Abstract
We examined cross-sectional and longitudinal associations between neighborhood socioeconomic status, social cohesion and safety and features of the diurnal cortisol curve including: area under the curve (AUC), wake-to-bed slope, wake-up, cortisol awakening response (CAR, wake-up to 30 minutes post-awakening), early decline (30 minutes to 2 hours post-awakening) and late decline (2 hours post-awakening to bed time). In cross-sectional analyses, higher neighborhood poverty was associated with a flatter early decline and a flatter wake-to-bed slope. Higher social cohesion and safety were associated with higher wake-up cortisol, steeper early decline and steeper wake-to-bed slope. Over 5 years, wake-up cortisol increased, CAR, early decline, late decline and wake-to-bed slope became flatter and AUC became larger. Higher poverty was associated with less pronounced increases in wake-up and AUC, while higher social cohesion was associated with greater increases in wake-up and AUC. Adverse neighborhood environments were cross-sectionally associated with flatter cortisol profiles, but associations with changes in cortisol were weak and not in the expected direction.
Keywords: Neighborhood poverty, social cohesion, safety, cortisol, stress, Hypothalmic-Pituitary-Adrenal axis
Introduction
Research has found that residents of more deprived neighborhoods have poorer health on multiple domains, including mental health and a range of chronic diseases (Diez Roux and Mair, 2010).
The stress pathway is one mechanism that may partly explain these findings. Psychosocial (violence, poor social cohesion) and physical environment (poor quality housing, lack of green space) stressors may be more frequently experienced by residents of deprived neighborhoods. Individuals who experience stress may adopt unhealthy behaviors, such as increased alcohol or tobacco consumption, unhealthy diets, or sedentary lifestyles, all of which have health consequences. In addition, neighborhood stressors may activate the hypothalamic-pituitary-adrenal axis (HPA), as well as other biological systems, with a range of physiological consequences.
Although a number of studies have examined how neighborhoods are related to behaviors (Diez Roux and Mair, 2010) fewer have directly examined the stress pathway. To date, most observational studies on neighborhoods and cortisol have employed a cross-sectional study design (Brenner et al., 2013; Chen and Paterson, 2006; Do et al., 2011; Karb et al., 2012; Roe et al., 2013; Rudolph et al., 2014) and have examined neighborhood socioeconomic status (NSES) as the main characteristic of interest (Brenner et al., 2013; Chen and Paterson, 2006; Dulin-Keita et al., 2012; Rudolph et al., 2014). To our knowledge, few studies have looked beyond NSES at other neighborhood social and physical characteristics like violence, social support and green space (Do et al., 2011; Karb et al., 2012; Roe et al., 2013) and only one has used a longitudinal approach (Dulin-Keita et al., 2012).
We used unique longitudinal data on daily cortisol profiles from a population sample to examine how neighborhood factors were related both to levels of cortisol and to longitudinal changes in cortisol. It is thought that the process of aging itself impacts cortisol rhythms, an indication of the natural wear and tear on the HPA (Karlamangla et al., 2013; Wang et al., 2014). Evaluating how additional chronic stressors, such as neighborhood factors, impact HPA functioning over time will contribute to our understanding of the relationship between stress and health. The neighborhood characteristics of interest in our study were measures of neighborhood socioeconomic status as well as safety and social cohesion. We investigated the following research questions: 1) Are stressful neighborhoods cross-sectionally associated with daily cortisol profiles? 2) Are neighborhood characteristics associated with changes in cortisol profiles over a five year period?
Daily cortisol patterns experience a sharp rise during the first 30–45 minutes after awakening, called the cortisol awakening response (CAR), followed by a gradual decline over the remainder of the day reaching the lowest point before bedtime (eFigure 2). Given this distinct pattern, we examine four pieces of the cortisol curve: wake up cortisol, CAR, early decline (slope between 30 minutes and 120 minutes after wake up) and late decline (slope between 120 minutes and bed time). We also examine two summary measures: the wake to bed slope and the area under the curve (AUC). As supported by existing evidence, we hypothesized neighborhood disadvantage, measured by higher poverty, less social cohesion and less safety, would be associated with lower wake up cortisol, steeper CAR, flatter early decline, late decline and wake to bed slope, and larger AUC (Do et al., 2011; Karb et al., 2012). We also hypothesized that changes associated with aging would be exacerbated in adverse neighborhood environments.
Methods
We used data from the Multi-Ethnic Study of Atherosclerosis (MESA). MESA, a longitudinal cohort study of cardiovascular disease (CVD), sampled 6814 CVD-free men and women age 44 – 84 years from six US sites. A baseline examination was held from 2000 – 2002 and four follow up exams from 2002 – 2012 (Bild et al., 2002).
The MESA Stress Study is an ancillary study that collected detailed measures of stress hormones at two time periods. MESA Stress Study I collected data over the third and fourth examination of the MESA parent study (2004 – 2006) from participants in the New York and Los Angeles study sites, (n= 1002). Six salivary cortisol samples were collected per day over three weekdays, resulting in 18 samples per participant. Cortisol was measured immediately after waking but before getting out of bed, 30 minutes later, around 1000 hours (h), around 1200h, around 1800h and before bed.
A follow up to Stress Study I was conducted from 2010 – 2012, in conjunction with MESA exam 5. Stress Study II recruited participants from Stress Study I, new participants at each of the two Stress I sites and participants from the Baltimore study site (n= 1082). Stress Study II collected eight saliva samples over two days (16 samples): upon awakening but before getting out of bed, 30 minutes later, 1 hour after breakfast, around 1000h, at noon, around 1600h, around 1800h and before bed. There were 514 participants who contributed to both Stress studies, with a maximum of 34 samples over 5 days available for analysis. Institutional review board approval was granted at each study site and written informed consent was obtained from participants.
Cortisol
Saliva samples were collected using Salivette collection tubes and stored at −20° C until analysis. Before biochemical analysis, samples were thawed and centrifuged at 3000 rpm for three minutes to obtain clear saliva with low viscosity. Salivary cortisol levels were determined by employing a commercially available chemi-luminescence assay (CLIA) with a high sensitivity of 0.16 ng/mL (IBL-Hamburg; Germany). Intra- and inter-assay coefficients of variation were below eight percent. All samples were assayed at a central laboratory in Dresden, Germany. Cortisol was measured in nmol per liter and log transformed for analysis.
