Abstract
Background
Implantable cardioverter-defibrillator (ICD) therapy is associated with improved outcomes in patients with heart failure (HF), but whether this association holds among older patients with multiple comorbid illnesses and worse HF burden remains unclear.
Methods and Results
Using the National Cardiovascular Data Registry’s ICD Registry and the Get With The Guidelines–Heart Failure (GWTG-HF) registry linked with Medicare claims, we examined outcomes associated with primary-prevention ICD versus no ICD among HF patients aged ≥65 years in clinical practice. We included patients with an ejection fraction ≤35% who received (ICD Registry) and who did not receive (GWTG-HF) an ICD. Compared with patients with an ICD, patients in the non-ICD group were older and more likely to be female and white. In matched cohorts, the 3-year adjusted mortality rate was lower in the ICD group versus the non-ICD group (46.7% versus 55.8%; adjusted hazard ratio [HR] 0.76; 95% CI 0.69 to 0.83). There was no associated difference in all-cause readmission (HR 0.99; 95% CI 0.92 to 1.08) but a lower risk of HF readmission (HR 0.88; 95% CI 0.80 to 0.97). When compared with no ICD, ICDs were also associated with better survival in patients with ≤3 comorbidities (HR 0.77; 95% CI 0.69 to 0.87) and >3 comorbidities (HR 0.77; 95% CI 0.64 to 0.93) and in patients with no hospitalization for HF (HR 0.75; 95% CI 0.65 to 0.86) and at least 1 prior HF hospitalization (HR 0.69; 95% CI 0.58 to 0.82). In subgroup analyses, there were no interactions between ICD and mortality risk for comorbidity burden (P=0.95) and for prior HF hospitalization (P=0.46).
Conclusion
Among older HF patients, ICDs for primary prevention were associated with lower risk of mortality even among those with high comorbid illness burden and prior HF hospitalization.
Keywords: aging, defibrillation, heart failure, morbidity, mortality
Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with heart failure (HF) and reduced ejection fraction,1–4 it is not clear if these devices are associated with improved outcomes among older patients with HF, especially those with significant comorbidities or multiple hospitalizations for HF. The landmark trials demonstrating the efficacy of ICD therapy3,4 among patients with HF and reduced ejection fraction were conducted at highly specialized centers and enrolled relatively young patients with few comorbid conditions. Consequently, the randomized trials may not apply to all patients seen in contemporary clinical practice, in which patient populations with left ventricular systolic dysfunction may be older and have a greater burden of coexisting illness.
Prior studies and risk-prediction models have found high mortality rates among older HF patients receiving ICD therapy.5–7 A recent study using the National Cardiovascular Data Registry’s (NCDR’s) ICD Registry linked with claims data found that many real-world, older patients with a heavy HF burden, as measured by the number of prior hospitalizations for HF, had higher mortality rates compared with participants in trials.8 Older patients, many of whom have several comorbidities and marked HF burden, make up >70% of the HF population in the United States,9 but no clinical trials are on the horizon to address the efficacy of ICDs in this population. Consequently, the question of whether real-world, medically complex patients with an ICD have better outcomes than those without an ICD remains largely unanswered.
To address this knowledge gap, we analyzed 2 large national registries linked with Medicare claims to examine the characteristics and outcomes of HF patients aged >65 years in clinical practice who received an ICD for primary prevention compared with eligible patients who did not receive an ICD. We also examined the associations between mortality and comorbidities and between mortality and HF burden to better inform clinical decision making in this population.
Methods
Data Sources
We conducted a retrospective cohort study using the NCDR’s ICD Registry (January 1, 2006, to December 31, 2007) and the Get With The Guidelines–Heart Failure (GWTG-HF; January 1, 2005, to December 31, 2009) national registry linked with Medicare claims data up to December 31, 2011.
