Abstract
Synapses are the primary means for transmitting information from one neuron to the next. They are formed during development of the nervous system, and formation of appropriate synapses is crucial for establishment of neuronal circuits that underlie behavior and cognition. Understanding how synapses form and are maintained will allow us to address developmental disorders such as autism, mental retardation and possibly also psychological disorders. A number of biochemical and proteomic studies have revealed a diverse and vast assortment of molecules present at the synapse. It is now important to untangle this large array of proteins and determine how it assembles into a functioning unit. Here we focus on recent reports describing how synaptic cell adhesion molecules interact with and organize the pre- and postsynaptic specializations of both excitatory and inhibitory central synapses.
Keywords: development, postsynaptic density, neuroligin, neurexin, SynCAM, ephrin
INTRODUCTION
As synapses are specialized sites of contact between neurons that facilitate neurotransmission, their organization must be tightly regulated. Cell adhesion molecules (CAMs) may serve to both facilitate the organization and the adhesion of the synapse. Indeed, similar to other adhesion sites such as adherens junctions and tight junctions, synapses contain some of the same CAMs. For example, integrins and cadherins, both key players at tight junctions, are prevalent at synapses and are integral to their correct structure and function (Benson et al., 2000; Chavis & Westbrook, 2001; Togashi et al., 2002; Arikkath & Reichardt, 2008). They help to recruit and organize key components such as synaptic vesicles at the presynaptic terminal and neurotransmitter receptors (NTRs) in the postsynaptic specialization.
However, many additional CAMs have evolved, adding great diversity to the types of adhesion molecules at the synapse; including the neuroligins (Nlgns), the nectin-like synaptic CAMs (SynCAMs), synaptic adhesion-like molecules (SALMs), netrin-G-ligands (NGLs), leucine-rich repeat transmembrane proteins (LRRTMs), ephrin receptors (Eph) and Sidekicks (Table 1; for reviews see Yamagata et al., 2003; Washbourne et al., 2004a; Gerrow & El-Husseini, 2006; Dalva et al., 2007; Brose, 2009). The Nlgns were identified as trans-synaptic partners of neurexins (Ichtchenko et al., 1995). Prior to this, the neurexins were only known to interact with the black widow spider toxin, α-latrotoxin (Davletov et al., 1995). Subsequent studies demonstrated that adhesion-induced clustering of neurexins in axons leads to synaptic vesicle immobilization (Scheiffele et al., 2000; Dean et al., 2003), while clustering of Nlgns results in recruitment of postsynaptic components (Nam & Chen, 2005; Barrow et al., 2009). Since the neurexin/Nlgn adhesion pair was the first such synaptogenic interaction to be characterized, it served as a template for the subsequent identification of other synaptic cell adhesion molecules (Biederer & Scheiffele, 2007).
Table 1.
This table lists the known postsynaptic CAMs and their presynaptic ligands. The citation list is not exhaustive: citations list one of the more pertinent references supporting this interaction and/or its relevance to synaptic physiology.
| Pre- | Post- | Citations |
|---|---|---|
| Cadherin | Cadherin | (Ranscht, 2000) |
| ephrinB | EphB | (Dalva et al., 2000) |
| ECM? | integrin | (Chavis & Westbrook, 2001) |
| Neurexin | LRRTM | (de Wit et al., 2009; Ko et al., 2009) |
| Neurexin1 | Neuroligin1 | (Scheiffele et al., 2000) |
| Reticulon3 | SALM | (Chang et al., 2010) |
| Sidekick2 | Sidekick2 | (Yamagata et al., 2002) |
| SynCAM2 | SynCAM1 | (Biederer et al., 2002; Fogel et al., 2007) |
| netrin G | NGL2 | (Kim et al., 2006) |
| LAR | NGL3 | (Woo et al., 2009) |
ECM, extracellular matrix; LAR, leukocyte common antigen-related.
The SynCAMs bind heterophilically to other SynCAM family members through their extracellular immunoglobulin (IG) domains (Fogel et al., 2007), although which SynCAM family member is localized to the pre- or postsynaptic side remains vague. Comparison of the synaptogenic activity of SynCAMs with Nlgns revealed that both are able to induce equivalent presynaptic structures, however, only Nlgn1 induces postsynaptic structures as detected by immunolabeling, whereas only SynCAM1 induces increased synaptic activity as measured by electrophysiological techniques (Sara et al., 2005). SALMs were identified as molecules that could interact with the postsynaptic density protein PSD95 and their overexpression results in the formation of additional excitatory synapses (Ko et al., 2006a). NGLs were identified using a very similar approach to SALMs (Kim et al., 2006). Both SALMs and NGLs are heterophilic molecules, binding a variety of presynaptically localized membrane proteins, notably netrins, LAR and reticulon3 (Table 1) (Woo et al., 2009b). An expression screen led to the identification of the LRRTM proteins as inducers of synaptic vesicle recruitment (Linhoff et al., 2009), and recent studies have identified these as being postsynaptic ligands to the neurexins (de Wit et al., 2009; Ko et al., 2009). The binding of ephrins to their receptors, the EphAs and EphBs, had long been known to direct axon pathfinding (Flanagan & Vanderhaeghen, 1998). However, the identification of the recruitment of NMDA receptors to synapses through a direct interaction with EphBs (Dalva et al., 2000) and the demonstration of trans-synaptic induction of presynaptic specializations unveiled the synaptogenic potential of the ephrin/EphB pair (Kayser et al., 2006). Finally, the sidekicks were identified as homophilic adhesion molecules that mediate lamina-specific targeting of synaptic connectivity in the retina (Yamagata et al., 2002).
Thus, over the past decade, the number of synaptic CAMs identified has steadily increased (with more potentially waiting to be uncovered) and they have all been subjected to increased scrutiny by means of biochemical, genetic and cell biological analyses (Brose, 2009). However, the major task ahead is now to elucidate the mechanisms by which these molecules may instruct the formation and maintenance of synapses. In this review, we focus our attention on recent studies which commence to shed light on the molecular interactions of CAMs that shape the developing synapse and determine the molecular organization of the mature synaptic contact. First, we will consider what is known about molecular interactions in the presynaptic terminal and then focus on the postsynaptic specialization.
