Abstract
Background
Patients with peripheral artery disease (PAD) experience significant morbidity and mortality. The OMEGA-PAD I Trial, a randomized, double-blinded, placebo-controlled trial, addressed the hypothesis that short-duration, high-dose n-3 polyunsaturated fatty acids (n-3 PUFA) oral supplementation improves endothelial function and inflammation in PAD.
Methods and Results
Eighty patients with stable claudication received 4.4 g of fish oil or placebo for 1 month. The primary end point was endothelial function as measured by brachial artery flow-mediated vasodilation. Secondary end points included biomarkers of inflammation, n-3 polyunsaturated fatty acids metabolome changes, lipid profile, and walking impairment questionnaires. Although there was a significant increase in FMD in the fish oil group following treatment (0.7±1.8% increase from baseline, P=0.04), this response was not different then the placebo group (0.6±2.5% increase from baseline, P=0.18; between-group P=0.86) leading to a negative finding for the primary endpoint. There was, however, a significant reduction in triglycerides (fish oil: −34±46 mg/dL, P<0.001; placebo −10±43 mg/dL, P=0.20; between-group differential P-value: 0.02), and an increase in the omega-3 index of 4±1% (P<0.001) in the fish oil group (placebo 0.1±0.9%, P=0.49; between-group P<0.0001). We observed a significant increase in the production of pathway markers of specialized pro-resolving mediators generated from n-3 polyunsaturated fatty acids in the fish oil group.
Conclusions
High-dose, short-duration fish oil supplementation did not lead to a different response in the primary end point of endothelial function between the treatment and placebo group, but improved serum triglycerides and increased the production of downstream n-3 polyunsaturated fatty acids–derived products and mediators in patients with PAD.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01310270.
Keywords: fish oil, n-3 polyunsaturated fatty acids, peripheral artery disease, specialized pro-resolving mediators, vascular function
Despite available medical and surgical therapies, patients with peripheral artery disease (PAD) continue to be threatened with an unacceptably high risk for cardiovascular (CV) events1–3 and suffer significant morbidity and a decrease in quality of life. Emerging evidence demonstrates that there is a dynamic relationship between inflammation and vascular dysfunction that likely contributes to the adverse outcomes in PAD. Elevated levels of circulating inflammatory markers, a consistent observation among PAD patients, have been associated with disease progression and mortality.4–6 Impaired endothelial function independently predicts CV events in patients suffering from PAD and undergoing vascular surgery.7–9 Furthermore, the degree of walking impairment in PAD is related to endothelial function, arterial stiffness, and markers of inflammation.10,11 Thus, interventions aimed at reducing vascular inflammation and improving endothelial function are likely to result in improved health outcomes in the PAD population.
n-3 polyunsaturated fatty acids (PUFA), which are a major component of fish oil, have long been recognized to have beneficial effects on CV health by decreasing total mortality, CV death, sudden cardiac death, and nonfatal CV events.12–15 Furthermore, current evidence suggests that n-3 PUFA can impact factors critical to PAD: inflammation and vascular function. For example, n-3 PUFA supplementation has been associated with a dramatic reduction in inflammation in diverse patient populations16–20 and an improvement in arterial stiffness in smokers.20 However, recent clinical trials have yielded conflicting data on the effects of n-3 PUFA supplementation in CV diseases.21–23 Differences in study results are likely attributable to the relatively low doses studied (averaging 1.5 g/day) and heterogeneity among cohorts.21 Very few studies have been conducted in symptomatic PAD patients,24 who exhibit the most pronounced inflammatory phenotype among all of the clinical subtypes of atherosclerosis. Our group recently demonstrated that the omega-3 index, a measure of n-3 PUFA content in red blood cells, is inversely related to inflammation in patients with PAD,25 and others have shown that n-3 PUFA supplementation can improve endothelial function26 and arterial stiffness.20
Recent work suggests new hypotheses relating PUFAs to cardiovascular health and disease. It is now well established that n-3 PUFA, namely, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are enzymatically converted into lipid mediators that have stereospecific and receptor-mediated biological actions.27 Specifically, lipid mediators generated from EPA and DHA are actively involved in the resolution phase of inflammation and as such have been collectively termed “specialized pro-resolving mediators (SPM).” These SPM families include the resolvins, protectins, and maresins that are structurally distinct and are produced by unique biosynthetic pathways (for review, see 27). Given their potent receptor-mediated biological roles in the resolution of inflammation, the elucidation of these mediators offers a new mechanism for the beneficial effects of n-3 PUFA in inflammatory pathologies. As several recent studies have demonstrated that healthy individuals taking n-3 PUFA supplements generate SPM in their bioactive concentration ranges,28–30 we also sought to determine whether n-3 PUFA supplementation would increase generation of SPM and/or their biosynthetic pathway markers in PAD patients.
The OMEGA-PAD I trial aimed to assess the impact of high-dose, short-term (1 month) n-3 PUFA supplementation on endothelial function and inflammation in patients with symptomatic PAD, and whether this is directly associated with augmentation of downstream SPM pathways.
Methods
Study Population, Intervention, and Protocol
The methods of the OMEGA-PAD study have been previously published.31 Briefly, the OMEGA-PAD trial was a randomized, double-blinded, and placebo-controlled trial. The study included 80 patients aged 50 and older with lower-extremity PAD (defined by intermittent claudication and an ankle–brachial index of <0.9), who presented to the vascular surgery clinic at the Veterans Affairs Medical Center in San Francisco (SFVAMC). The number of patients enrolled was based on a 0.9 power to detect an increase of 40% in flow-mediated vasodilation (FMD),31 based on existing literature.26 The patients targeted were those with intermittent claudication (Rutherford grade 1 to 3). Inclusion criteria included intermittent claudication (mild-severe) associated with rest or exercise ankle–brachial index <0.9. Exclusion criteria included critical limb ischemia (rest pain or tissue loss); hypersensitivity/allergies to fish or seafood; significant renal, hepatic, or inflammatory disorder; concurrent severe infections; acute illness or major surgery within 30 days; and use of immunosuppressive medications.
Subjects were randomized to 2.2 g oral n3-PUFA (Pro-Omega; Nordic Naturals, Watsonville, CA) twice daily (totaling 4.4 g/day) or a matched placebo for 1 month. Each ProOmega capsule contained 325 mg of EPA and 225 mg of DHA. Treatment corresponded to 4 capsules twice daily, totaling an amount of 2.6 g of EPA and 1.8 g of DHA daily. Individuals were randomized by a block randomization process, with the study pharmacist maintaining the key. Four subjects were randomized per block (total of 20 blocks) with a ratio of 1:1 for each block. Measurements were completed at baseline and after 1 month.
Subjects came to the hospital for an initial visit for baseline measurements and were then started on the study drug or placebo for 1 month. At the end of the month, they returned for a second visit, at which point study measurements were repeated. Prior to participating in the study, all subjects provided informed consent. The institutional review board approval was granted for this study by the Committee on Human Research at the University of California, San Francisco (UCSF). The study was registered with https://www.clinicaltrials.gov/show/NCT01310270.
Primary and Secondary End Points
The primary end point was change in endothelial function measured by brachial artery flow-mediated, endothelium-dependent vasodilation (FMD). Secondary end points included a change in inflammatory and pro-resolution markers, improvement in lipid profile (low-density lipoprotein, triglycerides, high-density lipoprotein), blood pressure, and patient-perceived walking impairment as determined by the Walking Impairment Questionnaire.32 The omega-3 index was measured to correlate observed effects with changes in red blood cell content of n-3 PUFA.
Measurements
Demographics, anthropometrics, medical history, and hemodynamic measurements
Demographic data, CV history, risk factors for PAD, medications, and examination findings pertinent to CV history were recorded. Systolic and diastolic blood pressures were measured by sphygmomanometry.
Vascular reactivity of brachial arteries
Flow-mediated vasodilation was performed according to current guidelines and standards33,34 and as already described by our group35 and other investigators.36 Briefly, subjects were allowed to rest for 10 minutes in a supine position in a darkened room at 23°C. A 5-cm tourniquet blood pressure cuff was placed on the upper arm distal to the insertion of the deltoid. The length of the brachial artery was surveyed by B-mode ultrasound (Philips HD11) using a broadband linear-array transducer with a 3- to 12-MHz range (Philips L12-3) until a straight segment with a visible registration structure could be located. Prior to cuff inflation, the baseline diameter of the vessel and blood-flow velocity were recorded for 60 seconds using ECG-gated image capture software (Brachial Imager, Medical Imaging Applications LLC, Coralville, IA). The blood pressure cuff was then inflated to the greater of 250 or 50 mm Hg above the subject’s systolic blood pressure for a period of 5 minutes. Recording of the B-mode images began 10 seconds prior to cuff release. Blood-flow velocity was assessed for a period of 30 seconds post–cuff release using the methods described above. B-mode images were recorded until 3 minutes post–cuff release. Analysis of the images was performed using continuous edge-detection software (Brachial Analyzer, Medical Imaging Applications LLC, Coralville, IA). Hyperemia diameter was calculated using a predetermined time window (55 to 65 seconds post–cuff release). FMD% was calculated as [(60-second Hyperemia diameter−Avg Baseline diameter/Avg Baseline diameter)×100]. The brachial stimulus ratio corresponds to hyperemia flow divided by baseline flow. FMD in healthy subjects is expected to be above 7%33 and has been reported to range between 0.20% and 19.2%.37
Time-averaged velocity measurements were obtained using the peak-velocity method. Average velocity at baseline was obtained from 60 seconds of data. Velocity of the hyperemia stimulus was calculated as the mean velocity of the first 4 heartbeats following cuff-release. Both mean velocity and the velocity time integral were recorded.