Neighborhood Features
Various features of neighborhoods were obtained by another ancillary study, the MESA Neighborhood Study. During 2003–2005, a total of 6,191 MESA participants were enrolled in the Neighborhood ancillary study (91% of baseline sample).Socioeconomic indicators from the US Census and survey-based assessments of the social environment were assigned to each participant. Participant’s home addresses at time of Stress I and Stress II were geocoded using the TeleAtlas geocoding software (TeleAtlas Lebanon, NH) which assigned each address to a census tract (Census 2000 boundaries), a proxy for neighborhood of residence.
Neighborhood Socioeconomic Status
Neighborhood socioeconomic status was accessed using data from the American Community Survey (ACS) 2005–2009 (Stress I) and ACS 2007–2011 (Stress II). Measures utilized were percent of persons below poverty level, median household income, percent of unemployed persons 16 years and older and median value of housing units. In addition, a summary measure of socioeconomic status (SES) was derived from principal components analysis (PCA); 16 census variables were included in the PCA and results yielded a summary index that included 6 indicators of household income, wealth, poverty, employment and housing. More information on the NSES index can be found elsewhere (Hajat et al., 2013; Moore et al., 2013). All NSES indicators were measured at the census tract level.
Social cohesion and safety
Questionnaires on neighborhood characteristics were administered to a random sample of residents living in selected census tracts where MESA Stress I participants resided in New York and Los Angeles in 2006–2008, referred to as Community Survey II. Responses from Community Survey II were used to create the social cohesion and safety measures corresponding to Stress I. A similar survey was administered to another random sample of residents living in selected census tracts where MESA participants lived from all six MESA study sites in 2011–2012, referred to as Community Survey III. In addition, the same survey was administered to the MESA participants at Exam 5 in 2010–2012. The responses from Community Survey III and MESA Exam 5 were combined to increase sample size in creating the survey based measures for Stress II.
On the basis of a conceptual model (Diez Roux, 2003) and prior work(Echeverria et al., 2004), safety and social cohesion were selected as the relevant survey based measures (see eAppendix for questions). Safety was derived from a 3 item scale and social cohesion from a 4 item scale and responses for each item of the scale ranged from 1 (strongly agree) to 5 (strongly disagree). Scales were based on previous work (Mujahid et al., 2007) and have acceptable internal consistency (Cronbach alpha 0.64–0.82). Conditional empirical Bayes (CEB) estimates were derived for each census tract from three level hierarchical linear models (i.e. scale items nested within individuals nested within neighborhoods) using HLM version 7.0. Estimates were adjusted to the mean gender, age, site and study source (MESA or Community Survey) distribution of respondents to account for any systematic differences in these factors. Information on CEB estimation has been previously published (Mujahid et al., 2008).
Covariates
In order to identify potential confounders of the cortisol – neighborhood characteristics association we employed a directed acyclic graph (DAG) (eFigure 1). All models were adjusted for the following individual level covariates: age, gender, race/ethnicity, income-wealth index, wake-up time (on the day of sampling), and sequential day the sample was collected (i.e. 1, 2 or 3) which were obtained via self-reported questionnaire. Race/ethnicity was classified as non-Hispanic white, non-Hispanic black, and Hispanic. We created an income-wealth index which ranges from 0–8 points, where 0 represents lowest income- wealth (Hajat et al., 2010).
Age, income-wealth index, gender, race/ethnicity and day were mean centered to facilitate interpretation of main effects for variables included in interactions. Wake-up time was centered at 7:00 AM. Additional covariates were included in sensitivity analysis (see eAppendix).
Statistical Analysis
We used mixed effects regression models with repeated measures of log transformed cortisol. To capture the non-linear pattern of cortisol (described above and seen in eFigure 2), piecewise linear regression models with two fixed knots at 30 and 120 minutes after wake-up were used (Hajat et al., 2010). The main effect of neighborhood exposures and interactions with the various slopes allowed for estimation of associations of neighborhood characteristics with different features of the cortisol profile: wake-up, CAR, early decline and late decline. In addition, to calculate the summary measure AUC, we used linear splines to connect the values from each of the sample times and then calculated the area under the linear spline based on the trapezoid rule, (Yeh and Kwan, 1978) using all available data and restricting estimates to a 16-hour day duration. Lastly, the wake-to-bed slope excluded the second sample and then calculated the overall slope of cortisol over the day. It was presented as the rate per 8 hours. In all models, neighborhood exposures were standardized to mean of 0 and standard deviation (SD) of 1 and each was modeled separately.
Cross-sectional analysis
To increase power, data from Stress I and Stress II were combined for cross-sectional analyses. After combining the two studies, we had 1458 unique white, black and Hispanic individuals, 5007 days and 32257 samples. We excluded samples that had missing or incomplete cortisol data (including time since wake-up), samples with cortisol values equal to 0 nmol/L or > 100nmol/L (generally considered to be outliers), persons on steroidsor hormone replacement therapy, those who did not participate in the neighborhoods study and those who had missing covariates and were left with 1297 persons, 4351 days and 27955 samples from 708 census tracts.
Neighborhood characteristics and covariates were assigned at the time of the corresponding Stress study. The main effects of neighborhood exposures and interactions of exposures with each spline piece were included to estimate associations of exposures with features of the cortisol curve. The main effects of covariates and interactions of covariates with each spline piece were included to adjust the associations of the neighborhood exposures for these possible confounders. Within-person correlations and between-person variation in slopes were modeled as random components for the intercept and slopes of the cortisol curve for the first and third slopes. In addition, a random effect for Stress Study indicator was included to account for within study correlation. Day level variability was accounted for using fixed effects for day of sample.
