Abstract
Background
Genomewide association studies have identified several loci associated with atrial fibrillation (AF) and have been reportedly associated with response to catheter ablation for AF in patients of European ancestry; however, associations between top susceptibility loci and AF recurrence after ablation have not been examined in Asian populations. We examined whether the top single nucleotide polymorphisms (SNPs) at chromosomes 4q25 (PITX2), 16q22 (ZFHX3), and 1q21 (KCNN3) were associated with AF in a Korean population and whether these SNPs were associated with clinical outcomes after catheter ablation for AF.
Methods and Results
We determined the association between 4 SNPs and AF in 1068 AF patients who underwent catheter ablation (74.6% male, aged 57.5±10.9 years, 67.9% paroxysmal AF) and 1068 age- and sex-matched controls. The SNPs at the PITX2 and ZFHX3 loci, but not the KCNN3 locus, were significantly associated with AF (PITX2/rs6843082_G: odds ratio 3.41, 95% CI 2.55 to 4.55, P=1.32×10−16; PITX2/rs2200733_T: odds ratio 2.05, 95% CI 1.66 to 2.53, P=2.20×10−11; ZFHX3/rs2106261_A: odds ratio 2.33, 95% CI 1.87 to 2.91, P=3.75×10−14; KCNN3/rs13376333_T: odds ratio 1.74, 95% CI 0.93 to 3.25, P=0.085). Among those patients who underwent catheter ablation for AF, none of the top AF-associated SNPs were associated with long-term clinical recurrence of AF after catheter ablation.
Conclusions
SNPs at the PITX2 and ZFHX3 loci were strongly associated with AF in Korean patients. In contrast to prior reports, none of the 4 top AF-susceptibility SNPs predicted clinical recurrence after catheter ablation.
Keywords: atrial fibrillation, catheter ablation, genetic polymorphism, phenotype, recurrence
Atrial fibrillation (AF) is the most commonly found sustained arrhythmia, with a lifetime risk of 25%.1 Risk factors include advancing age, hypertension, structural heart disease, and congestive heart failure, yet a subset of younger persons develop AF in the absence of established risk factors. Genetic factors play an important role in the pathogenesis of AF. Parental history of AF increases risk of AF by 1.4 to 1.9 times.2,3 Familial cases of AF underscore a genetic basis for disease,4 and research has implicated mutations and polymorphisms in the development of AF.5
Recently, several common genetic variants have been shown to be associated with AF in genomewide association studies performed in populations of European ancestry6–10; however, ethnic differences exist in the frequency of AF-related single nucleotide polymorphisms (SNPs) between European and Asian populations,11,12 and thus the relationship between these SNPs and AF in non-European populations remains unclear. Furthermore, despite the identification of these AF-associated loci, data regarding the association between variants at these loci and clinical outcomes remain limited. Some reports suggested that these variants were associated with an increased risk of AF recurrence after a radiofrequency catheter ablation (RFCA),13–15 a common therapy in symptomatic patients with lone AF. These reports, however, were conducted in patients of European ancestry, and the associations between genetic variants and responses to RFCA in other ancestral groups remain unclear.
The goal of the current study was 2-fold. First, we sought to determine whether the top 4 AF SNPs identified in European patients were also associated with AF in Korean patients. These SNPs reside on chromosomes 4q25 (PITX2 locus), 16q22 (ZFHX3 locus), and 1q21 (KCNN3 locus). Second, we examined whether these AF SNPs were associated with AF recurrence after RFCA.
Methods
Patient Inclusion
The study protocol was approved by the institutional review board of Severance Cardiovascular Hospital, Yonsei University Health System, and adhered to the Declaration of Helsinki. This study is registered at ClinicalTrials.gov (identifier NCT02138695). All 2136 subjects (1068 with AF patients and 1068 with age- and sex-matched control subjects) provided written informed consent. All AF patients who underwent RFCA were included in the Yonsei AF Ablation Cohort registry. The exclusion criteria of this study were as follows: (1) permanent AF refractory to electrical cardioversion, (2) AF with valvular disease, (3) structural heart disease other than left ventricular hypertrophy, and (4) prior AF ablation. Age- and sex-matched control DNA was obtained from the Korea Centers for Disease Control and Prevention, National Biobank of Korea (KOBB-2012-00).16,17 All control subjects had a 12-lead electrocardiogram to exclude the presence of AF.
SNP Selection and Genotyping
We evaluated selected SNPs from the top 3 AF-associated genetic loci in European patients from the meta-analysis of genomewide association studies reported by Ellinor and colleagues in 2012 and in other previous papers.6,10,18 Specifically, we selected the top variant or a proxy SNP at each locus. For the locus at PITX2, the top SNP reported was rs6817105,6 and the SNP rs2200733 is a perfect proxy for this SNP (r2=1.0). Because SNP rs6843082 has also been reported to be strongly associated with AF10 and is in only modest linkage disequilibrium with rs6817105, we included this SNP in our analyses (r2=0.434). For the locus at ZFHX3 on chromosome 16q22, the top SNP selected was rs2106261. At the KCNN3 locus on chromosome 1q21, the top SNP reported was rs6666258,6 and we selected a perfect proxy for genotyping: SNP rs13376333 (r2=1.0).