Ankle–brachial index
Ankle–brachial indices were measured using current guidelines and standards.38 Systolic blood pressures of the brachial, posterior tibial, and dorsalis pedis arteries were measured bilaterally. For each lower extremity, the highest systolic pressure of the 2 pedal pulses was divided by the highest systolic pressure of the 2 brachial arteries.
Blood sample collection
Blood samples were collected from subjects at baseline and at follow-up after the intervention. The samples were collected from subjects in a fasting state by a trained phlebotomist according to institutional guidelines and were processed immediately. Samples collected for inflammatory markers were collected in a serum-separator tube and allowed to clot for 30 minutes and centrifuged at 1665g, 4°C for 10 minutes (Beckman Coulter Allegra 6R Centrifuge, Brea, CA). Samples collected for lipidomics, metabolic hormones, and the Omega-Index assays were collected in plasma-EDTA tubes and centrifuged immediately at 1665g, 4°C for 10 minutes. Whole blood sample used for the Omega-Index was collected in a plasma-EDTA tube, aliquoted to a cryovial, and immediately frozen at −80°C.
Inflammatory markers
To assess systemic inflammation, pro-inflammatory mediators including high-sensitivity C-reactive protein, interleukin-6, soluble intracellular adhesion molecule-1, and tumor necrosis factor-α were measured. For this, 10 mL of whole venous blood was collected in a tiger-top tube from subjects in a fasting state and was allowed to clot for a minimum of 30 minutes at room temperature, and centrifuged at 2800 rpm for 10 minutes at 4°C. Serum was stored at −80°C until assayed for interleukin-6, soluble intracellular adhesion molecule-, and tumor necrosis factor-α per standard kit protocol (R&D Systems Inc, Minneapolis, MN). The typical coefficients of variation for interleukin-6, soluble intracellular adhesion molecule-1, and tumor necrosis factor-α are 7.4%, 4.6%, and 5.4%, respectively. The lower limits of detection are 0.04 pg/mL, 0.1 ng/mL, and 0.11 pg/mL, respectively. Plasma was assayed for high-sensitivity C-reactive protein the same day as collection by the SFVAMC lab per standard methodology (Beckman Coulter Analyzer, Miami, FL). The coefficient of variation for high-sensitivity C-reactive protein using this procedure is 5.1%. In healthy individuals, CRP is <1.0 mg/dL.
Lipidomics
Lipid mediators in plasma samples were measured by liquid chromatography–tandem mass spectrometry using a Shimadzu high-performance liquid chromatography system coupled to a triple quadrupole mass spectrometer (AB Sciex; API2000) as previously described.39,40 Briefly, frozen plasma was diluted with 2 volumes of cold-methanol containing deuterium-labeled internal standards (ie, d4-prostaglandin E2 and d8-5-HETE) and butylated hydroxytoluene (to prevent nonenzymatic oxidation during extraction). After solid phase extraction (C18), lipid mediators were profiled by liquid chromatography–tandem mass spectrometry using established multiple reaction monitoring transitions and retention time as criteria to identify and quantitate lipid mediators generated from arachidonic acid (AA), EPA, and DHA.29 Lipid mediators were quantified based on external calibration curves for each mediator using authentic standards (Cayman Chemical, Ann Arbor, MI) and after determination of extraction recovery based on internal standards. The limits of quantitation for the lipid mediators under these conditions are ≈10 pg on-column.
Omega-3 fatty acid measurements
The omega-3 index represents the proportional red blood cell content of the 2 major long-chain n-3 PUFA, EPA, and DHA.41 Ten milliliters of whole venous blood was collected in an EDTA tube from subjects in a fasting state, and centrifuged at 2800 rpm for 10 minutes at 4°C within 30 minutes of collection. Packed red blood cells were stored at −80°C until assayed for n-3 PUFA content of EPA and DHA, the omega-3 index, analyzed according to the HS-Omega-3 index® methodology.42
Renal, lipid, and metabolic measurements
Blood samples were collected from subjects in a fasting state for measurement of creatinine, estimated glomerular filtration rate, albumin, hemoglobin A1C as well as total cholesterol, triglycerides, low-density lipoprotein, and high-density lipoprotein. Plasma was assayed for these analytes the same day as collection by the SFVAMC lab per standard methodology (Beckman Coulter Analyzer). Serum was isolated at the same time points for homocysteine and assayed the same day as collection by the SFVAMC lab per standard methodology (Abbott Diagnostics Architect i1000 Analyzer, Lake Forest, IL).
Walking Impairment Questionnaire
The Walking Impairment Questionnaire is a validated instrument that assesses the patient-perceived walking impairment.32 The questionnaire was administered by research staff and measures patient-perceived walking capacity and limitation due to claudication across 3 domains: distance, speed, and stair climbing, as previously described.31
Statistical Analysis
The intervention group was compared to the placebo group for homogeneity of demographic and baseline clinical variables via the χ2 test or Student t test (depending on the type of variable). The generalized estimating equation was used for an intention-to-treat analysis (all participants randomized were analyzed based upon their assigned group). The significance level was preset at 0.05 and 2-sided t tests were performed. There was no a-priori adjustment for multiple hypothesis testing on the secondary end points. Statistical analyses were performed using Stata/SE 13 (StataCorp, College Station, TX). Hierarchical clustering, correlation analysis, and partial least-squares discriminant analysis were carried out using Metaboanalyst 2.5 software (http://www.metaboanalyst.ca). For this, levels of lipid mediators in placebo and fish oil groups at the 1-month follow-up were assessed. Products in which >75% of the values were missing were removed from the analysis, and the remaining missing values were imputed by replacing them with half of the minimum positive value for each mediator. The data were log transformed and autoscaled (mean centered and divided by the square root of the SD for each variable) before analysis.
Results
Eighty patients participated in the study, with 40 randomized to the fish oil group and 40 to the placebo group. Baseline characteristics of the patient cohort are shown in Table 1. The 2 groups were balanced, with the exception of their coronary artery disease history and intake of β-blockers. There was a trend toward less prevalent aspirin and statin use among participants in the fish oil group compared to the placebo group. No adverse reactions occurred secondary to receiving 4.4 g/day of fish oil for 1 month. The dropout rate was 10%. Five patients dropped out for “travel distance” or the size of pills, 1 patient was found “ineligible” based on age, 1 patient was hospitalized for a fall, and 1 patient was withdrawn for substance abuse (Figure 1).
Table 1.
