Abstract
Background
Reduced left ventricular (LV) ejection fraction increases the risk of ventricular arrhythmias; however, LV ejection fraction has a low sensitivity to predict ventricular arrhythmias. LV dilatation and mass may be useful to further risk-stratify for ventricular arrhythmias.
Methods and Results
Patients from the Genetic Risk of Assessment of Defibrillator Events (GRADE) study (N =930), a study of heart failure subjects with defibrillators, were assessed for appropriate implantable cardioverter-defibrillator shock and death, heart transplant, or ventricular assist device placement by LV diameter and mass. LV mass was divided into normal, mild, moderate, and severe classifications. Severe LV end-diastolic diameter had worse shock-free survival than normal and mild LV end-diastolic diameter (P =0.0002 and 0.0063, respectively; 2-year shock free, severe 74%, moderate 80%, mild 91%, normal 88%; 4-year shock free, severe 62%, moderate 69%, mild 72%, normal 81%) and freedom from death, transplant, or ventricular assist device compared with normal and moderate LV end-diastolic diameter (P<0.0001 and 0.0441, respectively; 2-year survival: severe 78%, moderate 85%, mild 82%, normal 89%; 4-year survival: severe 55%, moderate 64%, mild 63%, normal 74%). Severe LV mass had worse shock-free survival than normal and mild LV mass (P =0.0370 and 0.0280, respectively; 2-year shock free: severe 80%, moderate 81%, mild 91%, normal 87%; 4-year shock free: severe 68%, moderate 73%, mild 76%, normal 76%) but no association with death, transplant, or ventricular assist device (P =0.1319). In a multivariable Cox proportional hazards analysis adjusted for LV ejection fraction, LV end-diastolic diameter was associated with appropriate implantable cardioverter-defibrillator shocks (hazard ratio 1.22, P =0.020). LV end-diastolic diameter was associated with time to death, transplant, or ventricular assist device (hazard ratio 1.29, P =0.0009).
Conclusions
LV dilatation may complement ejection fraction to predict ventricular arrhythmias.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02045043.
Keywords: heart failure, ventricular arrhythmias
Sudden death and ventricular arrhythmias are common in heart failure patients, and implantable cardioverter-defibrillators (ICDs) have been shown to decrease mortality related to sudden death.1,2 Left ventricular (LV) ejection fraction (LVEF) is the predominant measure used to risk-stratify patients.3–5 However, using the single measure of LVEF ignores other variables that may add predictive value, such as LV dimensions. Clearly, identification of risk factors for ventricular arrhythmias is important given that ICD placement is associated with some degree of risk, from both the initial placement and associated long-term risks, such as device malfunction or infection.
LV dilatation reflects adverse ventricular remodeling and may be associated with an increased risk of ventricular arrhythmias. The goal of the current analysis was to determine the ability of LV dilatation to predict the risk of ventricular arrhythmias in a cohort of heart failure patients with severely depressed EF from the Genetic Risk Assessment of Defibrillator Events (GRADE) study. Given that patients with decompensated or more advanced heart failure may have a greater risk of sudden death, LV dilatation may complement LVEF to risk-stratify patients’ risk of ventricular arrhythmias. We sought to use measures easily obtained with echocardiography, namely LV end-diastolic diameter (LVEDD) and LV mass (LV mass), to stratify patients’ risk of ventricular arrhythmias.
Methods
Patients from the GRADE study, a prospective multicenter observational study that recruited 1808 subjects who received a new ICD implant or had an ICD generator change within the past 5 years, were retrospectively analyzed. Local institutional review board approval was obtained at all institutions, and all subjects gave informed consent. The inclusion criteria for GRADE included age ≥18, being able to give informed consent, and an LVEF <30% as assessed with echocardiography or nuclear imaging. Exclusion criteria included patient refusal, life expectancy <6 months, ongoing New York Heart Association (NYHA) class IV heart failure symptoms, a previous heart transplant, or ventricular assist device (VAD) placement. Enrolling centers were the University of Pittsburgh Medical Center, the Pittsburgh VA Healthcare System, Emory University Hospitals, Atlanta VA Medical Center, Massachusetts General Hospital, Mid-Ohio Cardiology Group, and the Ohio State Medical Center. Results from electrocardiograms and clinical imaging studies (echocardiograms, multigated acquisition scans, cardiac magnetic resonance imaging, right and left heart catheterizations, nuclear or echo-based stress tests) were recorded. Blood was drawn at the time of enrollment for DNA extraction to investigate the correlation between genetic polymorphisms and outcomes. Patients were followed yearly for up to 5 years, with the primary end point being freedom from appropriate first ICD shock (a surrogate reflecting risk of sudden cardiac death) and a secondary end point being freedom from a combined outcome of death/heart transplant/VAD placement, a surrogate for death unrelated to arrhythmias in this population with ICDs.
Assessment of LV Dimensions
LV dimensions were obtained from echocardiograms that were available on 930 patients at the time of enrollment or within 6 months before enrollment. Echocardiograms included 2-dimensional, Doppler, and M-mode modalities. The following measurements were independently performed at each center according to the American Society of Echocardiography (ASE) guidelines6 in the parasternal long axis: LVEDD, LV end-systolic diameter (LVESD), interventricular septal thickness, and posterior wall thickness. LVEF was calculated from LV diastolic and systolic dimensions. Patients were classified according to the degree of LV dilatation by using the sex-based LVEDD as specified by the ASE guidelines into not severe dilation (no, mild, or moderate dilation) or severe dilatation as shown in Table 1. LV mass was calculated as follows: LV mass =0.8×{1.04[LVIDd+PWTd+SWTd]3−(LVIDd)3]}+0.6 g where LVIDd was the LV internal diameter at end diastole, PWTd is the posterior wall thickness at end diastole, and SWTd is the septal wall thickness at end diastole.6 Patients were classified into the degree of LV mass according to the ASE guidelines into normal, mild, moderate, and severely abnormal (Table 1).
Table 1.