HOOKING UP WITH SYNAPTIC VESICLES
The majority of synaptic CAMs were identified based on their ability to cluster synaptic vesicles in axons at sites of contact (Scheiffele et al., 2000; Biederer et al., 2002; Linhoff et al., 2009). Such an assay is performed by immunolabeling neurons cocultured with non-neuronal cells, such as HEK293 cells, expressing potential synaptic CAMs (Scheiffele et al., 2000). Quantification of the intensity or area of synaptic markers on the transfected cells versus non-transfected cells gives a measure of how well the CAM can cause immobilization of synaptic vesicles (Biederer & Scheiffele, 2007). However, while the majority of the synaptic CAMs are capable of recruiting synaptic vesicles (Dalva et al., 2007), it remains unclear exactly how this recruitment occurs. A few studies which show interactions of synaptic CAMs with presynaptic components converge on the Veli/CASK/Mint1 complex. This extremely stable tripartite complex is highly enriched in synaptic membrane fractions (Butz et al., 1998). Veli, CASK and Mint1 bind to each other through their N-terminal domains, leaving their PDZ domains free to interact with other proteins, such as Munc18-1, a key regulator of synaptic vesicle exocytosis (Butz et al., 1998). Although Mint1 and Munc18-1 localize to active zones and to a lesser extent synaptic vesicles in the molecular layer of the cerebellum (Okamoto et al., 2000), a clear function for the tripartite complex in presynaptic assembly has yet to be demonstrated. Nevertheless, β-catenin, the principle interacting protein of cadherins (Yap et al., 2007; Arikkath & Reichardt, 2008), can bind to Veli through a PDZ interaction motif and this interaction is important for synaptic localization of the reserve pool of synaptic vesicles (Bamji et al., 2003).
Neurexins, the presynaptic ligands of Nlgns, can bind to CASK also through a PDZ binding motif (Hata et al., 1996). This interaction links the neurexin-Nlgn synaptic adhesion complex to synaptic vesicle exocytosis through the tripartite complex (Butz et al., 1998). More recent studies have gone on to show that Mint1 can be displaced by caskin1, and that the two resulting alternative tripartite complexes (i.e. Veli/CASK/Mint1 and Veli/CASK/caskin1) are equivalent in their relative amounts in brain homogenates (Tabuchi et al., 2002). It remains unclear, though, whether these alternative tripartite complex have different functional significances for organization of the presynaptic terminal. In addition, it is important to note that CASK can also potentially interact with rabphilin3a, a protein important for regulating exocytosis of synaptic vesicles, through the guanylate kinase-like domain of CASK (Zhang et al., 2001). Many more questions remain regarding presynaptic assembly. How does the interaction with the Veli/CASK/Mint1 or Veli/CASK/caskin1 complex attract and stabilize presynaptic components at a nascent synaptic site? This question is particularly relevant now, since the Veli/CASK/Mint1 complex has been implicated in postsynaptic assembly, as well as at the presynaptic terminal. The Veli/CASK/Mint1 complex forms the link between NMDA-type ionotropic glutamate receptors and the motor protein KIF17 (Setou et al., 2000). Furthermore, how is the recruitment and maintenance of synaptic vesicles, their fusion machinery and other active zone components regulated?
One indication of how the active zone cytomatrix may be recruited to synapses comes from a recent study of the presynaptic effects of ephrin-B1 and B2 (McClelland et al., 2009). These transmembrane receptors of the EphBs bind to syntenin-1 through a PDZ domain interaction and cause syntenin-1 to accumulate at synaptic contacts (McClelland et al., 2009). Syntenin-1 has been linked to presynaptic maturation via ERC2/CAST1 (Ko et al., 2006b). ERC2/CAST1 interacts with a number of very large proteins, including Piccolo and Bassoon, which form part of the active zone cytomatrix (Jin & Garner, 2008). This suggests that each presynaptic CAM family may contribute to the recruitment of different integral components of the presynaptic terminal.
THE LINK TO NEUROTRANSMITTER RECEPTORS
In contrast to this relative paucity in the understanding of interactions between CAMs and presynaptic components, many more studies have revealed a wealth of potential mechanisms through which CAMs may organize the postsynaptic specialization. There are several synaptic characteristics for which CAMs may be important determinants, ranging from the size of the synaptic cleft to the cytoskeletal structure of the spines of excitatory synapses. However, the key functional property of the postsynaptic specialization is the presence of NTRs. Thus, a major synaptic CAM function, that has a direct impact on neuronal physiology, is the ability to direct or maintain the aggregation of NTRs or of NTR-associated proteins at the synapse. There are at least three ways in which CAMs could perform this organizational function (Figure 1). They can (a) interact directly with NTRs, (b) interact with scaffolding molecules which in turn bind NTRs or (c) initiate signaling cascades which lead to the recruitment or maintenance of NTRs at synapses. Recent studies demonstrate that synaptic CAMs have co-opted all of these possibilities in organizing the postsynaptic specialization.
Figure 1.
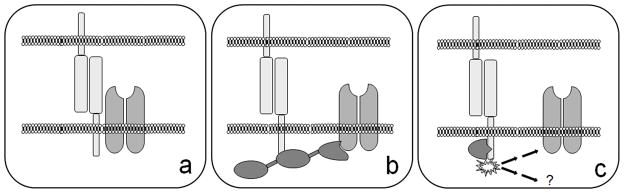
Schematic demonstrating three mechanisms by which a trans-synaptic adhesion complex can stabilize NTRs at synapses: (a) a direct interaction between the CAMs and NTRs, (b) an indirect interaction between the CAM and the NTRs via a single or multiple scaffolding molecules and (c) trans-synaptic adhesion activates a signaling cascade that ultimately results in the recruitment or stabilization of NTRs at synapses.
Direct interaction
To date, the EphB receptors are the only synaptic CAMs for which a direct interaction with NTRs has been demonstrated. These receptor tyrosine kinases (RTK) bind to NMDA-type glutamate receptor subunit 1 (NMDAR1; Dalva et al., 2000). Interestingly, this interaction is entirely dependent on EphB2 binding to its trans-synaptic ligand, ephrinB2 (Figure 1a). The interaction is mediated by the extracellular domain of EphB2 binding to the extracellular N-terminus of NMDAR1 (Dalva et al., 2000). As expected, this direct interaction stabilizes NMDARs at synaptic sites; overexpression of EphB2 in cultured cortical neurons or incubation with aggregates of soluble ephrinB2 results in a higher density of NMDAR1 immunoreactive clusters along neuronal dendrites (Dalva et al., 2000). While binding of ephrinB2 to EphB2 results in activation of the RTK, its enzymatic activity is not necessary for the interaction with the NMDA receptor.