We used whole blood samples for DNA extraction and genetic analyses. The SNPs were genotyped using validated TaqMan assays (Applied Biosystems, Life Technologies). Polymerase chain reaction product was amplified using 0.9 μm each of the forward and reverse primers, 0.2 μm each of the fluoresce in amidite and VIC minor groove binder sequence–specific probes, 3 ng DNA, 5.0 mmol/L MgCl2, and 1× TaqMan Universal PCR Master Mix containing AmpliTaq gold DNA polymerase in a 5.5-μL reaction volume. All SNPs had a call rate of >99%.
Radiofrequency Catheter Ablation
During the procedure, intracardiac electrograms were recorded using a Prucka CardioLab electrophysiology system (General Electric Health Care System Inc). Ablation was guided by 3-dimensional electroanatomical mapping (NavX system; St. Jude Medical Inc). An open irrigation 3.5-mm-tip deflectable catheter (Celsius, Johnson & Johnson Inc; Cool Flex, St. Jude Medical Inc; 30 to 35 W; 47°C) was used for RFCA (Stockert generator; Biosense Webster Inc). All patients initially underwent circumferential pulmonary vein (PV) isolation and cavotricuspid isthmus ablation. For patients with persistent AF, we added a roof line, posterior inferior line, and anterior line19 as a standard lesion set. At the operator’s discretion, additional ablations were delivered to the superior vena cava, non-PV foci, or regions of complex fractionated electrograms. We confirmed the PV isolation by both entrance and exit block and rechecked it under an isoproterenol infusion before finishing the procedure. In addition, we attempted to reinduce AF by isoproterenol infusion with rapid atrial pacing before finishing the procedure. The end point of our procedure was defined as no immediate recurrence of AF after cardioversion while receiving an isoproterenol infusion (5 to 10 μg/min). If there was immediate recurrence of AF after cardioversion, we then ablated these non-PV foci.
Postablation Management and Follow-up
Patients were seen in the outpatient clinic at 1, 3, 6, and 12 months and then every 6 months thereafter or whenever symptoms occurred after RFCA. An electrocardiogram was performed on every visit. A 24- or 48-hour Holter recording and/or event recorder was obtained at the 3- and 6-month visits as well as every 6 months afterward in accordance with the 2012 Heart Rhythm Society, European Heart Rhythm Association, and European Cardiac Arrhythmia Society expert consensus statement guidelines.20 Whenever patients reported symptoms of palpitations, Holter monitor or event monitor recordings were obtained and evaluated for possible arrhythmia recurrences. We defined recurrence of AF as any episode of AF or atrial tachycardia of at least 30 seconds in duration.21 Early recurrence was defined as having a documented AF electrocardiogram within 3 months after ablation. Clinical recurrence was defined as (1) any documented recurrence of AF after 3 months21 or (2) the initiation of an antiarrhythmic medication for AF.
Statistical Analyses
Odds ratios (ORs) were calculated using logistic regression with an additive genetic model. In case-only analysis, multiple parameters including clinical features and echocardiography parameters were compared depending on genotype distribution. Univariate analysis was performed for continuous variables using the t test and for nominal variables using the chi-square test. The log-rank test with Bonferroni correction for multiplicity of comparisons was applied to control the type 1 error. Multivariable Cox regression analyses were conducted to evaluate the associations between genetic variants and AF recurrence, with adjustment for age, sex, left atrium size, AF subtype (persistent AF), and early recurrence (≤3 months). Cases and controls were matched using the variables age and sex to reduce confounding effects. Conditional logistic regression models were used to assess the association between each SNP and AF risk to take matching situation into account. Potential confounding factors that were statistically significant at P<0.05 in the univariate analysis were included in the final analysis. ORs and 95% CIs were estimated using conditional logistic regression analysis with adjustments for potential confounding factors, body mass index, hypertension, diabetic mellitus, and congestive heart failure. Additional analyses were performed in 4 different subgroups. All statistical analyses were performed using SPSS version 19.0 (IBM Corp).
Results
Baseline characteristics of the study population are summarized in Table 1. We studied 1068 patients who underwent an AF ablation and 1068 controls who were matched on the basis of age and sex. The mean age of our study population was 57.5±11.2 years, and 74.6% of participants were male. As expected, the AF group had a higher prevalence of hypertension, diabetes mellitus, congestive heart failure, stroke, and coronary artery disease than the control group. To exclude the possible confounding effects caused by the imbalance of disease prevalence between cases and controls, we sequentially performed subgroup analyses excluding patients with the following risk factors: (1) hypertension; (2) diabetes mellitus; (3) congestive heart failure; and (4) strokes, coronary artery disease, and congestive heart failure simultaneously. All subgroup analyses were performed by using multivariable logistic regression, adjusting for age, sex, body mass index, hypertension, diabetic mellitus, and congestive heart failure, as appropriate, and the results were consistent (Table S1).
Table 1.