Degree of Dilatation as Defined by the American Society of Echocardiography Based on Left Ventricular End-Diastolic Dimension
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| LVEDD (cm) | Patients (No.) | LV Mass (g) | Patients (No.) | LVEDD (cm) | Patients (No.) | LV Mass (g) | Patients (No.) | |
| Normal | 4.2 to 5.9 | 197 | 88 to 224 | 109 | 3.9 to 5.3 | 29 | 67 to 162 | 14 |
| Mild | 6.0 to 6.3 | 118 | 225 to 258 | 87 | 5.4 to 5.7 | 32 | 163 to 186 | 19 |
| Moderate | 6.4 to 6.8 | 138 | 259 to 292 | 75 | 5.8 to 6.1 | 38 | 187 to 210 | 16 |
| Severe | ≥6.9 | 184 | ≥293 | 366 | ≥6.2 | 69 | ≥211 | 119 |
LVEDD indicates left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
Determination of Ventricular Arrhythmias
Ventricular arrhythmias were identified at the time of clinical events and by routine device interrogations performed throughout the study period. ICD shocks were adjudicated as either appropriate or inappropriate by an electrophysiologist at the initial center and then reviewed independently by 2 cardiologists at the University of Pittsburgh Medical Center. A third electrophysiologist was employed for dispute resolution. There was no standardization to ICD programming for treatment of ventricular tachycardia or fibrillation and, therefore, the threshold for ICD shocks and antitachycardia pacing (ATP) varied among patients. For the present analysis, only appropriate ICD shocks for ventricular tachycardia (VT) or fibrillation (VF) were recorded and used as an end point, while ATP was not used as an end point for this analysis.
Data Analysis
The primary end point was freedom from appropriate ICD shock, and the secondary end point was freedom from a composite end point of death/transplant/VAD. For this analysis, we examined patients who had transthoracic echocardiograms available where LV dimensions could be obtained and LVEF, LVEDD, and LV mass were measured (930 of the total of 1808 subjects in GRADE).
Statistical analysis was performed by using R statistical package version 3.1.1. Baseline characteristics were compared between patients with and without severe LVEDD, as defined by the ASE sex-based definitions (Table 2), as well as between those with and without echocardiograms (Table 3) by using t and χ2 tests. Cox proportional hazards modeling was used to assess whether LVEF, LVEDD, and LV mass were each independently associated with risk of shock and risk of death/transplant/VAD. We used the following modeling strategy. First, 3 sets of Kaplan–Meier survival curves, 1 for each of the 3 variables, were fitted to assess general patterns in association with the primary and secondary end points and the proportional hazard assumption. Then, 3 multivariable models were fitted, 1 for each of the 3 primary explanatory variables. Other covariates in the multivariable analysis model included: sex, age at enrollment, NYHA class, diabetes mellitus, hypertension, QRS, heart rate, treatment with an angiotenin-converting enzyme inhibitor or angiotensin II receptor blocker and treatment with a β-blocker. LV mass had a nonlinear association with shock and was modeled categorically as ASE sex-specific categories. LVEF and LVEDD were modeled continuously, with LVEDD quantified by centimeter. To assess whether each of the measures independently predicted shock, a final model was developed for each outcome considering LVEF, LVEDD, LV mass, and other confounders. To account for other variables that could affect outcomes, this process was repeated for 3 additional sets of models: the first adding device type and its interaction with LVEDD; a second adding sodium, blood urea nitrogen, creatinine, and ischemic cardiomyopathy; and the third using only shocks due to VT >180 bpm or VF.7 The first 2 were fit to both time to first shock and to death/transplant/VAD. An assessment of changes in magnitude of parameter estimates, in combination with Akaike Information Criteria, were used as criteria. Hazard ratios with 95% CIs and P values are reported from the multivariable analysis. A P value <0.05 is considered significant.
Table 2.
Baseline Demographics for the Entire Group, No Severe LV Dilatation and Severe LV Dilatation
| All Patients (n =930) | Not Severely Dilated (n =647) | Severely Dilated (n =243) | P Value, Comparing Severe/No Severe LV Dilatation | |
|---|---|---|---|---|
| Age, y | 62.3±11.7 | 63.7±11.6 | 59.4±11.5 | <0.0001 |
| Race, % black | 129 (14.2) | 75 (12.0) | 54 (18.8) | 0.0085 |
| Sex, % male | 740 (79.6) | 528 (82.9) | 212 (72.4) | 0.0003 |
| Tobacco use, % | 515 (55.6) | 355 (55.9) | 160 (54.8) | 0.8064 |
| NYHA III to IV, % | 274 (29.9) | 173 (27.7) | 101 (34.8) | 0.0146 |
| Ischemic cardiomyopathy, % | 661 (71.1) | 479 (75.2) | 182 (62.1) | <0.0001 |
| LVEF, %±SD | 20.5±6.0 | 21.8±5.6 | 17.8±5.8 | <0.0001 |
| Diabetes, % | 318 (34.3) | 229 (36.1) | 89 (30.5) | 0.1085 |
| Primary prevention, % | 670 (74.6) | 457 (74.7) | 213 (74.5) | 1.00 |
| β-Blocker, % | 791 (85.4) | 534 (84.2) | 257 (88.0) | 0.1565 |
| ACEI/ARB, % | 746 (80.3) | 511 (80.2) | 235 (80.5) | 0.9972 |
| Hypertension | 575 (62.0) | 416 (65.4) | 159 (54.5) | 0.0018 |
| Dual chamber device, % | 251 (27.2) | 195 (30.9) | 56 (19.2) | 0.0003 |
| Biventricular device, % | 429 (46.5) | 260 (41.2) | 169 (57.9) | <0.0001 |
| De novo implant, % | 546 (59.0) | 382 (60.0) | 167 (57.0) | 0.4328 |
LV indicates left ventricular; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Table 3.
