Abstract
Background
Plasma adiponectin levels have previously been inversely associated with carotid intima-media thickness (IMT), a marker of subclinical atherosclerosis. In this study, we used a sex-stratified Mendelian randomization approach to investigate whether adiponectin has a causal protective influence on IMT.
Methods and Results
Baseline plasma adiponectin concentration was tested for association with baseline IMT, IMT progression over 30 months, and occurrence of cardiovascular events within 3 years in 3430 participants (women, n =1777; men, n =1653) with high cardiovascular risk but no prevalent disease. Plasma adiponectin levels were inversely associated with baseline mean bifurcation IMT after adjustment for established risk factors (β =−0.018, P<0.001) in men but not in women (β =−0.006, P =0.185; P for interaction =0.061). Adiponectin levels were inversely associated with progression of mean common carotid IMT in men (β =−0.0022, P =0.047), whereas no association was seen in women (0.0007, P =0.475; P for interaction =0.018). Moreover, we observed that adiponectin levels were inversely associated with coronary events in women (hazard ratio 0.57, 95% CI 0.37 to 0.87) but not in men (hazard ratio 0.82, 95% CI 0.54 to 1.25). A gene score of adiponectin-raising alleles in 6 loci, reported recently in a large multi-ethnic meta-analysis, was inversely associated with baseline mean bifurcation IMT in men (β =−0.0008, P =0.004) but not in women (β =−0.0003, P =0.522; P for interaction =0.007).
Conclusions
This report provides some evidence for adiponectin protecting against atherosclerosis, with effects being confined to men; however, compared with established cardiovascular risk factors, the effect of plasma adiponectin was modest. Further investigation involving mechanistic studies is warranted.
Keywords: adiponectin, atherosclerosis, carotid intima-media thickness, genetics, Mendelian randomization
Adiponectin, a hormone with paracrine and endocrine effects, is secreted from adipose tissue and circulates in large amounts (3 to 30 mg/L) in plasma. In experimental studies, it enhanced insulin sensitivity and exerted atheroprotective effects.1 Adiponectin effects are mediated by 3 receptors, adiponectin receptor 1, adiponectin receptor 2, and T-cadherin, of which the first 2 have intracellular domains2,3 and diverse abilities to regulate downstream inflammatory cytokine responses.4
Low adiponectin levels are associated with obesity and related cardiovascular risk factors (type 2 diabetes mellitus [T2D],1 endothelial dysfunction,1 and dyslipidemia5). Low adiponectin is also inconsistently6–9 associated with increased risk of myocardial infarction, whereas high adiponectin is associated with increased mortality in populations with high cardiovascular risk.10–12 Moreover, adiponectin is inversely associated with carotid intima-media thickness (IMT), a marker of cardiovascular disease (CVD) risk,13–16 independent of established risk factors. Evidence suggests that IMT may be used as a surrogate marker for atherosclerotic processes16,17 and future cardiovascular events.18–20
It has yet to be demonstrated whether adiponectin levels have a direct (rather than an indirect) effect on CVD. In addition, it is unclear whether the significantly higher adiponectin levels observed in women compared with men contribute to the striking sex difference in CVD risk. By combining extensive ultrasound measures of IMT with plasma adiponectin levels and adiponectin-associated genetic variants identified in a multiethnic genomewide meta-analysis (n =45 891),21 we used a Mendelian randomization approach22 to explore whether adiponectin has a causal influence on carotid IMT in men and women in a large (n =3430) European cohort with high CVD risk.
Materials and Methods
The IMPROVE Cohort
IMPROVE has been described previously.23 Briefly, persons with at least 3 classic CVD risk factors who were free of clinical CVD at enrollment were recruited. Blood samples were drawn at baseline and stored appropriately. A structured medical history was obtained, and standard clinical and biochemical phenotyping was carried out. Plasma adiponectin concentration was analyzed with a double-antibody radioimmunoassay (Millipore). The total coefficients of variation were 15.2% at low levels (2 to 4 μg/mL) and 8.8% at high levels (26 to 54 μg/mL). T2D was defined as a diagnosis of diabetes, antidiabetic therapy, or fasting glucose ≥7 mmol/L at the baseline examination. In addition, persons who started insulin treatment before the age of 50 years were excluded. The Framingham risk score was calculated for all participants.24 A total of 3711 participants were recruited from 7 centers in Finland, Sweden, the Netherlands, France, and Italy between 2002 and 2004.
Carotid Ultrasound Examination
The carotid ultrasound protocol and precision of the ultrasonographic measurements have been reported previously.23 The far walls of the left and right common carotid artery (CC) and carotid bifurcation (Bif) were visualized in anterior, lateral, and posterior projections and recorded on VHS videotapes. IMT measurements were performed in a centralized laboratory (Department of Pharmacological Sciences, University of Milan, Italy). A dedicated software (M′Ath; Metris, SRL) that allowed semiautomatic edge of the echogenic lines of the intima-media complex was used. The entire lengths of the far walls of the CCs and the Bifs were measured in at least 3 different frames. The mean IMT (IMTmean) of each segment was calculated (based on 6 measurements for each segment), and the maximum IMT (IMTmax) for each segment was identified. Measurements were taken at baseline and 30 months. Progression at 30 months, expressed in mm/year, was calculated by linear regression of IMT changes over time. All scans for each patient were assigned to a single reader after coding and were read blindly. As reported previously,23 the intrasonographer intraclass correlation coefficents were 0.95 and 0.92 for CC-IMTmean and Bif-IMTmean, respectively. The intersonographer intraclass correlation coefficents for the same carotid segments were 0.89 and 0.95, respectively.
Cardiovascular Events and Follow-up
Occurrence of cardiovascular end points (myocardial infarction, angioplasty, diagnosis of angina pectoris, angioplasty, coronary artery bypass grafting and/or sudden cardiac death, ischemic stroke, transient ischemic attack, peripheral revascularization, and/or diagnosis of intermittent claudication) was monitored after 30 months. Diagnoses of incident angina pectoris, myocardial infarction, and ischemic stroke in the course of the study were based on European Society of Cardiology guidelines.1,2 Surgery or endovascular procedures on the carotid arteries were not included as study end points because they might be related to the baseline ultrasound examination. All events were validated by local specialists using medical records and death certificates and were adjudicated subsequently by a designated specialist who was blinded to the clinical history and IMT data. Coronary events were defined as myocardial infarction, sudden cardiac death, angina pectoris, percutaneous coronary angioplasty, or coronary artery bypass grafting.
Ethics Committee Approval
All participants provided written informed consent. The study was approved by local ethics committees at the participating institutions.
Single-Nucleotide Polymorphism Selection and Genotyping
Adiponectin-associated single-nucleotide polymorphisms (SNPs) from a large recent report by Dastani et al21 were considered for inclusion in an allelic score. SNPs and proxies used in the allelic score are presented in Table 1. SNPs associated with T2D, diabetes-related traits, lipid traits, and SNPs in the IRS1 locus25 were excluded to avoid analyzing pleiotropic effects, leaving rs2791553, rs3001032, rs925735, rs12051272, rs1597466, rs6810075, rs998584, and rs592423 to be included in the allelic score (SNPs marked with an asterisk in Table 1).
Table 1.