It is entirely possible that the EphBs are not the only synaptic CAMs to bind NTRs through their extracellular domains; however, this possibility has remained underexplored. The SynCAMs possess 3 IG domains and a highly variably spliced domain (Biederer, 2006). The first two IG domains are necessary for the trans-synaptic interaction (Fogel et al., 2007), leaving an orphan IG and variable domain in the extracellular space. It will be interesting to determine whether these regions can potentially interact with other synaptic proteins such as NTRs. Similarly, Nlgns possess a stem region between the acetylcholinesterase domain and the transmembrane domain for which a function has not yet been determined. Studies in cultured neurons suggest that known intracellular protein-protein interactions do not explain all possible activities for the recruitment of NMDA receptors to synapses. Deletion of the PDZ motif of Nlgn1 which can interact with a multitude of scaffolding proteins (see below) does not completely abrogate its ability to induce the clustering of NMDA receptors (Chih et al., 2005) or trafficking with NMDA receptors (Barrow et al., 2009). This may suggest a potential interaction with this receptor through the extracellular domain of Nlgn1. Also, the extracellular domains of other synaptic CAMs, such as the netrin-G-ligands (NGLs) or the leucine-rich repeat transmembrane proteins (LRRTMs), contain multiple protein interaction domains for which ligands have yet to be determined, and which could, therefore, potentially interact with key synaptic proteins such as NTRs.
Scaffolding protein interactions
Interaction via PDZ motifs
An intracellular interaction domain that has long been recognized as a major player in organizing the postsynaptic zone is the PDZ domain (Garner et al., 2000). This domain, which was named for the first three proteins identified to contain the domain (PSD95, discs large and zona occludens-1), is comprised of around 90 amino acids in two α helices and six β sheets (Garner et al., 2000). A great number of intracellular proteins that have been localized specifically to the postsynaptic compartment contain either single or multiple copies (up to 7) of the PDZ domain (Garner et al., 2000). As many of these proteins contain multiple protein-protein interaction domains, they have become collectively termed scaffolding proteins (Sheng & Kim, 2000). Furthermore, a subset of these intracellular scaffolding proteins, which present an inactive guanylate kinase domain, are classed together as membrane associated guanylate kinase proteins (MAGUKs; Funke et al., 2005).
PDZ domains most commonly bind to short motifs located at the C-termini of other proteins. These motifs can be loosely divided into two categories. Type I motifs have a small hydrophobic residue at the very C-terminus (0 position) and either a serine or threonine (or possibly cysteine) at the −2 position, whereas type II motifs have hydrophobic residues at both −2 and 0 positions (Kang et al., 2003). The majority of synaptic CAMs studied to date present PDZ motifs at their C-termini (Table 2). Furthermore, a large number of NTRs present PDZ binding motifs in their intracellular tails. Thus, one can envisage trimer complexes between a CAM and an NTR linked by a multiple PDZ domain containing scaffolding molecule (Figure 1b). However, a number of studies make it clear that interactions are overlapping and plentiful (Table 2; Torres et al., 1998; Garner et al., 2000; Meyer et al., 2004). Consequentially, one has to imagine a complex web of interactions incorporating different CAMs, scaffold proteins and NTRs at a single synapse (Garner et al., 2000).
Table 2.
This table lists the scaffolding proteins that have been characterized to interact with synaptic CAMs via their PDZ binding motifs. PDZ binding motifs can be categorized as either Type I or Type II based on the nature of the −2 and 0 amino acids (see text). The three most C-terminal amino acids (−2, −1 and 0) of the murine protein sequences are reported here.
|
|
||||
|---|---|---|---|---|
| CAM | PDZ motif | Interacting Proteins | Citations | |
|
|
||||
| Type I | LRRTM2 | -CEV | PSD95 | (Linhoff et al., 2009) |
| NGL2 | -TQI | PSD95 | (Kim et al., 2006) | |
| Nlgn1 | -TRV | PSD95, SAP102, Chapsyn110, MAGI1-3, Shank1, Shank3, Pick1, GOPC, SPAR, Semcap3, RGS3 | (Irie et al., 1997; Meyer et al., 2004) | |
| Nlgn2 | -TRV | PSD95, SAP102, Chapsyn110, MAGI1-3, Shank1, Shank3, Pick1, GOPC, SPAR, RGS3 | (Meyer et al., 2004) | |
| SALM2 | -STV | PSD95 PSD95, SAP102, Chapsyn110, MAGI1-3, Shank | (Ko et al., 2006) | |
| Sidekick2 | -SFV | 2, Shank3, Pick1, GOPC, SPAR, Semcap3, RGS3, scribble | (Meyer et al., 2004) | |
| Type II | EphB2 | -VEV | Pick1, GRIP | (Torres et al., 1998) |
| EphB7 | -IQV | Pick1, GRIP, syntenin | (Torres et al., 1998) | |
| ephrinB1 | -YKV | Pick1, GRIP, syntenin | (Torres et al., 1998) | |
| ephrinB2 | -YKV | PSD95, Pick1, SPAR, Semcap3, RGS3, GRIP, syntenin | (Meyer et al., 2004) | |
| SynCAM1 | -YFI | syntenin, GRIP*, CASK | (Biederer et al., 2002; Meyer et al., 2004; Hoy et al., 2009) | |
designates an interaction not validated in cultured mammalian cells.
A few studies have employed a yeast two hybrid (Y2H) approach to uncover potential PDZ interactions of synaptic CAMs (Torres et al., 1998; Meyer et al., 2004). These studies have permitted the comparison of PDZ interactions across multiple CAMs (Nlgn1 and 2, SynCAM1, ephrinB2, Sidekick2; Meyer et al., 2004). Indeed, they have provided a wealth of potential interactions (Table 2), which must now be validated. It is possible that interactions within the nuclei of yeast cells do not recapitulate the interactions seen at the plasma membrane in mammalian cells. For example, while GRIP was identified as an interacting protein of the PDZ motif of SynCAM1 (called IGSF4 in that study) through a Y2H screen (Meyer et al., 2004), this interaction has not been reproduced in a mammalian cell interaction assay (Hoy et al., 2009).