Baseline Demographics Comparing Patients Included in the Analysis With Those Excluded Due to Missing Outcome Values
| All Patients (n =1808) | Excluded (n =878) | Included (n =930) | P Value, Comparing Excluded/Included | |
|---|---|---|---|---|
| Age, y | 62.5±12.2 | 62.7±12.7 | 62.3±11.7 | 0.5361 |
| Race, % black | 327 (18.4) | 192 (22.9) | 129 (14.2) | <0.0001 |
| Gender, % male | 1438 (79.5) | 698 (79.5) | 740 (79.6) | 1.0000 |
| Tobacco use, % | 883 (52.2) | 368 (48.2) | 515 (55.6) | 0.0029 |
| NYHA III to IV, % | 485 (29.2) | 211 (28.2) | 274 (29.9 | 0.3351 |
| Ischemic cardiomyopathy, % | 1271 (70.4) | 610 (69.6) | 661 (71.1) | 0.5362 |
| LVEF, %±SD | 20.8±6.1 | 21.1±6.1 | 20.5±6.0 | 0.0331 |
| Diabetes, % | 601 (35.6) | 283 (37.1) | 318 (34.3) | 0.2613 |
| Primary prevention, % | 1244 (75.6) | 574 (76.8) | 670 (74.6) | 0.3215 |
| β-Blocker, % | 1529 (85.4) | 738 (85.3) | 791 (85.4) | 1.0000 |
| ACEI/ARB, % | 1350 (79.6) | 604 (78.7) | 746 (80.3) | 0.4658 |
| Hypertension | 1080 (63.8) | 505 (66.0) | 575 (62.0) | 0.0938 |
| Dual chamber device, % | 511 (28.4) | 260 (29.7) | 251 (27.2) | 0.2642 |
| Biventricular device, % | 801 (44.5) | 372 (42.5) | 429 (46.5) | 0.2241 |
| De novo implant, % | 1049 (58.0) | 500 (56.9) | 549 (59.0) | 0.3953 |
NYHA indicates New York Heart Association; LVEF, left ventricular ejection fraction; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Results
Demographics
A total of 930 of the 1808 subjects from GRADE had complete echocardiograms available for analysis. The baseline demographic and electrocardiographic characteristics are listed in Tables2 and 4, respectively. Follow-up for the cohort averaged 38.1±23.3 months for appropriate ICD shocks. During the follow-up period, 208 (22.4%) patients received at least 1 ICD shock. The majority of patients were male (79.6%, n =740), were of white race (white 83.98%, black 13.44%, other 2.6%), and had a primary prevention indication for ICD placement (74.61%). The QRS was widened (137.7±36.6 ms) and QTc duration averaged in the normal range (428.8±53.9 ms). The patients who did not have echocardiograms and thus were excluded were compared with the included patients to demonstrate that the entire GRADE was uniform. In the included sample, there are fewer African Americans, greater tobacco use, and slightly lower LVEF, but the patients are otherwise comparable to the excluded patients, as shown in Table 3.
Table 4.
Baseline Electrocardiogram Characteristics for the Entire Group, No Severe LV Dilatation and Severe LV Dilatation
| All Patients (n =930) | Not Severely Dilated (n =669) | Severely Dilated (n =270) | P Value, Comparing Severe/No Severe LV Dilation | |
|---|---|---|---|---|
| Heart rate, bpm | 75.3±15.6 | 75.5±16.0 | 74.7±14.8 | 0.4770 |
| PR, ms | 170.9±44.3 | 172.7±46.2 | 167.2±39.8 | 0.1210 |
| QRS, ms | 137.7±36.6 | 133.4±34.7 | 146.9±38.7 | <0.0001 |
| QTc, ms | 428.8±53.9 | 425.9±51.9 | 435.3±57.7 | 0.0219 |
| LBBB, % | 110 (12.8%) | 65 (11.1%) | 45 (16.6%) | 0.0313 |
| RBBB, % | 64 (7.4%) | 46 (7.8%) | 18 (6.6%) | 0.6410 |
LV indicates left ventricular; LBBB, left bundle branch block; RBBB, right bundle branch block.
Effect of LVEF and NYHA Class on Arrhythmias and Survival
Given that LVEF and NYHA class are known risk factors for increased mortality and sudden death in heart failure patients with reduced LVEF, we first sought to establish that these same risk factors were associated with sudden death in our cohort. Lower LVEF, when stratified by tertiles (≤18%, 18.1% to 25%, >25%), was associated with ICD shocks with the LVEF ≤18% category associated with worse survival free of ICD shocks compared with the other categories (Figure1A, P =0.0002). Cox proportional hazards modeling confirmed that decreased LVEF is associated with an increased hazard for shock (HR 1.05, 95% CI 1.02 to 1.08, P =0.0002) when LVEF was modeled as a continuous variable. These same LVEF categories demonstrated a stepwise decrease in survival free of death/transplant/VAD (P<0.0001, Figure1B). NYHA class did not show an association with survival free of ICD shock (Figure2A, P =0.21). Higher NYHA class was associated with a stepwise worsening in death/transplant/VAD-free survival (P<0.0001, Figure2B). Therefore, in this cohort, LVEF, but not NYHA class, was associated with both survival free of death/transplant/VAD and survival free of appropriate ICD shock.
Figure 1.
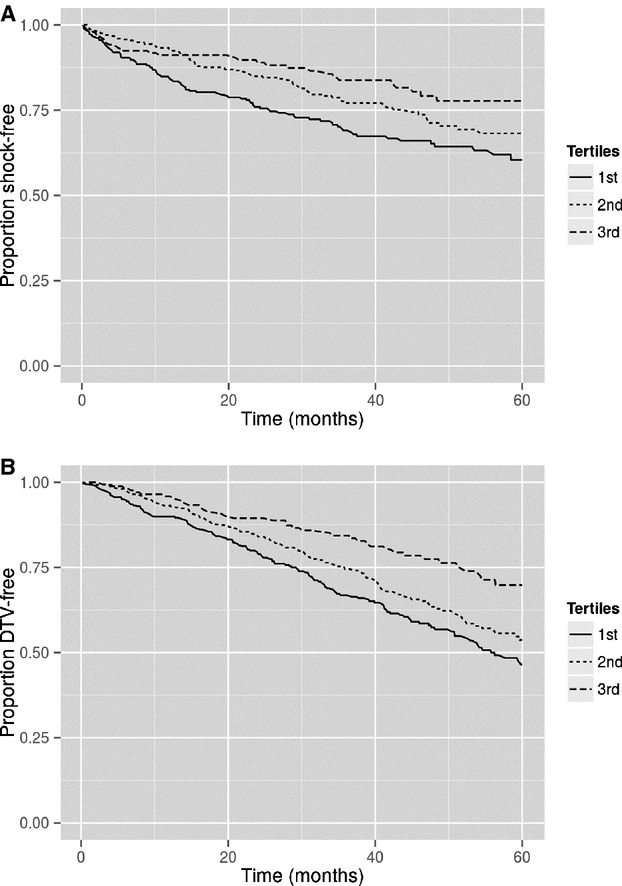
A, Survival free of ICD shock stratified by LVEF tertile as follows: 1, <18%, 2, 18% to 25%; 3, >25%. Lower LVEF was associated with worse shock free survival (P =0.0002). B, Survival free of death/transplant/VAD stratified by LVEF category (1, <18%; 2, 18% to 25%; 3, >25%). Lower LVEF was associated with worse survival free of death/transplant/VAD (P<0.0001). ICD indicates implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; VAD, ventricular assist device.