Baseline Characteristics of the Study Population
| AF (n=1068) | Controls (n=1068) | |
|---|---|---|
| Age, y | 57.5±10.9 | 57.5±11.5 |
| Male sex, % | 74.6 | 74.6 |
| PAF, % | 67.9 | — |
| Body mass index, kg/m2† | 24.7±3.2 | 23.6±3.1* |
| Hypertension | 510 (47.8) | 19 (1.8%)* |
| Diabetes mellitus | 139 (13.0) | 14 (1.3%)* |
| Congestive heart failure | 70 (6.6) | 1 (0.1%)* |
| Stroke | 106 (9.9) | 0 (0%)* |
| Coronary artery disease | 136 (12.7) | 0 (0%)* |
| CHADS2 score | 0.95±1.09 | — |
| Echocardiography | ||
| LA dimension, mm | 41.6±6.5 | — |
| LA volume index | 35.0±12.3 | |
| LVEF, % | 63.1±9.0 | — |
| E/Em | 9.7±5.1 | — |
| LVEDD, mm | 49.3±6.6 | — |
| LVESD, mm | 33.2±5.8 | — |
| LVMI, g/m2 | 75.3±41.2 | — |
| Early recurrence, % | 28.5 | — |
| Clinical recurrence, % | 19.0 | — |
Data are shown as mean±SD or n (%), except as noted. AF (n=1068) and control (n=1068). For the CHADS2 score, 1 point is given for congestive heart failure, hypertension, age >75 years, and diabetes mellitus; 2 points are given for previous stroke and transient ischemic attack. AF indicates atrial fibrillation; LA, left atrium; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; LVMI, left ventricular mass index; PAF, paroxysmal atrial fibrillation.
P<0.001 compared with AF.
16% of body mass index in controls are missing.
PITX2 and ZFHX3 Were Strongly Associated With AF in Korean Patients
We found that 2 of the 3 known AF-associated loci were strongly associated with AF in Korean patients (Table 2). Two variants at the PITX2 locus and 1 at the ZFHX3 locus were significantly associated with AF (rs6843082_G: OR 3.41, 95% CI 2.55 to 4.55, P=1.32×10−16; rs2200733_T: OR 2.05, 95% CI 1.66 to 2.53, P=2.20×10−11; rs2106261_A: OR 2.33, 95% CI 1.87 to 2.91, P=3.75×10−14) In contrast, genetic variants at the KCNN3 locus were not associated with AF in Korean patients (rs13376333_T: OR 1.74, 95% CI 0.93 to 3.25, P=0.085). Interestingly, the frequency of the AF risk allele at this locus (rs13376333_T) was very low in the Korean population, at only 1.9% compared with 29.5% in European patients.10
Table 2.
Association Between AF and SNPs From 3 Known AF Loci
| Nearest Gene | SNP | Chr | Position | Minor Allele/Major Allele | MAF | AF Associated Allele | OR | 95% CI | P Value* |
|---|---|---|---|---|---|---|---|---|---|
| PITX2 | rs6843082 | 4 | 111937516 | A/G | 0.204 | G | 3.41 | 2.55 to 4.55 | 1.32×10−16 |
| PITX2 | rs2200733 | 4 | 111929618 | C/T | 0.394 | T | 2.05 | 1.66 to 2.53 | 2.20×10−11 |
| ZFHX3 | rs2106261 | 16 | 71609121 | A/G | 0.395 | A | 2.33 | 1.87 to 2.91 | 3.75×10−14 |
| KCNN3 | rs13376333 | 1 | 153080977 | T/C | 0.019 | T | 1.74 | 0.93 to 3.25 | 0.085 |
AF indicates atrial fibrillation; Chr, chromosome; MAF, minor allele frequency; OR, odds ratio; SNPs, single nucleotide polymorphisms.
P value was calculated by using conditional logistic regression after adjusting for body mass index, hypertension, diabetes mellitus, and congestive heart failure.
Relationship Between AF-Risk SNPs and Baseline Clinical Characteristics of Patients Undergoing AF Ablation
Next we sought to determine whether any baseline differences existed in an extensive set of clinical and echocardiographic parameters based on AF genotype (Table 3). Among the 1068 AF ablation patients, the only notable difference was that carriers of the AF-associated risk allele for SNP rs6843082 on chromosome 4q25 were younger (50.8±15.3 versus 59.0±11.1, P=0.005).
Table 3.
