collected by submandibular bleed without anesthesia 6 weeks after BMT, as reported previously.19 Engraftment of donor BM cells was confirmed by double staining of peripheral leukocytes with a cocktail of CD45.1-FITC and CD45.2-PE antibodies (1:500; eBiosciences). In a separate experiment, we collected spleens and blood from SJL and C3HeB mice with or without tail-cuff training (n=8 per group) and processed the samples for flow cytometry analyses, as reported.19 Briefly, mouse spleens were minced in PBS supplemented with 3% FBS. Cells were isolated from the suspension by centrifugation and then resuspended in 3% FBS. Similarly, blood was incubated with 5 mL of ammonium-chloride-potassium lysis buffer for 5 minutes, washed, and resuspended in 3% FBS. Cell viability and cell number were assessed in aliquoted samples (10 μL) using trypan blue staining. Cells (1×106) were incubated with a cocktail of antibodies (eBiosciences) containing Ly6C-PE (1:500), Ly6G-FITC (1:500), CD11c-APC (1:200), and CD11b-PE CY7 (1:1500). For compensation controls, we used IgG beads stained with a single-color antibody. Immunofluorescence was detected using an Accuri C6 flow cytometer (BD Biosciences) and analyzed with the FlowJo software, as described previously.20.
Carotid Artery Ligation
C3HeB and SJL mice (n=5 to 6 per group) with or without tail-cuff training underwent ligation of the left external and internal branches of the carotid artery, as described previously.21 The ligation procedure was performed in C3HeB/SJL chimeras (n=3 to 6) after tail-cuff training. The mice were housed individually under pathogen-free conditions for 7 days after surgery.
Morphometry and Immunohistochemistry
After ligation, all animals were perfusion fixed, carotid arteries were harvested and embedded in paraffin, and cross-sections were prepared, as described.21 Serial cross-sections were stained with hematoxylin and eosin (Dako) and morphometry analyzed using MCID image software (Imaging Research Inc), as described.21 We measured carotid lumen, intima, media, and adventitia areas at 200-μm intervals from the carotid bifurcation and calculated compartment volumes for 1600 μm of the carotid length. Carotid sections from experimental animals (≈1000 μm proximal to carotid bifurcation) were stained with Ly6C rat anti-mouse antibody (1:100; Santa Cruz Biotechnology) or VCAM-1 rabbit anti-mouse antibody (1:1000; Santa Cruz Biotechnology) and counterstained with hematoxylin (Dako). We inhibited endogenous peroxidase activity in slides with 3% H2O2. We performed high-temperature antigen retrieval with a Decloaker buffer (pH 6.0; Biocare Medical) before staining with VCAM-1 antibody. Primary antibodies were incubated at 4°C overnight, followed by incubation with polymer Rat-on-Mouse or Rabbit-on-Rodent horseradish peroxidase kits (Biocare Medical). The peroxidase-binding sites were shown by 3,3′-diaminobenzidine (Dako). Immunostained carotid sections were captured by SPOT Insight FireWire camera (Diagnostic Instruments). We uniformly adjusted size and contrast of the images to meet journal guidelines (Adobe Photoshop CS3). We analyzed positively stained cells in 3 mice (2 to 3 sections per mouse) using Image-Pro software (Media Cybernetics).19
Statistics
Data are presented as mean±SEM. We used JMP 5.1.2 software (SAS Institute) for evaluation of statistical significance. Levels of significance between 2 groups were determined by the Student t test. Differences among ≥3 groups were determined by 1-way ANOVA with post hoc comparisons of all means by the Student t test. Hemodynamic differences between the mouse strains of tail-cuff training over time were analyzed by 2-way ANOVA and followed by Fisher’s post hoc test. Inflammatory cell population numbers were analyzed by nonparametric Wilcoxon/Kruskal–Wallis tests and followed by each pair comparison using a Wilcoxon method. The level of P<0.05 was regarded as significant.
Results
Hemodynamic Parameters Between Mouse Strains Without Stress
Direct radiotelemetry recordings in SJL mice showed lower HR14 than our data by tail-cuff method.11 Systolic, diastolic, and mean BP and RR intervals recorded by radiotelemetry were similar between the strains (Table1). Circadian variations of HR and systolic BP were the same in SJL and C3HeB mice (not shown). We also observed no differences in ECG intervals and peaks between the strains (not shown). These findings indicate that baseline HR was similar between SJL and C3HeB mice without stress.
Table 1.
Averaged Diurnal Changes in Hemodynamic Parameters Between Inbred Mouse Strains at Baseline
| Strain Parameter | C3HeB, n=6 | SJL, n=6 | ||
|---|---|---|---|---|
| Lights On | Lights Off | Lights On | Lights Off | |
| Activity, counts | 3.8±0.4 | 10.0±1.0* | 5.1±0.6 | 8.9±0.6* |
| Heart rate, beats/min | 554±14 | 627±6* | 557±13 | 621±18* |
| RR interval, ms | 122±4 | 95±3* | 114±5 | 102±5 |
| Systolic BP, mm Hg | 106±2 | 116±3* | 106±2 | 118±3* |
| Diastolic BP, mm Hg | 81±2 | 91±1* | 80±3 | 91±4* |
| Mean pressure, mm Hg | 94±2 | 106±2* | 93±2 | 103±2* |
Parameters are shown as mean±SEM. BP indicates blood pressure.
P<0.05 vs lights on period (Student t test).
Hemodynamic Reactivity to Stress Between Mouse Strains
To investigate stress susceptibility of SJL mice, we used a tail-cuff training protocol as a model of cumulative stress (Figure 1). On the first day of tail-cuff training, SJL mice showed significantly higher HR during 30-minute restraining, whereas 60 minutes of recovery showed only a trend (P=0.10) compared with C3HeB mice (Figure 1A). There was a 2-fold increase in HR in SJL versus C3HeB mice at day 5 (Figure 1B). The systolic BP responses were similar during 5 days of training in C3HeB mice (Figure 1C and 1D); however, systolic BP slightly increased in SJL mice compared with C3HeB mice on day 5 of training (Figure 1D). Because the tail-cuff software recorded measurements only between 15 and 30 minutes of restraining, we calculated areas under the curves for HR and systolic BP during the first 15 minutes of restraining measured by telemetry (Figure 1E and 1F). Early HR responses to stress in SJL mice were ≈2-fold greater than in C3HeB mice and significantly increased from days 1 to 5 (Figure 1E). In contrast, early systolic BP changes were noticeably less than changes in HR in both strains (Figure 1F versus 1E). There were no significant differences in early systolic BP areas across experimental groups (Figure 1F). We also tested hemodynamic reactivity of C3HeB and SJL mice to a novel cage test. In particular, mice were picked up by the tail for 5 seconds and returned into a new cage with fresh bedding and nesting material. There was significant increase in HR but not systolic BP during the first 5 minutes of the novel cage test in SJL mice compared with C3HeB mice (not shown). Finally, plasma corticosterone levels corroborated HR changes in inbred strains with or without tail-cuff restraining. Corticosterone levels were similar between the 2 inbred strains without stress (≈20 ng/mL); however, SJL mice tended to have higher corticosterone in plasma with stress compared with C3HeB (62±32 versus 16±9 ng/mL, respectively; P=0.09). Altogether, these data indicate that SJL mice are susceptible to mild stress, as revealed through greater HR responses compared with C3HeB mice.