The ICD Registry is an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society. It became the official repository of ICD implantation data for Medicare beneficiaries in April 2006, and a large number of participating hospitals submit data on all ICD implants. It is used in >1400 hospitals in the United States. Details of the registry have been published previously.10 Data quality is ensured using rigorous annual electronic quality checks and random onsite audits in up to 10% of participating sites.11 The GWTG-HF registry succeeded the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) registry in March 2005 and is an ongoing Web-based quality-improvement registry for patients hospitalized with HF.12 Patients are eligible if they are hospitalized with a primary diagnosis of HF or if they develop significant HF symptoms during a hospitalization for which HF was not the reason for admission. Data quality is monitored via electronic data checks, and reports from these checks ensure the completeness and accuracy of submitted data. Only sites and variables with a high degree of completeness are used in data analyses. Quintiles is the data collection coordination center, and the Duke Clinical Research Institute has an agreement to analyze the aggregate deidentified data for research purposes.
Both registries include data on demographic characteristics, medical history, and discharge medications as well as clinical and procedural information. To analyze long-term follow-up data, we obtained research-identifiable files for 100% fee-for-service Medicare (Parts A and B) inpatient claims and the corresponding denominator files through 2011 from the US Centers for Medicare and Medicaid Services. We linked records from both registries to the Medicare inpatient files using methods that have been described and validated previously.13 After linking the data, we used Medicare beneficiary identifiers to obtain subsequent events for beneficiaries with eligible hospitalizations.
Study Population
Indications for primary-prevention ICDs included: (1) left ventricular ejection fraction (LVEF) ≤35%, ischemic or nonischemic cardiomyopathy, and New York Heart Association (NYHA) class II or III (Sudden Cardiac Death in Heart Failure Trial [SCD-HeFT] criteria) and (2) LVEF ≤30%, prior myocardial infarction, and NYHA class I (Multicenter Automatic Defibrillator Implantation Trial II [MADIT II] criteria). Consequently, we used the ICD Registry to identify patients who received ICD therapy for MADIT II or SCD-HeFT criteria, and we used GWTG-HF to identify a comparison group of eligible patients who did not receive an ICD during the hospital stay. From both registries, we included patients who were aged ≥65 years, were enrolled in fee-for-service Medicare for at least 12 months before the index admission, were discharged alive but not to a skilled nursing facility or hospice, and did not leave against medical advice. In addition, we required that the patients have an LVEF ≤35%, consistent with guideline recommendations for primary-prevention ICD therapy.14–16
We included patients from the ICD Registry who were admitted between January 1, 2006, and December 31, 2007, and who underwent ICD implantation for primary prevention during the hospital stay. To ensure comparability with the GWTG-HF population, we included only patients from the ICD Registry who were admitted primarily for HF and not specifically for the device implantation. As in MADIT II and SCD-HeFT, we excluded patients who had NYHA class I or IV HF or who received an ICD within 40 days of a myocardial infarction or within 30 days of coronary revascularization. We also excluded patients who received biventricular devices or an ICD for secondary prevention. We included patients from GWTG-HF who were admitted between January 1, 2005, and December 31, 2009, and who did not receive an ICD during the hospital stay. We excluded patients from GWTG-HF who had new-onset HF, a contraindication or other physician-documented reason for not receiving an ICD, or missing medical history or outcome data.
Treatment
The treatment of interest was receipt of ICD, as recorded in the ICD Registry.
Outcomes
The primary outcome was all-cause mortality. Other outcomes of interest were all-cause readmission and HF readmission after the index hospitalization. We determined all-cause mortality on the basis of death dates in the Medicare denominator files. We determined all-cause readmission on the basis of any new nonelective inpatient claim not including the index hospitalization claim, transfers to or from another hospital, and admissions for rehabilitation. We determined HF readmission using inpatient claims to identify patients who had a hospital admission for a primary diagnosis of HF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 402.x1, 404.x1, 404.x3, and 428.x).