Longitudinal analysis
Participants who attended both Stress studies (n = 514 prior to exclusions) were included in the analyses of changes in cortisol features over time . We used the same exclusions described in the cross-sectional analysis above and were left with 465 persons, 2316 days and 14988 samples from 319 census tracts. Neighborhood characteristics for the longitudinal analysis corresponded to Stress I (i.e. they were not time-varying), while some person-level characteristics (e.g. age, BMI, smoking, individual SES) were time-varying. The main effects and interactions of the neighborhood characteristics with each spline piece were included to calculate the cortisol features of interest. The interactions of time between visits with each of the slope pieces were used to assess the average change in each of the features over time and three-way interactions between the exposure, spline pieces, and time between visits were used to assess deviation from overall change over time based on levels of neighborhood exposures. Covariate main effects and interactions of covariates with each spline piece were included to adjust for confounding. Three-way interactions of covariates, spline pieces, and time between visits were included only if covariates were statistically significant with a 0.2 p-value (three-way interactions involving race/ethnicity and smoking status were retained). Person-to-person variation in the effect of time between visits was accounted for with the inclusion of random components for time between visits and interactions of time and the first and third slope pieces. Within-person correlations and between-person variation in slopes were modeled as random components for the intercept and slopes of the cortisol curve for the first and third slopes. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Results were similar across census-based neighborhood SES characteristics; therefore, we only present data on poverty. Results pertaining to median household income, median home values, percent unemployed and the neighborhood SES index can be found in eTables 1 and 2.
Cross-sectional results
In the cross sectional analysis, the average age over both Stress studies was 67.6 years, with 49% male, 48% Hispanic and 30% African-American (Table 1). For census tracts in our study the average percent poverty was 19% and the mean of the social cohesion and safety scales were 3.4 and 3.3 respectively (range 1 – 5, 5 was the highest). In the bivariate cross sectional analysis (Table 2), persons in low poverty neighborhoods (−1 SD) had higher wake-up cortisol (2.58 nmol/L), and steeper early decline (−0.47), steeper late decline (−0.81 nmol/L/8 hr) and steeper overall slopes (−0.87 nmol/L/8 hours) compared to those in high poverty areas (+1 SD) (2.45, −0.41, −0.75, −0.77 respectively). Wake-up cortisol was higher and the early decline, late decline and slope were steeper for persons with higher neighborhood social cohesion (+1 SD). We also observed higher AUC for those in safer compared to less safe neighborhoods.
Table 1.
Descriptive characteristics of participants included in the pooled cross-sectional and longitudinal analysis, Multi-Ethnic Study of Atherosclerosis (MESA) Stress Study
| Stress Study I a |
Stress Study II a |
Sample in Pooled Cross- section |
Sample in longitudinal analysis |
|||
|---|---|---|---|---|---|---|
| At stress 1 | At stress 2 | |||||
| N Persons | 866 | 893 | 1297 | 465 | 465 | |
| N Days | 2569 | 1782 | 4351 | 1389 | 927 | |
| N Samples | 14633 | 13322 | 27955 | 8012 | 6976 | |
| N Census tracts | 489 | 534 | 708 | 319 | 319 | |
| Age (Mean (SD)) | 65.6 (9.8) | 69.6 (8.9) | 67.6 (9.6) | 64.2 (9.2) | 69.8 (9.2) | |
| Male (%) | 50 | 47 | 49 | 50 | 50 | |
| Race/Ethnicity (%) | ||||||
| White | 19 | 26 | 23 | 18 | 18 | |
| Black | 27 | 32 | 30 | 26 | 26 | |
| Hispanic | 54 | 42 | 48 | 56 | 56 | |
| Income/Wealth Index (Mean (SD)) |
3.8 (2.3) | 4.5 (2.2) | 4.1 (2.3) | 4.0 (2.3) | 4.3 (2.2) | |
| Cigarette Smoking Status (%) | ||||||
| Never | 45 | 46 | 45 | 49 | 46 | |
| Former | 47 | 48 | 48 | 44 | 49 | |
| Current | 8 | 6 | 7 | 7 | 5 | |
| Body Mass Index (Mean (SD)) |
29.1 (5.6) | 29.4 (5.5) | 29.3 (5.5) | 29.1 (5.2) | 29.3 (5.6) | |
| Center for Epidemiology Depression Scale (Mean (SD)) |
8.8 (9.0) | 8.3 (7.7) | 8.6 (8.3) | 8.7 (9.3) | 8.6 (7.9) | |
| Total moderate or vigorous physical activity METS/week (Mean (SD)) |
4846.4 (4369.9) |
5218.4 (6752.7) |
5035.2 (5706.8) |
5081.5 (4492.5) |
4829.3 (5512.7) |
|
| Study Site (%) | ||||||
| New York, NY | 53 | 42 | 47 | 53 | 53 | |
| Baltimore, MD b | n/a | 25 | 13 | n/a | n/a | |
| Los Angeles, CA | 47 | 34 | 40 | 47 | 47 | |
| Neighborhood Exposures c | ||||||
| % Poverty (Mean (SD)) | 19.2 (11.9) |
17.7 (11.6) |
18.5 (11.8) |
18.8 (11.6) | 18.8 (11.6) | |
| Social Cohesion (Mean (SD)) | 3.4 (0.2) | 3.5 (0.2) | 3.4 (0.2) | 3.4 (0.2) | 3.4 (0.2) | |
| Safety (Mean (SD)) | 3.3 (0.5) | 3.4 (0.3) | 3.3 (0.4) | 3.3 (0.4) | 3.3 (0.4) | |
| Outcome | ||||||
| Cortisol nmol/L (Mean (SD)) | 9.3 (9.4) | 11.4 (11.4) |
10.3 (10.5) |
9.0 (9.1) | 10.8 (10.7) | |
| Log cortisol (Mean (SD)) | 1.77 (1.03) |
1.95 (1.11) |
1.86 (1.07) |
1.73 (1.04) | 1.91 (1.10) | |
| Wake-to-bed slope d (Mean (SE)) |
−0.88 (0.01) |
−0.76 (0.01) |
−0.82 (0.01) |
−0.89 (0.02) | −0.76 (0.02) |
|
| Area under the curve d (Mean (SE)) |
1.55 (0.02) |
1.75 (0.02) |
1.66 (0.01) |
1.51 (0.02) | 1.72 (0.03) | |
Participants in Stress I and Stress II that were included in the pooled cross-sectional analysis. Some participants were included in both Stress I and II.