Baseline Characteristics of Patients
| General Characteristics | Fish Oil (n=40) | Placebo (n=40) | P Value |
|---|---|---|---|
| Age, y | 68±7 | 69±9 | 0.41 |
| Male sex, % | 39 (98) | 39 (98) | 1.0 |
| White, % | 27 (68) | 31 (78) | 0.32 |
| Index ABI | 0.73±0.12 | 0.71±0.14 | 0.74 |
| Rutherford (respondents/category), % | |||
| Mild claudication | 10 (25) | 10 (25) | 0.75 |
| Moderate claudication | 10 (25) | 12 (30) | |
| Severe claudication | 20 (50) | 18 (45) | |
| BMI, kg/m2 | 28±5 | 27±4 | 0.17 |
| Waist–hip ratio | 1.01±0.05 | 1.02±0.06 | 0.18 |
| Omega-3 index, % | 5.2±1.7 | 4.6±1.4 | 0.13 |
| Comorbidities | |||
| Coronary artery disease, % | 13 (33) | 22 (55) | 0.04* |
| Hypertension, % | 38 (95) | 35 (88) | 0.24 |
| Hyperlipidemia, % | 32 (80) | 36 (90) | 0.21 |
| Type II diabetes mellitus, % | 11 (28) | 14 (35) | 0.47 |
| Surgical history, % | 5 (13) | 7 (18) | 0.53 |
| Lower extremity bypass | |||
| Lower extremity percutaneous | 9 (23) | 13 (33) | 0.32 |
| Systolic blood pressure, mm Hg | 135±17 | 139±18 | 0.31 |
| Diastolic blood pressure, mm Hg | 75±9 | 75±9 | 0.97 |
| Medications | |||
| Aspirin, % | 24 (60) | 31 (78) | 0.09 |
| ACE inhibitor, % | 16 (40) | 18 (45) | 0.65 |
| β-Blocker, % | 14 (35) | 28 (70) | 0.002* |
| Statin, % | 31 (78) | 37 (93) | 0.06 |
| PAD risk factors | |||
| History of smoking, % | 38 (95) | 36 (90) | 0.40 |
| Total cholesterol, mg/dL | 175±48 | 161±37 | 0.15 |
| Triglycerides, mg/dL | 157±99 | 150±69 | 0.71 |
| HDL, mg/dL | 45±14 | 44±12 | 0.77 |
| LDL, mg/dL | 100±42 | 87±33 | 0.12 |
| Serum Cr, mg/dL | 1.1±0.4 | 1.1±0.4 | 0.42 |
| eGFR, mL/min | 79±23 | 71±20 | 0.14 |
| Albumin, g/dL | 4.0±0.3 | 4.1±0.4 | 0.75 |
| Homocysteine, μmol/L | 13±4 | 14±5 | 0.50 |
| HbA1c, % | 6.2±1.0 | 6.1±1.2 | 0.85 |
| Vitamin D, ng/mL | 24±11 | 23±12 | 0.64 |
| Inflammation | |||
| hsCRP, mg/L | 4.3±4.6 | 4.2±4.1 | 0.91 |
| IL-6, pg/mL | 1.3±0.7 | 1.4±0.7 | 0.59 |
| ICAM-1, ng/mL | 250±77 | 281±101 | 0.13 |
| TNF-α, pg/mL | 2.0±0.6 | 2.3±0.8 | 0.05 |
| Fibrinogen, mg/dL | 389±77 | 389±105 | 1.0 |
| Brachial FMD and tonometry | |||
| Brachial FMD, % | 7.3±3.9 | 6.6±3.4 | 0.37 |
| Brachial artery baseline diameter, cm | 0.38±0.06 | 0.38±0.07 | 0.66 |
| Brachial artery mean velocity, m/s | 0.16±0.07 | 0.17±0.08 | 0.46 |
| Brachial artery baseline flow, mL/min | 112±61 | 123±76 | 0.46 |
| Brachial artery baseline shear stress, dynes/cm2 | 12±5 | 13±9 | 0.40 |
| Brachial artery reactive hyperemia diameter, cm | 0.40±0.06 | 0.41±0.08 | 0.69 |
| Brachial artery reactive hyperemia velocity, m/s | 0.66±0.28 | 0.72±0.25 | 0.31 |
| Brachial reactive hyperemia flow, mL/min | 514±236 | 595±306 | 0.19 |
| Brachial reactive hyperemia shear stress, dynes/cm2 | 48±21 | 51±18 | 0.46 |
| Stimulus ratio | 5±2 | 5±2 | 0.75 |
| Patient-perceived walking performance | |||
| Walking distance (score, from 0 to 100) | 25±30 | 32±27 | 0.31 |
| Walking speed (score, from 0 to 100) | 22±23 | 30±27 | 0.18 |
| Stairs (score, from 0 to 100) | 32±31 | 34±26 | 0.72 |
Values as “mean±SD” or “n (%)”. ABI indicates ankle–brachial index; ACE, angiotensin-converting enzyme; BMI, body mass index; Cr, creatinine; eGFR, estimated glomerular filtration rate; FMD, flow-mediated dilatation; HDL, high-density lipoprotein; HbA1c, hemoglobin A1c; hsCRP, high-sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule; IL-6, interleukin-6; LDL, low-density lipoprotein; PAD, peripheral artery disease; TNF-α, tumor necrosis factor-α.
P<0.05 by an unpaired Student’s t test.
Figure 1.
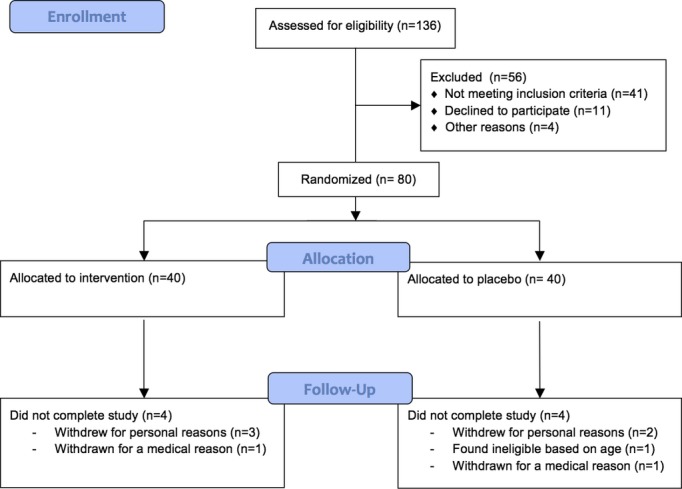
Study recruitment and enrollment schema.
With regard to the primary study end point, brachial artery FMD improved in the fish oil group (0.7±1.8% increase from baseline, P=0.04) and trended toward improvement in the placebo group (0.6±2.5% increase from baseline, P=0.18) (Table 2). There was, however, no significant difference between the 2 treatment groups over the course of the study (P=0.86), leading to a negative finding for the primary end point.
Table 2.
Changes in Endothelial Function During Study Period
| Brachial FMD and Tonometry | Fish Oil | Placebo | Difference Between Groups | ||
|---|---|---|---|---|---|
| Change Compared to Baseline | P Value | Change Compared to Baseline | P Value | ||
| Brachial FMD, % | 0.7±1.8 | 0.04* | 0.6±2.5 | 0.18 | 0.86 |
| Brachial artery baseline diameter, cm | 0.004±0.03 | 0.37 | −0.00003±0.04 | 1.0 | 0.59 |
| Brachial artery mean velocity, m/s | −0.003±0.08 | 0.83 | −0.002±0.09 | 0.87 | 0.98 |
| Brachial artery baseline flow, mL/min | −5±66 | 0.69 | −6±62 | 0.57 | 0.93 |
| Brachial artery baseline shear stress, dynes/cm2 | −0.2±6 | 0.86 | −0.5±8 | 0.71 | 0.84 |
| Brachial artery reactive hyperemia diameter, cm | 0.007±0.03 | 0.16 | 0.002±0.04 | 0.72 | 0.56 |
| Brachial artery reactive hyperemia velocity, m/s | 0.04±0.3 | 0.41 | −0.003±0.2 | 0.95 | 0.85 |
| Brachial reactive hyperemia flow, mL/min | 50±258 | 0.27 | −32±236 | 0.44 | 0.57 |
| Brachial reactive hyperemia shear stress, dynes/cm2 | 1±19 | 0.75 | 0.5±19 | 0.87 | 1.0 |
| Stimulus ratio | 0.5±3.2 | 0.42 | 0.2±2.5 | 0.96 | 0.53 |
Values as “mean±SD”. FMD indicates flow-mediated vasodilation.
P<0.05 by a paired Student’s t test (within group) or unpaired Student’s t test (across groups).
With regard to the secondary end points, the omega-3 index increased significantly in the treatment group by 4±1% during the month-long supplementation period (P<0.001) compared with the placebo group (0.1±0.9, P=0.49; between-group P<0.0001) (Table 3). An improvement in lipid profiles was also observed, most notably characterized by a decrease in triglycerides (fish oil: −34±46, P<0.001; placebo −10±43, P=0.20; between-group differential P-value: 0.02) (Table 3). No changes were noted in inflammatory cytokines or self-reported walking tolerance.
Table 3.
Changes in Lipid, Inflammatory, and Hemodynamic Profile With Treatment
| Parameter | Fish Oil | Placebo | Difference Between Groups | ||
|---|---|---|---|---|---|
| Change Compared to Baseline | P Value | Change Compared to Baseline | P Value | ||
| Omega-3 index | |||||
| Omega-3 index | 4±1 | <0.0001* | 0.1±0.9 | 0.49 | <0.0001* |
| Lipid profile | |||||
| Total cholesterol, mg/dL | −2±24 | 0.65 | −5±18 | 0.11 | 0.51 |
| Triglycerides, mg/dL | −34±46 | 0.0001* | −10±43 | 0.20 | 0.02* |
| HDL, mg/dL | 2±6 | 0.03* | 0.5±10 | 0.78 | 0.41 |
| LDL, mg/dL | 3±20 | 0.37 | −4±18 | 0.22 | 0.14 |
| Pro-inflammatory markers | |||||
| hsCRP, mg/L | 0.9±6.2 | 0.41 | 2±11 | 0.19 | 0.46 |
| IL-6, pg/mL | 0.1±0.9 | 0.49 | 0.12±1.0 | 0.53 | 0.98 |
| ICAM-1, ng/mL | 4.6±46 | 0.56 | 6±63 | 0.62 | 0.94 |
| TNF-α, pg/mL | −0.03±0.50 | 0.69 | −0.02±0.5 | 0.79 | 0.92 |
| Hemodynamic parameters | |||||
| Systolic blood pressure, mm Hg | 4±15 | 0.09 | −0.4±14 | 0.86 | 0.16 |
| Diastolic blood pressure, mm Hg | −0.7±9.7 | 0.64 | −2±9 | 0.24 | 0.63 |
| Index ABI | −0.02±0.1 | 0.60 | −0.03±0.1 | 0.18 | 0.65 |
| Patient-perceived walking performance | |||||
| Walking distance (score, from 0 to 100) | 2±21 | 0.54 | −3±10 | 0.13 | 0.21 |
| Walking speed (score, from 0 to 100) | 4±17 | 0.21 | −1±18 | 0.71 | 0.25 |
| Stairs (score, from 0 to 100) | −1±20 | 0.71 | 0.09±18 | 0.98 | 0.77 |
Values as “mean±SD” or “n (%)”. ABI indicates ankle–brachial index; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; ICAM-1, intracellular adhesion molecule-1; IL-6, interleukin-6; LDL, low-density lipoprotein; TNF-α, tumor necrosis factor-α.