SNPs and Proxies Included in the Allelic Score
| CHR | Dastani et al21 | IMPROVE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lead | Minor/Major Alleles | MAF | β of Minor Allele | Association With T2D, T2D-Related Traits, or Lipids* | Proxy | LD With Lead SNP r2 (D′) | Minor/Major Alleles | MAF | |
| 1 | rs2791553* | A/G | 0.40 | 0.02 | No | rs2494195 | 1 (1) | T/C | 0.38 |
| 1 | rs3001032* | C/T | 0.30 | 0.02 | No | rs4846567 | 0.96 (1) | T/G | 0.27 |
| 2 | rs925735* | C/G | 0.36 | 0.02 | No | rs2673141 | 1 (1) | G/A | 0.37 |
| 3 | rs1108842 | A/C | 0.49 | 0.03 | WHR | A/C | 0.48 | ||
| 3 | rs12051272* | T/G | 0.03 | −0.26 | No | T/G | 0.03 | ||
| 3 | rs1597466* | T/G | 0.10 | −0.03 | No | rs4301033 | 0.90 (1) | A/G | 0.09 |
| 3 | rs6810075* | C/T | 0.40 | −0.06 | No | rs1648707 | 0.90 (0.97) | C/A | 0.41 |
| 6 | rs998584* | A/C | 0.50 | 0.03 | No | rs1358980 | 0.84 (0.93) | T/C | 0.48 |
| 6 | rs592423* | A/C | 0.46 | −0.02 | No | A/C | 0.47 | ||
| 8 | rs2980879 | A/T | 0.31 | −0.03 | HDL-C, LDL-C, TG, and total chol. | rs2954030 | 0.80 (1) | T/C | 0.39 |
| 12 | rs601339 | G/A | 0.19 | 0.03 | HDL-C | rs2454722 | 1 (1) | G/A | 0.19 |
| 12 | rs7133378 | A/G | 0.30 | 0.02 | HDL-C =c, TG | A/G | 0.32 | ||
| 16 | rs2925979 | T/C | 0.30 | −0.04 | HDL-C, TG | T/C | 0.32 | ||
| 19 | rs731839 | G/A | 0.35 | −0.35 | HDL-C, TG | G/A | 0.35 | ||
| 19 | rs4805885 | T/C | 0.39 | −0.03 | HDL-C | rs8182584 | 0.86 (1) | T/G | 0.40 |
chol. indicates cholesterol; CHR, chromosome; HDL-C, high-density lipoprotein cholesterol; LD, linkage disequilibrium; LDL-C, low-density lipoprotein cholesterol; MAF, minor allele frequency; major allele, noneffect allele; minor allele, effect allele; SNP, single-nucleotide polymorphism; TG, triglycerides; WHR, waist–hip ratio.
Used in the allelic gene score.
DNA was available for all participants. High-throughput genotyping was performed using the Illumina 200K CardioMetabo chip (SNP Technology Platform, Uppsala University, Sweden), and standard quality control procedures were applied: SNPs were excluded for failing Hardy-Weinberg equilibrium (P<1×10−6) or call rate (95%) tests. Participants were excluded because of low call rate (<95%), ambiguous sex, cryptic relatedness, or non-European descent. Multidimensional scaling components were calculated using PLINK,26 and components 1 to 3 were included as covariates in genetic analyses to control for population structure. SNPs not present on the CardioMetabo chip were genotyped using TaqMan SNP genotyping assays (Applied Biosystems), and consistent quality control parameters were applied.
After quality control, 3430 subjects with genetic information, adiponectin levels, and IMT measures were included (women, n =1777; men, n =1653; age range 54 to 79 years).27 Cohort characteristics are described in Table 2.
Table 2.
Baseline Characteristics and Measurements of Carotid IMT in IMPROVE and Replication Cohorts
| Women | Men | |
|---|---|---|
| n | 1777 | 1653 |
| Age, y | 64.6 (59.9 to 67.3) | 64.5 (59.5 to 67.1) |
| SBP, mm Hg | 140 (130 to 152) | 141 (130 to 154) |
| DBP, mm Hg | 80 (75 to 88) | 83 (77 to 90) |
| Body mass index, kg/m2 | 26.5 (23.6 to 29.7) | 27.1 (24.9 to 29.3) |
| LDL-C, mmol/L | 3.6 (2.9 to 4.4) | 3.4 (2.7 to 4) |
| HDL-C, mmol/L | 1.3 (1.1 to 1.6) | 1.1 (0.93 to 1.3) |
| Triglycerides, mmol/L | 1.26 (0.91 to 1.79) | 1.38 (0.97 to 2.03) |
| Creatinine, mmol/L | 70 (63 to 79) | 89 (80 to 99) |
| C-reactive protein, mg/L | 2.10 (0.92 to 3.95) | 1.63 (0.67 to 3.23) |
| Fasting glucose, mmol/L | 5.3 (4.8 to 6.0) | 5.7 (5.2 to 6.6) |
| Adiponectin, μg/mL | 14.1 (8.7 to 22.0) | 8.2 (5.0 to 12.2) |
| Ultrasonographic variables | ||
| Baseline | ||
| CC-IMTmean, mm | 0.70 (0.64 to 0.77) | 0.74 (0.66 to 0.83) |
| CC-IMTmax, mm | 1.03 (0.94 to 1.19) | 1.13 (0.98 to 1.44) |
| Bif-IMTmean, mm | 1.00 (0.81 to 1.25) | 1.12 (0.91 to 1.44) |
| Bif-IMTmax, mm | 1.57 (1.26 to 2.13) | 1.77 (1.39 to 2.41) |
| Progression | ||
| CC-IMTmean, mm | 0.006 (−0.006 to 0.020) | 0.008 (−0.006 to 0.024) |
| CC-IMTmax, mm | 0.004 (−0.019 to 0.039) | 0.011 (−0.023 to 0.050) |
| Bif-IMTmean, mm | 0.027 (−0.004 to 0.066) | 0.031 (−0.006 to 0.073) |
| Bif-IMTmax, mm | 0.036 (−0.012 to 0.112) | 0.037 (−0.026 to 0.114) |
| Smoking habits | ||
| Never | 31.2 (515) | 63.9 (1136) |
| Former | 52.3 (864) | 22.7 (403) |
| Current | 16.6 (274) | 13.4 (238) |
| Type 2 diabetes mellitus* | 22.0 (386) | 32.0 (514) |
| Drugs at inclusion | ||
| Antiplatelet therapy | 14.6 (259) | 18.7 (309) |
| Oral antidiabetic drugs | 13.7 (241) | 21.7 (352) |
| Insulin | 3.1 (55) | 4.5 (74) |
| Lipid-lowering drugs | 51.1 (896) | 47.7 (774) |
| Antihypertensive drugs | 59.1 (1051) | 54.3 (898) |
Values are median (interquartile range) or percentage (number). Bif indicates bifurcation of the carotid artery; CC, common carotid artery; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; IMTmax, highest of all maximal IMT values obtained from left and right measurements; IMTmean, average of mean IMT values obtained from left and right measurements; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
Defined as a diagnosis of diabetes, antidiabetic therapy, or fasting glucose ≥7 mmol/L at the baseline examination.
Statistical Methods
Differences in plasma adiponectin across centers were analyzed by Kruskal–Wallis nonparametric 1-way analysis of variance, and the Jonckheere–Terpstra test for ordered alternatives was used to assess trends by latitude.
Associations among adiponectin levels, population structure (multidimensional scaling components [MDS] 1 to 3), and established CVD risk factors were investigated by calculation of Spearman rank correlation coefficients. Following this, skewed variables were natural log-transformed for normalization prior to further statistical analysis. Analysis of factors associated with plasma adiponectin was performed by multiple linear regression analysis (IBM SPSS Statistics 19.0).
Linear regression analysis was conducted to assess associations between adiponectin levels and IMT variables. Analyses were stratified for sex because there were significant differences in adiponectin levels between men and women. Adjustments were made for age (basic model) or for age, body mass index, T2D, systolic blood pressure, current smoking, triglycerides, high-density lipoprotein cholesterol, and C-reactive protein (full model). Inclusion of waist–hip ratio in the full model instead of body mass index made little or no difference for the findings. Similarly, replacing current smoking with quintiles of pack-years had negligible effects on the results. Tests for sex–adiponectin interaction were performed for the entire cohort.