Participants were not recruited from the Baltimore, MD study site for Stress I, therefore they do not have repeat cortisol measures and cannot be included in the longitudinal analysis.
In the change analysis, neighborhood exposures were not time-varying.
Standard errors are provided for wake to bed slope and area under the curve because it was calculated from an unadjusted linear mixed model.
Table 2.
Mean (SE) for features of the log cortisol curve at the mean and +/− 1 standard deviation (SD) of neighborhood characteristics, pooled cross sectional analysis a
| Wake-up level (nmol/L) |
Cortisol awakening response (CAR) (nmol/L/hr) |
Early decline (nmol/L/hr) |
Late decline (nmol/L/8 hr) |
Wake-to-bed slope (nm ol/L /8 hr) |
Area under the curve (AUC) |
|
|---|---|---|---|---|---|---|
| % Poverty (N persons=1,297; N Days=4,347; N samples=27,928 | ||||||
| Mean −1 SD | 2.58 (0.03) | 0.69 (0.04) | −0.47 (0.02) | −0.81 (0.02) | −0.87 (0.0 2) | 1.65 (0.03) |
| Mean | 2.52 (0.02) | 0.71 (0.03) | −0.44 (0.01) | −0.78 (0.01) | −0.82 (0.0 1) | 1.66 (0.01) |
| Mean +1 SD | 2.45 (0.03) | 0.73 (0.04) | −0.41 (0.02) | −0.75 (0.02) | −0.77 (0.0 2) | 1.67 (0.03) |
| P for trend | 0.007 | 0.51 | 0.017 | 0.032 | <0.0 01 | 0.64 |
| Social Cohesion (N persons=1,177; N Days=3,720; N samples=24,267) | ||||||
| Mean −1 SD | 2.45 (0.03) | 0.67 (0.05) | −0.39 (0.02) | −0.73 (0.02) | −0.75 (0.0 2) | 1.69 (0.03) |
| Mean | 2.53 (0.02) | 0.70 (0.03) | −0.44 (0.01) | −0.77 (0.01) | −0.81 (0.0 1) | 1.68 (0.02) |
| Mean +1 SD | 2.60 (0.03) | 0.73 (0.04) | −0.49 (0.02) | −0.81 (0.02) | −0.87 (0.0 1) | 1.67 (0.02) |
| P for trend | <0.001 | 0.32 | <0.001 | <0.001 | <0.0 01 | 0.63 |
| Safety (N persons=1,177; N Days=3,720; N samples=24,267) | ||||||
| Mean −1 SD | 2.40 (0.03) | 0.74 (0.05) | −0.42 (0.02) | −0.76 (0.02) | −0.79 (0.0 2) | 1.60 (0.03) |
| Mean | 2.53 (0.02) | 0.70 (0.03) | −0.44 (0.01) | −0.77 (0.01) | −0.81 (0.0 1) | 1.68 (0.02) |
| Mean +1 SD | 2.66 (0.03) | 0.66 (0.04) | −0.46 (0.02) | −0.78 (0.02) | −0.84 (0.0 1) | 1.75 (0.03) |
| P for trend | <0.001 | 0.19 | 0.057 | 0.64 | 0.00 9 | <0.001 |
Estimates at means, mean +1 SD and mean −1 SD were calculated from unadjusted piecewise models where neighborhood characteristic of interest was specified as continuous. The p for trend is calculated using neighborhood characteristics specified as continuous variables and interacted with each spline to calculate the pieces of the curve.
After adjustment for potential confounders, one SD higher poverty was associated with 2.34% (95% CI: −0.22%, 4.96%) flatter early decline, 2.32% (CI: 0.09%, 4.60%) flatter wake-to-bed slope and 2.41% (CI: −0.38%, 5.28%) larger AUC (Table 3). One SD higher social cohesion was associated with 5.39% (CI: 1.88%, 9.01%) higher wake-up cortisol, 4.98% (CI: −7.79%, −2.24%) steeper early decline and 3.25% (CI: −5.52%, −1.02%) steeper wake-to-bed slope. Safety showed similar results. There was no association between CAR or late decline and any of the neighborhood characteristics.
Table 3.
Percent difference (95% confidence interval) in cortisol associated with one standard deviation unit higher neighborhood characteristic for features of the cortisol curve in the pooled cross-sectional analysis a
| M od el | Percent difference at wake-up |
Percent difference in CAR (per 1 hr) |
Percent difference in early decline (per 1 hr) |
Percent difference in late decline (per 8 hrs) |
Percent difference in wake-to-bed slope (per 8 hrs) |
Percent difference in area under the curve (AUC) |
|---|---|---|---|---|---|---|
| % Poverty | −2.78 (−6.39, 0.71) | 2.87 (−3.46, 9.62) | 2.34 (−0.22, 4.96) | 0.69 (−2.01, 3.47) | 2.32 (0.09, 4.60) | 2.41 (−0.38, 5.28) |
| Socialcohesion | 5.39 (1.88, 9.01) | 3.95 (−2.98, 11.39) | −4.98 (−7.79, −2.24) | −1.12 (−3.82, 1.52) | −3.25 (−5.52, −1.02) | −0.41 (−3.28, 2.38) |