The most extensively studied intracellular interactions belong to the Nlgns (Irie et al., 1997; Meyer et al., 2004). Here, also, the validation of these interactions lags considerably behind the biochemical interaction possibilities, with only the interaction of the third PDZ domain of PSD95 having received significant attention in a neuronal context (Prange et al., 2004; Chih et al., 2005; Nam & Chen, 2005; Heine et al., 2008; Barrow et al., 2009). Clustering of Nlgn1 at the surface of neurons either by overexpression (Chih et al., 2005), by presentation of neurexin from a non-neuronal cell (Nam & Chen, 2005), by providing neurexin-coated beads (Heine et al., 2008) or by incubation with soluble neurexin multimers (Barrow et al., 2009) results in the aggregation of PSD95. This indicates that trans-synaptic binding of the neurexin/Nlgn complex is instructional for the recruitment of the postsynaptic protein PSD95 with a rapid time course (Heine et al., 2008; Barrow et al., 2009). The time delay between clustering of Nlgn1 and the recruitment of PSD95 (on the order of 1 hour) indicate that PSD95 is not pre-bound to the Nlgn1 intracellular tail (Barrow et al., 2009). Interestingly, palmitoylation is necessary for PSD95 aggregation at synaptic sites (El-Husseini et al., 2002) and at Nlgn1 clusters (Barrow et al., 2009), and this slow enzymatic step may account for the delay in recruitment. However, it is important to note that Nlgn1 and PSD95 may exist in pre-existing clusters at non-synaptic dendritic locations (Gerrow et al., 2006). These observations suggest that bringing Nlgn1 into clusters at the neuronal cell surface may trigger a signal that results in the palmitoylation of PSD95, allowing PSD95 to interact with the plasma membrane and increasing the likelihood of interacting with the PDZ motif of Nlgn1 (Washbourne, 2004; Huang & El-Husseini, 2005). The exact order of events is still not clear and resolving this will be highly informative of how recruitment of scaffold proteins to synapses occurs.
Clustering of Nlgn1 results in the accumulation of NMDA-type and AMPA-type glutamate receptors, too (Nam & Chen, 2005; Heine et al., 2008). Given that PSD95 binds the NMDA receptor through its first and second PDZ domains (Kornau et al., 1995), one could imagine a hypothetical trimeric complex consisting of Nlgn1, PSD95 and the NMDA receptor (Figure 1b). However, the NMDA receptor remains associated with Nlgn1 during trafficking and aggregates at the synapse even when the C-terminal PDZ motif of Nlgn1 has been deleted (Chih et al., 2005; Barrow et al., 2009). This redundancy may be explained by an interaction between synaptic scaffolding molecule (S-SCAM, or MAGI2), which can bind Nlgn1 via a WW domain (Iida et al., 2004), and NMDA receptors (Iida et al., 2007). Alternatively, there is the possibility of a yet unidentified interaction between Nlgn1 and NMDARs. Additional experiments monitoring aggregation of PSD95 to neurexin-coated beads coupled with electrophysiological recordings suggest that neurexin-Nlgn1 adhesion results in the recruitment of AMPA-type glutamate receptors (Heine et al., 2008). The specific interactions downstream of Nlgn1 that might be mediating the recruitment of AMPA receptors remain unclear, but may involve either PSD95 (El-Husseini et al., 2000) or an association with transmembrane AMPA receptor regulatory proteins (TARPs) through S-SCAM (Deng et al., 2006).
Induction of PSD95 aggregation by clustering synaptic CAMs appears to be a common theme (Table 2) as NGLs, SALMs and LRRTMs all interact with PSD95 and cause it to form puncta at synapses when the cognate trans-synaptic ligand is applied to neurons in culture (Kim et al., 2006; Ko et al., 2006a; Han & Kim, 2008; Linhoff et al., 2009). It is unclear whether PSD95 is the primary PDZ domain protein that these synaptic CAMs interact with or whether it has been chosen due to its status as postsynaptic marker extraordinaire for the glutamatergic synapse. If PSD95 is the major interacting protein for at least 4 different families of synaptic CAMs, PSD95 would be a linchpin for the formation and maintenance of the postsynaptic density (Han & Kim, 2008). Overexpression of PSD95 drives AMPA receptors to the synapse (El-Husseini et al., 2000; Beique et al., 2006), and ablation of the PSD95 gene in mice results in enhanced NMDA receptor-dependent LTP (Migaud et al., 1998). While these data suggest an important role for PSD95 in regulating synaptic strength, they do not point to PSD95 being the central organizer of glutamatergic synapses. Given the large number of MAGUKs with a very similar domain structure to PSD95 (such as PSD93, SAP97 etc), it is possible that PSD95 is only one of a host of scaffolding proteins that all act together as central organizers of glutamatergic synapses. Future studies will have to examine more closely whether the identity of the PSD95-like MAGUK that is primarily recruited by a particular synaptic CAM gives a synapse a particular ‘flavor’, i.e. an underlying property such as synaptic strength or signaling capabilities.
It is important to consider the consequence of the binding of PDZ domain-containing proteins for CAMs. As mentioned above, it is the binding of the trans-synaptic ligands of Nlgn1, LRRTM1 and 2, SALM2 and NGL1 which drives the formation of PSD95 clusters. However, disruption of the PDZ motif in Nlgn1, SALMs, NGLs and LRRTMs does not affect the synaptic localization of the CAMs themselves (Rosales et al., 2005; Kim et al., 2006; Ko et al., 2006a; Linhoff et al., 2009). In contrast, removal of additional sequences in the C-terminal tails of the Nlgn1 and LRRTM1/2 does disrupt their localization to synapses (Rosales et al., 2005; Linhoff et al., 2009). Thus, interactions via alternative protein motifs, for example with the WW domain of S-SCAM in the case of Nlgn1 (Iida et al., 2004), are important for directing the localization of CAMs to synapses. Also, PDZ interactions with MAGUK proteins other than PSD95, such as SAP102 or SAP97, mediate cotransport of glutamate receptors with Nlgn1 (Sans et al., 2003; Washbourne et al., 2004b; Barrow et al., 2009). Thus, deletion of the PDZ motif in Nlgn1 does not disrupt the localization of Nlgn1 (Rosales et al., 2005), but does significantly change co-transport with glutamate receptors (Barrow et al., 2009).
Interaction via non-PDZ motifs
While PDZ domain interactions are numerous within the postsynaptic specialization (Garner et al., 2000), a number of protein-protein interactions through other domains have recently been uncovered at synaptic CAMs. Importantly, an interaction between Nlgn2 and gephyrin has been characterized at inhibitory synapses (Poulopoulos et al., 2009). Nlgn2 is one of the few synaptic CAMs that localizes almost exclusively to GABAergic synapses (Graf et al., 2004; Varoqueaux et al., 2004; Chih et al., 2005; Levinson et al., 2005). Gephyrin, a key scaffolding molecule of glycinergic and GABAergic synapses (Kneussel & Betz, 2000), has the potential to bind to a 15 amino acid stretch in the intracellular C-tail present in all Nlgns via the E-domain of gephyrin (Poulopoulos et al., 2009). However, it was recently uncovered that a specific interaction between collybistin and Nlgn2 activates the protein collybistin (Poulopoulos et al., 2009). This activation involves collybistin’s src homology domain 3 (SH3), and enables gephyrin to be recruited to the plasma membrane (Harvey et al., 2004). The interaction with collybistin generates the specificity necessary for Nlgn2 to be the only Nlgn with the ability to interact with inhibitory NTRs.