Comparisons of Phenotypes and Clinical Outcomes According to SNPs From 3 Known AF Loci
| rs6843082 (PITX2) | rs2200733 (PITX2) | rs2106261 (ZFHX3) | rs13376333 (KCNN3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA (n=17) | GA (n=215) | GG (n=836) | CC (n=84) | CT (n=447) | TT (n=527) | GG (n=297) | GA (n=520) | AA (n=251) | CC (n=1020) | CT (n=48) | TT (n=0) | |
| Age, y | 50.8±15.3 | 59.0±11.1* | 57.2±10.7 | 56.7±12.7 | 58.1±10.7 | 57.1±10.8 | 58.2±11.8 | 57.6±10.5 | 56.4±10.7 | 57.5±11.0 | 56.2±9.4 | — |
| Male sex, % | 58.8 | 71.2 | 75.8 | 71.4 | 72.0 | 77.2 | 72.4 | 75.6 | 75.3 | 74.4 | 79.2 | — |
| PAF, % | 70.6 | 67.4 | 67.9 | 70.2 | 66.7 | 68.3 | 64.3 | 67.9 | 72.1 | 67.5 | 77.1 | — |
| Body mass index, kg/m2 | 26.0±3.5 | 24.6±2.8 | 24.9±2.8 | 24.8±2.8 | 24.7±2.9 | 25.0±2.7 | 24.7±2.8 | 24.8±2.8 | 24.9±2.6 | 24.8±2.8 | 25.5±2.5 | — |
| CHADS2 score | 0.88±0.78 | 1.06±1.14 | 0.93±1.08 | 0.95±1.04 | 0.95±1.10 | 0.97±1.09 | 0.98±1.10 | 0.97±1.12 | 0.88±1.01 | 0.96±1.09 | 0.73±0.94 | — |
| Hypertension | 9 (52.9%) | 108 (50.2%) | 393 (47.0%) | 44 (52.4%) | 202 (45.2%) | 261 (49.5%) | 147 (49.5%) | 256 (49.2%) | 107 (42.6%) | 493 (48.3%) | 17 (35.4%) | — |
| Diabetes mellitus | 0 (0%) | 26 (12.1%) | 113 (13.5%) | 6 (7.1%) | 62 (13.9%) | 71 (13.5%) | 31 (10.4%) | 75 (14.4%) | 33 (13.1%) | 134 (13.1%) | 5 (10.4%) | — |
| Congestive heart failure | 2 (11.8%) | 21 (9.8%) | 47 (5.6%) | 7 (8.3%) | 31 (6.9%) | 32 (6.1%) | 20 (6.7%) | 34 (6.5%) | 16 (6.4%) | 67 (6.6%) | 3 (6.3%) | — |
| Stroke | 2 (11.8%) | 24 (11.2%) | 80 (9.6%) | 6 (7.1%) | 43 (9.6%) | 56 (10.6%) | 29 (9.8%) | 52 (10.0%) | 25 (10.0%) | 101 (9.9%) | 5 (10.4%) | — |
| Coronary artery disease | 1 (5.9%) | 28 (13.0%) | 107 (12.8%) | 8 (9.5%) | 67 (15.0%) | 60 (11.4%) | 35 (11.8%) | 69 (13.3%) | 32 (12.7%) | 130 (12.7%) | 6 (12.5%) | — |
| Echocardiography | ||||||||||||
| LA dimension, mm | 40.2±7.1 | 41.6±6.5 | 41.7±6.0 | 41.1±6.6 | 41.5±6.1 | 41.9±6.0 | 41.4±6.3 | 41.8±6.4 | 41.0±5.7 | 41.7±6.1 | 41.5±5.8 | — |
| LA volume index | 38.3±18.9 | 35.5±13.1 | 35.0±11.9 | 34.4±12.5 | 35.2±12.6 | 34.9±11.9 | 35.2±12.4 | 35.5±12.5 | 33.7±11.5 | 35.1±12.3 | 32.8±10.6 | |
| LVEF, % | 62.8±11.3 | 63.4±8.2 | 63.3±8.3 | 62.8±9.0 | 63.5±8.5 | 63.1±8.1 | 64.0±7.6 | 63.5±8.0 | 62.7±9.0 | 63.3±8.4 | 62.7±7.7 | — |
| E/Em | 9.8±3.4 | 10.8±5.4 | 10.3±4.3 | 10.5±4.4 | 10.5±4.9 | 10.3±4.2 | 9.8±3.8 | 10.7±4.4 | 9.5±3.4 | 10.4±4.6 | 9.9±3.5 | — |
| LVEDD, mm | 50.1±3.7 | 49.8±4.6 | 49.9±4.2 | 50.7±4.7 | 49.6±4.5 | 50.0±4.1 | 49.6±4.3 | 49.9±4.3 | 50.0±4.6 | 49.8±4.3 | 50.4±4.7 | — |
| LVESD, mm | 33.8±4.7 | 33.8±5.1 | 33.6±4.6 | 34.4±5.1 | 33.4±4.7 | 33.7±4.6 | 33.3±4.6 | 33.5±4.4 | 33.8±5.0 | 33.6±4.7 | 34.1±4.9 | — |
| LVMI, g/m2 | 93.4±16.8 | 95.9±22.8 | 92.3±20.9 | 97.3±24.3 | 92.8±20.3 | 92.8±21.7 | 93.5±23.0 | 93.8±20.2 | 91.3±20.4 | 93.3±21.4 | 67.4±18.4 | — |
| Early recurrence, % | 0 | 28.8 | 28.9 | 25.0 | 28.2 | 29.4 | 29.6 | 28.1 | 27.9 | 27.6 | 45.8 | — |
CHADS2 score, 1 point is given for cardiac heart failure, hypertension, age >75 years, and diabetes mellitus; 2 points are given for previous stroke and transient ischemic attack. AF indicates atrial fibrillation; LA, left atrium; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; LVMI, left ventricular mass index; PAF, paroxysmal atrial fibrillation; SNPs, single nucleotide polymorphisms.
p<0.05 compared between AA and GA.
Relationship Between AF Genotype and Outcomes After AF Ablation
After ablation, 304 patients (28.5%) had early recurrence during the 3-month blanking period. We did not observe any significant associations between AF-risk SNPs and early AF recurrence. During 18.3±13.9 months (range 0 to 55 months) of follow-up, 203 patients (19.0%) had a clinical recurrence >3 months from RFCA. Figure 1 displays the freedom from AF recurrence for each of the 4 SNPs. None of the 4 SNPs were associated with a long-term clinical recurrence after RFCA (Table 4).
Figure 1.