Figure 1.
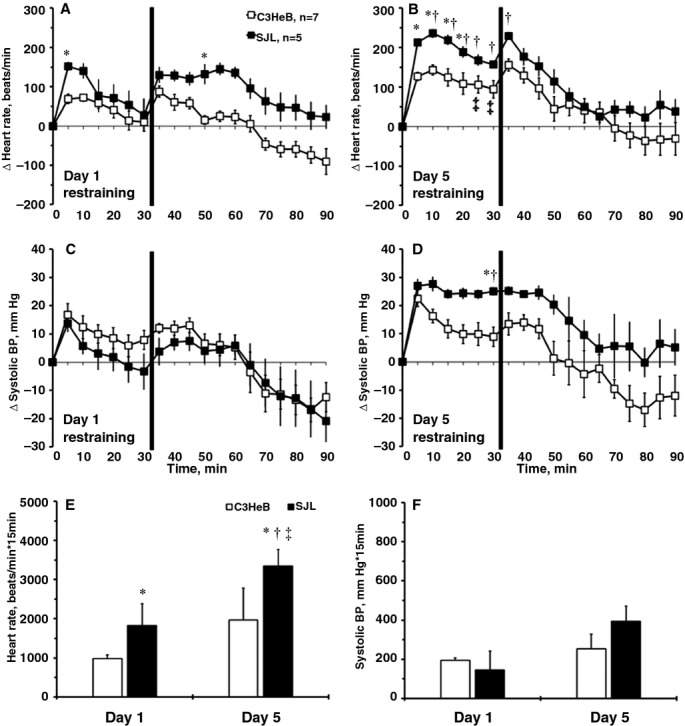
Hemodynamic responses to repeated tail-cuff measurements in mouse strains. A, Changes in HR on day 1. B, Changes in HR on day 5. C, Changes in systolic BP on day 1. D, Changes in systolic BP on day 5. Perpendicular black lines mark the end of the 30-minute restraining period of mice on the tail-cuff platform. E, Area under the curve for HR during the first 15 minutes of restraining (beats/min×15 minutes). F, Area under the curve for systolic BP during the first 15 minutes of restraining (mm Hg×15 minutes). Values are mean±SEM; *P<0.05 vs C3HeB mice at day 1; †P<0.05 vs SJL mice at day 1; ‡P<0.05 vs C3HeB mice at day 5 (ANOVA). BP indicates blood pressure; HR, heart rate.
Carotid Inflammation in Mouse Strains With or Without Stress
Vascular complications in patients with elevated HR are well documented.5 We previously reported that intimal cell proliferation in SJL mice is associated with a dramatic increase in leukocytes (CD45+) compared with C3HeB mice 14 days after carotid ligation.10 Time-course experiments in mice suggested that vascular inflammation peaked at 7 days after the surgery and was strain dependent.21,22 We found that carotid compartments and carotid thickening (intima-media volume) were similar across experimental groups of inbred strains (Table2). Among monocyte subsets, proinflammatory Ly6C+ cells are specifically involved in early inflammation and vascular complications.23 Immunohistochemistry showed that Ly6C+ cells localized predominantly to the intima and adventitia compartments in all experimental groups (Figure 2). Stress had no effect on Ly6C+ staining in C3HeB mice (Figure 2A versus 2C). In contrast, the number of Ly6C+ cells in the intima was significantly increased in SJL mice with stress compared with other groups (Figure 2A through 2C versus 2D). Quantitative analyses supported elevated vascular inflammation in SJL mice with stress (Figure 2E). We recently reported that SJL mice have greater aortic expression of VCAM-1 compared with C3HeB mice.13 We found that expression of VCAM-1 was higher in carotid arteries with or without injury from SJL versus C3HeB mice (Figure 3A versus 3B; insets are injured arteries). Mild stress had little effect on strain-dependent differences (Figure 3A and 3B versus 3C and 3D); however, we noticed higher VCAM-1 immunoreactivity in intima of SJL mice with stress after injury compared with other groups (Figure 3D, inset). Collectively, we showed that SJL mice were susceptible to stress-induced vascular inflammation in response to injury.
Table 2.
Morphometry Analyses of Carotid Arteries Across Inbred and Chimeric Mice
| Parameter Group | Lumen Volume, ×106 μm3 | Intima-Media Volume, ×106 μm3 | Adventitia Volume, ×106 μm3 | EEL Volume, ×106 μm3 |
|---|---|---|---|---|
| Inbred strains | ||||
| C3HeB, no stress, n=5 | 45±9 | 40±3 | 37±4 | 84±11 |
| SJL, no stress, n=6 | 40±3 | 48±9 | 42±8 | 85±16 |
| C3HeB, stress, n=6 | 54±8 | 38±3 | 36±2 | 93±8 |
| SJL stress, n=6 | 35±8 | 35±3 | 33±4 | 70±10 |
| Chimeras | ||||
| C3HeB/C3HeB, n=6 | 61±8 | 41±9 | 38±3 | 103±14 |
| SJL/SJL, n=5 | 73±8 | 54±7 | 45±6 | 89±7 |
| SJL/C3HeB, n=6 | 54±6 | 35±4 | 37±2 | 90±11 |
| C3HeB/SJL, n=3 | 63±11 | 27±1 | 29±2 | 127±14 |
Parameters are shown as mean±SEM. EEL indicates external elastic lamina.
Figure 2.