Cox proportional hazards analysis adjusting for baseline characteristics in basic and full models, as specified, was used to determine association of plasma adiponectin with cardiovascular events and coronary events only. The proportionality assumption was tested with time-dependent variables.
For the Mendelian randomization, an unweighted allelic score was constructed by calculating the sum of alleles associated with increased plasma adiponectin divided by the maximum number of possible adiponectin-raising alleles. To account for possible population structure, MDS 1 to 3 were included as covariates in regression models. MDS (comparable with principal component analysis) was performed using largely uncorrelated CardioMetabochip SNPs obtained by applying a filter of pairwise correlations of r2<0.5 within a 50-SNP window that iteratively shifted 5 SNPs along the sequence. The first component corresponded well with the latitude of the recruitment center, whereas the second approximated longitude. Analysis of allelic score associations with IMT were performed with basic (adjusting for population structure and age) and full (adjusting for population structure, age, body mass index, systolic blood pressure, high-density lipoprotein cholesterol, triglycerides, C-reactive protein, current smoking, T2D) linear regression models. Tests of sex–allelic score interactions were performed for the entire cohort. Multiple testing correction was not applied to the analysis of IMT variables because the phenotypes are closely interrelated.23
Results
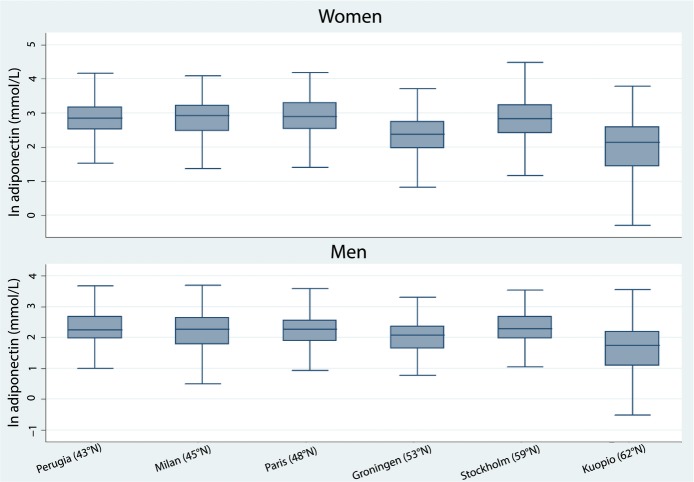
Baseline characteristics and ultrasonographic variables are presented in Table 2. Men and women differed for most variables including adiponectin levels, which were higher in women (median 14.1 [interquartile range 8.7 to 22.0] versus 8.2 [interquartile range, 5.0 to 12.2] μg/mL). Adiponectin levels also differed across centers, with significantly lower adiponectin levels in the north observed in both men and women (Figure 1). Of note, MDS component 1 (to a large degree reflecting south-to-north population structure) was associated with adiponectin and accounted for 16.5% and 8.0% of adiponectin variation in women and men, respectively (Table 3).
Figure 1.
Lower plasma adiponectin concentrations were observed in northern recruitment centers.
Table 3.
Effect Size Estimates for Variables Associated With Plasma Adiponectin in Multivariable Models
| Women | Men | |||
|---|---|---|---|---|
| Partial η2 | P Value | Partial η2 | P Value | |
| Age | 0.007 | <0.001 | 0.002 | 0.089 |
| SBP | 0.000 | 0.881 | 0.000 | 0.890 |
| Body mass index | 0.002 | 0.075 | 0.005 | 0.004 |
| HDL-C | 0.036 | <0.001 | 0.018 | <0.001 |
| Triglycerides | 0.023 | <0.001 | 0.010 | <0.001 |
| Type 2 diabetes mellitus | 0.017 | <0.001 | 0.013 | <0.001 |
| Current smoking | 0.000 | 0.502 | 0.000 | 0.671 |
| C-reactive protein | 0.003 | 0.025 | 0.001 | 0.317 |
| MDS1 | 0.165 | <0.001 | 0.080 | <0.001 |
| MDS2 | 0.011 | <0.001 | 0.010 | <0.001 |
| MDS3 | 0.003 | 0.027 | 0.002 | 0.047 |
| η2 for model | 0.320 | <0.001 | 0.192 | <0.001 |
HDL-C indicates high-density lipoprotein cholesterol; MDS, multidimensional scaling component; SBP, systolic blood pressure.
Sex-specific associations between adiponectin and established cardiovascular risk factors are shown in Table 4. Adiponectin levels were inversely associated with blood pressure, body mass index, triglycerides, creatinine, C-reactive protein, and T2D and were positively associated with age and high-density lipoprotein cholesterol.
Table 4.
Correlations Between Plasma Adiponectin Concentration and Established Cardiovascular Risk Markers
| Men (n =1653) | Women (n =1777) | |||
|---|---|---|---|---|
| r | P Value | r | P Value | |
| Age | 0.081 | 0.001 | 0.065 | 0.006 |
| SBP | −0.083 | 0.001 | −0.152 | <0.001 |
| DBP | −0.104 | <0.001 | −0.135 | <0.001 |
| Body mass index | −0.188 | <0.001 | −0.311 | <0.001 |
| LDL-C | 0.12 | <0.001 | 0.194 | <0.001 |
| HDL-C | 0.206 | <0.001 | 0.323 | <0.001 |
| Triglycerides | −0.171 | <0.001 | −0.285 | <0.001 |
| Creatinine | −0.084 | 0.001 | −0.095 | <0.001 |
| C-reactive protein | −0.062 | 0.012 | −0.159 | <0.001 |
| Fasting glucose | −0.255 | <0.001 | −0.36 | <0.001 |
| Type 2 diabetes mellitus | −0.192 | <0.001 | −0.257 | <0.001 |
| Current smoking | −0.036 | 0.142 | −0.055 | 0.021 |
| MDS1 | −0.246 | <0.001 | −0.324 | <0.001 |
| MDS2 | −0.114 | <0.001 | 0.005 | 0.846 |
| MDS3 | 0.038 | 0.120 | −0.078 | 0.001 |
Values are Spearman rank correlation coefficients. DBP indicates diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MDS, multidimensional scaling component; SBP, systolic blood pressure.
Adiponectin Levels and Baseline IMT
In men, adiponectin levels were inversely associated with CC-IMTmean and Bif-IMTmean and Bif-IMTmax, but only the associations with Bif-IMTmean remained significant after adjustment for established CVD risk factors (Table 5). In women, adiponectin was inversely associated with the means of both CC-IMT and Bif-IMT in the basic model; however, further adjustment attenuated the association. Sex interacted with adiponectin to significantly influence Bif-IMTmax in the fully adjusted model but not in the basic model (Table 5).
Table 5.
