Abstract
Background
Current abdominal aortic aneurysm (AAA) screening in men age 65 might have limited impact on overall AAA death rates if incidence is moving to older ages. Up-to-date population-based studies of age-specific incidence, risk factors, and outcome of acute AAA are needed to inform screening policy.
Methods and Results
In a prospective, population-based study (Oxfordshire, UK, 2002–2014), the incidence and outcome of acute AAA events were determined. Based on population projections and current incidence trends, the impact of screening strategies in the UK was estimated. Over the 12-year period, 103 incident acute AAA events occurred in the study population of 92 728. Incidence/100 000/year was 55 in men ages 65 to 74 years, but increased to 112 at 75 to 85 and 298 at ≥85, with 66.0% of all events occurring at age ≥75 years. Incidence at ages 65 to 74 was highest in male smokers (274), with 96.4% of events in men <75 years occurring in ever-smokers. Extrapolating rates to the UK population, using trial evidence of screening efficacy, the current UK screening program would prevent 5.6% of aneurysm-related deaths (315 200 scans/year: 1426/death prevented, 121/year-of-life saved). Screening only male smokers age 65 and then all men at age 75 would prevent 21.1% of deaths (247 900 scans/year; 297/death prevented, 34/year-of-life saved). By 2030, 91.0% of deaths will occur at age ≥75, 61.6% at ≥85, and 28.6% in women.
Conclusions
Given that two thirds of acute AAA occurred at ≥75 years of age, screening older age groups should be considered. Screening nonsmokers at age 65 is likely to have very little impact on AAA event rates.
Keywords: abdominal aortic aneurysms, population projection analysis, risk factor profiling, screening and prevention
The increase in life expectancy and the consequent rapid and ongoing increase in the elderly population have important implications for the effective targeting of preventive medicine. A key issue for screening and prevention of vascular disease is the extent to which disease incidence is also moving to older ages. Abdominal aortic aneurysms (AAAs) cause over 175 000 deaths globally, accounting for 1% of deaths in men over 65 years.1,2 Rupture carries an 80% mortality,3–5 compared with only a 2% to 6% 30-day mortality for elective surgical repair of screen-detected AAAs,6,7 suggesting that population screening to detect and repair AAAs before rupture may reduce mortality. In fact, the UK Multicenter Aneurysm Screening Study (MASS)4,8,9 showed that ultrasound screening of men ages 65 to 74 halved 10-year AAA-related mortality and national screening programs have recently been initiated for men age 65 in England10 (national implementation completed April 2013), Wales, Northern Ireland, Scotland,11 and Sweden.12 In the United States, following the Preventive Services Task Force recommendation,13 Medicare screening of men age 65 who have ever smoked at least 100 cigarettes has also commenced under the Screening Abdominal Aortic Aneurysms Very Efficiently Act (2007).
The MASS trial, carried out in the UK from 1997 to 1999, estimated that screening of all men age 65 would be cost-effective, with 1 death prevented per 216 men invited to screening.9 However, this estimate was based on a 4.9% prevalence of AAAs in men ages 65 to 74 in the trial cohort, whereas early results from the National Health Service (NHS) screening program10 suggest a 1.5% AAA prevalence in screened men age 65, with similar findings reported in Sweden14 and New Zealand.15 This, together with evidence suggesting that the greatest decline in AAA events and mortality has been in the younger age groups, suggests that the incidence of rupture may be shifting to older age groups.14–18 Although one-off screening of men at age 65 is a major step in preventing acute AAA, these findings raise concerns that it may have a limited impact on overall AAA-related mortality at the population level, particularly in light of increasing life expectancy and the limited duration (10 to 15 years) of “protection” afforded by screening.9,19,20
A potential solution would be more-targeted screening then that currently undertaken in Europe and the United States. Smoking, hypertension (HTN), male gender, and age are the key risk factors for aneurysm formation,21–26 and smoking and HTN may additionally increase risk of rupture,27–29 but the potential impact of risk-based screening has not been determined and previous screening trials have excluded women and older men.4,19,30,31 Indeed, there have been no prospective population-based studies of event rates, incidence, outcome, or projected future burden of acute AAA on which to base assessments of the most appropriate screening strategy. Previous studies have been based on routinely collected hospital coding data,3,14–18,28,32–34 retrospective registries,35,36 or restricted to prevalent groups, limited by age and gender, or cohorts of volunteers with specific risk-factor profiles.4,19,22,26,30,31,37–40 We therefore determined event rates, incidence, early case fatality, and long-term outcome of all acute aortic events during 2002–2014 (before the introduction of national screening in our region) in a prospective, population-based study in Oxfordshire, UK, both overall and in relation to the 4 main risk factors. Using population projections, we also predict acute AAA incidence rates over the next 2 decades and estimate the likely impact of current and potential alternative screening programs.
Methods
The Oxford Vascular Study (OXVASC) study population comprises all individuals (12-year average =92 728), irrespective of age, registered with ≈100 family physicians in 9 general practices in Oxfordshire, UK.41 In the UK, the vast majority of individuals register with a general practice, which provides their primary health care and holds a lifelong record of all medical consultations, and details of medications, blood pressure (BP) measurements, and investigations. All participating practices held accurate age-sex patient registers and allowed regular searches of their computerized diagnostic coding systems. All practices refer patients to only 1 secondary care center. The OXVASC has been approved by the local research ethics committee.
Case ascertainment was by prospective daily searches for acute events in hospital (“hot pursuit”) and retrospective searches of hospital, primary care administrative and diagnostic coding data, and centralized death certification (“cold pursuit”) for cases missed by hot pursuit and deaths in the community. Hot pursuit was based on the daily assessment of all patients with a possible vascular event identified by: (1) daily searches of emergency department admission and symptom/diagnosis registers; (2) daily listing from the central admissions department of all patients from our general practices admitted to hospital, as well as assessment of these patients in hospital; (3) daily visits to the cardiac surgery and vascular surgery wards and review of daily lists of all patients referred to vascular surgery; (4) daily identification by bereavement officers of patients dead on arrival at the hospital or who died soon after; and (5) daily assessment of all patients undergoing diagnostic angiographic, angioplasty/stenting, or arterial surgical procedures in any territory.
The methods of cold pursuit were: (1) weekly review of all listed surgical procedures undertaken by vascular and cardiovascular (CV) surgery; (2) direct collection of general practice records and diagnoses from each individual practice on a monthly basis. All relevant vascular diagnoses made in primary care are assessed by a senior clinician within the study and the patient event is ascertained; (3) monthly practice-specific list of all patients with relevant diagnostic codes from the coding departments covering all acute and community hospitals (Hospital Episode Statistics [HES] data); (4) monthly visits to the coroner’s office to review out-of-hospital deaths; (5) review of all death certificates and relevant clinical details in the study practices; (6) practice-specific listings of all International Statistical Classification of Diseases, 10th revision (ICD-10) death codes from the local department of public health; and (7) review of vascular surgery outpatient clinic letters to identify patients who were not admitted to the hospital. For all cases not initially identified by HES data and death certification, these data sources were researched using NHS number and other identifiers, where possible.
After ascertainment, a study clinician assessed patients as soon as possible after the event. Informed consent was sought, where possible, or assent was obtained from a relative. Standardized clinical history and examination were recorded, along with details of medication, past medical history, all investigations relevant to the admission, and all interventions occurring subsequent to the event. All diagnoses were subsequently reviewed by a senior clinician. After this, aortic events with any ongoing diagnostic uncertainty were assessed by a separate consultant vascular surgeon to ensure agreement on classification. If a patient died before assessment or was only identified by cold pursuit, eyewitness accounts were obtained and relevant records reviewed. If death occurred outside the hospital, or before investigation, autopsy results were also reviewed. Clinical details were sought from primary care physicians or other clinicians on all deaths of possible vascular etiology.
All surviving patients were followed up by a research nurse at 6 months or by their family doctor, with recurrent events also identified by the ongoing study surveillance. If a recurrent vascular event was suspected, the patient was assessed by a study physician. Premorbid disability and disability on follow-up were assessed with the modified Rankin Scale (mRS).42 All study clinicians were trained in performing mRS assessments by completing the approved digital training course.
To assess the impact of screening in relation to risks factors documented in primary care, data from primary health care records for all cases, and for all of the underlying study population, were obtained (individual patient data for cases and age-/sex-specific tabular data for the population) on history of HTN and smoking status. The accuracy of these data was tested in cases by direct questioning of patients, relatives and review of records. In addition, all pre-morbid BP measurements were obtained from primary care records of incident cases (available in 98.1%). These were taken using automated sphygmometers with patients in the seated position. A cut-off value of 140/90 was used to define HTN. Current smoking was defined as daily smoking within the previous 12 months.