| Safety | 4.50 (1.16, 7.95) | −2.15 (−9.10, 4.35) | −2.90 (−5.61, −0.26) | 0.21 (−2.35, 2.85) | −1.86 (−4.08, 0.31) | −0.44 (−3.41, 2.45) |
Models adjusted for stress study indicator, wake-up time, day of sampling, age, gender, race, and income/wealth index
Longitudinal results
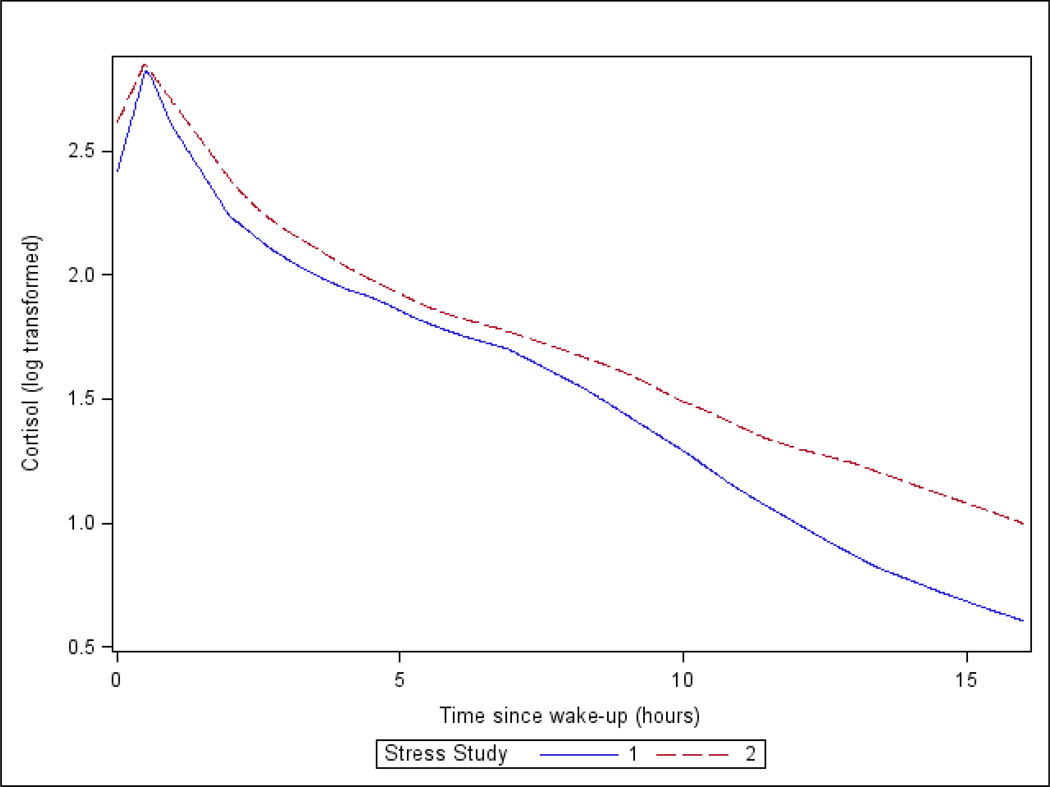
In the longitudinal analysis (n=465) about 50% of participants were male, 26% were African-American and 56% were Hispanic (Table 1). The average time between visits was 5.9 years (SD: 1.3). Over time, the AUC became larger (1.51 to 1.72) and the wake-to-bed slope became flatter (−0.89 to −0.76). On average participants enrolled in Stress II had flatter curves with higher cortisol at every time point compared to participants enrolled at Stress I (Figure 1).
Figure 1.
Average diurnal curves for Stress I (n = 866) and Stress II (n=893) participants. Participants enrolled in both studies, contribute data to each curve. Stress I participants are shown in the blue solid line and Stress II in the red dashed line.
Wake-up values increased over time but the increase was smaller at higher (0.10 log nmol/L) than at lower poverty levels (0.22 log nmol/L) (Table 4). AUC also increased over time but the increase was greater at higher (0.13) than at lower safety levels (0.06). The wake-to-bed slope became flatter over time. This flattening was less pronounced for residents of higher poverty compared to lower poverty neighborhoods and less pronounced for lower safety and social cohesion areas compared to higher safety and social cohesion areas (P trends <0.001, <0.001, 0.07 respectively). Similar patterns were observed for the early and late decline but confidence intervals contained the null.
Table 4.
Mean (SE) of 5 year change in features of the cortisol curve (derived from logged cortisol values) at the mean and +/− 1 standard deviation (SD) of neighborhood characteristics, longitudinal analysis a
| Category | Wake-up level (nmol/L) |
Cortisol awakening response (CAR) (nmol/L/hr) |
Early decline (nmol/L/hr) |
Late decline (nmol/L/8 hr) |
Wake-to-Bed Slope (nmol/L/8 hr) |
Areaunder the curve (AUC) |
|---|---|---|---|---|---|---|
| % Poverty (N persons=465; N days=2,316, N samples=14,988) | ||||||
| 5 year change at −1 SD |
0.22 (0.05) | −0.41 (0.11) | 0.10 (0.04) | 0.15 (0.03) | 0.27 (0.03) | 0.12 (0.02) |
| 5 year change at mean |
0.16 (0.03) | −0.39 (0.08) | 0.07 (0.02) | 0.14 (0.02) | 0.17 (0.03) | 0.10 (0.02) |
| 5 year change at +1 SD |
0.10 (0.05) | −0.38 (0.11) | 0.04 (0.03) | 0.13 (0.03) | 0.07 (0.04) | 0.07 (0.02) |
| P for trend | 0.088 | 0.86 | 0.24 | 0.71 | <0.001 | 0.19 |
| Social Cohesion (N persons=368; N days=1,831, N samples=11,867) | ||||||
| 5 year change at −1 SD |
0.09 (0.06) | −0.44 (0.13) | 0.06 (0.04) | 0.12 (0.04) | 0.05 (0.04) | 0.07 (0.03) |
| 5 year change at mean |
0.14 (0.04) | −0.37 (0.08) | 0.06 (0.03) | 0.14 (0.02) | 0.14 (0.03) | 0.10 (0.02) |
| 5 year change at +1 SD |
0.18 (0.04) | −0.30 (0.10) | 0.06 (0.04) | 0.15 (0.03) | 0.24 (0.03) | 0.12 (0.03) |
| P for trend | 0.22 | 0.36 | 0.93 | 0.54 | <0.001 | 0.11 |
| Safety (N persons=368; N days=1,831, N samples=11,867) | ||||||
| 5 year change at −1 SD |
0.15 (0.05) | −0.43 (0.11) | 0.04 (0.04) | 0.12 (0.04) | 0.10 (0.04) | 0.06 (0.03) |
| 5 year change at mean |
0.14 (0.04) | −0.37 (0.08) | 0.06 (0.03) | 0.14 (0.02) | 0.14 (0.03) | 0.10 (0.02) |
| 5 year change at +1 SD |
0.12 (0.05) | −0.32 (0.11) | 0.07 (0.04) | 0.16 (0.03) | 0.19 (0.04) | 0.13 (0.03) |
| P for trend | 0.59 | 0.45 | 0.58 | 0.40 | 0.066 | 0.027 |
Means, mean +1 SD and mean −1 SD were calculated from unadjusted piecewise models with time since Stress 1 and where neighborhood characteristic of interest was specified as continuous. The p for trend is calculated as the continuous neighborhood characteristic interacted with time since Stress 1 and the appropriate knots.