Associations Between Plasma Adiponectin and Baseline IMT
| Model | Men (n =1653) | Women (n =1777) | P Value Int* | |||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |||
| CC-IMTmean | Basic | −0.007 | −0.012 to −0.002 | 0.006 | −0.005 | −0.009 to −0.002 | 0.007 | 0.737 |
| Full | −0.003 | −0.008 to 0.002 | 0.233 | −0.001 | −0.005 to 0.003 | 0.644 | 0.261 | |
| CC-IMTmax | Basic | −0.007 | −0.015 to 0.001 | 0.080 | −0.004 | −0.010 to 0.003 | 0.228 | 0.661 |
| Full | −0.003 | −0.011 to 0.005 | 0.471 | 0.002 | −0.005 to 0.009 | 0.613 | 0.367 | |
| Bif-IMTmean | Basic | −0.020 | −0.029 to −0.011 | <0.001 | −0.013 | −0.021 to −0.005 | 0.002 | 0.240 |
| Full | −0.018 | −0.027 to −0.009 | <0.001 | −0.006 | −0.015 to 0.003 | 0.185 | 0.061 | |
| Bif-IMTmax | Basic | −0.023 | −0.033 to −0.012 | <0.001 | −0.011 | −0.021 to −0.001 | 0.029 | 0.121 |
| Full | −0.019 | −0.030 to −0.008 | 0.001 | −0.006 | −0.017 to 0.004 | 0.247 | 0.037 | |
Basic model was adjusted for age. Full model was adjusted for age, body mass index, type 2 diabetes mellitus, systolic blood pressure, current smoking, triglycerides, high-density lipoprotein cholesterol, and C-reactive protein. Bif indicates bifurcation of the carotid artery; CC, common carotid artery; IMT, intima-media thickness; IMTmax, highest of all maximal IMT values obtained from left and right measurements; IMTmean, average of mean IMT values obtained from left and right measurements; Int, interaction.
Sex–adiponectin interaction.
Adiponectin Levels and Progression of IMT
In men, adiponectin levels were inversely associated with progression of CC-IMTmean and CC-IMTmax, despite adjustment for established cardiovascular risk factors (Table 6), whereas the association with Bif-IMTmean was lost when adjusting for the full model. In women, an inverse association with progression of Bif-IMTmax was observed in the basic model only. Sex interacted significantly with adiponectin to influence progression of CC-IMTmean and CC-IMTmax but not Bif-IMTmean or Bif-IMTmax.
Table 6.
Associations Between Plasma Adiponectin and Progression of IMT
| Model | Men (n =1653) | Women (n =1777) | P Value Int* | |||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |||
| CC-IMTmean | Basic | −0.003 | −0.005 to −0.0007 | 0.008 | 0.0002 | −0.002 to 0.0019 | 0.867 | 0.034 |
| Full | −0.002 | −0.004 to 3.0×10−5 | 0.047 | 0.0007 | −0.001 to 0.0025 | 0.475 | 0.018 | |
| CC-IMTmax | Basic | −0.007 | −0.014 to −0.0007 | 0.031 | 0.0029 | −0.003 to 0.0089 | 0.347 | 0.020 |
| Full | −0.007 | −0.014 to −0.0001 | 0.045 | 0.0031 | −0.004 to 0.0097 | 0.354 | 0.024 | |
| Bif-IMTmean | Basic | −0.007 | −0.012 to −0.0013 | 0.015 | −0.0040 | −0.008 to 0.0011 | 0.135 | 0.309 |
| Full | −0.004 | −0.010 to 0.0019 | 0.186 | −0.0020 | −0.008 to 0.0028 | 0.371 | 0.229 | |
| Bif-IMTmax | Basic | −0.006 | −0.017 to 0.0061 | 0.349 | −0.0120 | −0.022 to −0.0010 | 0.026 | 0.523 |
| Full | −0.002 | −0.014 to 0.0104 | 0.789 | −0.0110 | −0.022 to 0.0005 | 0.061 | 0.582 | |
Basic model was adjusted for age. Full model was adjusted for age, body mass index, type 2 diabetes mellitus, systolic blood pressure, current smoking, triglycerides, high-density lipoprotein cholesterol, and C-reactive protein. Bif indicates bifurcation of the carotid artery; CC, common carotid artery; IMT, intima-media thickness; IMTmax, highest of all maximal IMT values obtained from left and right measurements; IMTmean, average of mean IMT values obtained from left and right measurements; Int, interaction.
Sex–adiponectin interaction.
Adiponectin Levels and Cardiovascular Events
During follow-up, there were 74 and 117 cardiovascular events (45 and 75 coronary events) among women and men, respectively. In univariate analysis, age, body mass index, high-density lipoprotein cholesterol, triglycerides, creatinine, C-reactive protein, and T2D were associated with cardiovascular events in women, whereas age, systolic blood pressure, creatinine, and current smoking were associated with cardiovascular events in men (Table 7).
Table 7.
Univariable Associations With Cardiovascular Events in Women and Men
| Women | Men | |||||
|---|---|---|---|---|---|---|
| HR* | 95% CI | P Value | HR* | 95% CI | P Value | |
| Age | 1.24 | 1.02 to 1.52 | 0.034 | 1.32 | 1.12 to 1.56 | 0.001 |
| SBP | 1.11 | 0.89 to 1.39 | 0.346 | 1.26 | 1.06 to 1.49 | 0.007 |
| DBP | 0.92 | 0.73 to 1.17 | 0.504 | 1.10 | 0.92 to 1.31 | 0.286 |
| Body mass index | 1.23 | 1.03 to 1.48 | 0.025 | 1.08 | 0.87 to 1.33 | 0.483 |
| LDL-C | 0.99 | 0.79 to 1.24 | 0.922 | 1.11 | 0.91 to 1.35 | 0.312 |
| HDL-C | 0.76 | 0.59 to 0.97 | 0.031 | 0.88 | 0.70 to 1.10 | 0.263 |
| Triglycerides | 1.30 | 0.98 to 1.72 | 0.065 | 1.07 | 0.98 to 1.16 | 0.120 |
| Creatinine | 1.24 | 0.98 to 1.57 | 0.076 | 1.21 | 1.02 to 1.44 | 0.028 |
| C-reactive protein | 1.18 | 1.02 to 1.35 | 0.022 | 1.17 | 1.02 to 1.35 | 0.024 |
| Fasting glucose | 1.21 | 0.99 to 1.48 | 0.065 | 1.02 | 0.87 to 1.20 | 0.769 |
| Current smoking | 1.32 | 0.73 to 2.41 | 0.361 | 1.64 | 1.07 to 2.50 | 0.023 |
| Type 2 diabetes mellitus | 2.14 | 1.33 to 3.45 | 0.002 | 1.16 | 0.79 to 1.71 | 0.450 |
DBP indicates diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
HR for 1 SD increase is reported for continuous variables except age, for which HR per 5-year increase is reported.
Adiponectin levels were associated with coronary events in women in the basic model (hazard ratio [HR] per 1 SD increase of plasma adiponectin 0.48, 95% CI 0.32 to 0.72) and in the full model (HR 0.57, 95% CI 0.37 to 0.87). In men, no association was detected between adiponectin levels and coronary events (HR 0.74 [95% CI 0.50 to 1.09] and 0.82 [95% CI 0.54 to 1.25], respectively). When considering all cardiovascular events in women, an association with adiponectin was observed in the basic model (HR 0.64, 95% CI 0.49 to 0.85), but this was lost on further adjustment (HR 0.76, 95% CI 0.57 to 1.02). In men, no association was observed between adiponectin and all cardiovascular events (HR 0.76 [95% CI 0.56 to 1.03] and 0.81 [95% CI 0.58 to 1.13], respectively).
Mendelian Randomization Analysis
To assess causality (and avoid reverse causation), a Mendelian randomization approach22 was used. If higher adiponectin levels have a direct causal role in reducing IMT measures, then genetic variants that increase adiponectin levels throughout life would be expected to demonstrate an association with lower IMT measures. Consequently, associations between an allelic score of adiponectin-increasing SNPs21 and IMT measures were investigated.
The allelic score explained 1.7% and 1.2% of variation in baseline plasma adiponectin levels in men and women, respectively. The allelic score was inversely associated with systolic blood pressure (men and women) and glucose (women only) (Table 8). It is worth noting that women with the least adiponectin-increasing alleles had higher levels of adiponectin than the men with the highest allelic scores (Table 9).
Table 8.