Analysis
All patients with acute vascular events affecting the aorta from April 1, 2002 to March 31, 2014 were assessed. Patients who had an event while temporarily away from Oxfordshire were included, but visitors to Oxfordshire who were not registered with one of the study practices were excluded. Acute AAAs were classified as either ruptured or symptomatic and defined by involvement of the aorta at or below the level of the renal arteries. Symptomatic cases were defined as acutely symptomatic aneurysms resulting in the need for emergency medical attention. These normally present as acute severe pain in the abdomen, back, or flank not obviously attributable to another cause and without evidence of aneurysm rupture on imaging.43 No strict aortic diameter was required for inclusion, but all cases did have aortic diameters of greater than 5.0 cm.
All events were categorized as first-ever incident or recurrent. A rupture in a patient with a previous symptomatic event was classified as a recurrent event if the delay to rupture was greater than 48 hours. The population structure was derived from the general practice age/sex registers and was based on the mean of the 10 mid-year population age-sex structures. Age- and sex-specific rates were calculated for all events of each type and for incident events. Analyses were done with SPSS software (version 20.0; SPSS, Inc., Chicago, IL). Numeric data are expressed as means (SD) or proportions, as appropriate. Group differences in continuous variables were examined with the Student t test or the Mann–Whitney U test for parametric and nonparametric variables, respectively. Group differences in categorical variables were examined with Fisher’s exact test or the chi-square test, as appropriate. Survival rates were derived by Kaplan-Meier analysis. P<0.05 was considered statistically significant.
Event- and aneurysm-related death (death within 30 days of acute presentation8,9) rates were projected to the whole UK population based on the 2010 census population. Future rates were also projected for 2020 and 2030 based on the Office of National Statistics (ONS) national population projections by age and sex for the UK,44 based on current incidence rates and separately by linear extrapolation of 2000–2012 time trends in age-/sex-specific incidence and mortality of acute AAA events in the UK extracted from ONS and HES data.
Estimates of the effectiveness of screening strategies were obtained by using MASS trial data on aneurysm screening efficacy in combination with our calculated event- and aneurysm-related death rates for the UK population. To calculate the number of life-years lost owing to acute AAA events in the UK, we used age- and sex-specific UK population life expectancies stratified by smoking status,45,46 obtained from the ONS and Institute and Faculty of Actuaries (IFoA). These expected life expectancies were reduced (10-year relative survival of 72.2% for men and 53.6% for women) to take into account the presence of atherosclerotic aneurysmal disease based on published registry data.47 It was thus possible to estimate the likely impact of current and potential alternative screening programs in terms of deaths prevented and life-years saved in 2010 based on the following key assumptions.
Key assumptions made when estimating screening strategy efficacy:
The time-period of “protection” afforded by screening was assumed to be 10 or 15 years in separate analyses, consistent with the range of estimates from the MASS trial which found a 48% (95% confidence interval [CI], 37 to 57) relative risk reduction (RRR) in aneurysm-related death up to 10 years from screening scan.8,9 We assumed a 50% RRR in 10-year risk for our primary analysis.
Estimates of screening efficacy in the MASS trial were based on prevention of deaths related to both elective and emergency presentations of AAA. Overall, 417 of 451 (92.5%) AAA-related deaths at 10-year follow-up in both treatment groups of the trial were the result of emergency presentations, with very similar RRRs found for the prevention of both emergency events (ruptured and symptomatic AAAs) and deaths related to emergency AAA presentations as found for overall AAA-related deaths. We therefore applied the 50% RRR to our estimates of the reduction in both acute events and deaths when calculating the effectiveness of screening strategies.
Screening in women was assumed to be as effective as shown in men.
Uptake of screening was assumed to be similar to that achieved in the MASS trial (80%).
The efficacy of screening was assumed to be the same at ages 65 and 75 as the overall results in the MASS trial, which included subjects from ages 65 to 74, and this result was also used to estimate the impact of targeting screening in smokers and hypertensives.
The overall benefit of screening at older ages (such as at age 75) will be dependent on the relative life expectancy of subjects with aneurysmal disease at this age, and this, in turn, is influenced by the competing risks for death from other diseases, such as coronary heart disease, cancer, and diabetes mellitus. In our analysis, we have calculated the estimated life expectancy of the UK population age 75 with known aortic aneurysmal disease stratified by smoking status. Although it is not possible to directly account for the competing risks of death resulting from individual diseases (given that these are not known), by calculating adjusted life expectancies, we have attempted to account for the overall impact of these competing risks in our analyses.
At 13-year follow-up in the MASS trial, 277 patients in the control group had undergone elective AAA repair for incidentally identified AAA, compared to 600 elective repairs in the screen-invited group, thereby reducing the effectiveness of screening. Overall, 64 of 600 (10.7%) of elective repairs in the screen-invited group occurred for incidentally detected AAAs found outside the screening program, and these were included in the screening efficacy analysis. To appropriately apply the MASS trial screening efficacy estimates to our population, we therefore also included acute AAA events in patients with previously incidentally detected aneurysms under surveillance in our analysis. However, sensitivity analyses excluding these patients were also performed.
- Screening provides the opportunity to identify people at increased general vascular risk and commence appropriate primary prevention. Given that we have applied the overall benefit obtained in MASS in our analyses, we hope to have accounted for this potential screening benefit. We concentrated on the reduction in AAA-related death that was reported in MASS and did not address the effect on all-cause mortality. Our reasons for this approach were:
- MASS did not show a significant reduction in non-AAA-related deaths. The 3% relative reduction in all-cause mortality appeared to be owing mainly to the reduction in AAA-related deaths. Indeed, in their 10-year results paper, the MASS trialists concluded that screening had not resulted in general differences in health care between the intervention groups;
- The more widespread use of statins and BP lowering in primary prevention during the last 10 to 15 years since MASS recruited would probably somewhat reduce any broader health impact of screening, even if such a benefit had been detected in the trial; and
- Recent data from the NHS screening program indicate low levels of antiplatelet, statin, and antihypertensive medication prescription in men found to have small aneurysms with multiple CV risk factors.
To estimate the likely impact of current and potential alternative screening programs on deaths prevented and life-years saved in 2020 and 2030, IFoA and ONS estimates of life expectancy in 2020 and 2030 were used, and it was assumed that AAA event and death rates will continue to decline in keeping with the recent national trends described above. The same proportionate reductions in age- and sex-specific life expectancy for 2020 and 2030 were applied (as were used above for 2010).
Results
Of the 92 728 study population, 23 808 were ≥55 years of age, of which 8954 (37.6%) had diagnosed HTN and 11 480 (48.2%) had ever smoked. A total of 196 acute nonocclusive aortic events occurred in 179 patients, including 127 acute aneurysms (108 abdominal, 6 thoracoabdominal, and 13 thoracic) and 69 acute dissections (51 Stanford type A and 18 type B). 103 incident acute AAA events were analyzed (rate =9/100 000/year; 72.8% male; mean age =78.7 years), of which 79 (76.7%) were incident ruptured aneurysms (7 in 100 000), 24 (23.3%) were acutely symptomatic aneurysms (2 in 100 000; Table1), and 61 were fatal (5/100 000/year). Of the 103 incident events, 31 (30.1%) were sudden deaths in the community and a further 6 (5.8%) died in transit to the hospital or shortly after arrival. Of the remaining 66 cases, 43 (65.1%) had emergency surgery (37 open; 6 endovascular).
Table 1.