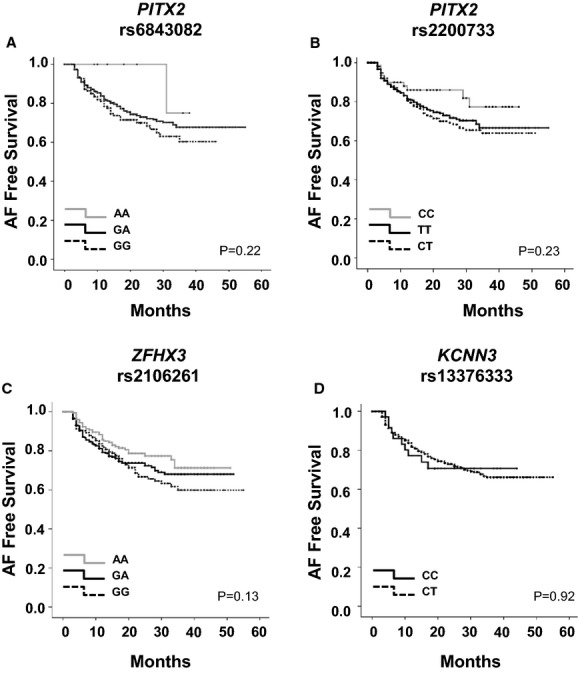
Kaplan–Meier curve showing relationship among recurrence and risk alleles of rs6843082 in PITX2 (A), rs2200733 in PITX2 (B), rs2106261 in ZFHX3 (C), and rs13376333 in KCNN3 (D). AF indicates atrial fibrillation.
Table 4.
Association Between Candidate Genes (SNPs) and Clinical Recurrence of AF Using Cox Regression Model
| Gene/SNP | Adjusted* | ||
|---|---|---|---|
| HR | 95% CI | P Value | |
| PITX2/rs6843082_G | 0.84 | 0.61 to 1.15 | 0.280 |
| PITX2/rs2200733_T | 1.01 | 0.80 to 1.26 | 0.963 |
| ZFHX3/rs2106261_A | 0.86 | 0.71 to 1.04 | 0.128 |
| KCNN3/rs13376333_T | 0.72 | 0.38 to 1.36 | 0.308 |
AF indicates atrial fibrillation; HR, hazard ratio; SNPs, single nucleotide polymorphisms.
Adjusted for sex, age, left atrium size, AF subtype (persistent AF), and early recurrence (≤3 months).
Discussion
In a well-characterized cohort of >1000 Korean patients with nonvalvular AF, we observed that 2 of the 3 main AF susceptibility signals discovered in European patients (chromosomes 4q25 [PITX2] and 16q22 [ZFHX3]) were similarly associated with AF, supporting a shared genetic basis for AF that transcends ancestry. Second, and in contrast to prior reports,13,14 we did not observe any significant associations between these top 4 AF susceptibility loci and AF recurrence following catheter ablation.
Interestingly, the AF risk allele at 1q21 was not associated with AF; however, the frequency of this variant in the Korean population was rare. Large-scale genomewide association studies showed the association of SNPs in PITX2, ZFHX3, and KCNN3 with AF in patients of European ancestry, and the results were replicated in a Japanese study.6 In Table 5, we summarized the published results of these 4 AF variants in patients of Asian descent. We noted that most published studies were smaller and studied only 1 or 2 of the top AF variants. Although SNPs at PITX2 and ZFHX3 were consistently associated with AF, there was less consistency among the studies at the KCNN3 locus. The lack of replication of the association at the KCNN3 locus may be due to the rarity of this risk allele among those of Asian descent. In Korean patients, the risk allele was quite rare, with a minor allele frequency of only 1.9%. Similarly, in Japanese and Chinese studies, this variant had a minor allele frequency of 1.5% and 4%, respectively. In contrast, European and Taiwanese populations had high prevalence of the AF risk allele (29.6% and 8.6%, respectively). Our findings highlight the variability in this KCNN3 AF risk allele, even among those of Asian descent. Our results also demonstrate the need for large-scale genetic studies in specific Asian subgroups to identify this and other regions uniquely associated with AF.
Table 5.
Summary of Association Results for the 3 AF Loci
| Ethnicity | European6 | Japanese6 | Hong Kong9 | Chinese11 | Taiwanese12 | Hong Kong22 | Chinese23 | Chinese24 | Korean |
|---|---|---|---|---|---|---|---|---|---|
| Total Number (AF Patients) | 59 133 (6707) | 4193 (843) | 3048 (285) | 2097 (650) | 428 (214) | 3169 (333) | 1234 (383) | 1593 (597) | 2136 (1068) |
| PITX2 | |||||||||
| OR | 1.64 | 1.84 | 1.42 | 1.81 | 3.06 | ||||
| 95% CI | 1.55 to 1.73 | 1.59 to 2.13 | 1.16 to 1.73 | 1.21 to 3.20 | 2.59 to 3.61 | ||||
| P value | 1.8×10−74 | 3.7×10−17 | 0.00064 | 3.7×10−11 | 4.3×10−14 | ||||
| SNP | rs6817105 | rs2634073 | rs2200733 | rs2200733 | rs6843082 | ||||
| ZFHX3 | |||||||||
| OR | 1.24 | 0.8 | 1.05 | 1.32 | 1.71 | 2.03 | |||
| 95% CI | 1.17 to 1.30 | 0.71 to 0.91 | 0.87 to 1.26 | 1.15 to 1.51 | 1.46 to 2.00 | 1.79 to 2.31 | |||
| P value | 3.2×10−16 | 6.8×10−4 | 0.63 | 1.97×10−4 | 1.9×10−11 | 5.3×10−14 | |||
| SNP | rs2106261 | rs12932445 | rs7193343 | rs2106261 | rs2106261 | rs2106261 | |||
| KCNN3 | |||||||||
| OR | 1.18 | 1.46 | 1.24 | 3.02 | 1.38 | ||||
| 95% CI | 1.13 to 1.23 | 0.85 to 2.51 | 0.88 to 1.75 | 1.54 to 6.29 | 0.89 to 2.14 | ||||
| P value | 2.0×10−14 | 0.17 | 0.333 | <0.001 | 0.152 | ||||
| SNP | rs6666258 | rs7514452 | rs13376333 | rs13376333 | rs13376333 | ||||
AF indicates atrial fibrillation; OR, odds ratio; SNP, single nucleotide polymorphism.