Independent Allelic Score in Relation to Baseline Characteristics in Women and Men
| Variable | Women | Men | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |
| Age | 0.0001 | −0.0204 to 0.0206 | 0.993 | −0.0055 | −0.0258 to 0.0149 | 0.599 |
| Body mass index | −0.0076 | −0.0256 to 0.0103 | 0.404 | −0.0129 | −0.0270 to 0.0011 | 0.071 |
| SBP | −0.1256 | −0.1951 to −0.0561 | <0.001 | −0.0752 | −0.1452 to −0.0053 | 0.035 |
| LDL-C | 0.0017 | −0.0023 to 0.0057 | 0.408 | 0.0031 | −0.0005 to 0.0067 | 0.095 |
| Triglycerides | 0.0010 | −0.0009 to 0.0029 | 0.296 | 0.0003 | −0.0019 to 0.0024 | 0.815 |
| HDL-C | −0.0003 | −0.0013 to 0.0007 | 0.542 | −0.0005 | −0.0015 to 0.0005 | 0.320 |
| C-reactive protein | 0.0023 | −0.0022 to 0.0068 | 0.309 | 0.0028 | −0.0020 to 0.0076 | 0.255 |
| Fasting glucose | −0.0012 | −0.0020 to −0.0004 | 0.004 | −0.0008 | −0.0017 to 0.0001 | 0.099 |
| Type 2 diabetes mellitus | −0.0031 | 0.9879 to 1.0059 | 0.497 | −0.0079 | 0.9839 to 1.0005 | 0.065 |
Linear regression analysis in for continuous variables and logistic regression analysis for type 2 diabetes mellitus. HDL-C indicates high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
Table 9.
Allelic Score in Relation to Plasma Adiponectin in Women and Men
| Allelic Score | 0 to 40 | >40 to 50 | >50 to 60 | >60 to 70 | >70 to 100 |
|---|---|---|---|---|---|
| Women, μg/mL | 12.7 (8.0 to 21.3) | 13.2 (8.7 to 20.8) | 13.9 (8.8 to 21.8) | 14.7 (9.0 to 22.2) | 17.9 (11.2 to 24.7) |
| Men, μg/mL | 6.5 (3.9 to 11.0) | 7.8 (4.8 to 11.9) | 8.1 (4.9 to 12) | 8.6 (5.7 to 12.8) | 9.7 (5.9 to 14.5) |
Values are median (interquartile range).
In men, the allelic score was inversely associated with baseline Bif-IMTmean and Bif-IMTmax in the basic and full models (Table 10; Figure 2). In women, no associations with IMT were detected. There was a sex–allelic score interaction for Bif-IMTmean and Bif-IMTmax (Table 10). No allelic score associations were observed with IMT progression measures (data not shown).
Table 10.
Association of the Allelic Score With Baseline IMT
| Model | Men (n =1653) | Women (n =1777) | P Value Int* | |||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |||
| CC-IMTmean | Basic | −0.0002 | −0.0005 to 0.0001 | 0.125 | 0.0001 | −0.0001 to 0.0003 | 0.425 | 0.158 |
| Full | −0.0002 | −0.0005 to 0.0001 | 0.120 | 0.0001 | −0.0001 to 0.0004 | 0.270 | 0.114 | |
| CC-IMTmax | Basic | −0.0002 | −0.0007 to 0.0002 | 0.312 | 0.0002 | −0.0002 to 0.0005 | 0.432 | 0.321 |
| Full | −0.0003 | −0.0007 to 0.0002 | 0.299 | 0.0002 | −0.0002 to 0.0006 | 0.278 | 0.255 | |
| Bif-IMTmean | Basic | −0.0008 | −0.0013 to −0.0003 | 0.003 | 0.0001 | −0.0004 to 0.0006 | 0.666 | 0.009 |
| Full | −0.0008 | −0.0013 to −0.0003 | 0.004 | 0.0002 | −0.0003 to 0.0007 | 0.522 | 0.007 | |
| Bif-IMTmax | Basic | −0.0009 | −0.0016 to −0.0003 | 0.004 | 0.0001 | −0.0005 to 0.0007 | 0.688 | 0.011 |
| Full | −0.0009 | −0.0015 to −0.0003 | 0.006 | 0.0002 | −0.0004 to 0.0008 | 0.556 | 0.011 | |
Basic model was adjusted for age and population structure. Full model was adjusted for age, population structure, body mass index, type 2 diabetes mellitus, systolic blood pressure, current smoking, triglycerides, high-density lipoprotein cholesterol, and C-reactive protein. Bif indicates bifurcation of the carotid artery; CC, common carotid artery; IMT, intima-media thickness; IMTmax, highest of all the maximal IMT values obtained from left and right measurements; IMTmean, average of mean IMT values obtained from left and right measurements; Int, interaction.
Sex–allelic score interaction.
Figure 2.
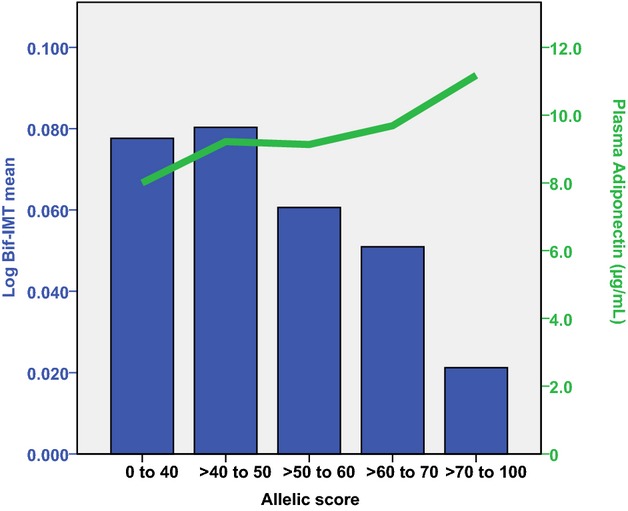
The allelic score in relation to log Bif-IMTmean (blue bars) and plasma adiponectin (green line) in men. Bif-IMT indicates bifurcation intima-media thickness.
Allelic Score and Incident CVD Events
The allelic score was inversely associated with coronary events in men (but not women) in the basic and full models (Table 11). Men with the lowest allelic scores, 0 to 40, had more incident coronary events than men with higher allelic scores (Figure 3).
Table 11.
Associations Between the Allelic Score and Coronary or Cardiovascular Events
| Model | Men (n =1653) | Women (n =1777) | |||||
|---|---|---|---|---|---|---|---|
| HR* | 95% CI | P Value | HR* | 95% CI | P Value | ||
| Coronary events | Basic | 0.73 | 0.58 to 0.93 | 0.012 | 0.96 | 0.71 to 1.31 | 0.798 |
| Full | 0.76 | 0.6 to 0.96 | 0.023 | 0.95 | 0.7 to 1.29 | 0.747 | |
| Cardiovascular events | Basic | 0.82 | 0.68 to 1.00 | 0.045 | 0.93 | 0.73 to 1.19 | 0.561 |
| Full | 0.83 | 0.68 to 1.01 | 0.059 | 0.93 | 0.73 to 1.18 | 0.562 | |
Basic model was adjusted for age and population structure. Full model was adjusted for age, population structure, body mass index, type 2 diabetes mellitus, systolic blood pressure, current smoking, triglycerides, high-density lipoprotein cholesterol, and C-reactive protein. HR indicates hazard ratio.
HR for 1 SD increase in allelic score.
Figure 3.