Demographics and Risk Factors for Incident Acute Abdominal Aortic Aneurysms by Gender and Type
| Total (n =103) | Male (n =75) | Female (n =28) | P Value | Ruptured (n =79) | Symptomatic (n =24) | P Value | |
|---|---|---|---|---|---|---|---|
| Mean (SD) age, y | 78.7 (8.6) | 78.0 (8.5) | 80.6 (8.8) | 0.17 | 78.8 (9.2) | 78.2 (6.5) | 0.76 |
| Male (%) | 75 (72.8) | 59 (74.7) | 16 (66.7) | 0.44 | |||
| Previous vascular disease (%) | |||||||
| Angina | 32 (31.1) | 23 (30.7) | 9 (32.1) | 0.89 | 25 (31.6) | 7 (29.2) | 0.82 |
| Acute coronary syndrome | 25 (24.3) | 21 (28.0) | 4 (14.3) | 0.20 | 19 (24.1) | 6 (25.0) | 0.92 |
| Transient ischaemic attack | 18 (17.5) | 14 (18.7) | 4 (14.3) | 0.77 | 14 (17.7) | 4 (16.7) | 0.91 |
| Stroke | 18 (17.5) | 14 (18.7) | 4 (14.3) | 0.77 | 12 (15.2) | 6 (25.0) | 0.27 |
| Peripheral arterial disease | 19 (18.4) | 12 (16.0) | 7 (25.0) | 0.30 | 12 (15.2) | 7 (29.2) | 0.12 |
| Any | 59 (57.3) | 41 (54.7) | 18 (64.3) | 0.38 | 45 (57.0) | 14 (58.3) | 0.91 |
| Risk factors (%) | |||||||
| Current smoker | 35 (34.0) | 29 (38.7) | 6 (21.4) | 0.10 | 25 (31.6) | 10 (41.7) | 0.36 |
| Ever smoked | 79 (76.7) | 62 (82.7) | 17 (60.7) | 0.02 | 60 (75.9) | 19 (79.2) | 0.74 |
| Hypertension | 70 (68.0) | 44 (58.7) | 26 (92.9) | 0.001 | 51 (64.6) | 19 (79.2) | 0.18 |
| Diabetes mellitus | 11 (10.7) | 8 (10.7) | 3 (10.7) | 1.00 | 8 (10.1) | 3 (12.5) | 0.72 |
| Cardiac failure | 14 (13.6) | 8 (10.7) | 6 (21.4) | 0.16 | 12 (15.2) | 2 (8.3) | 0.51 |
| Atrial fibrillation | 20 (19.4) | 15 (20.0) | 5 (17.9) | 0.81 | 17 (21.5) | 3 (12.5) | 0.39 |
| Medications (%) | |||||||
| Statin | 45 (43.7) | 32 (42.7) | 13 (46.4) | 0.73 | 33 (41.8) | 12 (50.0) | 0.48 |
| Aspirin | 44 (42.7) | 33 (44.0) | 11 (39.3) | 0.67 | 33 (41.8) | 11 (45.8) | 0.73 |
| Other antiplatelet agents | 4 (3.9) | 3 (4.0) | 1 (3.6) | 1.00 | 2 (2.5) | 2 (8.3) | 0.23 |
| Warfarin | 10 (9.7) | 8 (10.7) | 2 (7.1) | 0.72 | 9 (11.4) | 1 (4.2) | 0.45 |
| Antihypertensives (%) | |||||||
| 0 | 31 (30.1) | 29 (38.7) | 2 (7.1) | 26 (32.9) | 5 (20.8) | ||
| 1 | 24 (23.3) | 14 (18.7) | 10 (35.7) | 18 (22.8) | 6 (25.0) | ||
| ≥2 | 48 (46.6) | 32 (42.7) | 16 (57.1) | 0.004 | 35 (44.3) | 13 (54.2) | 0.43 |
Annual incidence was 55 in 100 000 in men ages 65 to 74 years, but was higher in older men (75 to 85: 112 in 100 000; ≥85: 298 in 100 000) and older women (≥85: 82 in 100 000), with only 22.3% of incident events and 13.1% of aneurysm-related deaths occurring in men ages 65 to 74 (Table2). Figure1 compares our incidence rates with those derived from HES data and death certification over the same time period for both our local Oxfordshire population and the UK. A total of 41.7% of incident acute AAA events were missed by routine HES and mortality coding when using the acute AAA code (I-713), mainly owing to incorrect coding as elective procedures, unspecified aneurysm, or thoracic aneurysm hospital episodes (n =35) or as acute coronary syndromes or aortic dissection events (n =8). Of cases that reached the hospital, only 34 of 66 were coded correctly. Coding was least accurate for acutely symptomatic aneurysms: 25.0% being correctly coded versus 67.1% of ruptured aneurysms (P<0.0001). Using all acute aneurysm codes (I-711, I-713, I-715, and I-718) increased the number of correctly identified cases to 67.0% (78.4% for aneurysm-related deaths), but at the expense of increased false positives (15.9%) owing mainly to inclusion of nonabdominal aneurysms. No significant improvement in coding analysis was detected throughout the study period.
Table 2.
Age- and Sex-Specific Rates Per Hundred Thousand Population (2002–2014) for Incident Acute Abdominal Aortic Aneurysm Events in: the Total Population; Current Smokers; Ex-Smokers; and Those With Diagnosed Hypertension
| Age, y | 45 to 54 | 55 to 64 | 65 to 74 | 75 to 84 | ≥85 | Total |
|---|---|---|---|---|---|---|
| Total population | ||||||
| Men | ||||||
| Cases | 0 | 5 | 23 | 28 | 19 | 75 |
| Rate/100 000 (95% CI) | — | 8 (3, 19) | 55 (35, 82) | 112 (75, 162) | 298 (179, 465) | 13 (10, 17) |
| Women | ||||||
| Cases | 0 | 2 | 5 | 10 | 11 | 28 |
| Rate/100 000 (95% CI) | — | 3 (0, 12) | 11 (4, 26) | 31 (15, 58) | 82 (41, 146) | 5 (3, 7) |
| Total | ||||||
| Cases | 0 | 7 | 28 | 38 | 30 | 103 |
| Rate/100 000 (95% CI) | — | 6 (2, 12) | 32 (22, 47) | 67 (47, 92) | 151 (102, 216) | 9 (8, 11) |
| Current smokers | ||||||
| Men | ||||||
| Cases | 0 | 4 | 17 | 6 | 2 | 29 |
| Rate/100 000 (95% CI) | — | 34 (9, 88) | 274 (159, 438) | 151 (56, 329) | 209 (25, 755) | 27 (18, 39) |
| Women | ||||||
| Cases | 0 | 1 | 2 | 3 | 0 | 6 |
| Rate/100 000 (95% CI) | — | 12 (0, 64) | 45 (5, 163) | 66 (14, 191) | — | 7 (3, 15) |
| Total | ||||||
| Cases | 0 | 5 | 19 | 9 | 2 | 35 |
| Rate/100 000 (95% CI) | — | 25 (8, 58) | 179 (108, 279) | 105 (48, 200) | 72 (9, 261) | 18 (12, 25) |
| Ex-smokers | ||||||
| Men | ||||||
| Cases | 0 | 1 | 5 | 14 | 13 | 33 |
| Rate/100 000 (95% CI) | — | 6 (0, 36) | 29 (9, 67) | 124 (68, 208) | 452 (240, 772) | 34 (24, 48) |
| Women | ||||||
| Cases | 0 | 0 | 3 | 4 | 4 | 11 |
| Rate/100 000 (95% CI) | — | — | 20 (4, 58) | 31 (9, 80) | 84 (23, 214) | 12 (6, 21) |
| Total | ||||||
| Cases | 0 | 1 | 8 | 18 | 17 | 44 |
| Rate/100 000 (95% CI) | — | 3 (0, 18) | 25 (11, 48) | 75 (44, 118) | 222 (129, 355) | 23 (17, 31) |
| Hypertensives | ||||||
| Men | ||||||
| Cases | 0 | 2 | 13 | 15 | 14 | 44 |
| Rate/100 000 (95% CI) | — | 13 (2, 48) | 72 (38, 124) | 119 (66, 196) | 360 (197, 604) | 71 (51, 95) |
| Women | ||||||
| Cases | 0 | 2 | 4 | 10 | 10 | 26 |
| Rate/100 000 (95% CI) | — | 15 (2, 56) | 21 (6, 55) | 57 (27, 104) | 128 (61, 235) | 37 (24, 55) |
| Total | ||||||
| Cases | 0 | 4 | 17 | 25 | 24 | 70 |
| Rate/100 000 (95% CI) | — | 14 (4, 37) | 46 (27, 74) | 82 (53, 122) | 205 (131, 305) | 53 (41, 67) |
CI indicates confidence interval.
Figure 1.
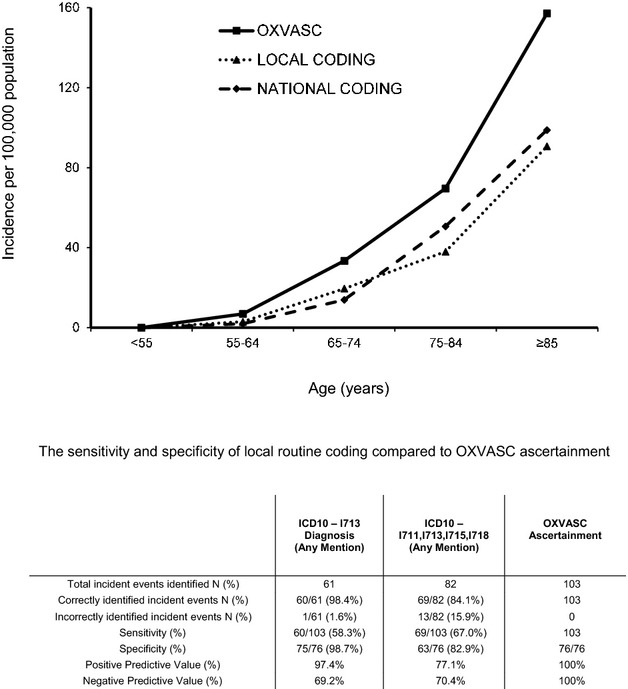
Efficacy of routine local and national coding (Hospital Episode Statistics data and death certification) in identifying acute abdominal aortic aneurysm events as compared to OXVASC ascertainment. OXVASC ascertainment is taken as the gold standard. ICD10 refers to the International Classification of Diseases, 10th Revision. I713 is the code for acute/ruptured abdominal aortic aneurysm. I711, I713, I715, and I718 codes refer to acute/ruptured aortic aneurysms at other anatomical locations. Only incident events were analyzed. For specificity calculations, the total number of OXVASC incident acute aortic events (179) during the 12-year study period was used. OXVASC indicates the Oxford Vascular Study.