Recently, another interaction at inhibitory synapses was discovered. It had previously been shown that S-SCAM (or MAGI2) has the potential to interact with Nlgn1 (Iida et al., 2004) and with the NMDA receptor (Iida et al., 2007), an interaction that may account for trafficking of NMDA receptors with Nlgn1 in dendrites (Barrow et al., 2009). However, one third of S-SCAM clusters in cultured hippocampal neurons are located at inhibitory synapses (Sumita et al., 2007). This localization is mediated by the binding of S-SCAM to β-dystroglycan, a glycoprotein located specifically at inhibitory synapses (Levi et al., 2002). This interaction is mediated by the three WW domains of S-SCAM. These domains are triple stranded beta sheets which bind to proline-rich motifs (Ilsley et al., 2002) and also mediate the interaction with Nlgn2 (Sumita et al., 2007). Thus, a versatile scaffold molecule links Nlgn1 to glutamate receptors at excitatory synapses and also links Nlgn2 to the dystroglycan complex at inhibitory synapses.
The SynCAMs, which are encoded by the CADM gene family (Biederer, 2006; Pietri et al., 2008), belong to the nectin-like molecules (Takai et al., 2008). These homophilic CAMs mediate cell adhesion events in many non-neuronal tissues (Fujita et al., 2006; Takai et al., 2008), but were also implicated in mediating synaptogenesis (Biederer et al., 2002). While it was clear that SynCAM1 could drive the differentiation of the presynaptic terminal by recruiting synaptic vesicles (Biederer et al., 2002) by an as yet unknown mechanism, it was only recently that a postsynaptic interaction was uncovered. Like other synaptic CAMs, SynCAMs possess a PDZ motif, which can potentially interact with CASK and syntenin (Table 2). However, it is the binding of members of the 4.1 family of proteins (4.1N and 4.1B) via their FERM domains to a juxtamembrane motif of SynCAM1 that drives the recruitment of glutamate receptors (Hoy et al., 2009). 4.1 proteins are known for their functions at cellular adhesion sites where they can recruit the actin cytoskeleton through a spectrin-actin binding domain and bind to transmembrane proteins via the FERM domain (named for its presence in 4.1 proteins, ezrin, radixin and moesin; Hoover & Bryant, 2000). Protein 4.1N interacts directly with AMPA receptor subunit GluR1 through the C-terminal domain (Shen et al., 2000), while 4.1B can specifically recruit NMDA receptors to SynCAM1 adhesions through an unknown interaction (Hoy et al., 2009). FERM binding motifs are present in other synaptic CAMs, notably in the intracellular tail of neurexins, where it was proposed that binding of 4.1 proteins would mediate an interaction with the actin cytoskeleton (Biederer & Sudhof, 2001). The binding of FERM domain-containing proteins to cell adhesion molecules is evolutionarily conserved: coracle and yurt, which are related to the 4.1 proteins, bind to neurexin IV in Drosophila melanogaster and act to establish epithelial polarity (Laprise et al., 2009). Thus, 4.1 protein interactions with synaptic CAMs have the potential to organize the synapse both through direct interactions with NTRs and also by recruiting the cytoskeleton.
Cadherins were thought, for a long time, to only contribute to the postsynaptic specialization in a structural capacity, by virtue of their localization at the periphery of postsynaptic densities (Fannon & Colman, 1996; Elste & Benson, 2006). However, a chain of scaffolding molecule interactions linking the cadherins to AMPA-type glutamate receptors was recently discovered. Cadherins bind δ-catenin, also known as neural plakophilin-related arm protein (NPRAP). This molecule, in turn, interacts with AMPA-receptor binding protein (ABP) and GRIP, multi-PDZ domain proteins that bind AMPA receptor subunits (Silverman et al., 2007). Thus, negatively affecting the link from cadherin to ABP/GRIP reduces the number of GluR2 subunits at synapses.
Signaling Interactions
The synaptic CAMs for which the transduction of cell-cell contact results in an enzymatic signaling cascade is most apparent are the EphBs. They possess an intracellular tyrosine kinase domain, which is activated on binding the trans-synaptic ligands, ephrinBs (Kullander & Klein, 2002). Furthermore, they can activate non-receptor tyrosine kinases and GTPases through guanine nucleotide exchange factors (GEFs; Kullander & Klein, 2002). EphrinB binding by EphB2 results in the phosphorylation of Rac1-GEF Tiam1 enabling spine formation (Tolias et al., 2007). Furthermore, activation of the Rho-GEF kalirin induces the localization of p21 activated kinase (PAK) to synapses, resulting in spine morphogenesis (Penzes et al., 2003). Recent work suggests that it is signaling by EphBs that maintains the mobility of dendritic filopodia and that, upon trans-synaptic contact with ephrinBs, the activity of PAK causes filopodia to then transition to stable spine structures (Kayser et al., 2008). Indeed, researchers were able to rescue both decreased filopodial motility and synaptogenesis in EphB mutant cortical slice cultures by introducing either wildtype EphB2 or mutant EphB2 presenting only the extracellular domain together with constitutively active PAK (Kayser et al., 2008). In addition, EphB2 can recruit focal adhesion kinase (fak) to modulate spine morphogenesis (Moeller et al., 2006). Thus, the EphBs are a prototypical synaptic CAM, in that they are able to drive differentiation of the postsynaptic specialization of glutamatergic synapses through all three molecular mechanisms (Figure 1): (a) direct interaction with NMDA receptors, (b) indirect interaction with AMPA receptors through GRIP (Torres et al., 1998), Table 2) and (c) activation of enzymatic signaling cascades through the GEFs kalirin and Tiam1. Importantly, EphBs also provide a molecular link between the necessity for both filopodial motility and trans-synaptic adhesion during synaptogenesis.
However, EphBs are not the only synaptic CAMs to signal through signaling cascades. Recently, cadherins were also shown to activate PAK through kalirin-7, resulting in spine growth and increased AMPA receptor content (Xie et al., 2008). Kalirin-7 is recruited to cadherins through the scaffolding protein afadin (or AF-6). This scaffolding protein binds to kalirin-7 through its single PDZ domain (Xie et al., 2008). It remains unclear, though, how afadin then binds to cadherins, as it had previously been suggested to bind to cadherins through this same PDZ domain (Mandai et al., 1997). It is possible that afadin forms a dimer to mediate this interaction.