The first identification of common genetic variants dates to 2007, yet data on the clinical utility of these variants remains relatively limited.25 Investigators have examined the risk of AF after cardioversion,26 the initiation of antiarrhythmic medication,27 and PV isolation ablation procedures.13,14 In our study of >1000 Korean patients who underwent PV ablation, we did not observe any significant associations between the AF risk alleles on chromosomes 4q25, 16q22, or 1q21 and recurrent AF after catheter ablation. Our findings are in contrast to the recent observations in 2 studies of patients with Europeans ancestry (Table 6).13,14 Many potential causes could underlie the discordant results observed between Asian and European populations. First, our results may point to a specific racial difference in the associations between AF genetic data and clinical recurrence after RFCA. It is possible that the genetic variants that we considered in Korean patients are only proxies rather than the truly causative SNPs at these loci. Second, because our study is nearly 3 times larger than the prior studies, it is possible that the findings in European patients reflected a relatively smaller sample size. Finally, the differences may also be due to technical variability of RFCA, surveillance for AF, or the length of the follow-up period among the studies. Ultimately, further large-scale multicenter studies will help resolve each of these issues.
Table 6.
Summary of AF Genetic Studies Related to AF Catheter Ablation
| N | Age | Male | PAF | Race | End Point | SNP | Closest Gene | Late Recurrence of AF | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Recurrence of AF | Late Recurrence of AF | HR (95% CI) | P Value | ||||||||
| Husser14 | 195 | 56±12 | 73% | 78% | European | Within 7 days | Between 3 and 6 months | rs10033464_T | PITX2 | 2.82 (1.29 to 6.15)* | 0.009 |
| rs2200733_T | PITX2 | 2.46 (1.06 to 5.69)* | 0.036 | ||||||||
| Shoemaker13 | 311 | 60 (52 to 66) | 72% | 47% | European | N/A | After the 3-month postablation blanking period | rs2200733_T | PITX2 | 0.76 (0.6 to 0.95)† | 0.016 |
| rs10033464_T | PITX2 | N/A | 0.97‡ | ||||||||
| Current study | 1068 | 58±11 | 75% | 68% | Asian | Within the 3-month postablation period | After the 3-month postablation blanking period | rs6843082_G | PITX2 | 0.84 (0.61 to 1.15) | 0.280 |
| rs2200733_T | PITX2 | 1.01 (0.80 to 1.26) | 0.963 | ||||||||
| rs2106261_T | ZFHX3 | 0.86 (0.71 to 1.04) | 0.128 | ||||||||
AF indicates atrial fibrillation; HR, hazard ratio; N/A, not available due to lack of data in published paper13; PAF, paroxysmal atrial fibrillation; SNP, single nucleotide polymorphism.
Odds ratio.
Survival time ratio.
Log-rank test.
Strengths of our study include the largest cohort of patients of Asian descent described to date, a well-characterized ablation population with detailed phenotypic data, and a standardized clinical protocol for follow-up after ablation. Our study was also subject to a number of potential limitations. First, we excluded patients with significant structural heart disease. Second, because we studied a Korean population, our results may not be generalizable to other races and ethnicities. This observational study included a highly selected group of patients referred for Yonsei AF Ablation Cohort; therefore, the results cannot be generalized to the entire AF population or to other ethnicities. The lack of association of the polymorphism to the recurrence of AF after ablation might be related to the low frequency of the variant in the selected population, a small effect of polymorphisms, heterogeneity of the population with varying underlying substrate, variable ablative techniques used, and follow-up, despite the relatively consistent protocols for inclusion, ablation, and follow-up.
Conclusions
Genetic variants at the PITX2 and ZFHX3 loci were strongly associated with AF among Korean patients, whereas there was no association at the KCNN3 locus. The clinical outcomes after a catheter ablation for AF could not be predicted by genetic variants in Korean patients.
Acknowledgments
We appreciate National Biobank of Korea (KOBB-2012-00) for kindly providing control DNA.
Sources of Funding
Dr Pak is supported by a grant (A085136) from the Korea Health 21 R&D Project, Ministry of Health and Welfare, and a grant (7-2013-0362) from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MSIP). Dr Choi is supported by grant no 2520140060 from the SNUH Research Fund and a Korea National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014R1A1A2A16055218). Dr Ellinor is supported by grants from the National Institutes of Health (R01HL092577, R01HL104156, K24HL105780, HL065962), the American Heart Association (13EIA14220013), and Fondation Leducq (14CVD01). Dr Lubitz is supported by a grant from the National Institutes of Health (K23HL114724) and a Doris Duke Charitable Foundation Clinical Scientist Development Award (2014105).