Covariates
We considered the following covariates for our analysis: patient demographic characteristics (age, sex, race), medical history (ischemic heart disease, prior atrial arrhythmia, diabetes, hypertension, chronic renal disease, chronic lung disease, cerebrovascular disease), laboratory tests and vital signs (LVEF, systolic blood pressure), and discharge medications (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, beta-blocker, diuretic, calcium channel blocker, digoxin, statin). NYHA class and QRS duration were not available in the GWTG-HF database.
Statistical Analysis
We described the baseline characteristics of the study population by treatment group using percentages for categorical variables and medians with 25th and 75th percentiles for continuous variables. We tested for differences between groups using the likelihood ratio chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables and by examining the standardized difference (defined as the absolute value of the difference in group means or proportions divided by the average SD and expressed as a percentage) between groups for each variable. Initial comparisons between patients in the ICD Registry and in GWTG-HF (non-ICD patients) showed appreciable imbalances for most baseline variables.
We proceeded with a matching process using the Rosenbaum and Rubin method to ensure valid comparisons of similar patients.17 First, for continuous variables, we excluded non-ICD patients whose value was below the minimum or above the maximum for ICD patients. Second, missing values were imputed using a Markov chain Monte Carlo method. Missing rates were generally low, <1% for variables in the ICD Registry and <3% for most variables in GWTG-HF. Third, a propensity model was created using multivariable logistic regression in which the dependent (outcome) variable was an indicator of whether each patient was an ICD or non-ICD patient, and the independent (predictor) variables were baseline variables available in both registries and had similar definitions, as listed above. An estimated propensity score (the probability of being an ICD patient) and a corresponding logit for the propensity score (loge[P/(1−P)]) were calculated for each patient. Fourth, a caliper width of 0.25 (SD of the logit) was used for matching. For a given ICD patient, all non-ICD patients were considered whose logit differed from the ICD patient’s logit by less than the caliper width. Among these patients, the non-ICD patient with the shortest (Mahalanobis) distance from the ICD patient was selected as a match. ICD patients for whom there were no non-ICD patients within the caliper width were omitted from the analysis. Each non-ICD patient was matched once at most.
All analyses except the initial baseline summaries used the matched cohort of patients. The primary analyses compared outcomes for matched patients between GWTG-HF and the ICD Registry. The secondary analyses examined associations between ICD and mortality risk in prespecified strata: (1) patients with ≤3 or >3 comorbidities (ie, chronic lung disease, prior atrial fibrillation, ischemic heart disease, diabetes, and renal disease defined as estimated glomerular filtration rate <60) and (2) patients with at least 1 HF hospitalization in the previous 6 months versus those without any hospitalizations. Cox proportional hazards models were used to evaluate the association of an ICD with the risk of all-cause mortality, all-cause readmission, and HF readmission among the matched patients. Competing risks methods (Fine and Gray models) were used for readmission end points, with death as the competing risk.18 We used covariate adjustment to control for any remaining imbalances in the matched comparisons. All of the baseline variables listed above for the propensity analysis were included as covariates in each model. Risk relationships are expressed as hazard ratios (HRs) with 95% CIs from the Cox models. A robust sandwich variance estimator was used in the Cox models to account for correlation among patients at the same hospital. The proportional hazards assumption for the ICD term was assessed and was met in all cases. For the subgroup analyses, models included a term for the interaction of subgroup with the presence of an ICD; risk relationships were derived from the Cox model, allowing for the interaction regardless of whether or not it was significant. Mortality, all-cause readmission, and HF readmission rates at 1 year and 3 years were presented both as unadjusted estimates (Kaplan–Meier rates for mortality and cumulative incidence rates for the readmission end points) and as adjusted rates derived from the Cox model. Differences were determined to be statistically significant at P<0.05, and all statistical tests were 2-sided. We used SAS version 9.2 (SAS Institute Inc) for all analyses. The institutional review board of the Duke University Health System approved the study.