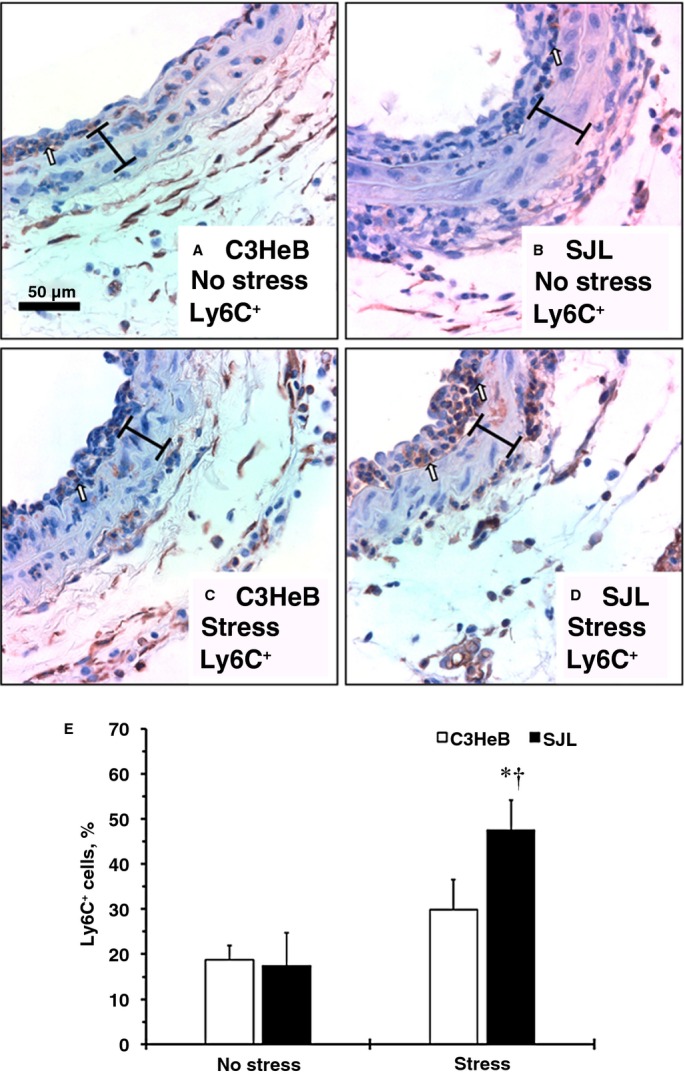
Infiltration of proinflammatory cells to the carotid artery after injury in mouse strains with or without stress. Representative Ly6C+ staining of carotids. A, C3HeB mice, no stress. B, SJL mice, no stress. C, C3HeB mice, with stress. D, SJL mice, with stress. Ly6C+ cells are stained dark brown (open arrows). Brackets show area between internal and external elastic lamina. Magnification bar is 50 μm. E, Relative number of Ly6C+ cells in the intima (percentage). Values are mean±SEM; *P<0.05 vs C3HeB mice, no stress; †P<0.05 vs SJL mice, no stress (Wilcoxon); n=3 per each experimental group.
Figure 3.
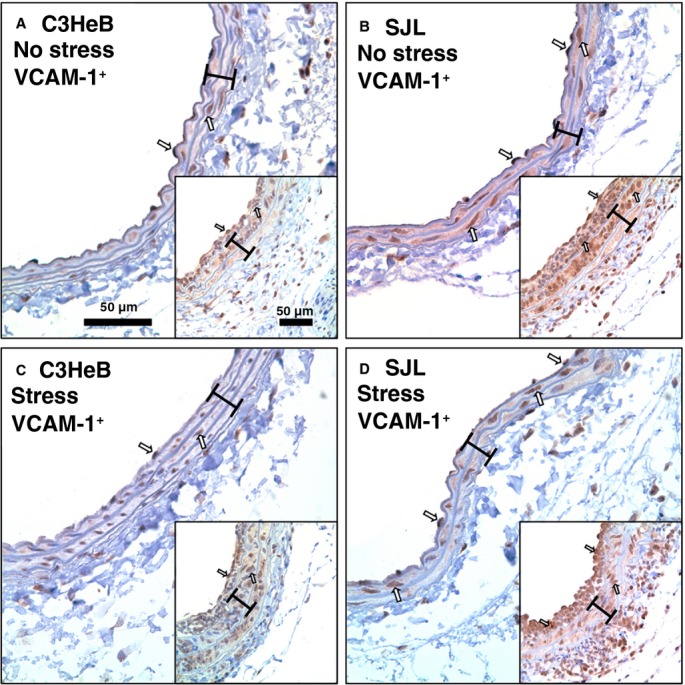
Carotid inflammation in mouse strains with or without stress. Representative vascular cell adhesion molecule 1 (VCAM-1)+ staining of noninjured carotids: A, C3HeB mice, no stress. B, SJL mice, no stress. C, C3HeB mice, with stress. D, SJL mice, with stress. Insets show VCAM-1 immunoreactivity in carotid arteries after the injury across respective groups (A through D). VCAM-1+ cells are stained dark brown (open arrows). Brackets show area between internal and external elastic lamina. Magnification bar is 50 μm.
Systemic Inflammation in Mouse Strains With or Without Stress
Clinical studies have demonstrated that individuals with elevated HR are at risk of subclinical inflammation with higher levels of circulating immune cells.3 Double-positive cells (CD11b+ and Ly6C+), which have higher expression of Ly6C (Ly6CHi), are proinflammatory monocytes that we measured in C3HeB and SJL mice (Figure 4A). There were no differences in the CD11b+Ly6CHi cells in spleen between mouse strains without stress (Figure 4B). Both mouse strains tended (P≈0.10) to have an increased percentage of the splenic CD11b+Ly6CHi cells after stress (Figure 4B); however, SJL mice had a lower percentage of proinflammatory monocytes in blood without stress and exhibited a significant increase in CD11b+Ly6CHi cells in blood after stress (Figure 4C). In contrast, C3HeB mice showed no change in blood CD11b+Ly6CHi after stress (Figure 4C). These findings indicate that SJL mice are susceptible not only to stress-induced increases in HR but also to elevation of proinflammatory monocytes in circulation compared with C3HeB mice.
Figure 4.
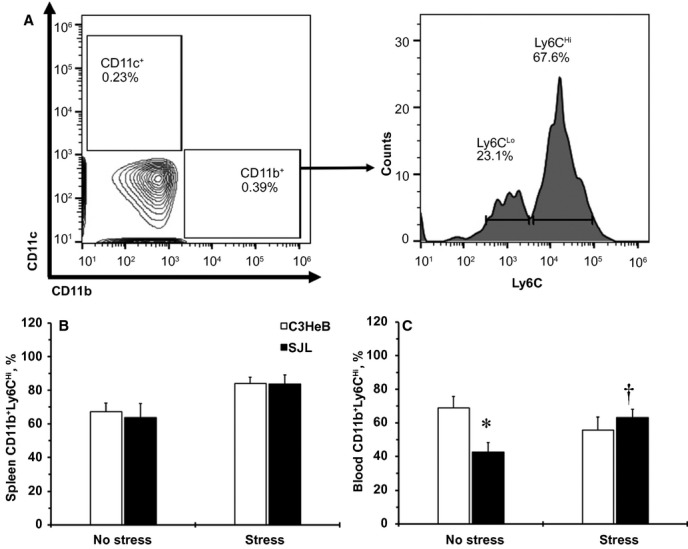
Systemic inflammation in mouse strains with or without stress. A, A flow cytometry gating strategy to identify proinflammatory cells in mouse strains. A representative double staining of CD11c+ and CD11b+ populations in blood from SJL mice after stress is shown on the left. A right-side histogram shows 2 subpopulations of Ly6C+ cells (low-Ly6CLo vs high-Ly6CHi) that are positive to CD11b+. Numbers indicate percentages of positive cells. B, Changes in CD11b+Ly6CHi cells in spleens from mouse strains. C, Changes in CD11b+Ly6CHi cells in blood from mouse strains. Values are mean±SEM; *P<0.05 vs CD11b+Ly6CHi to C3HeB mice, no stress; †P<0.05 vs CD11b+Ly6CHi to SJL mice, no stress (Wilcoxon); n=8 per each experimental group.