Figure 2.
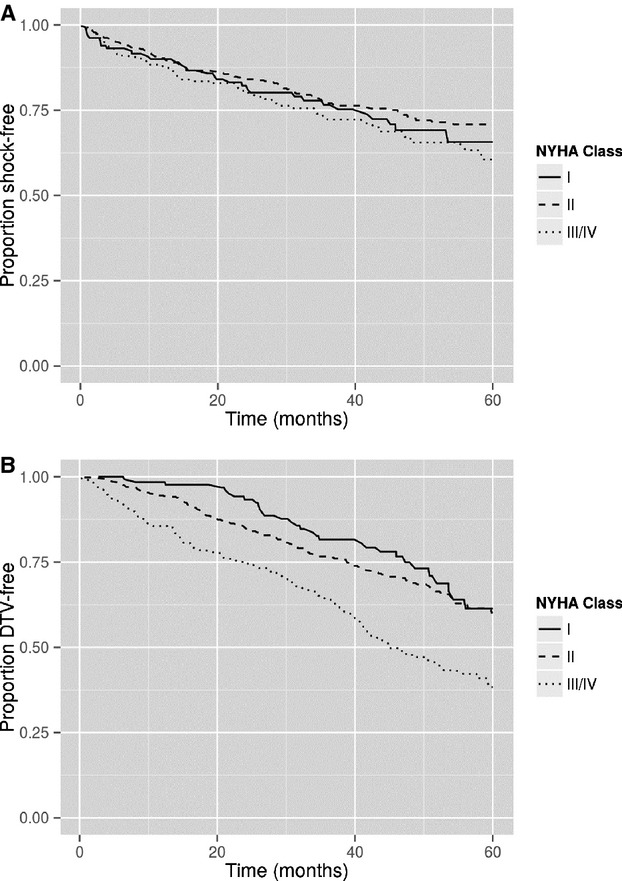
A, Survival free of ICD shock stratified by NYHA class. Increasing NYHA class was not associated with worse shock-free survival (P =0.2123). B, Survival free of death/transplant/VAD stratified by NYHA class. Increasing NYHA class was associated with worse survival free of death/transplant/VAD (P<0.0001). ICD indicates implantable cardioverter-defibrillator; NYHA, New York Heart Association; VAD, ventricular assist device.
Effect of LV Dilatation on Arrhythmias and Survival
Subjects who received an ICD shock or reached the combined end point of death/transplant/VAD placement had increased LVESD and LVEDD values than those who did not (ICD shock: LVESD 5.7 versus 5.3 cm, P<0.0001; LVEDD 6.6 versus 6.3 cm, P<0.0001; death/transplant/VAD: LVESD 5.7 versus 5.3 cm, P<0.0001; LVEDD 6.6 versus 6.2 cm, P<0.0001). Subjects with severe LV dilatation were younger (59.4 versus 63.7 years, P<0.0001), had wider QRS durations (146.9 versus 133.4 ms, P<0.0001), and were less likely to have ischemic cardiomyopathy (62.1% versus 75.2%, P<0.0001) compared with subjects without severe dilatation (Tables2 and 4). Furthermore, subjects with severe LV dilatation had decreased LVEF compared with those without severe dilatation (17.8% versus 21.8%, P<0.0001).
Shock-free survival was associated with LV dilatation (Figure3A, P =0.0002). Shock-free survival in the cohort with severe LV dilatation was worse compared with mild LV dilation (P =0.0063) and normal LV dimensions (P =0.0002); however, those with severe or moderate LV dilation did not differ (P =0.16) (2-year shock-free survival for severe LV dilation 74%, moderate 80%, mild 91%, and normal 88%; 4-year shock-free survival, severe LV dilation 62%, moderate 69%, mild 72%, and normal 81%). This was confirmed using Cox proportional hazards modeling (P =0.0004). For every 1-cm increase in the degree of LV dilation, there was an associated 33% increase in the hazard for the risk of ICD shock (95% CI 14% to 56%) as shown in Table 5. In addition, LV dilatation was associated with death/transplant/VAD (Figure3B, P =0.0002). Subjects with severe LV dilation had a lower freedom from death/transplant/VAD compared with those with normal and moderate LV dimensions (P<0.0001 and 0.0441), but survival did not differ from severe to mild (P =0.1246) (2-year survival: severe LV dilatation 78%, moderate 85%, mild 82%, normal 89%; 4-year survival: severe LV dilatation 55%, moderate 64%, mild 63%, normal 74%). These patterns were confirmed with Cox proportional hazards modeling (P<0.0001, HR 1.40, 95% CI 1.22 to 1.61).
Figure 3.