Table 5 presents the estimates for the mean 5 year change and the mean differences in 5 year change per 1 SD higher neighborhood characteristic for each feature of the log cortisol curve adjusted for covariates. The mean differences in 5 year change reflect the deviation from the average 5 year change associated with one SD higher value of the neighborhood exposure at Stress I.
Table 5.
Mean 5 year changes in selected features of the log cortisol curve and mean differences in 5 year changes per 1 standard deviation (SD) unit increase in neighborhood characteristic (95% confidence intervals)
| Wake-up c | Cortisol awakening response (CAR) (1 hour) d |
Early decline (1 hour) e |
Late decline (8 hour) e |
Wake-to- bed slope (8 hour) e |
Area under the curve (AUC) f |
|
|---|---|---|---|---|---|---|
| Mean 5 year change a |
0.14 (0.07, 0.21) | −0.36 (−0.51, −0.21) | 0.07 (0.02, 0.12) | 0.15 (0.11, 0.19) | 0.09 (0.05, 0.12) | 0.18 (0.13, 0.21) |
| Mean differences in 5 year change per 1 SD higher neighborhood characteristic b |
||||||
| % Poverty | −0.11 (−0.18, −0.05) | −0.02 (−0.16, 0.13) | 0.01 (−0.04, 0.06) | 0.00 (−0.05, 0.04) | −0.01 (−0.04, 0.03) | −0.12 (−0.17, −0.06) |
| Social cohesion | 0.08 (0.01, 0.15) | 0.12 (−0.05, 0.28) | −0.03 (−0.09, 0.02) | 0.01 (−0.05, 0.06) | 0.01 (−0.03, 0.05) | 0.09 (0.04, 0.15) |
| Safety | 0.02 (−0.05, 0.09) | 0.11 (−0.04, 0.26) | −0.03 (−0.09, 0.03) | 0.01 (−0.04, 0.06) | 0.02 (−0.02, 0.06) | 0.05 (−0.01, 0.11) |
Derived from model without any neighborhood characteristics and adjusted to the mean age, gender, race/ethnicity, income/wealth index, sequential day of sampling and time at wake-up centered at 7:00AM
Derived from model that also includes the neighborhood characteristic as the 3-way interaction of neighborhood exposure, time, and the appropriate knots.
A positive number in the mean 5 year change indicates an increase in the wake up value over time; a positive number in the mean difference in 5 year change indicates a larger increase in wake-up value for a 1 SD unit increase in the neighborhood exposure and a negative number indicates a smaller increase in wake-up value for a 1 SD unit increase in the neighborhood exposure.
A negative number in the mean 5 year change indicates a less pronounced slope (ie a flattening of the CAR) over time; a positive number in the mean difference in 5 year change indicates less flattening of the CAR over time for a 1 SD unit increase in neighborhood exposure and a negative number indicates more flattening of the CAR over time for a 1 SD unit increase in neighborhood exposure.
A positive number in the mean 5 year change indicates a less pronounced decline over time (i.e. a flattening of the slope); a positive number in the mean difference in 5 year change indicates greater flattening of the slope for a 1 SD unit increase in neighborhood exposure and a negative number indicates less flattening of the slope for a 1 SD unit increase in neighborhood exposure over time.
A positive number in the mean 5 year change indicates an increase in AUC; a positive number in the mean difference in 5 year change indicates a more pronounced increase in AUC for a 1 SD unit increase in neighborhood exposure and a negative number indicates a less pronounced increase in AUC for a 1 SD unit increase in neighborhood exposure over time.
Overall, wake-up values increased between Stress I and Stress II (mean 5 year change: 0.14). Higher poverty (+1 SD) was associated with a smaller increase in wake-up cortisol (mean difference in 5 year change: −0.11, CI: −0.18, −0.05) and better social cohesion (+1 SD) was associated with a larger increase in wake up cortisol (0.08 CI: 0.01, 0.15) relative to lower poverty and social cohesion. In other words, aging increases wake up cortisol by 0.14 over 5 years, but higher neighborhood poverty and lower social cohesion did not exacerbate this increase in wake up cortisol: in contrast, higher poverty was associated with a smaller increase in wake up cortisol while better social cohesion was associated with a larger increase in wake up cortisol.
Over time, the CAR, early decline, late decline and wake-to-bed slope became flatter, – 0.36, 0.07, 0.15 and 0.09 respectively. However, this flattening of cortisol features was not associated with neighborhood factors. The AUC became larger with time (0.18 CI: 0.13, 0.21); but again higher poverty did not further increase the AUC, instead it was associated with less pronounced increase in the AUC (−0.12 CI: −0.17, −0.06) and higher social cohesion was associated with a greater increase in the AUC (0.09 CI: 0.04, 0.15).
In an attempt to reduce bias from measured confounders, we conducted several sensitivity analyses. In addition to the covariates adjusted for above (our base model), we controlled for smoking status, BMI, depression and physical activity in case these were confounders instead of mediators (see eFigure 1). We also added education and occupational status to ensure against residual confounding by individual SES. And finally we added data on factors related to the day of sampling (e.g. number of cigarettes smoked, stress level and sleep quality); as these are thought to have an immediate impact on cortisol levels. Regardless of which variables were included, we observed little change in our parameter estimates (eTables 3 and 4).