The Nlgns have also been implicated in triggering signaling cascades. Epac2 (exchange factor directly activated by cAMP), a GEF, was found to modulate spine motility and to induce the removal of AMPA receptor subunits from the synapse (Woolfrey et al., 2009). This is triggered by an association with Nlgn3 and the downstream effects are mediated by the stimulation of Rap activity (Woolfrey et al., 2009). Interestingly, the spine shrinkage was dependent on dopamine receptor D1 activity. Thus, Nlgns may now be linked to cAMP-mediated and dopamine receptor-mediated long-term depression (LTD) and provide further insights into the etiology of autism spectrum disorders, as mutations in the Nlgn3 and Epac2 genes are associated with autism (Bacchelli et al., 2003; Jamain et al., 2003).
Additionally, Nlgns associate with the protein tyrosine phosphatase receptor T (PTPRT; Lim et al., 2009). Overexpression of this transmembrane protein phosphatase in cultured hippocampal neurons increased both excitatory and inhibitory synapse formation. This activity was dependent on an interaction between the extracellular domain of PTPRT with the extracellular domain of Nlgns (Lim et al., 2009). Furthermore, the activity of PTPRT was modulated by phosphorylation of the intracellular catalytic domain by the kinase Fyn. It remains unclear what the downstream substrates of the phosphatase activity of PTPRT are, however this study highlights how an extracellular interaction (Figure 1a) brings about a signaling cascade (Figure 1c) that can drive synaptogenesis. Further studies may uncover how this phosphatase may regulate both excitatory and inhibitory synapse formation.
Interestingly, the observation that collybistin interacts with Nlgn2 to specifically recruit gephyrin and therefore GABA A and glycine receptors to inhibitory synapses may hint at an additional signaling cascade through Nlgns, as collybistin is also a GEF protein (also known as ARHGEF9). In fact, it was first hypothesized that the GEF activity was necessary for recruitment of gephyrin and glycine receptors into aggregates (Kins et al., 2000). It is now understood that the interaction between collybistin and gephyrin is direct and does not require the guanine nucleotide exchange activity (Grosskreutz et al., 2001). It will be interesting to test whether the GEF activity is important for some other aspect of the development of inhibitory synapses.
SYNERGY OR REDUNDANCY?
Thus, CAMs are intricately entwined into the complex macromolecular structure that lies on the postsynaptic side of every synapse. They are connected to the NTRs, either directly or indirectly via scaffolding molecules or indirectly via signaling cascades (Figure 1). CAMs are presumably even connected to each other through these various modes of interaction. The question then arises as to whether they work in concert to bring about the formation and the maintenance of the synapse. Cooperativity between CAMs has been observed at adherens junctions between nectins and integrins (Sakisaka et al., 2007) and between nectins and cadherins at axo-dendritic contacts in hippocampal neuron cultures (Togashi et al., 2006). So, does each CAM, be it a Nlgn, an EphB or a SynCAM molecule, contribute a small interaction to building the entire postsynaptic specialization or do they work together? Is it possible that some CAMs synergize? Or are all their contributions small, thus making each individual CAM largely redundant.
Studies from knock-out mice lend weight to the hypothesis that, at least for the formation of synapses, synaptic CAMs are redundant. Removal of EphBs (Kayser et al., 2008), neurexins (Missler et al., 2003), Nlgns (Varoqueaux et al., 2006) or SynCAM1 (Fujita et al., 2006) does not abrogate the formation of synapses. However, the function of synapses in the adult animals is subtly altered. Neurexin knock-out mice present reduced release of synaptic vesicles due to impaired calcium entry (Missler et al., 2003). Reduction of Nlgn1 expression in the amygdala reduces long term potentiation and results in a reduction in associative fear memory (Kim et al., 2008). In knock-out Nlgn2 mice, inhibitory synaptic transmission from fast-spiking interneurons is reduced, while somatostatin-positive interneurons are spared (Gibson et al., 2009). Furthermore, mutations in Nlgn genes (Jamain et al., 2003; Laumonnier et al., 2004) and in the SynCAM gene (CADM1; (Zhiling et al., 2008) in humans may contribute to autism or mental retardation. While these disorders are traumatic, they underscore the fact that synapses do form and are still largely functional. We are still trying to grasp the subtle synaptic changes that might lead to the changes in behavior associated with autism. Thus, these observations would suggest that, for synaptic maintenance, the presence or absence of one specific CAM at a given developmental time point may fine-tune synapses. The key experiments to test the possible synergistic properties of synaptic CAMs remain to be performed. Crossing the mutant alleles for different CAM family members into a single mouse may start to shed light on this. However, interpretation of the resulting mice will be confounded by partially overlapping expression patterns; it will be necessary to concentrate on brain regions where the exact contribution of each CAM family member is well characterized. Perhaps, these experiments may have to first be confined to simpler systems, in which the assembly of an artificial postsynaptic density can be monitored and the individual or combined contributions of individual CAMs evaluated.
Acknowledgments
Research in the Washbourne Laboratory is supported by R01NS065795 from the National Institute of Neurological Disorders and Stroke.