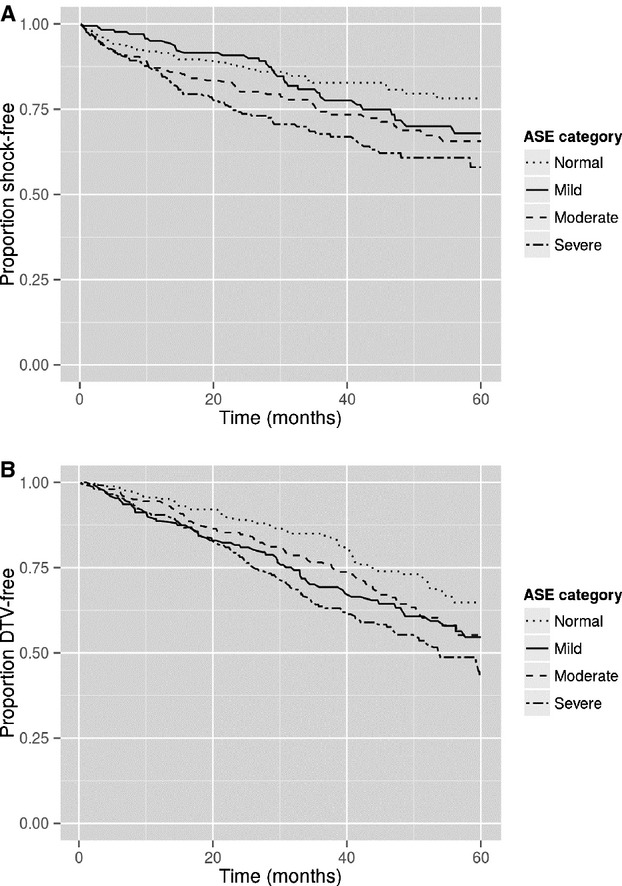
A, Survival free of ICD shock stratified by the LVEDD ASE classifications. Increasing LVEDD classification was associated with worse shock-free survival (P =0.0002). B, Survival free of death/transplant/VAD stratified by the LVEDD ASE classifications. Increasing LVEDD classification was associated with worse survival free of death/transplant/VAD (P =0.0002). ASE indicates American Society of Echocardiography; ICD, implantable cardioverter-defibrillator; LVEDD, left ventricular end-diastolic diameter; VAD, ventricular assist device.
Table 5.
Multivariate HRs to Predict ICD Shock
| LVEF Only HR (95% CI) | LVEDD Only HR (95% CI) | LV Mass Only HR (95% CI) | LVEF and LVEDD HR (95% CI) | |
|---|---|---|---|---|
| LVEF | 0.95 (0.93 to 0.98) | 0.96 (0.94 to 0.99) | ||
| LVEDD | 1.33 (1.14 to 1.56) | 1.22 (1.03 to 1.45) | ||
| LV mass severe vs normal | 1.57 (1.01 to 2.44) |
All models were also adjusted for sex, age, New York Heart Association class, diabetes, hypertension, QRS duration, heart rate, angiotensin-converting enzyme inhibitor use, β-blocker use. HR indicates hazard ratio; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter.
Effect of LV Mass on Arrhythmias and Survival
There were 159 patients with normal LV mass, 116 with mild increase, 115 patients with moderate increase, and 540 with severe increase in LV mass. Subjects who received an ICD shock and who reached the end point of death/transplant/VAD had greater LV mass (ICD shock: LV mass 334.3 versus 294.8, P<0.0001; death/transplant/VAD: LV mass 328.8 versus 291.7, P<0.0001). LV mass was associated with risk of shock (Figure4A, P =0.0316), with severe LV mass differing from both normal and mild (P =0.0370 and 0.0280, respectively) (2-year shock survival: severe LV mass 80%, moderate 81%, mild 91%, normal 87%; 4-year survival: severe LV mass 68%, moderate 73%, mild 76%, normal 76%). No significant difference was found in the Cox proportional hazards model after adjustment for confounders (P =0.12). Similarly, LV mass was not associated with death/transplant/VAD (Figure4B, P =0.1319) (2-year survival: severe LV mass 82%, moderate 80%, mild 90%, normal 86%; 4-year survival: severe LV mass 61%, moderate 70%, mild 67%, normal 68%).
Figure 4.
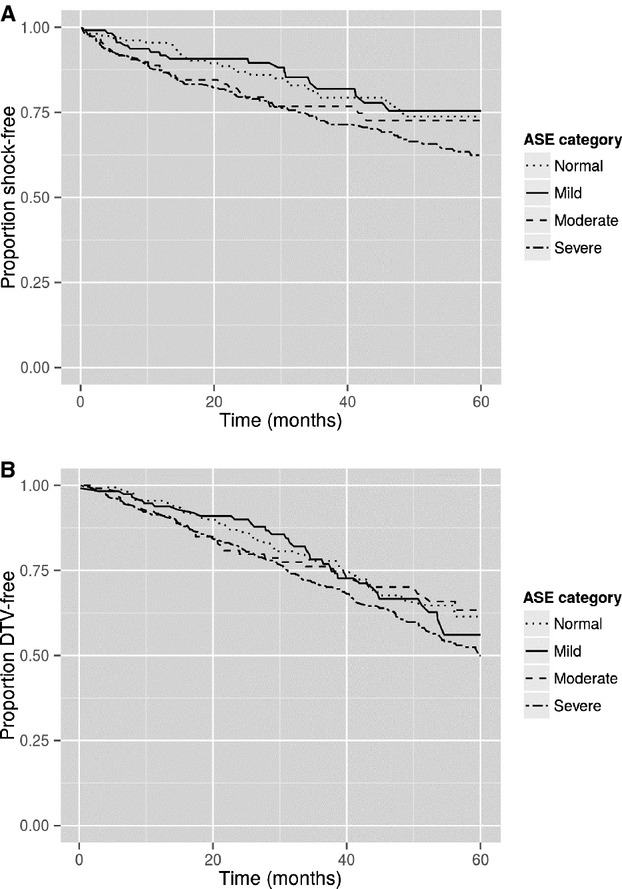
A, Survival free of ICD shock stratified by LV mass ASE classifications. Increasing LV mass classification was associated with worse shock-free survival (P =0.0316). B, Survival free of death/transplant/VAD stratified by LV mass ASE classifications. Increasing LV mass classification was not associated with worse survival free of death/transplant/VAD (P =0.1319). ASE indicates American Society of Echocardiography; ICD, implantable cardioverter-defibrillator; LV, left ventricular; VAD, ventricular assist device.
Multivariable Analysis
LVEDD was significantly associated with risk of shock after adjustment for LVEF (P =0.02, Table 5). The size of the effect of LVEDD when measured as a continuous variable was attenuated from an HR of 1.33 to 1.22 when accounting for LVEF. Therefore, there was an increased risk of ICD shock of 22% for every 1-cm increase in the LVEDD. The addition of LV mass resulted in an increased Akaike Information Criteria (2328 versus 2323) and was not associated with risk of shock (P =0.71); therefore, LV mass was excluded from the final model. Similarly, LVEDD was significantly associated with risk of death/transplant/VAD after adjustment for LVEF (P =0.00092, Table 6). The size of the effect was attenuated from 1.40 to 1.29. The addition of LV mass again resulted in an increased Akaike Information Criteria (3286 versus 3282) and was not associated with risk of death/transplant/VAD (P =0.56); it was, therefore, again excluded from the final model. Thus, LVEDD was associated with both shock risk and death/transplant/VAD risk above LVEF. LV mass does not provide additional information for either outcome.