P<0.05 by a paired Student’s t test (within group) or unpaired Student’s t test (across groups).
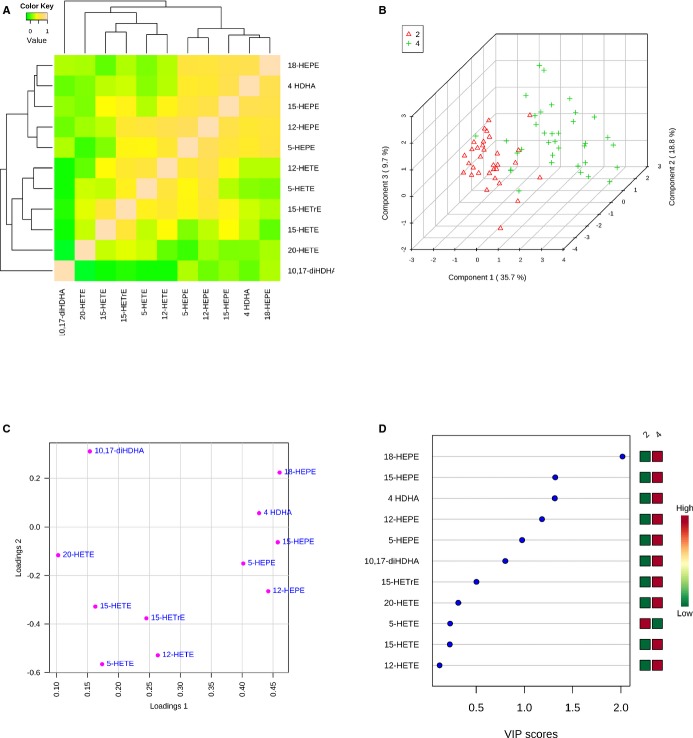
The results of the lipidomics analysis are summarized in Table 4 and Figures2 through 4. Using a targeted mass spectrometry–based approach, we identified several products of both EPA and DHA in plasma from PAD subjects. As shown in Figure 2 and Table 4, lipoxygenase products of EPA, including 5-hydroxyeicosapentaenoic acid (5-HEPE), 12-HEPE, and 15-HEPE were identified in plasma of both placebo and fish oil groups at baseline. Notably, fish oil supplementation for 1 month significantly increased the production of 5-HEPE and 15-HEPE, whereas their levels remained unchanged in the placebo groups (Table 4 and Figure 2). Similarly, 18-HEPE, which is a biosynthetic pathway marker of E-series resolvins27,43 and a product of aspirin-acetylated cyclooxygenase 2 and cytochrome P450 enzymes, increased significantly and substantially (>10-fold) in the fish oil group, but remained unchanged in the placebo group (Table 4 and Figure 2). Resolvin E1 (RvE1), a downstream product and proresolving lipid mediator generated from 18-hydroperoxy-EPE,43 was sparsely identified in the fish oil group, although there was no significant difference in the production of this short-lived and locally produced mediator throughout the duration of the study. Lastly, 3-series prostaglandins, such as prostaglandin E3 (PGE3 )and PGD3, were also sparsely detected, although no meaningful changes with treatment emerged (Table 4).
Table 4.
Changes in Lipid Mediator Profiles With n-3 PUFA Supplementation
| Product | Fish Oil | Placebo | P Value Between Groups (Delta) | ||||
|---|---|---|---|---|---|---|---|
| Pre [Range] | Post [Range] | P Value (Within Group) | Pre [Range] | Post [Range] | P Value (Within Group) | ||
| EPA products | |||||||
| 5-HEPE | 46±160 [0, 943] | 173±236 [0, 895] | 0.001* | 37±84 [0, 400] | 33±69 [0, 267] | 0.86 | 0.002* |
| 12-HEPE | 155±359 [0, 1361] | 1134±4072 [0, 23 186] | 0.13 | 129±298 [0, 1304] | 108±270 [0, 1152] | 0.75 | 0.14 |
| 15-HEPE | 25±47 [0, 180] | 180±276 [0, 1453] | 0.001* | 14±31 [0, 117] | 24±48 [0, 156] | 0.35 | 0.003* |
| 18-HEPE | 32±65 [0, 257] | 383±359 [0, 1566] | <0.00001* | 19±38 [0, 133] | 16±43 [0, 168] | 0.76 | <0.00001* |
| PGE3 | 0 [0, 0] | 0 [0, 0] | NA | 26±148 [0, 853] | 21±125 [0, 727] | 0.32 | 0.30 |
| PGD3 | 6±35 [0, 212] | 4±23 [0, 140] | 0.78 | 0 [0, 0] | 0 [0, 0] | NA | 0.79 |
| RvE1 | 50±286 [0, 1716] | 30±144 [0, 835] | 0.71 | 0 [0, 0] | 0 [0, 0] | NA | 0.72 |
| DHA products | |||||||
| 4-HDHA | 13±27 [0, 114] | 100±139 [0, 463] | 0.001* | 22±59 [0, 260] | 10±37 [0, 188] | 0.31 | 0.006* |
| 7-HDHA | 0 [0, 0] | 4±20 [0, 106] | 0.19 | 2±12 [0, 70] | 0 [0, 0] | 0.32 | 0.11 |
| 14-HDHA | 1496±3920 [0, 15 665] | 2995±11040 [0, 54 326] | 0.28 | 1428±3682 [0, 14 564] | 1436±4708 [0, 25 565] | 0.99 | 0.34 |
| 17-HDHA | 0 [0, 0] | 0 [0, 0] | NA | 256±1472 [0, 8456] | 551±2350 [0, 13 110] | 0.50 | 0.48 |
| 7S, 14S-diHDHA | 0 [0, 0] | 0 [0, 0] | NA | 0 [0, 0] | 0 [0, 0] | NA | NA |
| MaR1 | 0.6±4 [0, 23] | 0 [0, 0] | 0.32 | 0 [0, 0] | 0 [0, 0] | NA | 0.34 |
| 10,17-diHDHA | 40±56 [0, 156] | 53±63 [0, 217] | 0.19 | 29±53 [0, 180] | 21±35 [0, 99] | 0.11 | 0.06 |
| RvD1 | 9±51 [0, 309] | 7±42 [0, 249] | 0.32 | 0 [0, 0] | 0 [0, 0] | NA | 0.34 |
| RvD2 | 6±34 [0, 204] | 5±31 [0, 187] | 0.32 | 0 [0, 0] | 0 [0, 0] | NA | 0.34 |
| AA products | |||||||
| 5-HETE | 129±313 [0, 1647] | 79±130 [0, 520] | 0.33 | 148±295 [0, 1443] | 108±226 [0, 1146] | 0.61 | 0.79 |
| 12-HETE | 891±2159 [0, 9584] | 767±2246 [0, 9162] | 0.51 | 691±1782 [0, 8185] | 393±874 [0, 3950] | 0.35 | 0.63 |
| 15-HETE | 382±591 [0, 2157] | 295±542 [0, 2098] | 0.17 | 242±455 [0, 1923] | 168±279 [0, 1149] | 0.41 | 0.90 |
| 19-HETE | 497±2055 [0, 12 338] | 145±610 [0, 3506] | 0.16 | 81±285 [0, 1519] | 5±31 [0, 178] | 0.14 | 0.30 |
| 20-HETE | 161±406 [0, 2025] | 139±246 [0, 850] | 0.71 | 44±97 [0, 324] | 62±111 [0, 379] | 0.41 | 0.53 |
| 5,15- diHETE | 0 [0, 0] | 43±147 [0, 776] | 0.09 | 29±93 [0, 369] | 52±125 [0, 544] | 0.43 | 0.60 |
| 8,15- diHETE | 0 [0, 0] | 0 [0, 0] | NA | 0 [0, 0] | 0 [0, 0] | NA | NA |
| 20-OH LTB4 | 0 [0, 0] | 6±36 [0, 217] | 0.32 | 0 [0, 0] | 0 [0, 0] | NA | 0.34 |
| PGE2 | 1210±3208 [0, 14 700] | 2747±7134 [0, 36 468] | 0.24 | 0 [0, 0] | 0 [0, 0] | NA | 0.26 |
| PGD2 | 0 [0, 0] | 0 [0, 0] | NA | 0 [0. 0] | 0 [0, 0] | NA | NA |
| PGF2α | 0 [0, 0] | 8±49 [0, 293] | 0.32 | 0 [0, 0] | 0 [0, 0] | NA | 0.34 |
| LXA4 | 21±36 [0, 106] | 3±11 [0, 51] | 0.009* | 1±6 [0, 33] | 12±47 [0, 261] | 0.14 | 0.004* |
| LXB4 | 0 [0, 0] | 0 [0, 0] | NA | 68±390 [0, 2239] | 0 [0, 0] | 0.32 | 0.30 |
| DGLA products | |||||||
| 15-HETrE | 194±181 [0, 642] | 171±149 [0, 514] | 0.44 | 96±95 [0, 332] | 10±105 [0, 357] | 0.89 | 0.48 |
Values are mean±SD in units of pg/mL. 15-HETrE indicates 15-hydroxy eicosatrienoic acid; AA, arachidonic acid (n-6 PUFA); DGLA, dihomogamma linolenic acid; DHA, docosahexaenoic acid (n-3 PUFA); EPA, eicosapentaenoic acid (n-3 PUFA); HDHA, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; diHETE, dihydroxyeicosatetraenoic acid; LTB4, leukotriene B4; LXA4, lipoxin A4; LXB4, lipoxin B4; n-3 PUFA, n-3 polyunsaturated fatty acids; PGD2 (or D3), prostaglandin D2 (or D3); PGE2 (or E3), prostaglandin E2 (or E3); RvD1 (or 2), resolvin D1 (or D2); RvE1, resolvin E1.