References
- Arikkath J, Reichardt LF. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 2008;31:487–494. doi: 10.1016/j.tins.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchelli E, Blasi F, Biondolillo M, Lamb JA, Bonora E, Barnby G, Parr J, Beyer KS, Klauck SM, Poustka A, Bailey AJ, Monaco AP, Maestrini E. Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the cAMP-GEFII gene. Mol Psychiatry. 2003;8:916–924. doi: 10.1038/sj.mp.4001340. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow SL, Constable JR, Clark E, El-Sabeawy F, McAllister AK, Washbourne P. Neuroligin1: a cell adhesion molecule that recruits PSD-95 and NMDA receptors by distinct mechanisms during synaptogenesis. Neural Dev. 2009;4:17. doi: 10.1186/1749-8104-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Schnapp LM, Shapiro L, Huntley GW. Making memories stick: cell-adhesion molecules in synaptic plasticity. Trends Cell Biol. 2000;10:473–482. doi: 10.1016/s0962-8924(00)01838-9. [DOI] [PubMed] [Google Scholar]
- Biederer T. Bioinformatic characterization of the SynCAM family of immunoglobulin-like domain-containing adhesion molecules. Genomics. 2006;87:139–150. doi: 10.1016/j.ygeno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Biederer T, Scheiffele P. Mixed-culture assays for analyzing neuronal synapse formation. Nat Protoc. 2007;2:670–676. doi: 10.1038/nprot.2007.92. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sudhof TC. CASK and protein 4.1 support F-actin nucleation on neurexins. J Biol Chem. 2001;276:47869–47876. doi: 10.1074/jbc.M105287200. [DOI] [PubMed] [Google Scholar]
- Brose N. Synaptogenic proteins and synaptic organizers: “many hands make light work”. Neuron. 2009;61:650–652. doi: 10.1016/j.neuron.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Butz S, Okamoto M, Sudhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- Chang K, Seabold GK, Wang CY, Wenthold RJ. Reticulon 3 is an interacting partner of the SALM family of adhesion molecules. J Neurosci Res. 2010;88:266–274. doi: 10.1002/jnr.22209. [DOI] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Davletov BA, Krasnoperov V, Hata Y, Petrenko AG, Sudhof TC. High affinity binding of alpha-latrotoxin to recombinant neurexin I alpha. J Biol Chem. 1995;270:23903–23905. doi: 10.1074/jbc.270.41.23903. [DOI] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O’Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, Comoletti D, Taylor P, Ghosh A. LRRTM2 Interacts with Neurexin1 and Regulates Excitatory Synapse Formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F, Price MG, Davis CF, Mori M, Burgess DL. Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain. J Neurosci. 2006;26:7875–7884. doi: 10.1523/JNEUROSCI.1851-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini A, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Elste AM, Benson DL. Structural basis for developmentally regulated changes in cadherin function at synapses. J Comp Neurol. 2006;495:324–335. doi: 10.1002/cne.20876. [DOI] [PubMed] [Google Scholar]
- Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 2007;27:12516–12530. doi: 10.1523/JNEUROSCI.2739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Ozeki S, Tanabe Y, Toyama Y, Maekawa M, Kojima N, Senoo H, Toshimori K, Momoi T. Oligo-astheno-teratozoospermia in mice lacking RA175/TSLC1/SynCAM/IGSF4A, a cell adhesion molecule in the immunoglobulin superfamily. Mol Cell Biol. 2006;26:718–726. doi: 10.1128/MCB.26.2.718-726.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- Gerrow K, El-Husseini A. Cell adhesion molecules at the synapse. Front Biosci. 2006;11:2400–2419. doi: 10.2741/1978. [DOI] [PubMed] [Google Scholar]
- Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Huber KM, Sudhof TC. Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons. J Neurosci. 2009;29:13883–13897. doi: 10.1523/JNEUROSCI.2457-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz Y, Hermann A, Kins S, Fuhrmann JC, Betz H, Kneussel M. Identification of a gephyrin-binding motif in the GDP/GTP exchange factor collybistin. Biol Chem. 2001;382:1455–1462. doi: 10.1515/BC.2001.179. [DOI] [PubMed] [Google Scholar]
- Han K, Kim E. Synaptic adhesion molecules and PSD-95. Prog Neurobiol. 2008;84:263–283. doi: 10.1016/j.pneurobio.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, Lingenfelter SE, Pearce BR, Lundgren J, Owen MJ, Smart TG, Luscher B, Rees MI, Harvey RJ. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24:5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine M, Thoumine O, Mondin M, Tessier B, Giannone G, Choquet D. Activity-independent and subunit-specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc Natl Acad Sci U S A. 2008;105:20947–20952. doi: 10.1073/pnas.0804007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover KB, Bryant PJ. The genetics of the protein 4.1 family: organizers of the membrane and cytoskeleton. Curr Opin Cell Biol. 2000;12:229–234. doi: 10.1016/s0955-0674(99)00080-0. [DOI] [PubMed] [Google Scholar]
- Hoy JL, Constable JR, Vicini S, Fu Z, Washbourne P. SynCAM1 recruits NMDA receptors via protein 4.1B. Mol Cell Neurosci. 2009;42:466–483. doi: 10.1016/j.mcn.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, El-Husseini A. Modulation of neuronal protein trafficking and function by palmitoylation. Curr Opin Neurobiol. 2005;15:527–535. doi: 10.1016/j.conb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Iida J, Hirabayashi S, Sato Y, Hata Y. Synaptic scaffolding molecule is involved in the synaptic clustering of neuroligin. Mol Cell Neurosci. 2004;27:497–508. doi: 10.1016/j.mcn.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Iida J, Ishizaki H, Okamoto-Tanaka M, Kawata A, Sumita K, Ohgake S, Sato Y, Yorifuji H, Nukina N, Ohashi K, Mizuno K, Tsutsumi T, Mizoguchi A, Miyoshi J, Takai Y, Hata Y. Synaptic scaffolding molecule alpha is a scaffold to mediate N-methyl-D-aspartate receptor-dependent RhoA activation in dendrites. Mol Cell Biol. 2007;27:4388–4405. doi: 10.1128/MCB.01901-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilsley JL, Sudol M, Winder SJ. The WW domain: linking cell signalling to the membrane cytoskeleton. Cell Signal. 2002;14:183–189. doi: 10.1016/s0898-6568(01)00236-4. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Garner CC. Molecular mechanisms of presynaptic differentiation. Annu Rev Cell Dev Biol. 2008;24:237–262. doi: 10.1146/annurev.cellbio.23.090506.123417. [DOI] [PubMed] [Google Scholar]