Heart Rate in SJL/C3HeB Chimeras
To determine the role of BM-derived immune cells in cardiovascular complications, we generated SJL/C3HeB chimeric mice (Figures5 through 7). Repopulation of the donor BM cells (SJL–CD45.1+; C3HeB–CD45.2+) is shown in flow cytometry double staining of peripheral blood from chimeric mice 6 weeks after BMT (Figure 5A). We found that SJL/SJL chimeras had significantly higher HR than C3HeB/C3HeB and SJL/C3HeB chimeras (Figure 5B). The C3HeB/SJL chimeras tended to have a higher HR values (P=0.09) compared with SJL/C3HeB chimeras (Figure 5B). Our results suggest that BM cells derived from SJL mice are unable to increase HR in C3HeB recipient mice.
Figure 5.
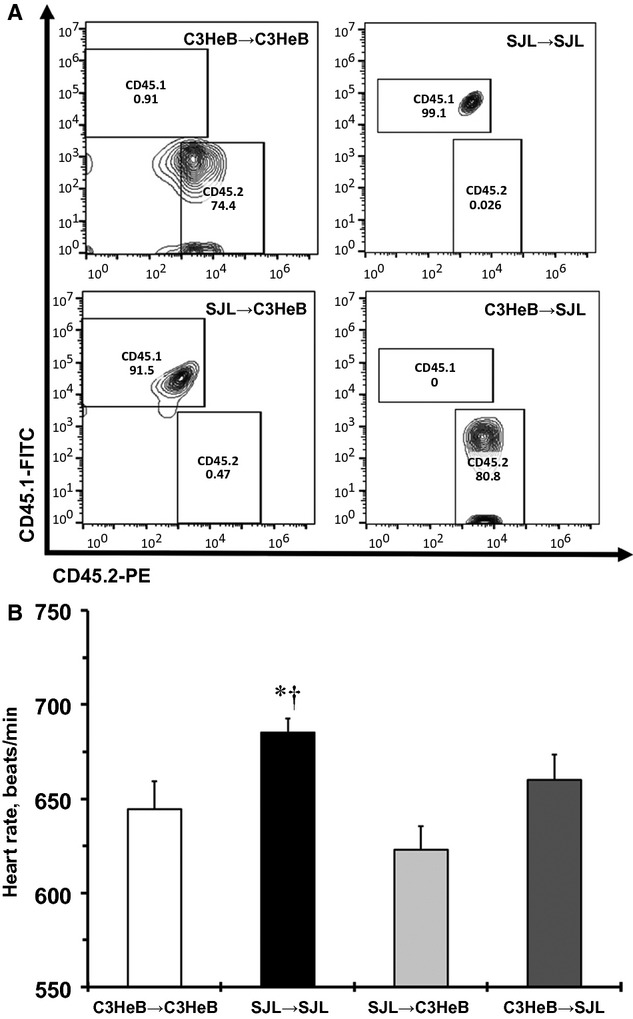
Heart rate measured by the tail-cuff method in SJL/C3HeB chimeras. A, Representative double staining of CD45.1+ (SJL) and CD45.2+ (C3HeB) in peripheral blood from chimeras. Numbers inside quadrants shows engraftment (percentage) following 6 weeks of bone marrow transplant. B, Heart rate values between SJL/C3HeB chimeras. Values are mean±SEM, n=3 for each group. *P<0.05 vs C3HeB/C3HeB chimeras; †P<0.05 vs SJL/C3HeB chimeras (ANOVA).
Figure 7.
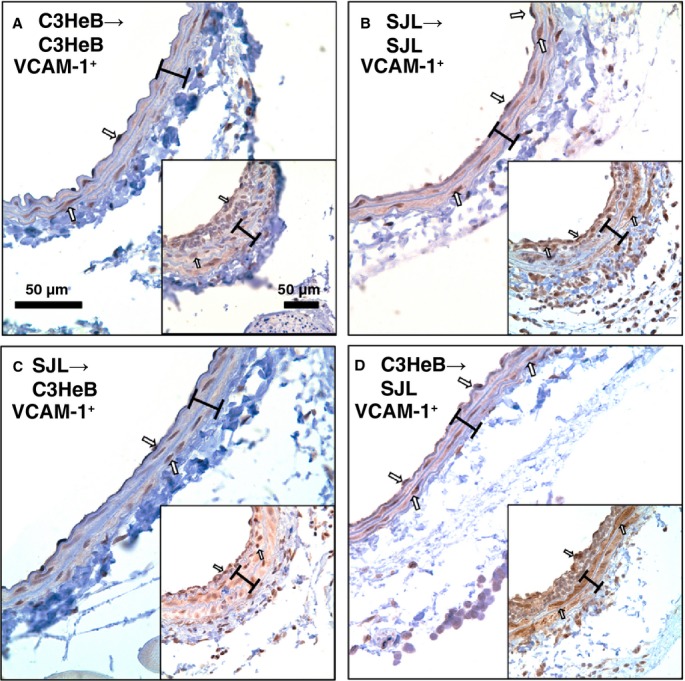
Carotid inflammation in SJL/C3HeB chimeras after injury. Representative vascular cell adhesion molecule 1 (VCAM-1)+ staining of noninjured carotids. A, C3HeB/C3HeB chimera. B, SJL/SJL chimera. C, SJL/C3HeB chimera. D, C3HeB/SJL chimera. Insets show VCAM-1 immunoreactivity in carotid arteries after injury across respective chimeras (A through D). VCAM-1+ cells are stained dark brown (open arrows). Brackets show area between internal and external elastic lamina. Magnification bar is 50 μm.