P<0.05 by a paired Student’s t test (within group) or unpaired Student’s t test (across groups).
Figure 2.
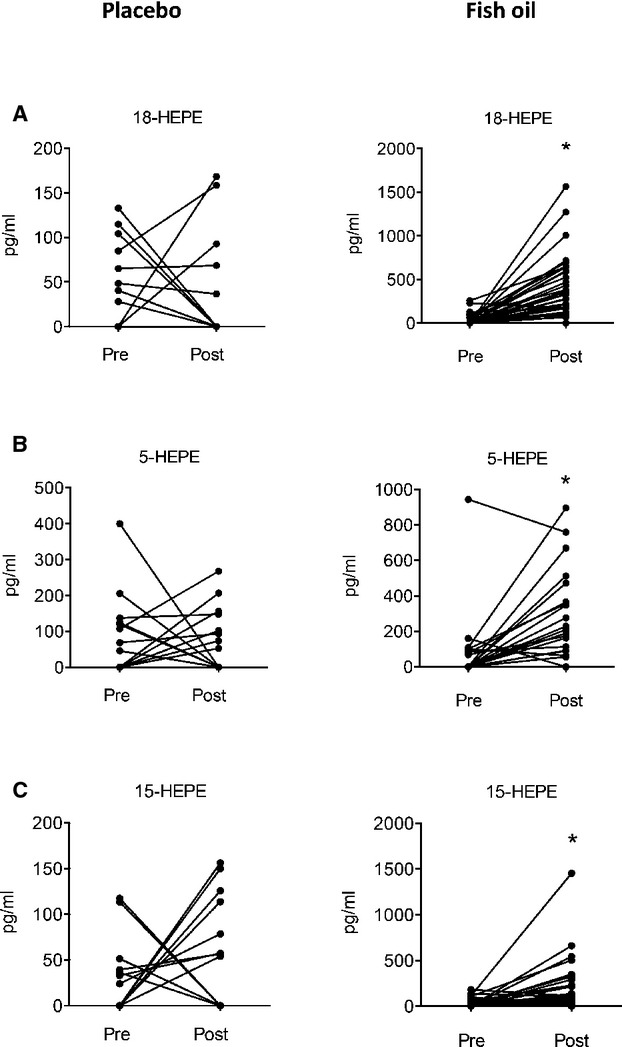
Omega-3 polyunsaturated fatty acid supplementation increases the production of EPA-derived lipid mediators in PAD patients. (A) Plasma levels of acetylated cyclooxygenase 2/cytochrome P450 product of EPA, 18-hydroxyeicosapentaenoic acid (18-HEPE) before (Pre) and after (Post) either placebo or fish oil treatment. (B, C) Plasma levels of 5-lipoxygenase (5-HEPE) and 15-lipoxygenase (15-HEPE) products of EPA as in panel A. *P<0.05 by an paired Student’s t test.
Figure 4.
Identification of omega-3 polyunsaturated fatty acid mediator signatures in PAD patients. (A) Hierarchical clustering and Spearman rank correlation analysis of omega-3 PUFA products based on their changes between placebo and fish oil groups 1 month post-treatment. (B) Partial least squares discriminant analysis (PLS-DA) score plot of placebo (2; red) and fish oil (4; green) groups 1 month post-treatment. (C) Loadings plot showing products related to group separations shown in panel B. (D) Variable importance in projection (VIP) scores (component 1) of products from placebo (2) and fish oil (4) groups.
In addition to EPA, DHA also serves as a substrate for lipoxygenase enzymes and gives rise to monohydroxydocosanoids that are markers of SPM biosynthesis, namely, D-series resolvins, protectins, and maresins.27 Lipidomics analysis of the DHA metabolome indicated that fish oil supplementation significantly increased the production of 4-hydroxydocosahexaenoic acid (4-HDHA), a potential product of 5-lipoxygenase44 (Figure 3 and Table 4). No significant increase in this product was observed in the placebo group, and the levels of 4-HDHA remained significant when comparing the 2 groups (Table 4). Other monohydroxy docosanoids, including 14-HDHA (a marker of maresin biosynthesis),45 17-HDHA (a marker of D-series resolvin and protectin biosynthesis), and 7-HDHA, were also identified in selected patient samples, although no significant patterns in their production were observed. D-series resolvins, including RvD1 and RvD2, were identified in selected patient samples, with no significant differences with treatment observed. In contrast, we identified 10,17-dihydroxyDHA (10,17-diHDHA) of the protectin family in both placebo and fish oil treatment groups at baseline. A trend toward an increase in the fish oil group and a trend toward a decrease in the placebo group were observed (Figure 3). Although these trends did not reach statistical significance, levels of 10,17-diHDHA were significantly higher in the fish oil group compared with the placebo group after 1 month of treatment (Figure 3) despite no significant differences between the 2 groups at baseline. We note that both authentic protectin D1 (PD1; 10R, 17S-dihydroxydocosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid) and its double-dioxygenation isomer (PDX; 10S, 17S-dihydroxydocosa-4Z, 7Z, 11E, 13Z, 15E, 19Z-hexaenoic acid) co-elute in our liquid chromatography method, precluding individual quantification of each product and potentially confounding the results.
Figure 3.
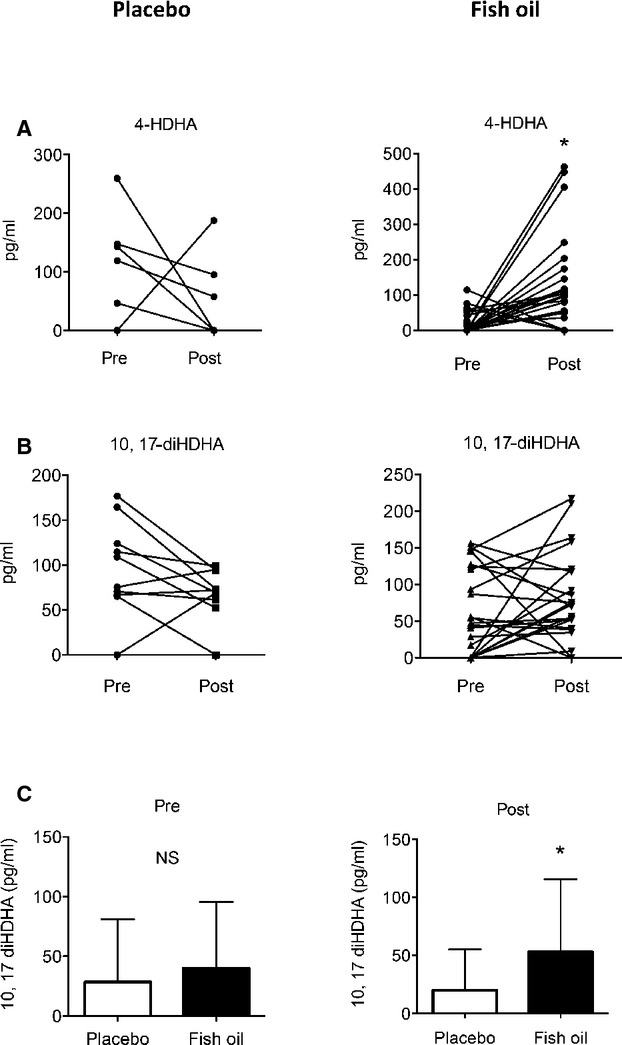
Increased production of DHA-derived lipid mediators in PAD patients. (A) Plasma levels of 4-hydroxydocosahexaenoic acid (4-HDHA) before (Pre) and after (Post) either placebo or fish oil treatment. (B) Plasma levels of 15-lipoxygenase product, 10,17-dihydroxydocosahexaenoic acid (10,17-diHDHA) as in panel A. (C) Comparison of 10,17-diHDHA levels at baseline (Pre; left panel) and 1 month after treatment of PAD patients with either placebo or fish oil (Post; right panel). NS indicate Non-significant; *P<0.05 by a paired (A, B) or unpaired (C) Student’s t test. Values in panel C are mean ± SD.