Discussion
In this study we examined the association between neighborhood characteristics and salivary cortisol cross-sectionally and longitudinally. In the cross-sectional analysis, as hypothesized, we found higher poverty was associated with flatter early decline and wake-to-bed slope and increased social cohesion and safety were associated with higher wake-up, steeper early decline and steeper wake-to-bed slope. In the longitudinal analysis, we did not find that adverse neighborhood environments exacerbated changes associated with aging. In fact in some case the opposite was observed: although wake up cortisol and AUC increased with age, higher poverty and lower social cohesion were associated with less pronounced (rather than more pronounced) increases in both parameters. No associations between neighborhood characteristics and changes in other features were observed
Our cross-sectional results were consistent with some previous literature. A study using MESA Stress I found participants in high poverty neighborhoods had flatter early declines and lower social cohesion was associated with lower wake-up cortisol (Do et al., 2011). Another study found flatter curves for persons in neighborhoods with higher perceived and observed stress and those in areas with low social support (Karb et al., 2012). A longitudinal study of fasting serum cortisol among children found neighborhood disadvantage was associated with lower total serum cortisol over time (Dulin-Keita et al., 2012). Given the younger population and use of only one cortisol sample these results are difficult to interpret. Lastly among laboratory based acute stress reactivity studies (Barrington et al., 2014; Hackman et al., 2012; Kapuku et al., 2002) and studies that sampled cortisol before and after an interview (which could be considered a novel stimulus), (Brenner et al., 2013; Rudolph et al., 2014) some found that higher neighborhood deprivation was associated with greater cortisol reactivity, (Barrington et al., 2014; Hackman et al., 2012; Rudolph et al., 2014) while others were null (Brenner et al., 2013; Kapuku et al., 2002). It is important to note that the effects of neighborhood stressors on the daily cortisol profile (possibly resulting from long term exposures and reflecting more chronic alterations of the HPA axis) may be very different from the effects of neighborhoods on short-term reactivity.
Existing evidence suggests flatter cortisol curves and higher AUC are associated with a host of stressors (e.g. low SES, minority status and disadvantaged neighborhoods) (Cohen et al., 2006; Do et al., 2011; Miller et al., 2007) as well as adverse health outcomes (e.g. diabetes and obesity) (Björntorp and Rosmond, 2000; Champaneri et al., 2012). In addition, studies have found higher wake-up cortisol levels, higher AUCs and flatter profiles as populations age (Heaney et al., 2012; Ice, 2005; Wang et al., 2014). A recent study found that blacks and Hispanics had larger increases in wake-up cortisol and less pronounced flattening in the early decline over a 6 year period (Wang et al., 2014). Given this evidence, we would expect to see greater increases in both wake-up cortisol and AUC and more flattening of the slope over time among persons in disadvantaged neighborhoods. However, our results for the slope and early and late declines were null, while we found that higher neighborhood poverty and low cohesion were associated with less pronounced (rather than more pronounced) increases in wake up cortisol and AUC. A sensitivity analysis using simple linear regression with pre-calculated features of the curve yielded similar results (data not shown).
Several factors could explain the unexpected findings in the longitudinal analysis. Although our study had richer data than most, measurement error is a major challenge in these analyses. Cortisol profiles are challenging to characterize and error is likely to be compounded when looking at change in cortisol versus cortisol at one point in time. There is some evidence to indicate that compliance with the sampling protocol was worse among lower SES individuals, (Hajat et al., 2010) that is the reliability of cortisol measures may be better among persons in more advantaged neighborhoods. This differential misclassification could also have limited our ability to detect what are likely to be weak associations. Second, there is a possibility of selection bias in our sample. In order to measure change in cortisol participants had to have samples collected at both Stress I and Stress II. Those who were lost to follow up between the two studies were on average older, of lower SES and more likely to engage in unhealthy behaviors than those who continued on in the study (data not shown). We attempted to account for this by controlling for several variables that are predictors of loss to follow up in sensitivity analysis (BMI, smoking, depression, exercise) and found similar results. Lastly, unmeasured confounding may be driving our results. Given that very few studies have followed participants to examine how cortisol changes over time, (Ross et al., 2014; Wang et al., 2014) there may be factors associated with the outcome (and the neighborhood exposures) that were not included in the models.
Our longitudinal analyses assume that the repeat days captured at each MESA Stress exam adequately capture relatively stable states (when measures over 2–3 days are pooled), but that these states can change over longer periods (years) as a result of aging and other exposures. The general patterns that we observed associated with aging (lower wake up, flattening of the declines, and higher AUC) are consistent with the idea that our measures at each exam are capturing features that change over time. However substantial error in using even multiple days to capture a relatively stable trait may have made it very difficult to detect associations of long term trends over time with other exposures whose effects are likely to be small. Very little research has examined the stability of cortisol features over short or long term periods. A recent study found that AUC was more stable over 3 and 12 months than the slope or CAR (Ross et al., 2014). In the MESA Stress study cohort, the late decline slope appeared to be more stable over a 6 year period compared to other features of the cortisol curve (Wang et al., 2014). In addition, both studies found that stability in the AUC and slope parameters was much greater for days within a given visit than across visits, (Ross et al., 2014; Wang et al., 2014) suggesting that it is reasonable to examine how factors are associated with changes in these two parameters over time. Additional research is needed to better determine the stability of various cortisol traits and how they change over time. An additional methodologic limitation of our analyses is that the measurement of social cohesion and safety (between 2006 – 2008) occurred slightly after the first Stress study (between 2004 – 2006).
In addition to the methodological reasons for the unexpected results we observed, it should be noted that there is some possibility that the longitudinal results reflect reality. As posited by the allostatic load theory the physiological response to chronic stress may take several different forms including forms involving a lack of normal responsivity to stressors resulting in lower cortisol levels overall (McEwen and Seeman, 1999). It is therefore plausible that chronic stress resulting from neighborhood stressors ultimately leads to an altered physiologic response resulting in a downward shift of the cortisol curve. It should also be noted that these findings may have been observed by chance alone. Additional research on different study populations is required in order to confirm or refute the patterns we observed.
Our study had several strengths. We have a large and multi-ethnic sample. Relative to other studies, our cortisol sampling is dense and few other studies have cortisol measurements on the same individuals over time (Dulin-Keita et al., 2012; Ross et al., 2014). Lastly, we used the MESA community surveys to measure social cohesion and safety which may provide more valid measurement of true neighborhood characteristics (Diez Roux, 2007; Mujahid et al., 2008). To conclude, our study found that adverse neighborhood environments are associated with generally flatter cortisol curves. Additional research is needed to better understand the impact of neighborhoods on changes in cortisol profiles over time.