Table 6.
Multivariate HRs to Predict Death/Transplant/VAD
| LVEF Only HR (95% CI) | LVEDD Only HR (95% CI) | LV Mass Only HR (95% CI) | LVEF and LVEDD HR (95% CI) | |
|---|---|---|---|---|
| LVEF | 0.95 (0.93 to 0.97) | 0.97 (0.95 to 0.99) | ||
| LVEDD | 1.40 (1.22 to 1.61) | 1.29 (1.11 to 1.50) | ||
| LV mass severe vs normal | 1.18 (0.83 to 1.67) |
All models were also adjusted for sex, age, New York Heart Association class, diabetes, hypertension, QRS duration, heart rate, angiotensin-converting enzyme inhibitor use, β-blocker use. HR indicates hazard ratio; VAD, ventricular assist device; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter.
Secondary Analyses
Additional analyses were performed given that patients were recruited from several different centers and that there may have been other variables that could have affected outcomes. When we analyzed the type of ICD (single chamber versus dual chamber versus biventricular pacemaker/ICDs), the device type was not significantly associated with either the risk of either ICD shock or the risk of death/transplant/VAD, as shown in Tables7 and 8. Additional adjustment for sodium, BUN, creatinine, and ischemic cardiomyopathy did not alter the primary findings (Tables7 and 8). We sought to investigate whether LVEF and LVEDD were also associated with ventricular arrhythmias >180 bpm given that there was no uniform ICD programming among all patients. Analysis considering only VT >180 bpm or VF also did not alter the primary findings (Tables7 and 8).
Table 7.
Multivariate HRs to Predict ICD Shock
| LVEF Only HR (95% CI) | LVEDD Only HR (95% CI) | LV Mass Only HR (95% CI) | LVEF and LVEDD HR (95% CI) | ||
|---|---|---|---|---|---|
| Original (base) models n =839 Event count =187 | LVEF | 0.95 (0.93 to 0.98) P =0.0002 | 0.96 (0.94 to 0.99) P =0.0098 | ||
| LVEDD | 1.33 (1.14 to 1.56) P =0.0004 | 1.22 (1.03 to 1.45) P =0.0196 | |||
| LV mass Severe vs normal | 1.57 (1.01 to 2.44) P =0.0469 | ||||
| Additional adjustment for sodium, blood urea nitrogen, creatinine, and ischemic cardiomyopathy n =543 Event count =132 | LVEF | 0.94 (0.91 to 0.97) P<0.0001 | 0.95 (0.92 to 0.98) P =0.0035 | ||
| LVEDD | 1.44 (1.20 to 1.74) P =0.0001 | 1.30 (1.07 to 1.58) P =0.0074 | |||
| LV mass severe vs normal | 2.00 (1.12 to 3.56) P =0.0190 | ||||
| Base models plus device type n =833 Event count =186 | LVEF | 0.95 (0.93 to 0.98) P =0.0001 | 0.96 (0.94 to 0.99) P =0.0074 | ||
| LVEDD | 1.34 (1.15 to 1.57) P =0.0003 | 1.23 (1.04 to 1.46) P =0.0175 | |||
| LV mass severe vs normal | 1.59 (1.02 to 2.48) P =0.04147 | ||||
| Fast shock on base covariates n =839 Event count =112 | LVEF | 0.95 (0.92 to 0.98) P =0.0018 | 0.96 (0.93 to 1.00) P =0.0395 | ||
| LVEDD | 1.42 (1.15 to 1.75) P =0.0009 | 1.30 (1.04 to 1.63) P =0.0204 | |||
| LV mass severe vs normal | 2.03 (1.10 to 3.76) P =0.0245 |
All models were also adjusted for sex, age, New York Heart Association class, diabetes, hypertension, QRS duration, heart rate, angiotensin-converting enzyme inhibitor use, β-blocker use. HR indicates hazard ratio; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter.
Table 8.
Multivariate HRs to Predict Death/Transplant/VAD
| LVEF Only HR (95% CI) | LVEDD Only HR (95% CI) | LV Mass Only HR (95% CI) | LVEF and LVEDD HR (95% CI) | ||
|---|---|---|---|---|---|
| Original (base) models n =834 Event count =271 | LVEF | 0.95 (0.93 to 0.97) P<0.0001 | 0.97 (0.95 to 0.99) P =0.0041 | ||
| LVEDD | 1.40 (1.22 to 1.61) P<0.0001 | 1.29 (1.11 to 1.50) P =0.0009 | |||
| LV mass severe vs normal | 1.18 (0.83 to 1.67) P =0.3603 | ||||
| Additional adjustment for sodium, blood urea nitrogen, creatinine, and ischemic cardionyopathy, n =542 Event count =186 | LVEF | 0.96 (0.93 to 0.98) P =0.0006 | 0.97 (0.94 to 0.99) P =0.0147 | ||
| LVEDD | 1.33 (1.12 to 1.56) P =0.0009 | 1.22 (1.03 to 1.46) P =0.0235 | |||
| LV mass severe vs normal | 1.22 (0.80 to 1.88) P =0.3526 | ||||
| Base models plus device type n =828 Event count =268 | LVEF | 0.95 (0.93 to 0.97) P<0.0001 | 0.97 (0.95 to 0.99) P =0.0049 | ||
| LVEDD | 1.39 (1.21 to 1.60) P<0.0001 | 1.28 (1.10 to 1.49) P =0.0011 | |||
| LV mass severe vs normal | 1.20 (0.84 to 1.72) P =0.3136 |
All models were also adjusted for sex, age, New York Heart Association class, diabetes, hypertension, QRS duration, heart rate, angiotensin-converting enzyme inhibitor use, β-blocker use. HR indicates hazard ratio; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; VAD, ventricular assist device.