In contrast to the increased formation of several EPA and DHA products, downstream enzymatic products of AA metabolism, such as 5-hydroxyeicosatetraenoic acid (5-HETE), 12-HETE, 15-HETE, 19-HETE, and 20-HETE, were not significantly altered by treatment group. Prostanoids, such as prostaglandin E2, prostaglandin D2, and prostaglandin F2, were also sparsely detected and not different between groups or with fish oil treatment. Interestingly, lipoxin A4, which is an SPM derived from AA, was identified in both treatment groups and its levels significantly decreased in the fish oil group (Table 4).
To further elucidate relationships between n-3 PUFA–derived mediators and determine which products were important in driving overall differences between the treatment groups, we next performed correlation analysis and partial least-squares discriminant analysis. As shown in Figure 4A, Spearman rank correlation analysis showed that changes in EPA and DHA products, including 18-HEPE and 4-HDHA, positively correlated with each other but not AA products, including 15-HETE and 5-HETE. Hierarchical clustering demonstrated that changes in monoHEPEs and 4-HDHA between the groups were closely related to each other, but were clearly separated from AA products (Figure 4A). The 2 treatment groups were separated in partial least-squares discriminant analysis (Figure 4B), and a loadings plot demonstrated that monoHEPEs and 4-HDHA were major contributors to group separation (Figure 4C). Variable importance in projection scores revealed that among these products, 18-HEPE was the most important in driving differences between the groups (Figure 4D). The reduction in triglycerides was significantly and inversely correlated with changes in 18-HEPE (r=−0.26, P=0.04), a relationship that was similar in extent to the omega-3 index (r=−0.26, P=0.03). The changes in the omega-3 index were most strongly correlated with changes in 18-HEPE (r=0.62, P<0.001) and were also significantly and positively correlated with levels of 15-HEPE (r=0.37, P=0.002), 5-HEPE (r=0.37, P=0.002), and 4-HDHA (r=0.49, P<0.001). Interestingly, the omega-3 index was inversely correlated with lipoxin A4 (r=−0.36, P=0.003), which is consistent with shunting of lipoxygenase substrates from AA to EPA and DHA. Overall, these analyses demonstrate a significant enrichment in n-3 PUFA–derived lipid mediators in PAD patients treated with fish oil for 1 month.
Discussion
The OMEGA-PAD-1 Trial assessed the effects of a month-long, high-dose n-3 PUFA supplement intervention on endothelial function and inflammation in patients with PAD. The primary end point of a change in endothelial function response between fish oil and placebo was negative. Changes were seen in secondary end points including the lipid profiles and the n-3 metabolome. These changes were also accompanied by a notable 4% increase in the omega-3 index of the treatment group. Although the primary end point of a change in endothelial function was negative, this study reports potential beneficial effects of n-3 PUFA on metabolic and physiologic parameters in patients with PAD, and provides a framework for further studies examining alternative dosing schemes and subgroups within the PAD population.
Primary End point: n-3 PUFA and Endothelial Function
Impaired endothelial function has been recognized as an inciting step in the development of atherosclerosis and is, therefore, a critical feature in the pathogenesis of PAD. Furthermore, endothelial dysfunction independently predicts CV events in individuals with PAD undergoing vascular surgery.7–9 FMD is a noninvasive, ultrasound-guided tool readily used as a reliable measure of nitric-oxide–mediated vascular endothelial function; decreasing FMD is a surrogate for worsening endothelial function. In our study, FMD improved significantly in the treatment group but not in the control group, and there was also no difference in the change between the groups, leading to a negative finding for the primary end point, which contradicts other studies.26 The lack of a difference between supplementation and placebo groups in our study was unexpected. The study was adequately powered to detect a difference based on the current literature; however, it is possible that in our patient population at the Veterans Affairs Medical Center, endothelial dysfunction was so profound that a significant difference between the fish oil group and placebo group could not be detected. Furthermore, the trend for an improvement in FMD in the control group was not expected. Hence, reasons why a significant difference in response between the 2 groups include (1) an overall improvement in both groups related to participation in the study, such as altered lifestyle behaviors or increased medical attention; (2) lack of power based on the profound depression in FMD at baseline; or (3) the need for a longer duration of treatment. Another trial is currently taking place to address the issue of a longer duration of treatment (OMEGA-PAD II Trial, NCT01979874—NIH funded), which will also serve to address questions related to functional improvement in walking.
Secondary End Points: Inflammation and Resolution of Inflammation
Several studies have demonstrated that the inflammatory markers C-reactive protein, IL-6, and intracellular adhesion molecule-1 are elevated in patients with PAD.46–48 Inflammation itself can increase the risk of progression to PAD, disease severity,49–52 and mortality.5,6,53 Despite available medical therapies, patients with PAD continue to have a higher risk of CV events compared to patients with coronary artery disease,2,3 and this difference is thought to be related to increased systemic inflammation.1 There is an important unmet need to optimize medical management for patients with PAD, in an effort to decrease their excess morbidity and mortality. Making attempts to reduce or resolve factors that are likely involved in this excess risk, such as inflammation, has potential to positively impact clinical outcomes for patients with PAD.
In recent years, it has become apparent that the resolution of inflammation involves active counter-regulation by distinct mediators, rather than a passive decrescendo of pro-inflammatory signals. With implementation of unbiased lipidomics in models of self-resolving inflammation, Serhan et al discovered that novel lipid mediators derived from PUFA are generated by specific biosynthetic pathways.27 There are 4 distinct classes of SPM that have been recognized: the lipoxins derived from the n-6 PUFA, AA, and the resolvins, protectins, and maresins derived from the n-3 PUFAs, EPA, and DHA.27 In the context of PAD, several investigators have demonstrated that SPM have potent biological effects on vascular cells.54 Lipoxins and resolvins regulate leukocyte–endothelial interactions, reduce the formation of reactive oxygen species, and regulate the production of prostacyclin and nitric oxide.55–60 More recently, resolvins were found to counter-regulate inflammatory signaling in vascular smooth muscle cells and to decrease neo-intimal formation in vivo.61 Importantly, recent studies have shown that following oral supplementation with n-3 PUFAs, the detected amounts of SPM in human blood meet threshold levels for having anti-inflammatory and proresolution activity.29,30,62 In the present study, we identified several members of these SPM families and determined that their biosynthetic pathway markers are significantly increased in PAD patients taking fish oil supplements.
The current study provides evidence that utilization of EPA and DHA by endogenous enzymatic pathways occurs in PAD patients and that generation of these mediators may relate to the beneficial effects of n-3 PUFA. Indeed, several SPM pathway markers, such as 18-HEPE, 5-HEPE, and 15-HEPE, were significantly elevated in PAD patients treated with fish oil. Whether 18-HEPE and other SPM biomarkers have direct biological activity in this context remains to be determined, although a recent study documented that 18-HEPE administration reduces maladaptive cardiac remodeling in mice.63 That SPM biosynthetic pathway markers, rather than bioactive SPM products (eg, resolvins), were the most robust outputs reliably detected in plasma of PAD patients is not surprising. These pathway markers, such as 18-HEPE, are intermediates that give rise to several downstream products, such as E-series resolvins, that are locally produced in microenvironments (ie, resolving exudates). Analogously, AA products such as 5-HETE are biosynthetic pathway markers of products such as the leukotrienes, which were also rarely detected in plasma in this study. While several studies have documented that resolvins and related SPM can be measured in human plasma and serum following oral n-3 PUFA supplementation, most of these studies were carried out in healthy human volunteers.29,30,62 Thus, sparse detection of SPM end products in this study could also be related to defective bioconversion of intermediates into resolvins, protectins, and maresins. Indeed, we have previously documented that plasma levels of 15-epimeric lipoxin A4 are significantly lower in patients with symptomatic PAD than in healthy volunteers, suggesting a “resolution deficit” in PAD.47 A similar deficit in SPM has also been documented in several other human studies, including obese patients with PAD, patients with asthma, and in murine atherosclerosis models.27,55,64 Further longer-term studies will be required to determine whether SPM pathways are enriched as disease progression slows and vascular function improves and whether these changes precede changes in inflammatory biomarkers. Along these lines, it is notable that a trend in increased production of 10,17-diHDHA was observed in the fish oil group, whereas its levels were declining in the placebo group during the 1-month study period.