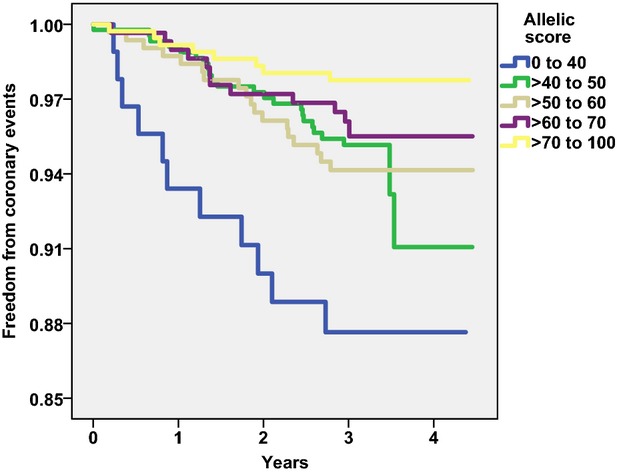
Kaplan–Meier plot of freedom from coronary events in men classified according to the allelic score.
Discussion
This study is the first to address the issue of whether adiponectin has direct effects on CVD, using molecular genetics, plasma adiponectin measurements, and repeated carotid IMT imaging in the longitudinal IMPROVE study.23 Furthermore, as this was the largest single study of adiponectin in relation to IMT to date, we were able to examine sex-specific effects of adiponectin on IMT in the CC and the Bif of the carotid artery.
Whereas other studies have reported associations between adiponectin and IMT,13–16 this report highlighted differences in the effect of adiponectin along the carotid tree: Adiponectin levels were associated with the Bif-IMT at baseline and with progression of the CC. It should be noted that baseline associations reflect lifetime (≥60 years) exposure to plasma adiponectin levels and the allelic score, whereas progression of CC-IMT reflects 30 months of exposure. These findings may be relevant in light of differences between CC-IMT and Bif-IMT; in general, CC-IMT is not a measure of atherosclerosis but rather a thickening of the media in response to age and high shear stress and is associated with hypertension and prevalent stroke.28,29 Carotid atherosclerosis occurs predominantly in the Bif19 in an area of low shear stress, and Bif-IMT is associated primarily with coronary heart disease risk factors and prevalent coronary heart disease.19,28
Of note, it is possible that our finding that levels of adiponectin showed a north–south trend (lower in the north), even after adjustment for established cardiovascular risk factors, might contribute to the previously demonstrated opposite north–south gradient in IMT (larger in the north)23; however, further work is required to confirm this finding.
To assess causality, we used Mendelian randomization to demonstrate causal effects of adiponectin on the carotid tree, using an allelic score of adiponectin-increasing SNPs determined in a large multiethnic analysis of 45 891 persons.21 To minimize pleiotropic effects, SNPs significantly associated with T2D, diabetes-related traits, and lipid traits were excluded from the allelic score. Despite this, associations with systolic blood pressure (in women and to some extent in men) and fasting glucose (in women only) were observed. In men, we could show that the allelic score was associated with Bif-IMT and could provide support for a protective role of adiponectin in early atherosclerosis, as assessed by IMT; however, it should be noted that the effect of adiponectin (plasma levels and score) was modest and limited to the Bif and thus cannot be generalized to the rest of the carotid artery.
It is worth noting that in this cohort with high CVD risk, the majority of participants were on lipid-lowering and/or antihypertensive medication. Analysis of untreated participants only is underpowered (n =372 men, n =368 women) but demonstrates effect sizes comparable to the whole cohort (data not shown). Similarly, stratification (rather than adjustment) for T2D status might be preferable but would severely limit power. Differences in effect of either adiponectin or allelic score on all studied phenotypes were minimal between the whole population and participants with or without T2D (data not shown).
The Framingham risk score24 suggests that certain CVD risk factors are more important than others. Calculation of the Framingham risk score indicates that 1358 men and 632 women in this study were classified as being at high risk of CVD (Framingham risk score >0.20). Considering only this subset of the population, effect sizes were generally stronger than in the whole population, with the same phenotypes demonstrating significance for baseline IMT (but not progression) in analysis of either adiponectin (data not shown) or allelic score (Table 12).
Table 12.
Association of the Allelic Score With Baseline IMT in Subjects With Framingham Risk Score >0.20
| Model | Men (n =1653) | Women (n =1777) | P Value Int* | |||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |||
| CC-IMTmean | Basic | −0.0001 | −0.0005 to 0.0002 | 0.478 | 0.0003 | −0.0002 to 0.0007 | 0.250 | 0.268 |
| Full | −0.0001 | −0.0005 to 0.0002 | 0.489 | 0.0003 | −0.0002 to 0.0007 | 0.249 | 0.221 | |
| CC-IMTmax | Basic | 0.0000 | −0.0006 to 0.0005 | 0.869 | 0.0004 | −0.0003 to 0.0011 | 0.283 | 0.435 |
| Full | 0.0000 | −0.0006 to 0.0005 | 0.875 | 0.0004 | −0.0004 to 0.0011 | 0.344 | 0.419 | |
| Bif-IMTmean | Basic | −0.0007 | −0.0013 to −0.0001 | 0.023 | 0.0004 | −0.0005 to 0.0013 | 0.388 | 0.017 |
| Full | −0.0007 | −0.0013 to −0.0001 | 0.020 | 0.0003 | −0.0006 to 0.0012 | 0.520 | 0.020 | |
| Bif-IMTmax | Basic | −0.0008 | −0.0015 to −0.0001 | 0.024 | 0.0005 | −0.0006 to 0.0015 | 0.398 | 0.019 |
| Full | −0.0008 | −0.0015 to −0.0001 | 0.026 | 0.0004 | −0.0007 to 0.0015 | 0.472 | 0.024 | |
Basic model was adjusted for age and population structure. Full model was adjusted for age, population structure, body mass index, type 2 diabetes mellitus, systolic blood pressure, current smoking, triglycerides, high-density lipoprotein cholesterol, and C-reactive protein. Bif indicates bifurcation of the carotid artery; CC, common carotid artery; IMT, intima-media thickness; IMTmax, highest of all the maximal IMT values obtained from left and right measurements; IMTmean, average of mean IMT values obtained from left and right measurements; Int, interaction.
Sex–allelic score interaction.
In addition, we demonstrated that there are sex-specific effects: Adiponectin levels and allelic scores were associated with IMT measures of the carotid Bif in men but not in women (even after adjustment for established cardiovascular risk factors), and adiponectin levels were associated with coronary events in women but not in men. That associations with IMT are not consistent with those for coronary events is not a surprise. As noted, baseline IMT measures of the carotid Bif can be considered a surrogate marker for the development of atherosclerosis from birth until enrollment in the study (over a time span of ≈65 years). In contrast, the cardiovascular events are acute incidents due to plaque rupture and atherosclerosis. Consequently, the 2 parameters studied reflect different components of CVD.
The sex-specific differences in effect of adiponectin on CVD may be due to the differences in levels of adiponectin between men and women. Because the effects of adiponectin-raising alleles in women on IMT measures were negligible, it could be hypothesized that women with low adiponectin still have enough adiponectin to prevent or slow atherosclerosis development. In contrast, men with few adiponectin-raising alleles (allelic score 0 to 40) had very low adiponectin levels (6.5 μg/mL, interquartile range 3.9 to 11.0 μg/mL), which may be permissive of the atherosclerosis process compared with men with higher allelic scores (allelic score >70 to 100) and higher adiponectin levels (9.7 μg/mL, interquartile range 5.9 to 14.5 μg/mL).