Results
Baseline Characteristics
The baseline characteristics of the patients from the ICD Registry and GWTG-HF (non-ICD patients) prior to matching are shown in Table 1. Patients in the non-ICD group were older and were more likely to be female and white compared with the ICD group. Prior atrial arrhythmias, diabetes, hypertension, chronic lung disease, and cerebrovascular disease were less common in the non-ICD group prior to matching. Rates of evidence-based medical therapy with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and beta-blockers were similar for the 2 groups. As shown in Table 1, 95% of the ICD Registry patients were matched, and propensity score matching resulted in well-matched samples with minimal residual differences. On average, the matched patients were aged 75 years and were predominantly white men with mean LVEF 25% and similar comorbidity profiles.
Table 1.
Baseline Characteristics for ICD (ICD Registry) and No ICD (GWTG-HF) Patients
| Baseline Characteristic | All Patients Qualifying for Analysis | 1:1 Matched Patients | ||||||
|---|---|---|---|---|---|---|---|---|
| No ICD (GWTG-HF) n=6138 | ICD (ICD Registry) n=1560 | % Standardized Difference | P Value | No ICD (GWTG-HF) n=1487 | ICD (ICD Registry) n=1487 | % Standardized Difference | P Value | |
| Age, y | 79 (72 to 84) | 75 (70 to 80) | 51 | <0.0001 | 75 (70 to 80) | 75 (70 to 80) | 1 | 0.96 |
| Male | 56% (3463) | 68% (1064) | 25 | <0.0001 | 67% (997) | 67% (997) | 0 | 0.99 |
| White race | 79% (4770) | 75% (1161) | 11 | <0.0001 | 75% (1107) | 75% (1112) | 1 | 0.72 |
| LVEF, % | 27 (20 to 30) | 25 (20 to 30) | 46 | <0.0001 | 25 (20 to 30) | 25 (20 to 30) | 5 | 0.23 |
| Ischemic heart disease | 69% (4230) | 72% (1124) | 7 | 0.016 | 72% (1078) | 72% (1066) | 2 | 0.62 |
| Prior atrial arrhythmia | 33% (2014) | 43% (673) | 21 | <0.0001 | 38% (571) | 41% (615) | 6 | 0.096 |
| Systolic BP | 134 (118 to 154) | 126 (110 to 142) | 33 | <0.0001 | 128 (115 to 143) | 127 (111 to 143) | 1 | 0.38 |
| Diabetes | 40% (2425) | 45% (702) | 11 | <0.0001 | 47% (695) | 45% (671) | 3 | 0.39 |
| Hypertension | 71% (4356) | 83% (1299) | 30 | <0.0001 | 83% (1231) | 82% (1226) | 1 | 0.81 |
| Chronic renal disease | 66% (4064) | 69% (1076) | 6 | 0.038 | 69% (1020) | 68% (1014) | 1 | 0.81 |
| Chronic lung disease | 27% (1653) | 34% (533) | 16 | <0.0001 | 32% (482) | 34% (500) | 3 | 0.48 |
| Cerebrovascular disease | 14% (865) | 19% (301) | 14 | <0.0001 | 17% (252) | 18% (272) | 4 | 0.34 |
| ACE inhibitor or ARB | 72% (4397) | 72% (1118) | 1 | 0.67 | 71% (1053) | 72% (1070) | 4 | 0.34 |
| Beta-blocker | 85% (5197) | 85% (1318) | 1 | 0.65 | 86% (1274) | 85% (1256) | 2 | 0.62 |
| Diuretic | 83% (4632) | 79% (1218) | 12 | <0.0001 | 80% (1081) | 79% (1167) | 3 | 0.51 |
| Calcium channel blocker | 17% (904) | 7% (115) | 30 | <0.0001 | 8% (101) | 8% (115) | 0 | 0.92 |
| Digoxin | 33% (1773) | 34% (526) | 2 | 0.51 | 33% (426) | 34% (507) | 4 | 0.31 |
| Statin | 35% (2099) | 61% (943) | 53 | <0.0001 | 57% (827) | 59% (871) | 4 | 0.30 |
ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; GWTG-HF, Get With The Guidelines–Heart Failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction.