Previously, several randomized trials in patients with coronary artery disease demonstrated that fish oil supplementation or dietary intake of oily fish correlated with a decrease in total mortality, decrease in CV death, decrease in sudden cardiac death, and a reduction in nonfatal CV events.12–15 In a recent review, our group observed that only a limited number of studies exist on the role of n-3 PUFA in PAD.24 We speculate that the present body of literature concerning n-3 PUFA and PAD is limited due to 2 important factors. First, there is marked variability of n-3 PUFA dosing in major trials, and many studies have had relatively low dosing (averaging 1.5 g daily), which likely has minimal effects in the setting of increased inflammation associated with PAD. These differences contribute to the heterogeneity of clinical outcomes. Second, the majority of clinical trials that evaluate clinical effects of n-3 PUFAs have included study subjects who are at relatively low risk on the CV risk spectrum when compared to symptomatic PAD patients. In reviewing the literature, however, many studies have shown that n-3 PUFA is associated with a dramatic reduction in inflammation in diverse patient populations.16–20 Furthermore, as described above, the most recent evidence supports the beneficial role of n-3 PUFA in the resolution of inflammation.27,65,66 In the future, it would be prudent to design studies that have sufficiently high n-3 PUFA dosing and duration to counteract the increased inflammatory state in PAD and to more accurately examine the nutrient’s clinical effects. With specific regard to the enhancement of SPM pathways, future studies might focus on subgroups of patients who experience a secondary inflammatory insult, to determine whether clinical resolution is improved.
Secondary End Point: An Improvement in Triglycerides
Many patients with PAD have hypertriglyceridemia, which contributes in part to their increased CV risk.67 N-3 PUFA are among the pharmacologic treatments indicated for reduction of elevated triglycerides.68 In fact, the American Heart Association recommends 2 to 4 g daily of EPA/DHA as treatment for hypertriglyceridemia, which is a higher dose than that recommended for primary and secondary prevention of CVD.69–71 This dosing suggests that higher-than-average levels are necessary to exert clinical effects.70,71 n-3 PUFA, in particular EPA and DHA, are thought to improve triglyceride levels by decreasing production of hepatic very-low-density lipoprotein and triglyceride carrying units, promoting β-oxidation of fatty acids, and inhibiting phosphatidic acid phosphatase/phosphohydrolase, an enzyme that catalyzes the conversion of phosphatidic acid to diacylglycerol, and diacylglycerol acyltransferase, the enzyme that catalyzes the last step in triglyceride synthesis.72 In clinical trials involving subjects with PAD, n-3 supplementation has repeatedly been shown to decrease serum triglyceride levels,68,73–75 a finding also upheld by our results. Interestingly, 18-HEPE was found to correlate with the reduction in triglycerides in PAD patients, although causality in this relationship needs to be evaluated in future mechanistic studies.
Clinical Implications
Although guidelines from the American Heart Association recommend that individuals with CVD incorporate fish into their diets and supplement daily with n-3 PUFA,69–71 the evidence for PAD remains elusive. Since this group of patients is at an extreme of the “inflammatory” spectrum of atherosclerosis, perhaps medical management should be further optimized to reduce their inflammatory burden. An innovative aspect of our study is the high-potency dosing of n-3 PUFA (4.4 g/day), which is markedly different from other randomized trials (which average doses of 1.5 g/day as per the most recent meta-analysis).31 This short-term intervention reduced triglyceride levels and augmented biosynthetic pathways of resolution mediators. It remains to be determined whether such alterations would correlate with clinical benefits over time, or in response to an acute insult such as a therapeutic vascular intervention.
Limitations
This trial has several limitations. With regard to the randomization, only 2 of 49 covariates were statistically significantly different between the groups (1 with P=0.04 and 1 with P=0.002). Although this could point to an imbalance between the groups, this can be entirely consistent with chance. Furthermore, it is important to stress that the findings presented here need to be interpreted as hypothesis generating in view of a negative primary end point and the fact that there was no a-priori adjustment for multiple hypothesis testing on the secondary end points. While recruitment was open to both women and men, men were primarily recruited, which reflects the patient population served by the SFVAMC. Our cohort is predominantly white. Furthermore, it is possible that the inflammatory phenotype of our overall cohort was elevated, given that veterans experience profound psychological and psychosocial stress compared to nonveteran individuals with PAD.76 It is possible that continued smoking during the trial could have blunted the effects of fish oil therapy. Since there was no formal reporting of smoking at follow-up, this represents a limitation of the study. Another major limitation relates to dosing scheme. A high-dose and short-duration intervention was chosen in this study. We are currently conducting a follow-up trial (OMEGA-PAD II Trial, NCT01979874) that will introduce a longer-duration intervention. Compliance with daily supplementation is another factor that may affect our results. In order to verify compliance, we performed a pill count when patients returned after the treatment period. Additionally, measuring the omega-3 index, which quantifies incorporation of n-3 PUFA into the red blood cell membrane, helped to confirm appropriate supplementation.
Conclusions
In this randomized, double-blinded, placebo-controlled trial including primarily male subjects with PAD, we investigated the biochemical and clinical effects of a month-long, high-dose intervention with fish oil. The current study did not demonstrate a significant difference in the response of endothelial function between the fish oil and placebo group, the primary end point. Our findings, however, suggest that, independent of any other lifestyle changes, a short course of high-potency n-3 PUFA supplementation is associated with an improvement of the omega-3 index, an increase in the production of downstream n-3 PUFA products that serve as biomarkers of the main SPM pathways (eg, 18-HEPE), and a decrease in serum triglycerides. Further studies are needed to evaluate the effects of fish oil supplementation on functional status and cardiovascular outcomes in PAD patients, as well as the role for longer duration, high-potency supplementation.
Acknowledgments
The authors would like to acknowledge the generous contribution of Nordic Naturals in providing Pro-Omega Capsules and placebo for this study.
Sources of Funding
This work was supported by start-up funds from the University of California San Francisco and the Northern California Institute for Research and Education. The project described was supported by Award Number KL2RR024130 from the National Center for Research Resources, Award Number 1K23HL122446-01 from the National Institute of Health/NHLBI, and a Society for Vascular Surgery Seed Grant and Career Development Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funding organizations were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2 TR000143. We also acknowledge the support of NIH grants HL106173 (to Spite) and HL116186 (to Hellmann). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosures
None.