Carotid Inflammation in SJL/C3HeB Chimeras
To investigate the effects of BM-derived monocytes on vascular inflammation, we performed carotid ligations in SJL/C3HeB chimeras after tail-cuff training (Figure 6). Similar to studies in inbred mouse strains, carotid compartments were comparable across SJL/C3HeB chimeras (Table2); however, Ly6C+ immunoreactivity in carotid arteries from SJL/C3HeB chimeras was reduced compared with SJL or C3HeB mice without irradiation (Figures6A through 6D versus 2A through 2D). Nevertheless, quantitative analyses showed that SJL/SJL and C3HeB/SJL chimeras had significantly higher Ly6C+ cells in the intima compared with SJL/C3HeB chimeras (Figure 6B). The SJL/SJL and C3HeB/SJL chimeras tended to have more Ly6C+ cells in the intima (P=0.09) compared with C3HeB/C3HeB chimeras (Figure 6B). Similar to our observations in inbred mice after stress (Figure 3), carotid expression of VCAM-1 was higher in carotid arteries from SJL/SJL and C3HeB/SJL chimeras (Figure 7B and 7D versus 7A and 7C; insets are injured arteries), although we found higher VCAM-1 immunoreactivity in media of C3HeB/SJL chimeras after the injury compared with other chimeras (Figure 7D, inset). Consequently, increases in vascular inflammation in SJL mice in response to stress are independent from BM-derived cells.
Figure 6.
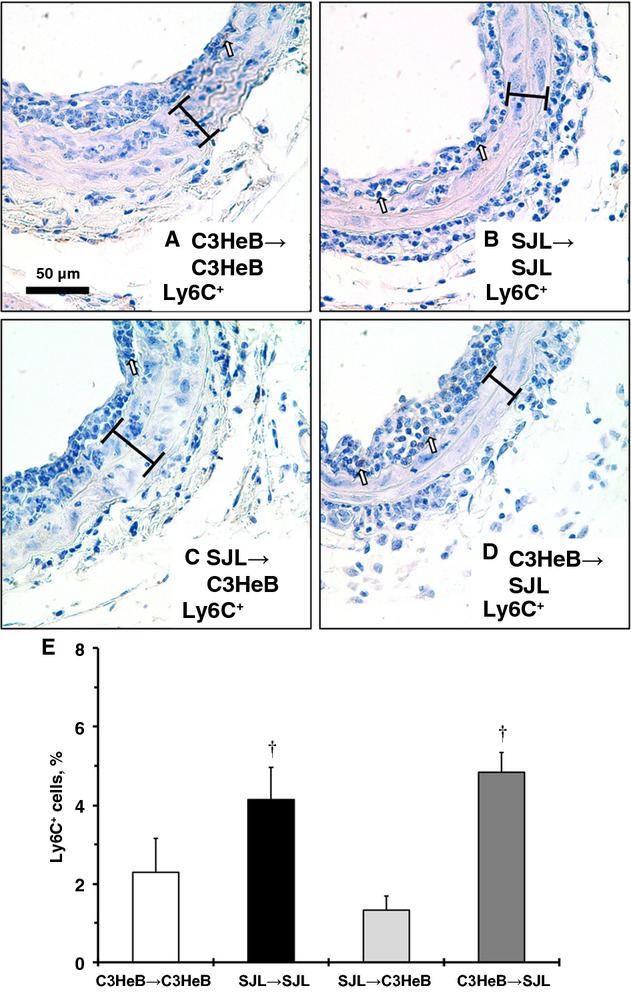
Infiltration of proinflammatory cells to the carotid artery in SJL/C3HeB chimeras after injury. Representative Ly6C+ staining of carotids: A, C3HeB/C3HeB chimera. B, SJL/SJL chimera. C, SJL/C3HeB chimera. D, C3HeB/SJL chimera. Ly6C+ cells are stained dark brown (open arrows). Brackets show area between internal and external elastic lamina. Magnification bar is 50 μm. E, Relative number of Ly6C+ cells in the intima (percentage). Values are mean±SEM. †P<0.05 vs SJL/C3HeB chimeras (Wilcoxon); n=3 for each experimental group.
Heart Rate Variability in Mouse Strains
Cardiovascular complications are more prevalent in type 2 diabetes patients with elevated HR.5 A potential mechanism is related to autonomic neuropathy in diabetes patients.4 We evaluated HR variability in the frequency domain between mouse strains during the 12-hour lights-on period (Figure 8). The high-frequency values that represent a parasympathetic tone were significantly lower in SJL mice compared with C3HeB mice (Figure 8A). There was no difference in low-frequency activity between mouse strains that reflects both sympathetic and parasympathetic tone (Figure 8B); however, the ratio of low/high-frequency power was significantly higher in SJL versus C3HeB mice, suggesting an imbalance between parasympathetic over sympathetic systems. In summary, SJL mice exhibited impaired parasympathetic regulation that could lead to cardiovascular complications in response to stress.
Figure 8.
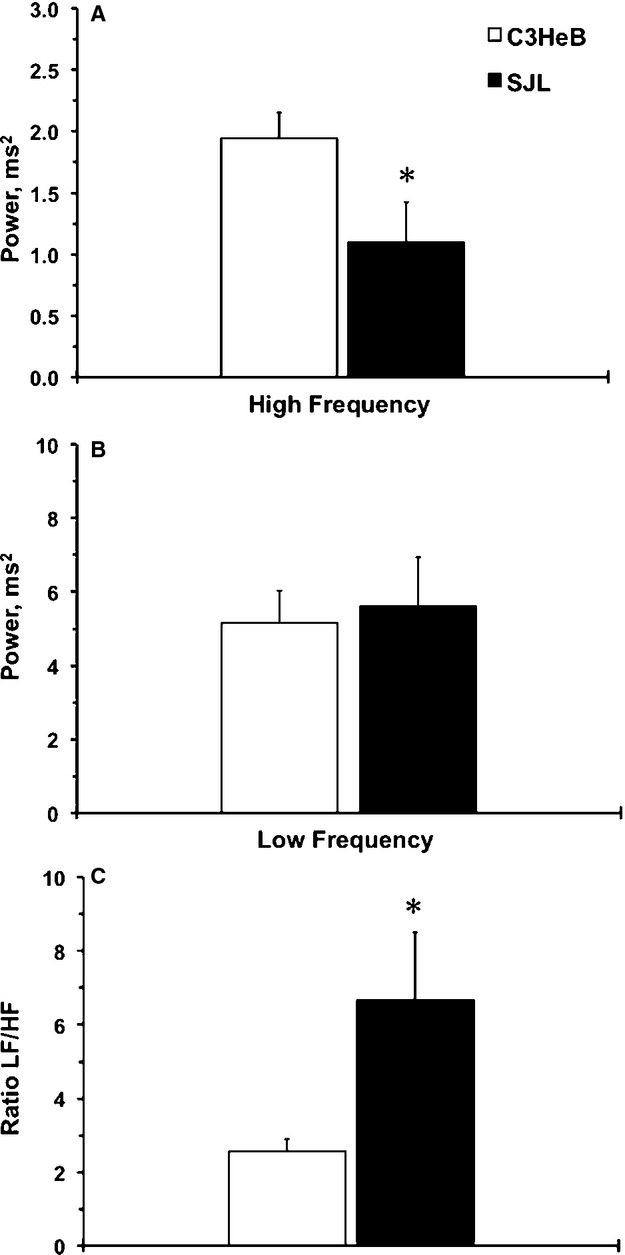
Frequency-domain analyses of heart rate regulation in mouse strains. A, High-frequency power (HF) shows parasympathetic tone in mouse strains. B, Low-frequency power (LF) shows sympathoparasympathetic tone in mouse strains. C, LF/HF ratio shows sympathoparasympathetic balance in mouse strains. Values are mean±SEM; *P<0.05 vs C3HeB (Student t test); n=5 per experimental group.