Extrapolating our incidence rates to the 2010 UK population (Figure2) yielded a total of 6905 incident events per year, 67.1% age ≥75 years, and 32.3% age ≥85 years. The corresponding number of deaths resulting from an incident acute AAA was 3957, 77.7% age ≥75 years, and 39.1% age ≥85 years (Figure3). Incorporating current UK age- and sex-specific life expectancies, stratified by smoking status, and adjusted for atherosclerotic aneurysmal disease, the life-years lost owing to acute AAA events were 21 800 (Table2).
Figure 2.
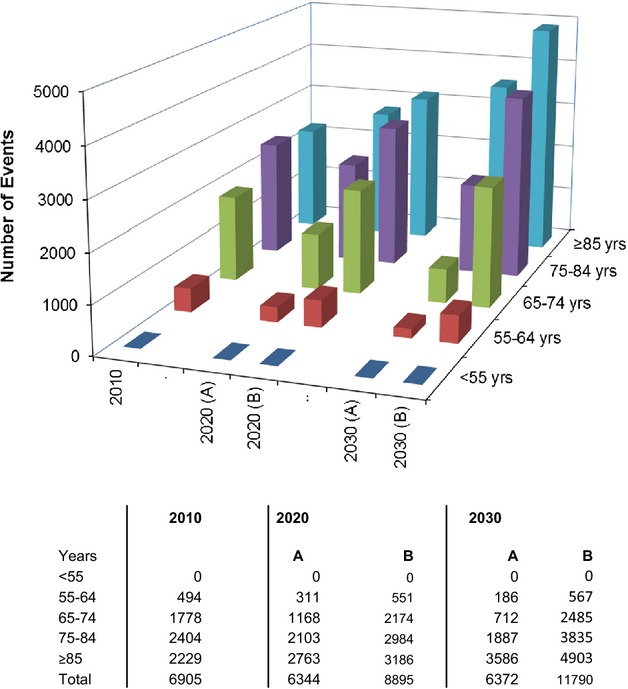
Projected number of incident acute abdominal aortic events occurring in the UK population in 2010, 2020, and 2030 stratified by age and incidence rates: A, if rates reduce by the same age-adjusted degree as they have for last decade; and B, if they remain unchanged from current rates.
Figure 3.
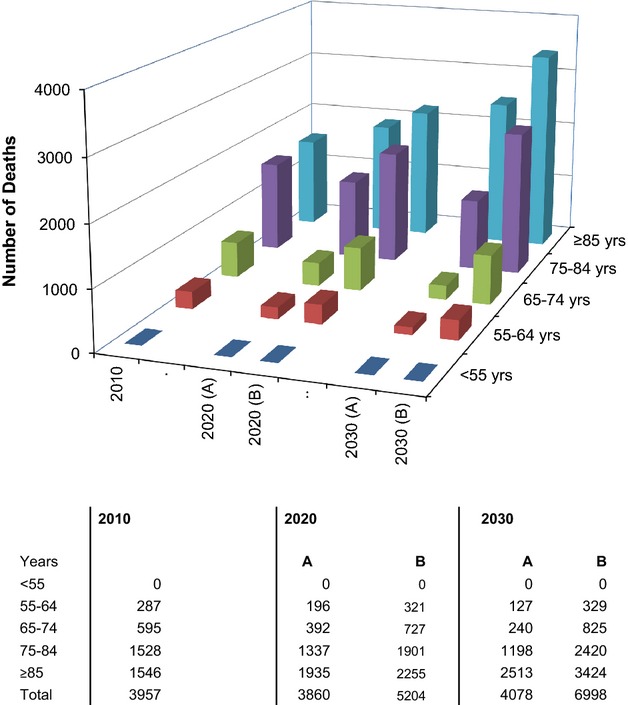
Projected number of acute abdominal aortic aneurysm-related deaths occurring in the UK population in 2010, 2020, and 2030 stratified by age and death rates: A, if rates reduce by the same age-adjusted degree as they have for last decade; and B, if they remain unchanged from current rates.
Based on projected changes in UK population structure and current time trends in incidence of acute AAA, the annual numbers of incident cases in the UK in 2030 should remain fairly static, but by then 85.9% of events will occur at age ≥75 years and 56.3% at age ≥85 years (Figure2). Likewise, by 2030, the total number of acute aneurysm-related deaths will be static, but over 90% will occur at age ≥75 years, 61.6% at ≥85 years, and 28.6% will be in women (Figure3), with a similar number of life-years lost.
Using our extrapolated event rates and trial evidence of a 50% reduction in 10-year aneurysm-related deaths from one-off screening of men age 65, currently the UK screening program would have prevented ≈10.7% of acute incident events, 5.6% of aneurysm-related deaths, and 12.0% of life-years lost (315 200 scans/year; 1426 per death prevented, 121 per year of life saved; Tables 3, 4, and S1 through S4). Taking into account future projected changes in UK population demographics and current trends in incidence, by 2030 the proportions prevented fall to 4.6% for incident events, 2.1% for related deaths, and 5.2% of life-years saved (421 700 scans/year; 4827 per death prevented, 369 per year of life saved; Tables 3, 4, and S1 through S4). If the current screening program were extended to include a repeat scan in all men age 75 and a first scan in all women age 75, this would prevent ≈28.1% of acute incident events, 24.9% of aneurysm-related deaths, and 37.7% of life-years lost, but require 752 100 scans/year (Tables S2 through S4).
Table 3.
Projected Impact of the Current UK Screening Program on Acute AAA Events Versus Alternative Strategies if Incidence Rates Reduce by the Same Age-Adjusted Degree as They Have for Last Decade
| 2010* | 2020* | 2030* | ||||
|---|---|---|---|---|---|---|
| Total UK population ≥55 years | 17 646 359 | 20 861 307 | 23 923 713 | |||
| Annual acute AAA events | 6905 | 6344 | 6372 | |||
| Length of screening efficacy, y | 10 | 15 | 10 | 15 | 10 | 15 |
| Screening program | ||||||
| Current: men age 65 | ||||||
| Acute AAA events expected in screened population, N (%) | 1472 (21.3) | 2411 (34.9) | 960 (15.1) | 1782 (28.1) | 581 (9.1) | 1306 (20.5) |
| Acute AAA events prevented, N (%) | 736 (10.7) | 1206 (17.5) | 480 (7.6) | 891 (14.0) | 291 (4.6) | 653 (10.3) |
| Annual scans | 315 200 | 315 200 | 325 400 | 325 400 | 421 700 | 421 700 |
| Scans required to prevent 1 event | 428 | 261 | 678 | 365 | 1449 | 646 |
| Alternative A: men age 65 current smokers†+all men age 75 | ||||||
| Acute AAA events expected in screened population, N (%) | 3130 (45.3) | 3779 (54.7) | 2459 (38.8) | 3301 (52.0) | 1945 (30.5) | 3038 (47.7) |
| Acute AAA events prevented, N (%) | 1565 (22.7) | 1889 (27.4) | 1230 (19.4) | 1650 (26.0) | 972 (15.3) | 1519 (23.8) |
| Annual scans | 247 900 | 247 900 | 307 200 | 307 200 | 336 800 | 336 800 |
| Scans required to prevent 1 event | 158 | 131 | 250 | 186 | 347 | 222 |
| Alternative B: men age 65 current smokers†+all men age 75+women age 75 with diagnosed hypertension | ||||||
| Acute AAA events expected in screened population, N (%) | 3654 (52.9) | 4727 (68.5) | 2919 (46.0) | 4252 (67.0) | 2381 (37.4) | 4111 (64.5) |
| Acute AAA events prevented, N (%) | 1827 (26.5) | 2363 (34.2) | 1460 (23.0) | 2126 (33.5) | 1191 (18.7) | 2055 (32.3) |
| Annual scans | 336 400 | 336 400 | 417 200 | 417 200 | 452 700 | 452 700 |
| Scans required to prevent 1 event | 184 | 142 | 286 | 196 | 380 | 220 |
Values are given as overall numbers with percentages in brackets, unless otherwise stated. AAA indicates abdominal aortic aneurysm; ONS, Office of National Statistics.
Incorporates 2010 UK population census and ONS Principal Population Projections for 2010–2030.
Current smoking was defined as daily smoking within the previous 12 months.
Table 4.