Discussion
Our results suggest that LV dilatation was associated with ICD shocks and freedom from death/transplant/VAD placement in heart failure patients with reduced EF. Only LV dilatation, and not LV mass, was associated with increased ICD shocks and the end point of death/transplant/VAD placement by time to event analysis. In a multivariable model that included LVEF and LV mass, LVEDD was associated with a significant increased risk of an ICD shock. The strong significance of LVEDD in the presence of LVEF suggests that in a population where all of the patients had an LVEF ≤30%, LV dilatation adds further information for both ICD shock and death. In GRADE, appropriate ICD shock was used as a surrogate for risk of sudden cardiac death. Thus, in this population with severe systolic dysfunction, LV dilatation may aid in the risk stratification for mortality due to ventricular arrhythmias.
Current Echocardiographic Parameters Used to Predict Outcomes
LVEF is the best studied structural parameter used to risk-stratify heart failure patients for sudden death. Multiple trials have shown that defibrillator placement in patients with decreased LV systolic function decreases the risk of sudden death.1–3 However, the majority of patients in these trials (eg, Multicenter Automatic Defibrillator Implantation Trial (MADIT-2) and Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT)) did not receive an ICD shock over the follow up period. Furthermore, other trials such as the Coronary Artery Bypass Graft (CABG) Patch, Defibrillator in Acute Myocardial Infarction and Immediate Risk Stratification Improves Survival Trials did not show any survival benefit to defibrillator placement, perhaps because the subjects in these trials had defibrillators placed soon after revascularization and there may have been additional competing risks of mortality.8–10 Additional risk stratification may better identify populations who would benefit from ICD placement.
LV Dilatation
LV dilatation is often the result of multiple pathological changes that occur during the adverse myocardial remodeling process in progressive heart failure. Acute ventricular dilatation in an animal model has been shown to decrease the ventricular effective refractory period and increase the dispersion of refractoriness, thus increasing the risk of reentrant ventricular arrhythmias.11,12 During chronic LV dilatation and failure, ion channel changes lead to action potential prolongation caused by changes in K+, Ca2+, and Na+ currents as well as alterations in the distribution and expression of connexin40 and connexin43.13 A recent report from the OREGON-Sudden Unexpected Death Study (SUDS) study by Narayan et al14 showed that severe LV dilation is associated with an increased risk of sudden cardiac death in subjects in the community with an EF <35%. In the current cohort of heart failure patients, LV dilatation was associated with defibrillator shocks even after adjustment for LVEF. LV dilatation may be an additional risk factor to consider in heart failure patients with reduced LVEF when risk-stratifying patients for sudden death. Furthermore, LV dilatation can be easily assessed on a transthoracic echocardiogram at the time of EF measurement.
We do acknowledge that an HR of 1.22 for LVEDD to predict increased risk of ICD shock may be difficult to use this parameter in clinical settings; however, it should be kept in mind that LVEDD remained a significant predictor in all the multivariable analyses. In addition, serial increases in LVEDD were associated with an increased risk of ICD shocks and freedom from death/transplant/VAD. While LVEDD was associated with both outcomes, we would suggest that further study of LV dilation is worthwhile to understand its ability to predict outcomes in heart failure.
LV Mass
Increased LV mass has been associated with adverse outcomes in diverse patient groups, such as those with hypertrophic cardiomyopathy and hypertensive heart disease, and is shown to increase the risk of sudden death.15–17 In several animal models, heart failure and LV hypertrophy has been associated with decreased K currents (Ito, Iks), altered connexin43 expression, and an increased QT interval.13,18–20 However, increased LV mass is not uniformly related to increased ventricular arrhythmias and sudden death.21,22 The current data from GRADE suggest that an increase in LV mass was not associated with increased ICD shocks.
Limitations
These data have several limitations. The echocardiographic parameters were determined at the time of enrollment to GRADE after the patient had already met accepted qualifications for ICD placement that included an initial waiting period post myocardial infarction and/or intervention. Therefore, these results do not reflect the degree of LV dilation at the initial diagnosis of heart failure. We also acknowledge that the degree of LV dilatation changes with changes in hemodynamics and progression of heart failure and the LVEDD measurement was taken at the initial enrollment into GRADE, usually prior to ICD placement. Second, patients recruited into the GRADE study all had an LVEF ≤30%, and therefore these results cannot be extrapolated to patients with lesser degrees of LV systolic dysfunction. In addition, this is a post-hoc analysis and LV dimensions were not collected for all patients as a significant number of patients were enrolled into GRADE based on nuclear imaging and did not have echocardiograms. We have compared patients who had an echocardiogram with those without an echocardiogram to show that the entire population was uniform despite the fact that not all patients had echocardiograms. There was nonuniform programming of ICD therapies and therefore some ICD therapies may have been avoided with current ICD programming.23 However, these programming modifications were less commonly used during the enrollment and follow-up periods of GRADE. We have addressed this concern by analyzing the lowest zone for which patients had ventricular arrhythmia therapy turned on (Table 9), and it is shown in Table 7 that LVEDD did still predict ventricular arrhythmias >180 bpm. Unfortunately, we do not have information on the time to detection for this analysis. While appropriate ICD therapies are a valid estimate of ventricular arrhythmias, it is recognized that appropriate ICD therapies are more frequent than sudden death and thus not a perfect surrogate for sudden cardiac death.24 Similarly, we do acknowledge that it may have been useful to include ATP as an end point; however, when GRADE was enrolling, ATP was usually programmed for VT at slower heart rates and with shorter detection times. Given that the goal of this study was to investigate sudden cardiac death and that programming for the VF zone was more uniform, we opted to only use shocks as an end point. ICD shock with ATP is the same primary end point that was used for the Prospective Observational Study of Implantable Cardioverter-Defibrillator (PROSE-ICD) study, which is a genetic database that has followed patients who received a primary prevention ICD.25 Finally, we acknowledge that there may be other echocardiographic parameters that may be predictive of outcomes, especially LV volume; however, we did not have this information available for this analysis.
Table 9.
Device Programming by Included/Excluded
| All Patients (n =544) | Excluded (n =205) | Included (n =339) | P Value, Comparing Excluded/Included | |
|---|---|---|---|---|
| Programmed zone | 0.5443 | |||
| <170 | 148 (27.2) | 61 (29.8) | 87 (25.7) | |
| 170 to 190 | 276 (50.7) | 102 (49.8) | 174 (51.3) | |
| >190 | 120 (22.1) | 42 (20.5) | 78 (23.0) | |
| ATP used, % | 322 (59.3) | 127 (62.3) | 195 (57.5) | 0.3188 |
ATP indicates antitachycardia pacing.