Discussion
The major finding of this study is that impaired parasympathetic regulation is critical for stress-induced cardiac and vascular complications (Figure 9). We showed that elevation of HR is robust in SJL versus C3HeB inbred mice in response to mild stress. In addition, SJL mice have higher reactivity to stress in circulating proinflammatory monocytes compared with C3HeB mice. A combination of mild stress with vascular injury significantly increased proinflammatory cell infiltration to the arterial wall in SJL but not in C3HeB mice. Yet, mild stress had few effects on our previously documented differences in arterial expression of VCAM-1 between SJL and C3HeB mouse strains. Repopulation of the immune cells from SJL/C3HeB or C3HeB/SJL chimeras had no effect on stress-dependent HR increase or vascular inflammation. Finally, autonomic nervous regulation was impaired in SJL mice. Taken together, we propose that genetic susceptibility to stress is determined by impaired parasympathetic regulation leading to elevation of HR and systemic and vascular inflammation. Stress-induced elevation in HR or vascular inflammation, however, is independent from circulating proinflammatory cells (Figure 9).
Figure 9.
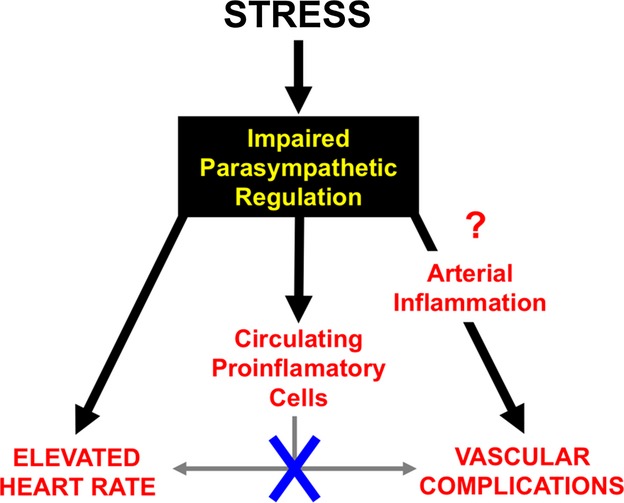
A proposed role of impaired parasympathetic regulation in cardiovascular complications in response to stress. There was no direct effect of circulating proinflammatory monocytes on heart rate or infiltration of inflammatory cells. Increase in arterial inflammation might be central for vascular complications due to impaired autoregulation.
Genetic studies on HR variation are important for understanding this complex phenotype. Despite recent technical advances, we can explain only a small portion (<2%) of the genetic contribution to HR variation in humans.24 A major challenge in large population studies is related to interindividual variability in hemodynamic parameters.25 The latter can be overcome by repeated measurements to improve reproducibility, as was shown in patients with heart failure.26 Experiments in animals significantly reduce effects of the environmental and methodological artifacts that are unavoidable in human studies; however, the method of hemodynamic measurement (direct or indirect) has an important impact on the results. In particular, the stress response has a confounding factor in the tail-cuff plethysmography (indirect) method. Previous studies using ApoE−/− mice on 129xC57BL/6 background reported comparable measurements between indirect (tail-cuff) or direct (carotid catheter) methods.27 In contrast, experiments in CD-1 and C57BL/6J mice showed hemodynamic discrepancies between tail-cuff and radiotelemetry methods.28 Our laboratory used a training procedure for each animal to reduce the effect of the stress associated with the indirect method of measurement.11,21 This tail-cuff training results in mild stress because the plasma corticosterone levels were significantly lower compared with more severe psychological stress models in mice.18 Of note, systolic BP was significantly less reactive to tail-cuff training, unlike HR, in our experiments. Consequently, it is important to use both direct and indirect methods when evaluating hemodynamic parameters in genetically altered mice or mice on mixed background.
A recent report5 showed that elevated HR increased vascular complications in type 2 diabetes patients in the ADVANCE study; however, the relationship between elevated HR and inflammation is not well understood. Data from ApoE−/− mice suggest that proinflammatory Ly6C+ monocytes in circulation play a key pathogenic role in recovery after cardiac injury.29 The same group reported that an increase in sympathetic nervous system is responsible for monocytopoesis in the spleen that accelerated atherosclerosis in ApoE−/− mice after myocardial infarction or stroke.23 We and others showed that SJL mice have greater circulating white blood cell levels compared with C3HeB mice.12,13 In the current study, we discovered that the SJL mouse strain is more reactive to stress not only for HR but also for proinflammatory monocytes in circulation and in the artery wall on injury. It is important to note that other subsets of immune cells (eg, T lymphocytes) might play a role in stress-dependent hypertension and vascular inflammation, as was recently shown in RAG1−/− mice30; however, our BMT experiments between SJL and C3HeB mice suggest that systemic repopulation of immune cells alone cannot cause elevation in HR or vascular complications after mild stress. This is in line with our recent data in congenic mice that carry an SJL locus on the C3HFeB background. In this study, we showed that systemic inflammatory cells had no effect on regulating vascular wall inflammation in response to injury.13,31 A potential caveat of BMT could be related to irradiation resistance of some subpopulations of leukocytes in the SJL mouse strain short term (up to 11 days after irradiation).32 Another long-term study (up to 80 days after irradiation) showed that SJL mice developed a severe wasting syndrome with a higher mortality rate.33 The authors showed that SJL mice died due to myeloproliferation, leukemic transformation, and infection. Likewise, we observed higher mortality in SJL recipient mice after a longer period of time (up to 60 days) after the irradiation and BMT (not shown). Flow cytometry analyses confirmed successful repopulation of the donor BM in SJL/C3HeB chimeras, yet SJL/C3HeB chimeras had reduced Ly6C+ immunoreactivity in carotid arteries compared with SJL or C3HeB mice without irradiation. Taken together, our new findings suggest that systemic manipulation with proinflammatory cells has no direct effect on increase in HR or vascular inflammation in response to stress.