Projected Impact on Aneurysm-Related Death of the Current UK Screening Program Versus Alternative Strategies if Incidence Rates Reduce by the Same Age-Adjusted Degree as They Have for Last Decade
| 2010* | 2020* | 2030* | ||||
|---|---|---|---|---|---|---|
| Total UK population ≥55 years | 17 646 359 | 20 861 307 | 23 923 713 | |||
| Annual AAA-related deaths | 3957 | 3860 | 4078 | |||
| Length of screening efficacy, y | 10 | 15 | 10 | 15 | 10 | 15 |
| Screening program | ||||||
| Current: men age 65 | ||||||
| AAA-related deaths expected in screened population, N (%) | 441 (11.2) | 1056 (26.7) | 288 (7.5) | 825 (21.4) | 174 (4.3) | 649 (15.9) |
| AAA-related deaths prevented, N (%) | 221 (5.6) | 528 (13.3) | 144 (3.7) | 413 (10.7) | 87 (2.1) | 324 (8.0) |
| Annual scans | 315 200 | 315 200 | 325 400 | 325 400 | 421 700 | 421 700 |
| Scans required to prevent 1 death | 1426 | 597 | 2260 | 788 | 4847 | 1302 |
| Alternative A: men age 65 current smokers†+all men age 75 | ||||||
| AAA-related deaths expected in screened population, N (%) | 1670 (42.2) | 2189 (55.3) | 1362 (35.3) | 2036 (52.7) | 1123 (27.5) | 1998 (49.0) |
| AAA-related deaths prevented, N (%) | 835 (21.1) | 1095 (27.7) | 681 (17.6) | 1018 (26.4) | 561 (13.8) | 999 (24.5) |
| Annual scans required | 247 900 | 247 900 | 307 200 | 307 200 | 336 800 | 336 800 |
| Scans required to prevent 1 death | 297 | 226 | 451 | 302 | 600 | 337 |
| Alternative B: men age 65 current smokers†+all men age 75+women age 75 with diagnosed hypertension | ||||||
| AAA-related deaths expected in screened population, N (%) | 1970 (49.8) | 2743 (69.3) | 1625 (42.1) | 2593 (67.2) | 1372 (33.7) | 2629 (64.5) |
| AAA-related deaths prevented, N (%) | 985 (24.9) | 1371 (34.7) | 813 (21.0) | 1296 (33.6) | 686 (16.8) | 1314 (32.2) |
| Annual scans required | 336 400 | 336 400 | 417 200 | 417 200 | 452 700 | 452 700 |
| Scans required to prevent 1 death | 342 | 245 | 513 | 322 | 660 | 345 |
AAA indicates abdominal aortic aneurysm.
Incorporates 2010 UK population census and ONS Principal Population Projections for 2010–2030.
Current smoking was defined as daily smoking within the previous 12 months.
The increase in number of scans required to screen all men at age 65 and all men and women at age 75 could be reduced by a risk factor-targeted approach. For example, 33 of 35 of all events at age <75 years occurred in current (n =24) or previous (n =9) smokers (Figure4), a much higher prevalence of current smoking (P<0.001) than in the underlying study population (Table S5), although overall prevalence of current or previous smoking among cases was lower in women (60.7% versus 82.7% in men; P =0.02; Table1). In addition, the mean age at event was lowest in current smokers, compared to ex-smokers and never-smokers (P<0.0001; Table5). For those who gave up smoking ≥10 years before event, age at event was similar to that of never-smokers (Table5). Incidence rates were greatest (274/100 000/year) in male current smokers men ages 65 to 74 years (Table2). In view of this, screening only male smokers age 65 and then all men at age 75 would have prevented twice the number of events (22.7%), 4 times the number of deaths (21.1%), and almost 3 times the number of life-years lost (33.3%) as the current screening strategy, with a 21.4% reduction in the number of scans/year required (247 900); 297 per death prevented; and 34 per year of life saved (Tables 3, 4, and S1 through S4). For this strategy, by 2030 the proportions prevented would be 15.3% for incident events, 13.8% for related deaths, and 24.7% for life-years saved.
Figure 4.
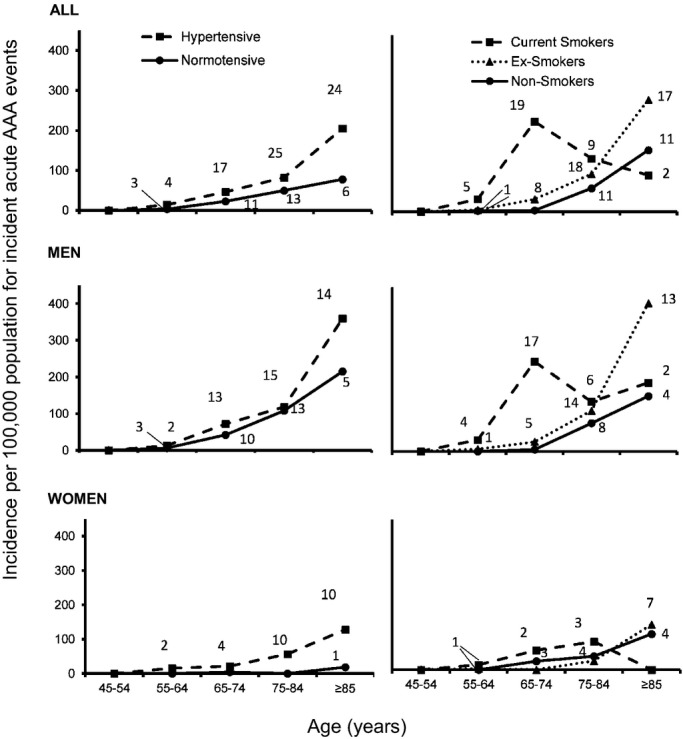
Age- and sex-specific rates per hundred thousand population (2002–2014) for incident acute events by risk factor status. The term “normotensive” refers to no diagnosis of hypertension made before event. Data labels refer to total number of incident events occurring in study period. AAA indicates abdominal aortic aneurysm.
Table 5.
Smoking Status and Age of Acute Event Stratified by Hypertensive Status
| Life-Long Nonsmoker | Ex-Smoker | Current Smoker | P Value | Total | |||
|---|---|---|---|---|---|---|---|
| Give Up >10 Years Before | Give Up <10 Years Before | All | |||||
| N (%) | 24 (23.3) | 29 (28.2) | 15 (14.6) | 44 (42.8) | 35 (34.0) | 103 | |
| Male, N (%) | 13 (54.2) | 24 (82.8) | 9 (60.0) | 33 (75.0) | 29 (82.9) | 0.03 | 75 (72.8) |
| Hypertension, N (%) | 16 (66.7) | 21 (72.4) | 11 (73.3) | 32 (72.7) | 22 (62.9) | 0.83 | 70 (68.0) |
| Mean (SD) age, y | 83.3 (7.9) | 83.7 (6.7) | 76.9 (5.1) | 81.2 (7.0) | 72.2 (7.2) | <0.001 | 78.7 (8.6) |
| Mean (SD) age, years in those with diagnosed hypertension | 84.4 (8.8) | 83.7 (5.6) | 77.1 (5.8) | 81.2 (6.5) | 72.9 (7.9) | <0.001 | 79.4 (8.7) |
| Mean (SD) age, years in those not diagnosed hypertensive | 81.2 (5.8) | 83.8 (9.4) | 76.3 (3.1) | 81.3 (8.5) | 70.8 (5.7) | 0.001 | 77.1 (8.4) |
In contrast to current smoking, which was a powerful risk factor for premature disease in both men and women (Figure4 and Tables S5 and S6), HTN was the predominant risk factor in women at all ages, but was less important in men, with only 7.1% of female cases being normotensive versus 41.3% of male cases (P<0.0001; Figure4). Consequently, among women with HTN, incidence of acute AAA reached 57/100 000/year at ages 75 to 85 and 128/100 000/year at age ≥85 (Table2 and Figure4). Premorbid control of BP was also worse in women. Over the 5 years preceding the incident event, the mean (SD) proportion of all BPs recorded in primary care (mean number of readings =12/patient) that were greater than 140/90 mm Hg averaged 53.0% (27.0) in women and 36.1% (31.7) in men (difference, P =0.02). The highest recorded SBP in the 5 years preceding the event averaged 197.2 (SD =27.8) mm Hg in women and 171.6 (25.6) in men (P<0.0001), despite more-intensive (P =0.004) use of antihypertensive drugs in women (Table1).
A screen of women age 75 with known HTN would have prevented 27.4% of events in women, 30.0% of deaths, and 16.8% of life-years lost (88 500 scans/year; 590 per death prevented, 93 per year of life saved), although impact on overall numbers of events would be more limited (3.8% of events prevented, 3.8% of deaths, and 4.4% of life-years lost overall; Tables S3 and S4). Further targeting screening by history of CV disease (any transient ischemic attack, stroke, myocardial infarction, angina, or peripheral vascular disease) would be unlikely to be effective given that only 18 of 38 (47.4%) acute AAA ages 75 to 84 had such a history.