Supplementary Material
Acknowledgements
MESA was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The MESA Stress Study was supported by R01HL076831, R01 HL10161-01A1 and R21 DA024273 and the MESA Neighborhoods Study was supported by 2R01 HL071759. AH was also funded by K99-ES-023498 from National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anjum Hajat, Email: anjumh@uw.edu.
Kari Moore, Email: kam642@drexel.edu.
D. Phuong Do, Email: dphoung@uwm.edu.
Sharon Stein Merkin, Email: SMerkin@mednet.ucla.edu.
Naresh M. Punjabi, Email: npunjabi@jhmi.edu.
Brisa Ney Sáñchez, Email: brisa@umich.edu.
Teresa Seeman, Email: TSeeman@mednet.ucla.edu.
Ana V. Diez Roux, Email: avd37@drexel.edu.
References
- Barrington WE, Stafford M, Hamer M, Beresford SA, Koepsell T, Steptoe A. Neighborhood socioeconomic deprivation, perceived neighborhood factors, and cortisol responses to induced stress among healthy adults. Health & Place. 2014;27C:120–126. doi: 10.1016/j.healthplace.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, Greenland P, JacobsJr DR, Kronmal R, Liu K. Multi-Ethnic Study of Atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Brenner AB, Zimmerman MA, Bauermeister JA, Caldwell CH. The physiological expression of living in disadvantaged neighborhoods for youth. J Youth Adolesc. 2013;42:792–806. doi: 10.1007/s10964-012-9838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, Roux AD, Golden SH. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2012;61:986–995. doi: 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychology. 2006;25:704–714. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV. Residential environments and cardiovascular risk. Journal of Urban Health. 2003;80:569–589. doi: 10.1093/jurban/jtg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV. Neighborhoods and health: where are we and were do we go from here? Revue d’epidemiologie et de sante publique. 2007;55:13–21. doi: 10.1016/j.respe.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Mair C. Neighborhoods and health. Annals of the New York Academy of Sciences. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Do DP, Diez Roux AV, Hajat A, Auchincloss AH, Merkin SS, Ranjit N, Shea S, Seeman T. Circadian rhythm of cortisol and neighborhood characteristics in a population-based sample: The Multi-Ethnic Study of Atherosclerosis. Health & Place. 2011;17:625–632. doi: 10.1016/j.healthplace.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin-Keita A, Casazza K, Fernandez JR, Goran MI, Gower B. Do neighbourhoods matter? Neighbourhood disorder and long-term trends in serum cortisol levels. Journal of Epidemiology and Community Health. 2012;66:24–29. doi: 10.1136/jech.2009.092676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria SE, Diez-Roux AV, Link BG. Reliability of self-reported neighborhood characteristics. Journal of Urban Health. 2004;81:682–701. doi: 10.1093/jurban/jth151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Front Hum Neurosci. 2012;6:277. doi: 10.3389/fnhum.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2010;35:932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O’Neill MS, Sheppard L, Kaufman JD. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA) Environmental Health Perspectives. 2013;121:1325–1333. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney JL, Phillips AC, Carroll D. Aging, health behaviors, and the diurnal rhythm and awakening response of salivary cortisol. Experimental Aging Research. 2012;38:295–314. doi: 10.1080/0361073X.2012.672134. [DOI] [PubMed] [Google Scholar]
- Ice GH. Factors influencing cortisol level and slope among community dwelling older adults in Minnesota. Journal of cross-cultural gerontology. 2005;20:91–108. doi: 10.1007/s10823-005-9085-5. [DOI] [PubMed] [Google Scholar]
- Kapuku GL, Treiber FA, Davis HC. Relationships among socioeconomic status, stress induced changes in cortisol, and blood pressure in African American males. Annals of Behavioral Medicine. 2002;24:320–325. doi: 10.1207/S15324796ABM2404_08. [DOI] [PubMed] [Google Scholar]
- Karb RA, Elliott MR, Dowd JB, Morenoff JD. Neighborhood-level stressors, social support, and diurnal patterns of cortisol: the Chicago Community Adult Health Study. Social Science and Medicine. 2012;75:1038–1047. doi: 10.1016/j.socscimed.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime trajectories of cortisol: demographic and socioeconomic differences--findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2585–2597. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moore K, Diez Roux AV, Auchincloss A, Evenson KR, Kaufman J, Mujahid M, Williams K. Home and work neighbourhood environments in relation to body mass index: the Multi-Ethnic Study of Atherosclerosis (MESA) Journal of Epidemiology and Community Health. 2013;67:846–853. doi: 10.1136/jech-2013-202682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. American Journal of Epidemiology. 2007;165:858–867. doi: 10.1093/aje/kwm040. [DOI] [PubMed] [Google Scholar]
- Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan TE, Cooper RS, Ni H, Shea S. Neighborhood characteristics and hypertension. Epidemiology. 2008;19:590–598. doi: 10.1097/EDE.0b013e3181772cb2. [DOI] [PubMed] [Google Scholar]
- Roe JJ, Thompson CW, Aspinall PA, Brewer MJ, Duff EI, Miller D, Mitchell R, Clow A. Green space and stress: evidence from cortisol measures in deprived urban communities. International Journal of Environmental Research and Public Health. 2013;10:4086–4103. doi: 10.3390/ijerph10094086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KM, Murphy ML, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KE, Gary SW, Stuart EA, Glass TA, Marques AH, Duncko R, Merikangas KR. The association between cortisol and neighborhood disadvantage in a U.S. population-based sample of adolescents. Health & Place. 2014;25:68–77. doi: 10.1016/j.healthplace.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sanchez BN, Golden SH, Shrager S, Kirschbaum C, Karlamangla AS, Seeman TE, Diez Roux AV. Stability and predictors of change in salivary cortisol measures over six years: MESA. Psychoneuroendocrinology. 2014;49C:310–320. doi: 10.1016/j.psyneuen.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. Journal of Pharmacokinetics and Biopharmaceutics. 1978;6:79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.