- Kang BS, Cooper DR, Devedjiev Y, Derewenda U, Derewenda ZS. Molecular roots of degenerate specificity in syntenin’s PDZ2 domain: reassessment of the PDZ recognition paradigm. Structure. 2003;11:845–853. doi: 10.1016/s0969-2126(03)00125-4. [DOI] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser MS, Nolt MJ, Dalva MB. EphB receptors couple dendritic filopodia motility to synapse formation. Neuron. 2008;59:56–69. doi: 10.1016/j.neuron.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SY, Lee YK, Park S, Choi JS, Lee CJ, Kim HS, Choi YB, Scheiffele P, Bailey CH, Kandel ER, Kim JH. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc Natl Acad Sci U S A. 2008;105:9087–9092. doi: 10.1073/pnas.0803448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Burette A, Chung HS, Kwon SK, Woo J, Lee HW, Kim K, Kim H, Weinberg RJ, Kim E. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–1301. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- Kins S, Betz H, Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H. Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations. J Physiol. 2000;525(Pt 1):1–9. doi: 10.1111/j.1469-7793.2000.t01-4-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Fuccillo MV, Malenka RC, Sudhof TC. LRRTM2 Functions as a Neurexin Ligand in Promoting Excitatory Synapse Formation. Neuron. 2009;64:791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Kim S, Chung HS, Kim K, Han K, Kim H, Jun H, Kaang BK, Kim E. SALM synaptic cell adhesion-like molecules regulate the differentiation of excitatory synapses. Neuron. 2006a;50:233–245. doi: 10.1016/j.neuron.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Ko J, Yoon C, Piccoli G, Chung HS, Kim K, Lee JR, Lee HW, Kim H, Sala C, Kim E. Organization of the presynaptic active zone by ERC2/CAST1-dependent clustering of the tandem PDZ protein syntenin-1. J Neurosci. 2006b;26:963–970. doi: 10.1523/JNEUROSCI.4475-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U. Yurt, Coracle, Neurexin IV and the Na(+),K(+)-ATPase form a novel group of epithelial polarity proteins. Nature. 2009;459:1141–1145. doi: 10.1038/nature08067. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthelemy C, Moraine C, Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Grady RM, Henry MD, Campbell KP, Sanes JR, Craig AM. Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. J Neurosci. 2002;22:4274–4285. doi: 10.1523/JNEUROSCI.22-11-04274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- Lim SH, Kwon SK, Lee MK, Moon J, Jeong DG, Park E, Kim SJ, Park BC, Lee SC, Ryu SE, Yu DY, Chung BH, Kim E, Myung PK, Lee JR. Synapse formation regulated by protein tyrosine phosphatase receptor T through interaction with cell adhesion molecules and Fyn. EMBO J. 2009;28:3564–3578. doi: 10.1038/emboj.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland AC, Sheffler-Collins SI, Kayser MS, Dalva MB. Ephrin-B1 and ephrin-B2 mediate EphB-dependent presynaptic development via syntenin-1. Proc Natl Acad Sci U S A. 2009;106:20487–20492. doi: 10.1073/pnas.0811862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Varoqueaux F, Neeb A, Oschlies M, Brose N. The complexity of PDZ domain-mediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology. 2004;47:724–733. doi: 10.1016/j.neuropharm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RGM, Morrison JH, O’Dell TJ, Grant SGN. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Moeller ML, Shi Y, Reichardt LF, Ethell IM. EphB receptors regulate dendritic spine morphogenesis through the recruitment/phosphorylation of focal adhesion kinase and RhoA activation. J Biol Chem. 2006;281:1587–1598. doi: 10.1074/jbc.M511756200. [DOI] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Matsuyama T, Sugita M. Ultrastructural localization of mint1 at synapses in mouse hippocampus. Eur J Neurosci. 2000;12:3067–3072. doi: 10.1046/j.1460-9568.2000.00200.x. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Pietri T, Easley-Neal C, Wilson C, Washbourne P. Six cadm/SynCAM genes are expressed in the nervous system of developing zebrafish. Dev Dyn. 2008;237:233–246. doi: 10.1002/dvdy.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B. Cadherins: molecular codes for axon guidance and synapse formation. Int J Dev Neurosci. 2000;18:643–651. doi: 10.1016/s0736-5748(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Rosales CR, Osborne KD, Zuccarino GV, Scheiffele P, Silverman MA. A cytoplasmic motif targets neuroligin-1 exclusively to dendrites of cultured hippocampal neurons. Eur J Neurosci. 2005;22:2381–2386. doi: 10.1111/j.1460-9568.2005.04400.x. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol. 2007;19:593–602. doi: 10.1016/j.ceb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, Vicini S, Wenthold RJ. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol. 2003;5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Sudhof TC, Kavalali ET. Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci. 2005;25:260–270. doi: 10.1523/JNEUROSCI.3165-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor- containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113 ( Pt 11):1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- Silverman JB, Restituito S, Lu W, Lee-Edwards L, Khatri L, Ziff EB. Synaptic anchorage of AMPA receptors by cadherins through neural plakophilin-related arm protein AMPA receptor-binding protein complexes. J Neurosci. 2007;27:8505–8516. doi: 10.1523/JNEUROSCI.1395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumita K, Sato Y, Iida J, Kawata A, Hamano M, Hirabayashi S, Ohno K, Peles E, Hata Y. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J Neurochem. 2007;100:154–166. doi: 10.1111/j.1471-4159.2006.04170.x. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Biederer T, Butz S, Sudhof TC. CASK participates in alternative tripartite complexes in which Mint 1 competes for binding with caskin 1, a novel CASK-binding protein. J Neurosci. 2002;22:4264–4273. doi: 10.1523/JNEUROSCI.22-11-04264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Togashi H, Miyoshi J, Honda T, Sakisaka T, Takai Y, Takeichi M. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J Cell Biol. 2006;174:141–151. doi: 10.1083/jcb.200601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci U S A. 2007;104:7265–7270. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, Bredt DS, Gale NW, Yancopoulos GD. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21:1453–1463. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Washbourne P. Greasing transmission; palmitoylation at the synapse. Neuron. 2004;44:901–902. doi: 10.1016/j.neuron.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Dityatev A, Scheiffele P, Biederer T, Weiner JA, Christopherson KS, El-Husseini A. Cell adhesion molecules in synapse formation. J Neurosci. 2004a;24:9244–9249. doi: 10.1523/JNEUROSCI.3339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004b;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, Sheng M, Kim E. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009a;12:428–437. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Kim E. The NGL family of leucine-rich repeat-containing synaptic adhesion molecules. Mol Cell Neurosci. 2009b;42:1–10. doi: 10.1016/j.mcn.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, Xie Z, Jones KA, Quilliam LA, Prakriya M, Penzes P. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci. 2009;12:1275–1284. doi: 10.1038/nn.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Photowala H, Cahill ME, Srivastava DP, Woolfrey KM, Shum CY, Huganir RL, Penzes P. Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7. J Neurosci. 2008;28:6079–6091. doi: 10.1523/JNEUROSCI.1170-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yap AS, Crampton MS, Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol. 2007;19:508–514. doi: 10.1016/j.ceb.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Luan Z, Liu A, Hu G. The scaffolding protein CASK mediates the interaction between rabphilin3a and beta-neurexins. FEBS Lett. 2001;497:99–102. doi: 10.1016/s0014-5793(01)02450-4. [DOI] [PubMed] [Google Scholar]
- Zhiling Y, Fujita E, Tanabe Y, Yamagata T, Momoi T, Momoi MY. Mutations in the gene encoding CADM1 are associated with autism spectrum disorder. Biochem Biophys Res Commun. 2008;377:926–929. doi: 10.1016/j.bbrc.2008.10.107. [DOI] [PubMed] [Google Scholar]