Overall 30-day case fatality rate was 59.2% for incident acute AAA and was 41.7% for patients reaching the hospital alive. Thirty-day fatality was higher for ruptured aneurysms (74.7%), compared with acutely symptomatic aneurysms (8.3%; P<0.0001; Figure5). For all cases, mean (SD) length of stay was 9.25 (±15.5) days and intensive care unit (ICU) stay was 4.8 (±10.7), 15.3 (±17.5), and 7.9 (±12.9) days, respectively, for those admitted to the hospital alive. For those discharged from the hospital alive (n =40), mean (SD) length of stay and ICU stay were 19.3 (±15.8) and 6.9 (±7.3) days and were similar for cases age ≥75 years (mean, 16.4±13.5) and age <75 years (21.8±17.6; P =0.36). Five patients required community hospital rehabilitation and 2 required permanent care home placement, all of whom were age ≥75 years.
Figure 5.
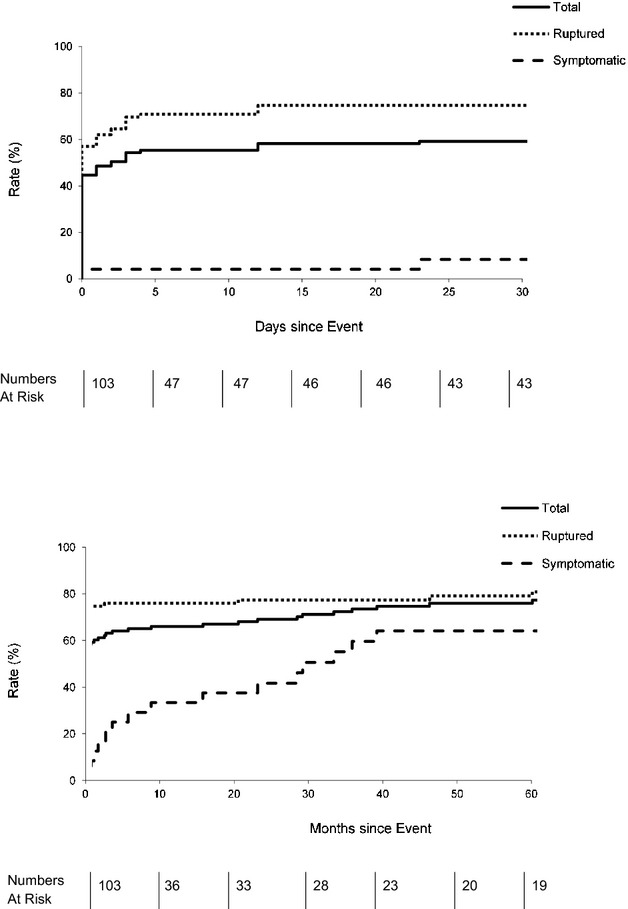
Mortality of acute abdominal aortic aneurysms with numbers at risk tabled below. Top: 30-day mortality; bottom: 5-year mortality.
Although 30-day case fatality for elective repair of AAA in the UK national audit increases with age (2% at age <75 years; 6% at ≥85 years; Table S6), there was no evidence that effectiveness of screening at older ages would be undermined by other factors. Thirty-day case fatality resulting from acute AAA increased from 40.0% at age <75 years to 69.1% at age ≥75 years (P =0.01; Table S6). Among patients with incident acute AAA, rates of premorbid disability also increased with age (Table S7), but 97.4% (76/78) of those age ≥75 years were mobile with or without walking aid (mRS, 0 to 3). The proportion of patients who were dead or had an increase in disability after the event increased with age was 48.6% (17 of 35) at age <75 years versus 86.8% (59 of 68) at age ≥75 years (P<0.0001).
Of the 103 patients with acute AAA, 15 (14.6%) had a known AAA under surveillance. For these cases, mean (SD) age at event was 81.3 (8.3) and at previous diagnosis was 77.0 (9.8). Ten patients presented with ruptured AAAs and 5 with acutely symptomatic aneurysms. Five (33.3%) died before arrival at the hospital and 8 (53.3%) died within 30 days of event. However, only 1 acute AAA event was in a male ages 65 to 74 and 7 were age ≥85 years. Our estimates of the effectiveness of screening strategies at ages 65 and 75 were not altered by excluding these cases (Tables S8 and S9).
Discussion
To our knowledge, this is the first prospective, population-based study of incidence, risk factors, and outcome of all acute AAA events without exclusion of patients by age or sex, thereby allowing estimation of the impact of screening in different groups and also taking into account the impact of risk factors on risk of acute AAA, as opposed to the prevalence of AAA. We reported several important findings. First, our incidence of acute AAA (9/100 000/year) and related death rates (5/100 000/year) were 30% to 40% higher than local and national estimates based on routine coding data.16–18 Second, however, we showed that incidence in men ages 65 to 74 years was lower than that found in the MASS trial (55/100 000/year vs. 96/100 000/year in the MASS control group, P =0.02), in keeping with recent time-trend data from coding studies, suggesting that AAA admission and death rates have fallen in younger age groups over the last 2 decades,16,17 and with current screening programs reporting a lower-than-expected prevalence of AAAs in men age 65.10,14,15 Third, we showed that nearly two thirds of all acute AAA now occur at age ≥75 years and that such events have a higher case fatality than at younger ages. Fourth, we showed that the total annual number of events, deaths, and life-years lost in the UK will remain fairly static over the next few decades if age-specific incidence continues to fall, but that the proportion of all AAA-related deaths that occur at age ≥75 will increase from 77.7% in 2010 to 91.0% by 2030, by which time 56.3% of events and 61.6% of deaths will occur at age ≥85. Fifth, we showed that, currently, the recently introduced UK screening program would prevent less than 10% of all aneurysm-related deaths, with this proportion likely to fall to around 2% by 2030. Finally, we estimated the population impact and logistic implications of several alternative screening strategies, supported by age-specific data on risk factors, premorbid disability rates, and outcomes, all of which should influence decisions about the optimal screening strategy.
Extension of the current UK screening program to all men and women at age 75 would result in a 3- to 4-fold increase in the proportion of deaths prevented and life-years lost, owing to the much higher incidence of acute AAA events at ages 75 to 84 years than at 64 to 75 and the higher case fatality at older ages. Although the uptake of screening might be lower at older ages and suitability for surgery of patients with screen-detected AAA might be lower than at age 65, we found no evidence that age alone should be a bar to screening. First, 97.4% of patients with acute AAA age ≥75 years were mobile (mRS, 0 to 3) before the event, but 86.8% were dead or more disabled after the event. Indeed, the increase in 30-day case fatality of acute AAA events with age was much greater in absolute terms than the increase in case fatality owing to elective AAA repair. One quarter of elective AAA repairs in the UK are already performed in patients over the age of 80,48 with excellent functional outcomes in the vast majority.49 Second, our estimates of effectiveness of screening took into account the shorter life expectancy in older individuals with known aneurysmal disease. A mean life expectancy after elective AAA surgery in all men age 75 of 9.2 years would not appear to be a bar to screening and is not markedly lower than that for male smokers age 65 (11.8 years), who are the de facto target of the current screening program. Indeed, screening only at age 65 years could be said to penalize nonsmokers, in whom the mean age of acute AAA was nearly 20 years older, by which time any benefit from previous screening would be lost.
We have also shown that a risk-factor–targeted screening strategy would both increase the number of deaths prevented and life-years saved and reduce the number of scans required per year. Current smoking was particularly strongly associated with occurrence of acute AAA at younger ages. The U.S. Medicare screening program limits screening to men age 65 years who have ever smoked, whereas our data suggest that screening could be further limited to current smokers. Screening only male current smokers age 65 and then all men at age 75 would result in an almost 4-fold increase in the number of deaths prevented and a 3-fold increase in the number of life-years saved, compared to the current UK strategy, with an approximate 20% reduction in the number of scans required.
Previous HTN was a particularly important risk factor in women, being present in 93% of those with incident acute AAA. Over one quarter of acute AAA events at all ages currently occur in women, rising to one third by 2030, and so consideration should be given to offering screening to women with risk factors, and a policy of screening all women age 75 with known HTN might be evaluated in randomized trials. We found that such a policy would potentially prevent 150 deaths per year in the UK, with 590 scans required to prevent each death and 93 scans per year of life saved.
The particularly strong influence of smoking and HTN on risk of rupture and their apparent interactions with age and sex are consistent with previous studies of rupture during follow-up of patients with prevalent AAA.27–30 Although current smoking increases rate of expansion of AAA by 15% to 20%, it doubles the risk of rupture.29 We have shown that men ages 65 to 74 who smoke have an approximate 3% 10-year risk of acute AAA, highlighting the need for screening campaigns to reach this very-high-risk group. However, we found a much lower risk in ex-smokers (Figure4), suggesting that smoking cessation be very strongly encouraged in men with small presurgical aneurysms or those unfit for surgery. HTN also appears to have little effect on rate of AAA expansion in cohort studies, but increases the risk of rupture by 30% per 10 mm Hg increase in mean BP.29 The particularly strong association between HTN and risk of acute AAA in women in our study might explain why although women have a lower aneurysm prevalence, and presumably therefore a reduced underlying susceptibility, they have a 4-fold increase in risk of subsequent rupture (adjusted for aneurysm diameter).29 Not only were almost all women with acute AAA in our study hypertensive, but also premorbid control of BP was also substantially worse than in men, highlighting the importance of aggressive management of HTN in women with presurgical AAA.