Many pathological conditions associated with chronic inflammation (eg, diabetes, hyperglycemia, hyperlipidemia, obesity) lead to autonomic dysregulation of HR.34 Impaired parasympathetic activity, for example, is well documented in experimental animal models of diabetes.6,7 In this study, we demonstrated autonomic dysfunction in the SJL mouse strain despite similar hemodynamic parameters compared with C3HeB mice. Imbalance in autonomic regulation could be central for stress-related susceptibility to cardiovascular complications.35 We recently reported greater activation of the aortic endothelium (measured by VCAM-1) in SJL mice.13 Interestingly, VCAM-1 is a key vascular inflammatory marker that increases under more severe stress protocols in ApoE−/− or C57BL/6 mice.36,37 The studies also suggest that stress and an atherogenic diet increase vascular inflammation but have different effects on atherogenesis. In the current study, we confirmed elevated expression of VCAM-1 in carotids from SJL inbred mice or recipient SJL chimeras. Mild stress had little effect on VCAM-1 immunoreactivity in SJL mice, which might suggest a potential link between vascular activation and impaired autoregulation in the SJL mouse strain.
We discovered that several genomic loci on mouse chromosomes 2, 7, 11, 17, and 18 control response to injury in a genetic cross between SJL and C3HeB inbred mouse strains.38 Mouse chromosome 7 might contribute to variation in HR and carotid remodeling; we identified a robust genetic locus on chromosome 7 that controls elevated HR in SJL mice.11 Our previous work indicated that the likely candidate genes in the locus are 3 γ-aminobutyric acid (GABA) receptor genes, encoding receptor subunits for the inhibitory neurotransmitter GABAA. It is well known that the GABAergic system in the hypothalamus inhibits stress-induced tachycardia.39 The human chromosome15q locus is syntenic to the mouse chromosome 7 locus and is important for HR variation40 and behavioral disorders.41–45 Future studies are required to assess the causative link between the GABAergic system and cardiovascular complications in response to stress.
Conclusions
We propose that impaired parasympathetic regulation is central for cardiac, vascular, and immune complications in genetically susceptible subjects under stress. We showed for the first time that mild stress increases HR and circulating and vascular proinflammatory cells in SJL mice; however, immune cells from stress-susceptible SJL mice had no effect on HR or vascular inflammation in stress-protected C3HeB chimeras. Our results suggest that imbalance of autonomic regulation could lead to greater stress reactivity of HR, proinflammatory blood profiles, and vascular complications. These clinical indications could become important diagnostic measures of evaluation of future cardiovascular morbidity and mortality.
Acknowledgments
The authors would like to thank Janice Gerloff, Kathy Donlon, and Martha Zettel for help with animal handling and histology.
Sources of Funding
This study was supported in part by funds from Russian Program “Scientific and scientific-pedagogical personnel of innovative Russia” for 2009–2013 GKNo14.740.11.0923, HL62826 and HL105623 (Korshunov).
Disclosures
None.
References
- Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- Inoue T, Iseki K, Iseki C, Kinjo K. Elevated resting heart rate is associated with white blood cell count in middle-aged and elderly individuals without apparent cardiovascular disease. Angiology. 2012;67:541–546. doi: 10.1177/0003319711428071. [DOI] [PubMed] [Google Scholar]
- Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Hillis GS, Hata J, Woodward M, Perkovic V, Arima H, Chow CK, Zoungas S, Patel A, Poulter NR, Mancia G, Williams B, Chalmers J. Resting heart rate and the risk of microvascular complications in patients with type 2 diabetes mellitus. J Am Heart Assoc. 2012;1:e002832. doi: 10.1161/JAHA.112.002832. doi: 10.1161/JAHA.112.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell TS, Chapleau MW, Hajduczok G, Abboud FM. Baroreflex dysfunction in diabetes mellitus. I. Selective impairment of parasympathetic control of heart rate. Am J Physiol. 1994;266:H235–H243. doi: 10.1152/ajpheart.1994.266.1.H235. [DOI] [PubMed] [Google Scholar]
- Park HJ, Zhang Y, Du C, Welzig CM, Madias C, Aronovitz MJ, Georgescu SP, Naggar I, Wang B, Kim YB, Blaustein RO, Karas RH, Liao R, Mathews CE, Galper JB. Role of SREBP-1 in the development of parasympathetic dysfunction in the hearts of type 1 diabetic Akita mice. Circ Res. 2009;105:287–294. doi: 10.1161/CIRCRESAHA.109.193995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24:1793–1798. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the “Glagov phenomenon” is genetically determined. Circulation. 2004;110:220–226. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- Korshunov VA, Nikonenko TA, Tkachuk VA, Brooks A, Berk BC. Interleukin-18 and macrophage migration inhibitory factor are associated with increased carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2006;26:295–300. doi: 10.1161/01.ATV.0000196544.73761.82. [DOI] [PubMed] [Google Scholar]
- Smolock EM, Ilyushkina IA, Ghazalpour A, Gerloff J, Murashev AN, Lusis AJ, Korshunov VA. A genetic locus on mouse chromosome 7 controls elevated heart rate. Physiol Genomics. 2012;44:689–698. doi: 10.1152/physiolgenomics.00041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile BT, Mason-Garrison CL, Justice MJ. Sex and strain-related differences in the peripheral blood cell values of inbred mouse strains. Mamm Genome. 2003;14:81–85. doi: 10.1007/s00335-002-2160-0. [DOI] [PubMed] [Google Scholar]
- Smolock EM, Burke RM, Wang C, Thomas T, Batchu SN, Qiu X, Zettel M, Fujiwara K, Berk BC, Korshunov VA. Intima modifier locus 2 controls endothelial cell activation and vascular permeability. Physiol Genomics. 2014;46:624–633. doi: 10.1152/physiolgenomics.00048.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Liu E, Miller-DeGraff L, Keener HL, Walker C, Clark JA, Myers PH, Rouse DC, Wiltshire T, Kleeberger SR. The genetic contribution to heart rate and heart rate variability in quiescent mice. Am J Physiol Heart Circ Physiol. 2008;295:H59–H68. doi: 10.1152/ajpheart.00941.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV—heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: a theoretical and practical guide. Exp Physiol. 2008;93:83–94. doi: 10.1113/expphysiol.2007.040733. [DOI] [PubMed] [Google Scholar]