References
- Grenon SM, Vittinghoff E, Owens CD, Conte MS, Whooley M, Cohen BE. Peripheral artery disease and risk of cardiovascular events in patients with coronary artery disease: insights from the Heart and Soul Study. Vasc Med. 2013;18:176–184. doi: 10.1177/1358863X13493825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter G, Cannon CP, McCabe CH, Michowitz Y, Kaluski E, Charlesworth A, Milo O, Bentley J, Blatt A, Krakover R, Zimlichman R, Reisin L, Marmor A, Lewis B, Vered Z, Caspi A, Braunwald E. Prior peripheral arterial disease and cerebrovascular disease are independent predictors of adverse outcome in patients with acute coronary syndromes: are we doing enough? Results from the Orbofiban in Patients with Unstable Coronary Syndromes-Thrombolysis in Myocardial Infarction (OPUS-TIMI) 16 study. Am Heart J. 2003;145:622–627. doi: 10.1067/mhj.2003.6. [DOI] [PubMed] [Google Scholar]
- Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- Owens CD, Ridker PM, Belkin M, Hamdan AD, Pomposelli F, Logerfo F, Creager MA, Conte MS. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45:2–9. doi: 10.1016/j.jvs.2006.08.048. ; discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidula H, Tian L, Liu K, Criqui MH, Ferrucci L, Pearce WH, Greenland P, Green D, Tan J, Garside DB, Guralnik J, Ridker PM, Rifai N, McDermott MM. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med. 2008;148:85–93. doi: 10.7326/0003-4819-148-2-200801150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criqui MH, Ho LA, Denenberg JO, Ridker PM, Wassel CL, McDermott MM. Biomarkers in peripheral arterial disease patients and near- and longer-term mortality. J Vasc Surg. 2010;52:85–90. doi: 10.1016/j.jvs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- Brevetti G, Silvestro A, Di Giacomo S, Bucur R, Di Donato A, Schiano V, Scopacasa F. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374–379. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- Coutinho T, Rooke TW, Kullo IJ. Arterial dysfunction and functional performance in patients with peripheral artery disease: a review. Vasc Med. 2011;16:203–211. doi: 10.1177/1358863X11400935. [DOI] [PubMed] [Google Scholar]
- McDermott MM, Lloyd-Jones DM. The role of biomarkers and genetics in peripheral arterial disease. J Am Coll Cardiol. 2009;54:1228–1237. doi: 10.1016/j.jacc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365–372. doi: 10.1002/clc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Liu K, Daviglus ML, Jenny NS, Mayer-Davis E, Jiang R, Steffen L, Siscovick D, Tsai M, Herrington D. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009;103:1238–1243. doi: 10.1016/j.amjcard.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef MA, Munro IA, Garg ML. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr. 2009;63:1154–1156. doi: 10.1038/ejcn.2009.20. [DOI] [PubMed] [Google Scholar]
- Ohsawa M, Itai K, Onoda T, Tanno K, Sasaki S, Nakamura M, Ogawa A, Sakata K, Kawamura K, Kuribayashi T, Yoshida Y, Okayama A. Dietary intake of n-3 polyunsaturated fatty acids is inversely associated with CRP levels, especially among male smokers. Atherosclerosis. 2008;201:184–191. doi: 10.1016/j.atherosclerosis.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis. 2009;205:538–543. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siasos G, Tousoulis D, Oikonomou E, Zaromitidou M, Verveniotis A, Plastiras A, Kioufis S, Maniatis K, Miliou A, Siasou Z, Stefanadis C, Papavassiliou AG. Effects of omega-3 fatty acids on endothelial function, arterial wall properties, inflammatory and fibrinolytic status in smokers: a cross over study. Int J Cardiol. 2013;166:340–346. doi: 10.1016/j.ijcard.2011.10.081. [DOI] [PubMed] [Google Scholar]
- Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B. Omega 3 fatty acids and cardiovascular outcomes: systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5:808–818. doi: 10.1161/CIRCOUTCOMES.112.966168. [DOI] [PubMed] [Google Scholar]
- Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R. n-3 Fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–1808. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- Grenon SM, Hughes-Fulford M, Rapp J, Conte MS. Polyunsaturated fatty acids and peripheral artery disease. Vasc Med. 2012;17:51–63. doi: 10.1177/1358863X11429175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenon SM, Conte MS, Nosova E, Alley H, Chong K, Harris WS, Vittinghoff E, Owens CD. Association between n-3 polyunsaturated fatty acid content of red blood cells and inflammatory biomarkers in patients with peripheral artery disease. J Vasc Surg. 2013;58:1283–1290. doi: 10.1016/j.jvs.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liang X, Wang L, Lu X, Huang J, Cao J, Li H, Gu D. Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221:536–543. doi: 10.1016/j.atherosclerosis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- Grenon SM, Owens CD, Alley H, Chong K, Yen PK, Harris W, Hughes-Fulford M, Conte MS. n-3 Polyunsaturated fatty acids supplementation in peripheral artery disease: the OMEGA-PAD trial. Vasc Med. 2013;18:263–274. doi: 10.1177/1358863X13503695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai SP, Kruidenier LM, Rouwet EV, Graffius K, Prins MH, Teijink JA. The walking impairment questionnaire: an effective tool to assess the effect of treatment in patients with intermittent claudication. J Vasc Surg. 2009;50:89–94. doi: 10.1016/j.jvs.2008.12.073. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley H, Owens CD, Gasper WJ, Grenon SM. Ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery in clinical research. J Vis Exp. 2014:e52070. doi: 10.3791/52070. . doi: 10.3791/52070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, Nelson M, Lloyd-Jones D, Van Horn L, Garside D, Kibbe M, Domanchuk K, Stein JH, Liao Y, Tao H, Green D, Pearce WH, Schneider JR, McPherson D, Laing ST, McCarthy WJ, Shroff A, Criqui MH. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bots ML, Westerink J, Rabelink TJ, de Koning EJ. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J. 2005;26:363–368. doi: 10.1093/eurheartj/ehi017. [DOI] [PubMed] [Google Scholar]
- Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- Hellmann J, Zhang MJ, Tang Y, Rane M, Bhatnagar A, Spite M. Increased saturated fatty acids in obesity alter resolution of inflammation in part by stimulating prostaglandin production. J Immunol. 2013;191:1383–1392. doi: 10.4049/jimmunol.1203369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, Hellmann J, Conte MS, Owens CD, Spite M, Grenon SM. Short-term physical inactivity impairs vascular function. J Surg Res. 2014;190:672–682. doi: 10.1016/j.jss.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. doi: 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, Sangiovanni JP, Gronert K, Smith LE. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen D, Rexrode KM, Creager MA, Ridker PM, Pradhan AD. Metabolic syndrome, inflammation, and risk of symptomatic peripheral artery disease in women: a prospective study. Circulation. 2009;120:1041–1047. doi: 10.1161/CIRCULATIONAHA.109.863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens CD, Kim JM, Hevelone ND, Gasper WJ, Belkin M, Creager MA, Conte MS. An integrated biochemical prediction model of all-cause mortality in patients undergoing lower extremity bypass surgery for advanced peripheral artery disease. J Vasc Surg. 2012;56:686–695. doi: 10.1016/j.jvs.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Preis O, Ridker PM, Gerhard-Herman M. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death) Am J Cardiol. 2005;96:1374–1378. doi: 10.1016/j.amjcard.2005.07.041. [DOI] [PubMed] [Google Scholar]
- Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- Carriere I, Dupuy AM, Lacroux A, Cristol JP, Delcourt C. Biomarkers of inflammation and malnutrition associated with early death in healthy elderly people. J Am Geriatr Soc. 2008;56:840–846. doi: 10.1111/j.1532-5415.2008.01677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezinski ME, Gimbrone MA, Jr, Nicolaou KC, Serhan CN. Lipoxins stimulate prostacyclin generation by human endothelial cells. FEBS Lett. 1989;245:167–172. doi: 10.1016/0014-5793(89)80214-5. [DOI] [PubMed] [Google Scholar]
- Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost. 2007;97:88–98. [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocytes recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, Serhan CN, Conte MS. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27:2220–2232. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden A, Mas E, Croft KD, Phillips M, Mori TA. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J Lipid Res. 2014;55:2401–2407. doi: 10.1194/jlr.M045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J Exp Med. 2014;211:1673–1687. doi: 10.1084/jem.20132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, Nguyen BT, Madenci AL, Ozaki CK, Serhan CN. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol. 2013;304:C1141–C1149. doi: 10.1152/ajpcell.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzamora MT, Fores R, Baena-Diez JM, Pera G, Toran P, Sorribes M, Vicheto M, Reina MD, Sancho A, Albaladejo C, Llussa J. The peripheral arterial disease study (PERART/ARTPER): prevalence and risk factors in the general population. BMC Public Health. 2010;10:38. [Google Scholar]
- Sadovsky R, Kris-Etherton P. Prescription omega-3-acid ethyl esters for the treatment of very high triglycerides. Postgrad Med. 2009;121:145–153. doi: 10.3810/pgm.2009.07.2020. [DOI] [PubMed] [Google Scholar]
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T. Pennathur S; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Bays HE, Tighe AP, Sadovsky R, Davidson MH. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev Cardiovasc Ther. 2008;6:391–409. doi: 10.1586/14779072.6.3.391. [DOI] [PubMed] [Google Scholar]
- Woodcock BE, Smith E, Lambert WH, Jones WM, Galloway JH, Greaves M, Preston FE. Beneficial effect of fish oil on blood viscosity in peripheral vascular disease. Br Med J (Clin Res Ed) 1984;288:592–594. doi: 10.1136/bmj.288.6417.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans RO, Bilo HJ, Weersink EG, Rauwerda JA, Fonk T, Popp-Snijders C, Donker AJ. Fish oil supplementation in patients with stable claudication. Am J Surg. 1990;160:490–495. doi: 10.1016/s0002-9610(05)81012-8. [DOI] [PubMed] [Google Scholar]
- Mori TA, Vandongen R, Mahanian F, Douglas A. Plasma lipid levels and platelet and neutrophil function in patients with vascular disease following fish oil and olive oil supplementation. Metabolism. 1992;41:1059–1067. doi: 10.1016/0026-0495(92)90286-j. [DOI] [PubMed] [Google Scholar]
- Grenon SM, Hiramoto J, Smolderen KG, Vittinghoff E, Whooley MA, Cohen BE. Association between depression and peripheral artery disease: insights from the Heart and Soul Study. J Am Heart Assoc. 2012;1:e002667. doi: 10.1161/JAHA.112.002667. doi: 10.1161/JAHA.112.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]