We also found that one third of all acute AAA events occur at age ≥85 years and that this proportion is likely to rise to over one half by 2030. Although rescreening at age 85 would be controversial, and life expectancy after surgery at this age is only around 5 years, longer-term survival might increase in the future and screening of selected individuals might be appropriate. In terms of targeting screening in this age group, we found that HTN continued to be a risk factor for acute AAA, but that smoking was less strongly associated (Figure4).
Although we consider our findings to be valid, our study has a number of potential shortcomings:
Although the OXVASC population covers a broad range of deprivation, with 22% of districts ranking in the lower third nationally, the population is 94% white and the proportions of other racial groups are too small (3.1% Asian, 1.5% Chinese, and 1.4% Afro-Caribbean) to determine separate event rates and outcomes.50 However, the proportion of whites is similar in the UK as a whole (88% white) and in many other western countries (Australia, 90%; France, 91%; Germany, 93.9%). Although the demographic differences between Oxfordshire and the rest of the UK might undermine the generalizability of our findings, the coding-based incidence of acute AAA in OXVASC was very similar to that for the UK as a whole (Figure2).
Despite our overlapping ascertainment methods, it is unlikely that we ascertained every single acute aortic event in our population. Although we identified many out-of-hospital deaths, some sudden deaths in the community resulting from acute AAA will undoubtedly go undiagnosed. However, such underascertainment is likely to be greater at older ages, where postmortem examination is less routine, such that our estimate of the proportion of deaths that occur at older ages might be conservative. Moreover, estimates of screening efficacy in the MASS trial would have been similarly affected. It is possible therefore that MASS may have underestimated the benefit of screening. However, MASS did not find a reduction in non-AAA-related death in the screening group, suggesting that any underestimation of events had a limited impact.
The Rankin scale is a handicap assessment tool that was originally formulated in 1957 as a validated method for assessing cerebrovascular event severity and outcome. Over the last 2 decades, it has been used to scale disability subsequent to a variety of acute and chronic conditions, including cardiac arrest and Parkinson’s disease. It is not specifically designed for use after AAA events, but the reasons for using it in this study are that it provides a simple generic measure of handicap, and it now allows the premorbid and postevent handicap after AAA to be compared with similar Rankin-based data in other acute and chronic conditions. We hope that the use of this scale adds important data necessary for assessing the effectiveness of AAA screening at older ages.
Our projections of future event rates will inevitably be prone to error given the uncertainty about changes in risk factors and preventive treatments in the future. However, extrapolation of the current rate of decline in age-specific incidence is not unreasonable and, following this continuation, will tend to push AAA incidence in a direction that would support our suggested screening changes, that is, increased life history of smoking in currently middle-aged and older women adds to the case for screening women at age 75 years, and reduced smoking in men is likely to mean that incidence in men will continue to move to older ages. To avoid potential errors in the calculation of absolute rates, we have focused on the relative proportions of events in various age and risk groups, which remain relatively constant independent of the absolute current incidence rates used in the analysis. Unfortunately, it is not possible to provide reliable confidence intervals for the absolute outcome numbers provided in Tables 3 and 4 owing to the fact that the ONS and IFoA do not provide these for their UK population projections.
There is still some uncertainty about the rate of decline in the protective effect of screening with time from last screen.9,20 We therefore reported separate analyses based on time periods of “protection” afforded by screening of 10 or 15 years, based on the latest follow-up estimates from the MASS trial, and used a 50% RRR for both acute AAA events and related deaths.9,20 In a similar fashion to MASS, we have used 10-year age groups (65 to 74 and 75 to 84 years) to estimate the effectiveness of screening at age 65 and 75.
Our estimates of the effectiveness of screening in men and women age 75 was based on assumptions that uptake of screening and willingness to undergo surgery for screen-detected AAA would be the same as that shown for men ages 65 to 74 in the MASS trial. However, our main finding that screening of male smokers at age 65 and all men at age 75 would prevent nearly 4 times as many deaths and 3 times as many life-years lost given that the current program with fewer scans required is sufficiently clear cut to be robust to alterations in the underlying assumptions.
Although our main analyses were based on only 103 acute AAA events, inevitably limiting the precision of some of our estimates of rates and risk factor associations, our study is still one of the largest-ever prospective studies of acute AAA events and is the first unselected population-based study not based only on routinely collected coding data. National-level studies of routine coding data have the advantage of large numbers, but we have shown that they are likely to miss over 40% of incident events, particularly acutely symptomatic AAAs, and they are unable to address other key issues, such as premorbid functional state and functional outcome, which are essential to considerations of the likely effectiveness and appropriateness of screening. Our access in OXVASC to the lifelong record of all medical consultations, medications details, BP measurements, and investigations for patients with acute events and the availability of age- and sex-specific risk factor data for the underlying study population (defined in the same way as in the cases) is also vital to any assessment targeted screening beyond consideration of only age and sex.
In conclusion, in the first prospective, unselected, population-based study of acute AAA events, we have shown a high incidence and case fatality in older ages. The majority of acute events, related deaths, and life-years lost now occur in those over age 75 and this will shift to age ≥85 over the next few decades. These observations have implications for health service provision and national screening strategies.
Sources of Funding
The Oxford Vascular Study is funded by the Wellcome Trust, Wolfson Foundation, UK Medical Research Council, the Dunhill Medical Trust, the Stroke Association, the National Institute for Health Research (NIHR), and the NIHR Biomedical Research Center, Oxford. Howard is funded by the NIHR Biomedical Research Center, Oxford. Rothwell has a Wellcome Trust Senior Investigator Award and an NIHR Senior Investigator Award.
Acknowledgments
The authors are grateful to all the staff in the general practices that collaborated in OXVASC: Abingdon Surgery, Stert St, Abingdon; Malthouse Surgery, Abingdon; Marcham Road Family Health Center, Abingdon; The Health Center, Berinsfield; Kidlington Health Center; Kidlington; Yarnton Health Center, Kidlington; 19 Beaumont Street, Oxford; East Oxford Health Center, Oxford; Church Street Practice, Wantage. In addition, the authors are grateful to Mr Edward Choke for supplying extracted data from ONS and HES for time-trend analysis.
Disclosures
None.
Supporting Information
Table S1. Expolated life expectancies for the general population and elective aneurysm repair survivors.
Table S2. Current and projected life-years gained per year for the current screening strategy and potential alternatives.
Table S3. Sensitivity analysis on the projected impact of screening programme alternative strategies on acute abdominal aortic events if incidence rates reduce by the same age-adjusted degree as they have for last decade.
Table S4. Sensitivity analysis on the projected impact on aneurysm-related death for screening program alternative strategies if incidence rates reduce by the same age-adjusted degree as they have for last decade.
Table S5. Comparison of the presence of risk factors in those with acute events versus the background study population expressed as age-/sex-adjusted risk ratios.
Table S6. OXVASC: events and aneurysm-related mortality rates by age and risk factor status.
Table S7. Changes in disability status for patients with incident acute events.
Table S8. Additional sensitivity analysis on the projected impact of the current UK screening program on acute AAA events versus alternative strategies if cases with known AAAs are excluded (and incidence rates reduce by the same age-adjusted degree as they have for last decade).
Table S9. Additional sensitivity analysis on the projected impact of the current UK screening program on aneurysm-related deaths versus alternative strategies if cases with known prevalent AAAs already under surveillance are excluded (and incidence rates reduce by the same age-adjusted degree as they have for last decade).
References
- Mortality Statistics Series. 2013. Office of National Statistics. Available at: www.statistics.gov.uk. Accessed 08.04.2014.
- Institute for Health Metrics and Evaluation. Global burden of disease study. Available at: http://www.healthmetricsandevaluation.org/gbd/visualizations/gbd-cause-patterns. Accessed 08.04.2014.
- Heikkinen M, Salenius JP, Auvinen O. Ruptured abdominal aortic aneurysm in a well-defined geographic area. J Vasc Surg. 2002;36:291–296. doi: 10.1067/mva.2002.125479. [DOI] [PubMed] [Google Scholar]
- Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA Multicentre Aneurysm Screening Study G. The multicentre aneurysm screening study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- Dueck AD, Kucey DS, Johnston KW, Alter D, Laupacis A. Survival after ruptured abdominal aortic aneurysm: effect of patient, surgeon, and hospital factors. J Vasc Surg. 2004;39:1253–1260. doi: 10.1016/j.jvs.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;2:CD002945. doi: 10.1002/14651858.CD002945.pub2. [DOI] [PubMed] [Google Scholar]
- Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess. 2012;16:1–218. doi: 10.3310/hta16090. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Ashton HA, Gao L, Scott RA. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ. 2009;338:b2307. doi: 10.1136/bmj.b2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RA. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649–1656. doi: 10.1002/bjs.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS. Abdominal aortic aneurysm (AAA) screening programme: annual report 2011–12. Available at: http://www.aaa.screening.nhs.uk/annual_report. Accessed 07.08.2013.