Strengths and Limitations
A limitation of this study is the lack of information regarding hormone replacement therapy. Accordingly, complete assessment of the effect of female sex hormones on adiponectin is not possible. We cannot rule out that this might contribute to the sex differences reported. In addition, our results are primarily informative for participants with high cardiovascular risk and may not pertain to the general population. Furthermore, during the review process, a number of reports were published demonstrating associations between T2D-relevant traits and the loci that previously did not show evidence of pleiotropy, hence we cannot exclude pleiotropic effects of the allelic score. The lack of association between the plasma adiponectin and coronary events in men might be due to lack of statistical power. Despite these limitations, the IMPROVE study has comprehensive measurements of IMT at baseline and after 30 months in addition to adiponectin levels and dense genotyping. Furthermore, information on cardiovascular events is complete for 94.5% of participants over 3 years, limiting any follow-up bias. Although consistent, the effects of plasma adiponectin levels and allelic scores on Bif-IMT were much smaller than those provided by established CVD risk factors. In summary, this study fills a gap in the field and adds some support for a causal relationship between adiponectin and IMT.
Conclusions
This report provides some evidence of adiponectin protecting against atherosclerosis; however, this effect is limited to a specific part of the carotid artery (the Bif) and only to men, and the magnitude is modest. Mechanistic studies are warranted to clarify the exact nature of the effect.
Acknowledgments
We thank all participants of this study.
Sources of Funding
IMPROVE was supported by the European Commission (Contract number: QLG1-CT-2002-00896), the Swedish Heart-Lung Foundation, the Swedish Research Council (projects 8691 and 0593), the Knut and Alice Wallenberg Foundation, the Foundation for Strategic Research, the Stockholm County Council (project 592229), the Strategic Cardiovascular and Diabetes Programmes of Karolinska Institutet and Stockholm County Council, the European Union Framework Programme 7 (FP7/2007-2013) for the Innovative Medicine Initiative under grant agreement no. IMI/115006 (the SUMMIT consortium), the Academy of Finland (Grant #110413), the British Heart Foundation (RG2008/08, RG2008/014) and the Italian Ministry of Health (Ricerca Corrente). The SNP Technology Platform is supported by Uppsala University, Uppsala University Hospital and the Swedish Research Council for Infrastructures. Persson is supported by the Stockholm County Council (clinical postdoctorial appointment). Strawbridge is supported by Swedish Heart-Lung Foundation (20120600), the Tore Nilsson, Gamla Tjänarinnor and Thurings foundations. Gertow acknowledges support from the Swedish Heart-Lung Foundation and Stiftelsen för Gamla Tjänarinnor. Sabater-Lleal is supported by the Swedish Heart-Lung Foundation (20130399), and acknowledges funding from Åke Wiberg and Tore Nilssons foundations. Sennblad acknowledges funding from the Magnus Bergvall Foundation and the Foundation for Old Servants. Rauramaa acknowledges the Ministry of Education and Culture in Finland. S.Sö. is supported by the Västerbotten County Council (ALF) and the Swedish Heart and Lung Foundation. AGT is supported by TÁMOP 4.2.4.A/1-11-1-2012-0001 National Excellence Program – research fellowship co-financed by the European Union and the European Social Fund. M.K. is supported by the UK Medical Research Council (K013351), the Economic and Social Research Council and the Academy of Finland. The University College London Genetics Institute supported S.Sh.
Disclosures
None.
Supporting Information
References
- Han SH, Sakuma I, Shin EK, Koh KK. Antiatherosclerotic and anti-insulin resistance effects of adiponectin: basic and clinical studies. Prog Cardiovasc Dis. 2009;52:126–140. doi: 10.1016/j.pcad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Tian L, Luo N, Zhu X, Chung BH, Garvey WT, Fu Y. Adiponectin-AdipoR1/2-APPL1 signaling axis suppresses human foam cell formation: differential ability of AdipoR1 and AdipoR2 to regulate inflammatory cytokine responses. Atherosclerosis. 2012;221:66–75. doi: 10.1016/j.atherosclerosis.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazumi T, Kawaguchi A, Hirano T, Yoshino G. Serum adiponectin is associated with high-density lipoprotein cholesterol, triglycerides, and low-density lipoprotein particle size in young healthy men. Metabolism. 2004;53:589–593. doi: 10.1016/j.metabol.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- Persson J, Lindberg K, Gustafsson TP, Eriksson P, Paulsson-Berne G, Lundman P. Low plasma adiponectin concentration is associated with myocardial infarction in young individuals. J Intern Med. 2010;268:194–205. doi: 10.1111/j.1365-2796.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- Persson J, Folkersen L, Ekstrand J, Helleberg J, Gabrielsen A, Lundman P, Hedin U, Paulsson-Berne G. High plasma adiponectin concentration is associated with all-cause mortality in patients with carotid atherosclerosis. Atherosclerosis. 2012;225:491–496. doi: 10.1016/j.atherosclerosis.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Saarikoski LA, Huupponen RK, Viikari JS, Marniemi J, Juonala M, Kahonen M, Raitakari OT. Adiponectin is related with carotid artery intima-media thickness and brachial flow-mediated dilatation in young adults—the Cardiovascular Risk in Young Finns Study. Ann Med. 2010;42:603–611. doi: 10.3109/07853890.2010.514284. [DOI] [PubMed] [Google Scholar]
- Iglseder B, Mackevics V, Stadlmayer A, Tasch G, Ladurner G, Paulweber B. Plasma adiponectin levels and sonographic phenotypes of subclinical carotid artery atherosclerosis: data from the SAPHIR Study. Stroke. 2005;36:2577–2582. doi: 10.1161/01.STR.0000190834.00284.fd. [DOI] [PubMed] [Google Scholar]
- Pilz S, Horejsi R, Moller R, Almer G, Scharnagl H, Stojakovic T, Dimitrova R, Weihrauch G, Borkenstein M, Maerz W, Schauenstein K, Mangge H. Early atherosclerosis in obese juveniles is associated with low serum levels of adiponectin. J Clin Endocrinol Metab. 2005;90:4792–4796. doi: 10.1210/jc.2005-0167. [DOI] [PubMed] [Google Scholar]
- Rundek T, Blanton SH, Bartels S, Dong C, Raval A, Demmer RT, Cabral D, Elkind MS, Sacco RL, Desvarieux M. Traditional risk factors are not major contributors to the variance in carotid intima-media thickness. Stroke. 2013;44:2101–2108. doi: 10.1161/STROKEAHA.111.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillesen H, Muntendam P, Adourian A, Entrekin R, Garcia M, Falk E, Fuster V. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging. 2012;5:681–689. doi: 10.1016/j.jcmg.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG, Bots ML. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, Wilsgaard T, Lochen ML, Njolstad I, Arnesen E. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromso Study. Stroke. 2007;38:2873–2880. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- Mathiesen EB, Johnsen SH, Wilsgaard T, Bonaa KH, Lochen ML, Njolstad I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromso Study. Stroke. 2011;42:972–978. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, Henneman P, Heid IM, Kizer JR, Lyytikainen LP, Fuchsberger C, Tanaka T, Morris AP, Small K, Isaacs A, Beekman M, Coassin S, Lohman K, Qi L, Kanoni S, Pankow JS, Uh HW, Wu Y, Bidulescu A, Rasmussen-Torvik LJ, Greenwood CM, Ladouceur M, Grimsby J, Manning AK, Liu CT, Kooner J, Mooser VE, Vollenweider P, Kapur KA, Chambers J, Wareham NJ, Langenberg C, Frants R, Willems-Vandijk K, Oostra BA, Willems SM, Lamina C, Winkler TW, Psaty BM, Tracy RP, Brody J, Chen I, Viikari J, Kahonen M, Pramstaller PP, Evans DM, St PourcainB, Sattar N, Wood AR, Bandinelli S, Carlson OD, Egan JM, Bohringer S, van Heemst D, Kedenko L, Kristiansson K, Nuotio ML, Loo BM, Harris T, Garcia M, Kanaya A, Haun M, Klopp N, Wichmann HE, Deloukas P, Katsareli E, Couper DJ, Duncan BB, Kloppenburg M, Adair LS, Borja JB Consortium D, Consortium M, Investigators G; Mu TC, Wilson JG, Musani S, Guo X, Johnson T, Semple R, Teslovich TM, Allison MA, Redline S, Buxbaum SG, Mohlke KL, Meulenbelt I, Ballantyne CM, Dedoussis GV, Hu FB, Liu Y, Paulweber B, Spector TD, Slagboom PE, Ferrucci L, Jula A, Perola M, Raitakari O, Florez JC, Salomaa V, Eriksson JG, Frayling TM, Hicks AA, Lehtimaki T, Smith GD, Siscovick DS, Kronenberg F, van Duijn C, Loos RJ, Waterworth DM, Meigs JB, Dupuis J, Richards JB, Voight BF, Scott LJ, Steinthorsdottir V, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Hofmann OM, Segre AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Bostrom KB, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Crawford G, Doney AS, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PR, Jorgensen T, Kao WH, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Petersen AK, Platou C, Proenca C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparso T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, Tichet J, Tuomi T, van Dam RM, van Haeften TW, van Herpt T, van Vliet-Ostaptchouk JV, Walters GB, Weedon MN, Wijmenga C, Witteman J, Bergman RN, Cauchi S, Collins FS, Gloyn AL, Gyllensten U, Hansen T, Hide WA, Hitman GA, Hofman A, Hunter DJ, Hveem K, Laakso M, Morris AD, Palmer CN, Rudan I, Sijbrands E, Stein LD, Tuomilehto J, Uitterlinden A, Walker M, Watanabe RM, Abecasis GR, Boehm BO, Campbell H, Daly MJ, Hattersley AT, Pedersen O, Barroso I, Groop L, Sladek R, Thorsteinsdottir U, Wilson JF, Illig T, Froguel P, van Duijn CM, Stefansson K, Altshuler D, Boehnke M, McCarthy MI, Soranzo N, Wheeler E, Glazer NL, Bouatia-Naji N, Magi R, Randall J, Elliott P, Rybin D, Dehghan A, Hottenga JJ, Song K, Goel A, Lajunen T, Doney A, Cavalcanti-Proenca C, Kumari M, Timpson NJ, Zabena C, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Roccasecca RM, Pattou F, Sethupathy P, Ariyurek Y, Barter P, Beilby JP, Ben-Shlomo Y, Bergmann S, Bochud M, Bonnefond A, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Crisponi L, Day IN, de Geus EJ, Delplanque J, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Grundy S, Gwilliam R, Hallmans G, Hammond N, Han X, Hartikainen AL, Hayward C, Heath SC, Hercberg S, Hillman DR, Hingorani AD, Hui J, Hung J, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Mahley R, Mangino M, Martinez-Larrad MT, McAteer JB, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Mukherjee S, Naitza S, Neville MJ, Orru M, Pakyz R, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tonjes A, Uitterlinden AG, van Dijk KW, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Ward KL, Watkins H, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC Consortium D, Consortium G, Global BPC; Borecki IB, Meneton P, Magnusson PK, Nathan DM, Williams GH, Silander K, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Serrano-Rios M, Lind L, Palmer LJ, Hu FBs, Franks PW, Ebrahim S, Marmot M, Kao WH, Pramstaller PP, Wright AF, Stumvoll M, Hamsten A, Procardis C, Buchanan TA, Valle TT, Rotter JI, Penninx BW, Boomsma DI, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Peltonen L, Mooser V, Sladek R Investigators M, Consortium G. Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Chasman DI, Johansen CT, Fouchier SW, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Feitosa MF, Orho-Melander M, Melander O, Li X, Li M, Cho YS, Go MJ, Kim YJ, Lee JY, Park T, Kim K, Sim X, Ong RT, Croteau-Chonka DC, Lange LA, Smith JD, Ziegler A, Zhang W, Zee RY, Whitfield JB, Thompson JR, Surakka I, Spector TD, Smit JH, Sinisalo J, Scott J, Saharinen J, Sabatti C, Rose LM, Roberts R, Rieder M, Parker AN, Pare G, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, McArdle W, Masson D, Martin NG, Marroni F, Lucas G, Luben R, Lokki ML, Lettre G, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Konig IR, Khaw KT, Kaplan LM, Johansson A, Janssens AC, Igl W, Hovingh GK, Hengstenberg C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Groop LC, Gonzalez E, Freimer NB, Erdmann J, Ejebe KG, Doring A, Dominiczak AF, Demissie S, Deloukas P, de Faire U, Crawford G, Chen YD, Caulfield MJ, Boekholdt SM, Assimes TL, Quertermous T, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Taylor HA, Jr, Gabriel SB, Holm H, Gudnason V, Krauss RM, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Strachan DP, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, Kathiresan S. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit RA, Trompet S, de Craen AJ, Jukema JW. Using genetic variation for establishing causality of cardiovascular risk factors: overcoming confounding and reverse causality. Neth Heart J. 2014;22:186–189. doi: 10.1007/s12471-014-0534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre D, Nyyssonen K, Rauramaa R, de Faire U, Hamsten A, Smit AJ, Mannarino E, Humphries SE, Giral P, Grossi E, Veglia F, Paoletti R, Tremoli E. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: the IMPROVE study. Eur Heart J. 2010;31:614–622. doi: 10.1093/eurheartj/ehp496. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Rung J, Cauchi S, Albrechtsen A, Shen L, Rocheleau G, Cavalcanti-Proenca C, Bacot F, Balkau B, Belisle A, Borch-Johnsen K, Charpentier G, Dina C, Durand E, Elliott P, Hadjadj S, Jarvelin MR, Laitinen J, Lauritzen T, Marre M, Mazur A, Meyre D, Montpetit A, Pisinger C, Posner B, Poulsen P, Pouta A, Prentki M, Ribel-Madsen R, Ruokonen A, Sandbaek A, Serre D, Tichet J, Vaxillaire M, Wojtaszewski JF, Vaag A, Hansen T, Polychronakos C, Pedersen O, Froguel P, Sladek R. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet. 2009;41:1110–1115. doi: 10.1038/ng.443. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertow K, Sennblad B, Strawbridge RJ, Ohrvik J, Zabaneh D, Shah S, Veglia F, Fava C, Kavousi M, McLachlan S, Kivimaki M, Bolton JL, Folkersen L, Gigante B, Leander K, Vikstrom M, Larsson M, Silveira A, Deanfield J, Voight BF, Fontanillas P, Sabater-Lleal M, Colombo GI, Kumari M, Langenberg C, Wareham NJ, Uitterlinden AG, Gabrielsen A, Hedin U, Franco-Cereceda A, Nyyssonen K, Rauramaa R, Tuomainen TP, Savonen K, Smit AJ, Giral P, Mannarino E, Robertson CM, Talmud PJ, Hedblad B, Hofman A, Erdmann J, Reilly MP, O’Donnell CJ, Farrall M, Clarke R, Franzosi MG, Seedorf U, Syvanen AC, Hansson GK, Eriksson P, Samani NJ, Watkins H, Price JF, Hingorani AD, Melander O, Witteman JC, Baldassarre D, Tremoli E, de Faire U, Humphries SE, Hamsten A. Identification of the BCAR1-CFDP1-TMEM170A locus as a determinant of carotid intima-media thickness and coronary artery disease risk. Circ Cardiovasc Genet. 2012;5:656–665. doi: 10.1161/CIRCGENETICS.112.963660. [DOI] [PubMed] [Google Scholar]
- Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GD. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–181. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.