Disclosures
None.
Supporting Information
Table S1. Subgroup association analyses of 3 known AF loci and atrial fibrillation excluding hypertension (n=558, n=1049); diabetes (n=929, n=1054); congestive heart failure (CHF; n=998, n=1067); and stroke, coronary artery disease, and CHF (n=796, n=1067). N=cases, n=controls.
References
- Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- Fox CS, Parise H, D’Agostino RB, Sr, Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, Villalon ML, Vasan RS, Pencina MJ, Levy D, Larson MG, Ellinor PT, Benjamin EJ. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269. doi: 10.1001/jama.2010.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, Hammill SC, Packer DL, Olson TM. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–2192. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- Sabeh MK, MacRae CA. The genetics of atrial fibrillation. Curr Opin Cardiol. 2010;25:186–191. doi: 10.1097/HCO.0b013e3283385734. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Muller-Nurasyid M, Krijthe BP, Lubitz SA, Bis JC, Chung MK, Dorr M, Ozaki K, Roberts JD, Smith JG, Pfeufer A, Sinner MF, Lohman K, Ding J, Smith NL, Smith JD, Rienstra M, Rice KM, Van Wagoner DR, Magnani JW, Wakili R, Clauss S, Rotter JI, Steinbeck G, Launer LJ, Davies RW, Borkovich M, Harris TB, Lin H, Volker U, Volzke H, Milan DJ, Hofman A, Boerwinkle E, Chen LY, Soliman EZ, Voight BF, Li G, Chakravarti A, Kubo M, Tedrow UB, Rose LM, Ridker PM, Conen D, Tsunoda T, Furukawa T, Sotoodehnia N, Xu S, Kamatani N, Levy D, Nakamura Y, Parvez B, Mahida S, Furie KL, Rosand J, Muhammad R, Psaty BM, Meitinger T, Perz S, Wichmann HE, Witteman JC, Kao WH, Kathiresan S, Roden DM, Uitterlinden AG, Rivadeneira F, McKnight B, Sjogren M, Newman AB, Liu Y, Gollob MH, Melander O, Tanaka T, Stricker BH, Felix SB, Alonso A, Darbar D, Barnard J, Chasman DI, Heckbert SR, Benjamin EJ, Gudnason V, Kaab S. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaab S, Darbar D, van Noord C, Dupuis J, Pfeufer A, Newton-Cheh C, Schnabel R, Makino S, Sinner MF, Kannankeril PJ, Beckmann BM, Choudry S, Donahue BS, Heeringa J, Perz S, Lunetta KL, Larson MG, Levy D, MacRae CA, Ruskin JN, Wacker A, Schomig A, Wichmann HE, Steinbeck G, Meitinger T, Uitterlinden AG, Witteman JC, Roden DM, Benjamin EJ, Ellinor PT. Large scale replication and meta-analysis of variants on chromosome 4q25 associated with atrial fibrillation. Eur Heart J. 2009;30:813–819. doi: 10.1093/eurheartj/ehn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, Smith AV, Schnabel RB, Bis JC, Boerwinkle E, Sinner MF, Dehghan A, Lubitz SA, D’Agostino RB, Sr, Lumley T, Ehret GB, Heeringa J, Aspelund T, Newton-Cheh C, Larson MG, Marciante KD, Soliman EZ, Rivadeneira F, Wang TJ, Eiriksdottir G, Levy D, Psaty BM, Li M, Chamberlain AM, Hofman A, Vasan RS, Harris TB, Rotter JI, Kao WH, Agarwal SK, Stricker BH, Wang K, Launer LJ, Smith NL, Chakravarti A, Uitterlinden AG, Wolf PA, Sotoodehnia N, Kottgen A, van Duijn CM, Meitinger T, Mueller M, Perz S, Steinbeck G, Wichmann HE, Lunetta KL, Heckbert SR, Gudnason V, Alonso A, Kaab S, Ellinor PT, Witteman JC. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, Thorgeirsson G, Gulcher J, Mathiesen EB, Njolstad I, Nyrnes A, Wilsgaard T, Hald EM, Hveem K, Stoltenberg C, Kucera G, Stubblefield T, Carter S, Roden D, Ng MC, Baum L, So WY, Wong KS, Chan JC, Gieger C, Wichmann HE, Gschwendtner A, Dichgans M, Kuhlenbaumer G, Berger K, Ringelstein EB, Bevan S, Markus HS, Kostulas K, Hillert J, Sveinbjornsdottir S, Valdimarsson EM, Lochen ML, Ma RC, Darbar D, Kong A, Arnar DO, Thorsteinsdottir U, Stefansson K. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, Sinner MF, de Bakker PI, Mueller M, Lubitz SA, Fox E, Darbar D, Smith NL, Smith JD, Schnabel RB, Soliman EZ, Rice KM, Van Wagoner DR, Beckmann BM, van Noord C, Wang K, Ehret GB, Rotter JI, Hazen SL, Steinbeck G, Smith AV, Launer LJ, Harris TB, Makino S, Nelis M, Milan DJ, Perz S, Esko T, Kottgen A, Moebus S, Newton-Cheh C, Li M, Mohlenkamp S, Wang TJ, Kao WH, Vasan RS, Nothen MM, MacRae CA, Stricker BH, Hofman A, Uitterlinden AG, Levy D, Boerwinkle E, Metspalu A, Topol EJ, Chakravarti A, Gudnason V, Psaty BM, Roden DM, Meitinger T, Wichmann HE, Witteman JC, Barnard J, Arking DE, Benjamin EJ, Heckbert SR, Kaab S. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang F, Yang Y, Fu F, Xu C, Shi L, Li S, Xia Y, Wu G, Cheng X, Liu H, Wang C, Wang P, Hao J, Ke Y, Zhao Y, Liu M, Zhang R, Gao L, Yu B, Zeng Q, Liao Y, Yang B, Tu X, Wang QK. Significant association of SNP rs2106261 in the ZFHX3 gene with atrial fibrillation in a Chinese Han GeneID population. Hum Genet. 2011;129:239–246. doi: 10.1007/s00439-010-0912-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Chang SN, Hwang JJ, Chiang FT, Tseng CD, Lee JK, Lai LP, Lin JL, Wu CK, Tsai CT. Significant association of rs13376333 in KCNN3 on chromosome 1q21 with atrial fibrillation in a Taiwanese population. Circ J. 2012;76:184–188. doi: 10.1253/circj.cj-11-0525. [DOI] [PubMed] [Google Scholar]
- Benjamin Shoemaker M, Muhammad R, Parvez B, White BW, Streur M, Song Y, Stubblefield T, Kucera G, Blair M, Rytlewski J, Parvathaneni S, Nagarakanti R, Saavedra P, Ellis CR, Patrick Whalen S, Roden DM, Darbar RD. Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation. Heart Rhythm. 2013;10:394–400. doi: 10.1016/j.hrthm.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2010;55:747–753. doi: 10.1016/j.jacc.2009.11.041. [DOI] [PubMed] [Google Scholar]
- Shoemaker MB, Bollmann A, Lubitz SA, Ueberham L, Saini H, Montgomery J, Edwards T, Yoneda Z, Sinner MF, Arya A, Sommer P, Delaney J, Goyal SK, Saavedra P, Kanagasundram A, Whalen SP, Roden DM, Hindricks G, Ellis CR, Ellinor PT, Darbar D, Husser D. Common genetic variants and response to atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2015;8:296–302. doi: 10.1161/CIRCEP.114.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, Cha SH, Kim JW, Han BG, Min H, Ahn Y, Park MS, Han HR, Jang HY, Cho EY, Lee JE, Cho NH, Shin C, Park T, Park JW, Lee JK, Cardon L, Clarke G, McCarthy MI, Lee JY, Lee JK, Oh B, Kim HL. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- Baik I, Kim J, Abbott RD, Joo S, Jung K, Lee S, Shim J, In K, Kang K, Yoo S, Shin C. Association of snoring with chronic bronchitis. Arch Intern Med. 2008;168:167–173. doi: 10.1001/archinternmed.2007.8. [DOI] [PubMed] [Google Scholar]
- Lubitz SA, Ozcan C, Magnani JW, Kaab S, Benjamin EJ, Ellinor PT. Genetics of atrial fibrillation: implications for future research directions and personalized medicine. Circ Arrhythm Electrophysiol. 2010;3:291–299. doi: 10.1161/CIRCEP.110.942441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak HN, Oh YS, Lim HE, Kim YH, Hwang C. Comparison of voltage map-guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm. 2011;8:199–206. doi: 10.1016/j.hrthm.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696.e621. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- Shi L, Li C, Wang C, Xia Y, Wu G, Wang F, Xu C, Wang P, Li X, Wang D, Xiong X, Bai Y, Liu M, Liu J, Ren X, Gao L, Wang B, Zeng Q, Yang B, Ma X, Yang Y, Tu X, Wang QK. Assessment of association of rs2200733 on chromosome 4q25 with atrial fibrillation and ischemic stroke in a Chinese Han population. Hum Genet. 2009;126:843–849. doi: 10.1007/s00439-009-0737-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ni B, Lin Y, Chen XG, Fang Z, Zhao L, Hu Z, Zhang F. Genetic polymorphisms in ZFHX3 are associated with atrial fibrillation in a Chinese Han population. PLoS One. 2014;9:e101318. doi: 10.1371/journal.pone.0101318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbar D, Roden DM. Genetic mechanisms of atrial fibrillation: impact on response to treatment. Nat Rev Cardiol. 2013;10:317–329. doi: 10.1038/nrcardio.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez B, Shoemaker MB, Muhammad R, Richardson R, Jiang L, Blair MA, Roden DM, Darbar D. Common genetic polymorphism at 4q25 locus predicts atrial fibrillation recurrence after successful cardioversion. Heart Rhythm. 2013;10:849–855. doi: 10.1016/j.hrthm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez B, Vaglio J, Rowan S, Muhammad R, Kucera G, Stubblefield T, Carter S, Roden D, Darbar D. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol. 2012;60:539–545. doi: 10.1016/j.jacc.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Subgroup association analyses of 3 known AF loci and atrial fibrillation excluding hypertension (n=558, n=1049); diabetes (n=929, n=1054); congestive heart failure (CHF; n=998, n=1067); and stroke, coronary artery disease, and CHF (n=796, n=1067). N=cases, n=controls.