- Scottish abdominal aortic aneurysm screening programme. Available at: http://www.scotland.gov.uk/Topics/Health/health/screening/AAAscreening. Accessed 11.02.2014.
- 2008. Screening for abdominal aortic aneurysm. Available at: http://www.sbu.se/en/Published/Alert/Screening-for-Abdominal-Aortic-Aneurysm. Accessed 11.02.2014.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142:198–202. doi: 10.7326/0003-4819-142-3-200502010-00011. [DOI] [PubMed] [Google Scholar]
- Svensjo S, Bjorck M, Gurtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124:1118–1123. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg. 2011;98:645–651. doi: 10.1002/bjs.7461. [DOI] [PubMed] [Google Scholar]
- Choke E, Vijaynagar B, Thompson J, Nasim A, Bown MJ, Sayers RD. Changing epidemiology of abdominal aortic aneurysms in England and Wales: older and more benign? Circulation. 2012;125:1617–1625. doi: 10.1161/CIRCULATIONAHA.111.077503. [DOI] [PubMed] [Google Scholar]
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg. 2012;43:161–166. doi: 10.1016/j.ejvs.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Norman PE, Spilsbury K, Semmens JB. Falling rates of hospitalization and mortality from abdominal aortic aneurysms in Australia. J Vasc Surg. 2011;53:274–277. doi: 10.1016/j.jvs.2010.08.087. [DOI] [PubMed] [Google Scholar]
- Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696–701. doi: 10.1002/bjs.5780. [DOI] [PubMed] [Google Scholar]
- Hafez H, Druce PS, Ashton HA. Abdominal aortic aneurysm development in men following a “normal” aortic ultrasound scan. Eur J Vasc Endovasc Surg. 2008;36:553–558. doi: 10.1016/j.ejvs.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Hammond EC, Horn D. Smoking and death rates: report on forty-four months of follow-up of 187,783 men. 2. Death rates by cause. JAMA. 1958;166:1294–1308. doi: 10.1001/jama.1958.02990110030007. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA, Scott RA. Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br J Surg. 2000;87:195–200. doi: 10.1046/j.1365-2168.2000.01353.x. [DOI] [PubMed] [Google Scholar]
- Hager J, Lanne T, Carlsson P, Lundgren F. Lower prevalence than expected when screening 70-year-old men for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2013;46:453–459. doi: 10.1016/j.ejvs.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Nelson DB, Joseph AM. Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg. 2003;38:329–334. doi: 10.1016/s0741-5214(03)00136-8. [DOI] [PubMed] [Google Scholar]
- Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ. Gelijns AC, Greco G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- Sode BF, Nordestgaard BG, Gronbaek M, Dahl M. Tobacco smoking and aortic aneurysm: two population-based studies. Int J Cardiol. 2013;167:2271–2277. doi: 10.1016/j.ijcard.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Sweeting MJ, Thompson SG, Brown LC, Powell JT. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- Lindholt JS, Sorensen J, Sogaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97:826–834. doi: 10.1002/bjs.7001. [DOI] [PubMed] [Google Scholar]
- Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, Parsons RW, Dickinson JA. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensjo S, Bjorck M, Wanhainen A. Current prevalence of abdominal aortic aneurysm in 70-year-old women. Br J Surg. 2013;100:367–372. doi: 10.1002/bjs.8984. [DOI] [PubMed] [Google Scholar]
- Acosta S, Ogren M, Bengtsson H, Bergqvist D, Lindblad B, Zdanowski Z. Increasing incidence of ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg. 2006;44:237–243. doi: 10.1016/j.jvs.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Filipovic M, Goldacre MJ, Roberts SE, Yeates D, Duncan ME, Cook-Mozaffari P. Trends in mortality and hospital admission rates for abdominal aortic aneurysm in England and Wales, 1979–1999. Br J Surg. 2005;92:968–975. doi: 10.1002/bjs.5118. [DOI] [PubMed] [Google Scholar]
- Bengtsson H, Bergqvist D. Ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg. 1993;18:74–80. doi: 10.1067/mva.1993.42107. [DOI] [PubMed] [Google Scholar]
- Melton LJ, III, Bickerstaff LK, Hollier LH, Van Peenen HJ, Lie JT, Pairolero PC, Cherry KJ, O’Fallon WM. Changing incidence of abdominal aortic aneurysms: a population-based study. Am J Epidemiol. 1984;120:379–386. doi: 10.1093/oxfordjournals.aje.a113902. [DOI] [PubMed] [Google Scholar]
- Pleumeekers HJ, Hoes AW, van der Does E, van Urk H, Hofman A, de Jong PT, Grobbee DE. Aneurysms of the abdominal aorta in older adults. The Rotterdam Study. Am J Epidemiol. 1995;142:1291–1299. doi: 10.1093/oxfordjournals.aje.a117596. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Larson JC, Margolis KL, Allison MA, Freiberg MS, Cochrane BB, Graettinger WF, Curb JD. Abdominal aortic aneurysm events in the Women’s Health Initiative: cohort study. BMJ. 2008;337:a1724. doi: 10.1136/bmj.a1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromso Study. Am J Epidemiol. 2001;154:236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- Darwood R, Earnshaw JJ, Turton G, Shaw E, Whyman M, Poskitt K, Rodd C, Heather B. Twenty-year review of abdominal aortic aneurysm screening in men in the county of Gloucestershire, United Kingdom. J Vasc Surg. 2012;56:8–13. doi: 10.1016/j.jvs.2011.12.069. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–1054. doi: 10.1136/jnnp.54.12.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug ES, Romundstad P, Aadahl P, Myhre HO. Emergency non-ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2004;28:612–618. doi: 10.1016/j.ejvs.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics. National population projections. National population projections by age and sex for the UK and constituent countries. Available at: http://www.statistics.gov.uk/hub/population/index.html. Accessed 10.01.2014.
- Office for National Statistics. 2010-based period and cohort life expectancy tables. Cohort expectations of life. Available at: http://www.ons.gov.uk/ons/rel/lifetables/period-and-cohort-life-expectancy-tables/2010-based/index.html. Accessed 10.03.2014.
- The Institute and Faculty of Actuaries. Continuous mortality investigation. Base mortality tables. Available at: http://www.actuaries.org.uk/research-and-resources/pages/base-mortality-tables-produced-cmi. Accessed 10.03.2014.
- Mani K, Björck M, Lundkvist J, Wanhainen A. Improved long-term survival after abdominal aortic aneurysm repair. Circulation. 2009;120:201–211. doi: 10.1161/CIRCULATIONAHA.108.832774. [DOI] [PubMed] [Google Scholar]
- Vascular services quality improvement programme. Available at: http://www.vsqip.org.uk/wp/wp-content/uploads/2013/07/NVR-2013-Report-on-Surgical-Outcomes-Consultant-Level-Statistics.pdf. Accessed 01.04.2014.
- Pol RA, Zeebregts CJ, van Sterkenburg SM, Reijnen MM. Thirty-day outcome and quality of life after endovascular abdominal aortic aneurysm repair in octogenarians based on the Endurant Stent Graft Natural Selection Global Postmarket Registry (ENGAGE) J Vasc Surg. 2012;56:27–35. doi: 10.1016/j.jvs.2011.12.080. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics. 2001 and 2011 census area statistics. Available at: http://www.ons.gov.uk/ons/guide-method/census/2011/index.html. Accessed 01.02.2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Expolated life expectancies for the general population and elective aneurysm repair survivors.
Table S2. Current and projected life-years gained per year for the current screening strategy and potential alternatives.
Table S3. Sensitivity analysis on the projected impact of screening programme alternative strategies on acute abdominal aortic events if incidence rates reduce by the same age-adjusted degree as they have for last decade.
Table S4. Sensitivity analysis on the projected impact on aneurysm-related death for screening program alternative strategies if incidence rates reduce by the same age-adjusted degree as they have for last decade.
Table S5. Comparison of the presence of risk factors in those with acute events versus the background study population expressed as age-/sex-adjusted risk ratios.
Table S6. OXVASC: events and aneurysm-related mortality rates by age and risk factor status.
Table S7. Changes in disability status for patients with incident acute events.
Table S8. Additional sensitivity analysis on the projected impact of the current UK screening program on acute AAA events versus alternative strategies if cases with known AAAs are excluded (and incidence rates reduce by the same age-adjusted degree as they have for last decade).
Table S9. Additional sensitivity analysis on the projected impact of the current UK screening program on aneurysm-related deaths versus alternative strategies if cases with known prevalent AAAs already under surveillance are excluded (and incidence rates reduce by the same age-adjusted degree as they have for last decade).