- Olfe J, Domanska G, Schuett C, Kiank C. Different stress-related phenotypes of BALB/c mice from in-house or vendor: alterations of the sympathetic and HPA axis responsiveness. BMC Physiol. 2010;10:2. doi: 10.1186/1472-6793-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff J, Korshunov VA. Immune modulation of vascular resident cells by Axl orchestrates carotid intima-media thickening. Am J Pathol. 2012;180:2134–2143. doi: 10.1016/j.ajpath.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchu SN, Hughson A, Gerloff J, Fowell DJ, Korshunov VA. Role of Axl in early kidney inflammation and progression of salt-dependent hypertension. Hypertension. 2013;62:302–309. doi: 10.1161/HYPERTENSIONAHA.113.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- Korshunov VA, Solomatina MA, Plekhanova OS, Parfyonova YV, Tkachuk VA, Berk BC. Plasminogen activator expression correlates with genetic differences in vascular remodeling. J Vasc Res. 2004;41:481–490. doi: 10.1159/000081804. [DOI] [PubMed] [Google Scholar]
- Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijgelsheim M, Newton-Cheh C, Sotoodehnia N, de Bakker PI, Muller M, Morrison AC, Smith AV, Isaacs A, Sanna S, Dorr M, Navarro P, Fuchsberger C, Nolte IM, de Geus EJ, Estrada K, Hwang SJ, Bis JC, Ruckert IM, Alonso A, Launer LJ, Hottenga JJ, Rivadeneira F, Noseworthy PA, Rice KM, Perz S, Arking DE, Spector TD, Kors JA, Aulchenko YS, Tarasov KV, Homuth G, Wild SH, Marroni F, Gieger C, Licht CM, Prineas RJ, Hofman A, Rotter JI, Hicks AA, Ernst F, Najjar SS, Wright AF, Peters A, Fox ER, Oostra BA, Kroemer HK, Couper D, Volzke H, Campbell H, Meitinger T, Uda M, Witteman JC, Psaty BM, Wichmann HE, Harris TB, Kaab S, Siscovick DS, Jamshidi Y, Uitterlinden AG, Folsom AR, Larson MG, Wilson JF, Penninx BW, Snieder H, Pramstaller PP, van Duijn CM, Lakatta EG, Felix SB, Gudnason V, Pfeufer A, Heckbert SR, Stricker BH, Boerwinkle E, O’Donnell CJ. Genome-wide association analysis identifies multiple loci related to resting heart rate. Hum Mol Genet. 2010;19:3885–3894. doi: 10.1093/hmg/ddq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov VA, Dyachenko IA, Murashev AN. Genetic determinants of heart rate variation and cardiovascular diseases. In: Puiu PDM, editor. Genetic Disorders. InTech; 2013. pp. 89–103. doi: 10.5772/53642. Available at: http://www.intechopen.com/books/genetic-disorders/genetic-determinants-of-heart-rate-variation-and-cardiovascular-diseases. [Google Scholar]
- Greenberg BH, Hermann DD, Pranulis MF, Lazio L, Cloutier D. Reproducibility of impedance cardiography hemodynamic measures in clinically stable heart failure patients. Congest Heart Fail. 2000;6:74–80. doi: 10.1111/j.1527-5299.2000.80140.x. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286:H2408–H2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, Harrison DG. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry. 2012;71:774–782. doi: 10.1016/j.biopsych.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolock EM, Machleder DE, Korshunov VA, Berk BC. Identification of a genetic locus on chromosome 11 that regulates leukocyte infiltration in mouse carotid artery. Arterioscler Thromb Vasc Biol. 2013;33:1014–1019. doi: 10.1161/ATVBAHA.112.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glineur S, Antoine-Moussiaux N, Michaux C, Desmecht D. Immune depression of the SJL/J mouse, a radioresistant and immunologically atypical inbred strain. Immunobiology. 2011;216:213–217. doi: 10.1016/j.imbio.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Sprangers B, Van Wijmeersch B, Luyckx A, Sagaert X, De Somer L, Rutgeerts O, Lenaerts C, Landuyt W, Boeckx N, Dubois B, De Wolf-Peeters C, Waer M, Billiau AD. Allogeneic bone marrow transplantation and donor lymphocyte infusion in a mouse model of irradiation-induced myelodysplastic/myeloproliferation syndrome (MD/MPS): evidence for a graft-versus-MD/MPS effect. Leukemia. 2009;23:340–349. doi: 10.1038/leu.2008.298. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Zhang T, Chen Y, Liu H, Zhou Z, Zhai Y, Yang J. Chronic unpredictable stress accelerates atherosclerosis through promoting inflammation in apolipoprotein E knockout mice. Thromb Res. 2010;126:386–392. doi: 10.1016/j.thromres.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Zhukov V, Bradlow H, Sanchez-Alavez M, Gonzalez AS, Curtiss LK, Conti B. Effects of chronic mental stress and atherogenic diet on the immune inflammatory environment in mouse aorta. Brain Behav Immun. 2011;25:1649–1657. doi: 10.1016/j.bbi.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov VA, Berk BC. Genetic modifier loci linked to intima formation induced by low flow in the mouse carotid. Arterioscler Thromb Vasc Biol. 2009;29:47–53. doi: 10.1161/ATVBAHA.108.178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMicco JA, Sarkar S, Zaretskaia MV, Zaretsky DV. Stress-induced cardiac stimulation and fever: common hypothalamic origins and brainstem mechanisms. Auton Neurosci. 2006;126–127:106–119. doi: 10.1016/j.autneu.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Singh JP, Larson MG, O’Donnell CJ, Tsuji H, Corey D, Levy D. Genome scan linkage results for heart rate variability (the Framingham Heart Study) Am J Cardiol. 2002;90:1290–1293. doi: 10.1016/s0002-9149(02)02865-5. [DOI] [PubMed] [Google Scholar]
- Maddox LO, Menold MM, Bass MP, Rogala AR, Pericak-Vance MA, Vance JM, Gilbert JR. Autistic disorder and chromosome 15q11-q13: construction and analysis of a BAC/PAC contig. Genomics. 1999;62:325–331. doi: 10.1006/geno.1999.6017. [DOI] [PubMed] [Google Scholar]
- Ashley-Koch AE, Mei H, Jaworski J, Ma DQ, Ritchie MD, Menold MM, Delong GR, Abramson RK, Wright HH, Hussman JP, Cuccaro ML, Gilbert JR, Martin ER, Pericak-Vance MA. An analysis paradigm for investigating multi-locus effects in complex disease: examination of three GABA receptor subunit genes on 15q11-q13 as risk factors for autistic disorder. Ann Hum Genet. 2006;70:281–292. doi: 10.1111/j.1469-1809.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- Dick DM, Edenberg HJ, Xuei X, Goate A, Kuperman S, Schuckit M, Crowe R, Smith TL, Porjesz B, Begleiter H, Foroud T. Association of GABRG3 with alcohol dependence. Alcohol Clin Exp Res. 2004;28:4–9. doi: 10.1097/01.ALC.0000108645.54345.98. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Zhou Z, Kimura M, Mash DC, Yuan Q, Goldman D. GABAergic gene expression in postmortem hippocampus from alcoholics and cocaine addicts; corresponding findings in alcohol-naive P and NP rats. PLoS One. 2012;7:e29369. doi: 10.1371/journal.pone.0029369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Navarro P, Vaquero-Lorenzo C, Blasco-Fontecilla H, Diaz-Hernandez M, Gratacos M, Estivill X, Costas J, Carracedo A, Fernandez-Piqueras J, Saiz-Ruiz J, Baca-Garcia E. Genetic epistasis in female suicide attempters. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:294–301. doi: 10.1016/j.pnpbp.2012.04.014. [DOI] [PubMed] [Google Scholar]