Conclusion
Increased LV dilatation and LVEF were associated with an increased risk of appropriate ICD shocks in subjects with severe LV dysfunction, but LV mass was not. LV dilatation can easily assessed during routine clinical practice and may provide a useful clinical tool for risk stratification for sudden cardiac death in heart failure patients.
Sources of Funding
This study was supported by National Institutes of Health (NIH)–National Heart, Lung, and Blood Institute (NHLBI) R01 HL77398 (Dr London, PI) NIH-NHLBI R01 HL103946 (Dr Cheng, PI), and NIH-NCATS UL1 TR001082 (Drs Mulvahill and Carlson).
Disclosures
None.
References
- Moss AJ, Zareba W, Hall WJ, Klein H, Wiber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML for the Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- Solomon S, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioini A, Kober L, White H, Van de Werf F, Pieper K, Califf RM, Pfeffer MA Valsartan in Acute Myocardial Infarction Trial (VALIANT) Investigators. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- Rouleau JL, Talajic M, Sussex B, Potvin L, Warnica W, Davies RF, Gardner M, Stewart D, Plante S, Dupuis R, Lauzon C, Ferguson J, Mikes E, Balnozan V, Savard P. Myocardial infarction patients in the 1990s—their risk factors, stratification and survival in Canada: the Canadian Assessment of Myocardial Infarction (CAMI) Study. J Am Coll Cardiol. 1996;27:1119–1127. doi: 10.1016/0735-1097(95)00599-4. [DOI] [PubMed] [Google Scholar]
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN. MUSTT: a randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- Bigger JT., Jr Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. N Engl J Med. 1997;37:1569–1575. doi: 10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, Kornacewicz-Jach Z, Sredniawa B, Lupkovics G, Hofgartner F, Lubinski A, Rosenqvist M, Habets A, Wegscheider K, Senger JL IRIS Investigators. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–1436. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- Reiter MJ, Synhorst DP, Mann DE. Electrophysiological effects of acute ventricular dilatation in the isolated rabbit heart. Circ Res. 1988;62:554–562. doi: 10.1161/01.res.62.3.554. [DOI] [PubMed] [Google Scholar]
- Reiter MJ, Landers M, Zetelaki Z, Kirchhof CJ, Allessie MA. Electrophysiological effects of acute dilatation in the isolated rabbit heart: cycle length-dependent effects on ventricular refractoriness and conduction velocity. Circulation. 1997;96:4050–4056. doi: 10.1161/01.cir.96.11.4050. [DOI] [PubMed] [Google Scholar]
- Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Curr Opin Cardiol. 2010;25:29–36. doi: 10.1097/HCO.0b013e328333d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Aleong R, Chugh H, Nichols GA, Gunson K, London B, Jui J, Chugh SS. Left ventricular diameter and risk stratification for sudden cardiac death. J Am Heart Assoc. 2014;3:e001193. doi: 10.1161/JAHA.114.001193. doi: 10.1161/JAHA.114.001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- Fox ER, Han H, Taylor HA, Walls UC, Samdarshi T, Skelton TN, Pan J, Arnett D. The prognostic value of the mitral diastolic filling velocity ratio for all-cause mortality and cardiovascular morbidity in African Americans: the Atherosclerotic Risks in Communities (ARIC) study. Am Heart J. 2006;152:749–755. doi: 10.1016/j.ahj.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Leonardi S, Raineri C, De Ferrari GM, Ghio S, Scelsi L, Pasotti M, Tagliani M, Valentini A, Dore R, Raisaro A, Arbustini E. Usefulness of cardiac magnetic resonance in assessing the risk of ventricular arrhythmias and sudden death in patients with hypertrophic cardiomyopathy. Eur Heart J. 2009;30:2003–2010. doi: 10.1093/eurheartj/ehp152. [DOI] [PubMed] [Google Scholar]
- Bacharova L, Plandorova J, Klimas J, Krenek P, Kyselovic J. Discrepancy between increased left ventricular mass and “normal” QRS voltage in early stage of left ventricular hypertrophy in spontaneously hypertensive rats. J Electrocardiol. 2008;41:730–734. doi: 10.1016/j.jelectrocard.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Oikarinen L, Nieminen MS, Toivonen L, Viitasalo M, Wachtell K, Papademetriou V, Jern S, Dahlöf B, Devereux RB, Okin PM LIFE Study Investigators. Relation of QT interval and QT dispersion to regression of echocardiographic and electrocardiographic left ventricular hypertrophy in hypertensive patients: the Losartan Intervention For Endpoint Reduction (LIFE) study. Am Heart J. 2003;145:919–925. doi: 10.1016/S0002-8703(02)94785-X. [DOI] [PubMed] [Google Scholar]
- Armoundas AA, Wu R, Juang G, Marbán E, Tomaselli GF. Electrical and structural remodeling of the failing ventricle. Pharmacol Ther. 2001;92:213–230. doi: 10.1016/s0163-7258(01)00171-1. [DOI] [PubMed] [Google Scholar]
- Dogra V, Oliver R, Lapidus J, Balaji S, Kron J, McAnulty J, Chugh SS. Apparent protective effect of increased left ventricular wall thickness in an ICD population. J Cardiac Fail. 2003;9:412–415. doi: 10.1054/s1071-9164(03)00131-3. [DOI] [PubMed] [Google Scholar]
- Reinier K, Dervan C, Singh T, Uy-Evanado A, Lai S, Gunson K, Jui J, Chugh SS. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm. 2011;8:1177–1182. doi: 10.1016/j.hrthm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, III, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W MADIT-RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with non-ischemic cardiomyopathy? Circulation. 2006;113:776–782. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- Cheng A, Dalal D, Butcher B, Norgard S, Zhang Y, Dickfeld T, Eldadah ZA, Ellenbogen KA, Guallar E, Tomaselli GF. Prospective observational study of implantable cardioverter-defibrillators in primary prevention of sudden cardiac death: study design and cohort description. J Am Heart Assoc. 2013;22:e000083. doi: 10.1161/JAHA.112.000083. doi: 10.1161/JAHA.112.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]