Of the 1487 matched ICD Registry patients, 82% (1215) had HF for >9 months, 17% (249) had HF for 3 to 9 months, and 1% (23) had HF for an unknown duration. The 23 with HF for an unknown duration were included in the analysis because they met the criteria for LVEF ≤35% and for myocardial infarction at least 40 days prior to receiving an ICD.
Mortality and Readmission
During a median follow-up of 4.5 years, 876 matched ICD patients died, and during a median follow-up duration of 3 years, 896 matched non-ICD patients died (Table 2). The ICD was associated with lower mortality compared with no ICD (adjusted HR 0.76; 95% CI 0.69 to 0.83; P<0.0001). The adjusted mortality differences between the ICD and non-ICD groups were evident at 1 year (23.4% for ICD versus 29.5% for no ICD) and 3 years (46.7% versus 55.8%) (Figure 1). All-cause readmission rates over time were similar for both groups (adjusted event rates ≈70% at 1 year and 85% at 3 years for both; HR 0.99; 95% CI 0.92 to 1.08; P=0.88). HF readmission rates were lower for the ICD group compared with the non-ICD group (adjusted event rates 36.8% versus 40.6% at 1 year and 49.6% versus 54.0% at 3 years; HR 0.88; 95% CI 0.80 to 0.97; P=0.013) (Figure 2).
Table 2.
Outcomes for ICD Versus No ICD
| ICD (ICD Registry) | No ICD (GWTG-HF) | |
|---|---|---|
| n | 1487 | 1487 |
| Follow-up duration among survivors, y | ||
| Median | 4.5 | 3.0 |
| 25th, 75th percentiles | 2.7, 5.0 | 2.0, 4.2 |
| Minimum, maximum | 0.014, 6.0 | 0.019, 6.7 |
| Mortality | ||
| Total deaths | 876 | 896 |
| Mortality rate at 1 year, % (95% CI) | 22.6 (20.6 to 24.9) | 30.1 (27.8 to 32.6) |
| Mortality rate at 3 years, % (95% CI) | 46.6 (44.0 to 49.3) | 56.0 (53.3 to 58.7) |
| Mortality rate at 3 years conditional on surviving 1 year, % (95% CI) | 31.0 (28.2 to 33.9) | 37.0 (33.8 to 40.3) |
| Adjusted mortality rate at 1 year, % (95% CI) | 23.4 (23.1 to 23.7) | 29.5 (29.2 to 29.9) |
| Adjusted mortality rate at 3 years, % (95% CI) | 46.7 (46.2 to 47.2) | 55.8 (55.3 to 56.3) |
| Adjusted HR (95% CI) for ICD vs no ICD | 0.76 (0.69 to 0.83) | |
| P value for HR | <0.0001 | |
| All-cause hospitalization | ||
| Total events | 1297 | 1243 |
| Event rate at 1 year, % (95% CI) | 67.7 (65.3 to 70.1) | 71.0 (68.7 to 73.4) |
| Event rate at 3 years, % (95% CI) | 85.8 (83.9 to 87.6) | 84.2 (82.3 to 86.1) |
| Adjusted event rate at 1 year, % (95% CI) | 69.2 (69.0 to 69.5) | 69.4 (69.2 to 69.7) |
| Adjusted event rate at 3 years (95% CI) | 84.9 (84.7 to 85.1) | 85.1 (84.9 to 85.2) |
| Adjusted HR (95% CI) for ICD vs no ICD | 0.99 (0.92 to 1.08) | |
| P value for HR | 0.88 | |
| HF hospitalization | ||
| Total events | 760 | 777 |
| Event rate at 1 year, % (95% CI) | 34.8 (32.4 to 37.3) | 42.5 (40.0 to 45.2) |
| Event rate at 3 years, % (95% CI) | 50.2 (47.7 to 52.9) | 53.1 (50.5 to 55.8) |
| Adjusted event rate at 1 year, % (95% CI) | 36.8 (36.5 to 37.1) | 40.6 (40.3 to 40.9) |
| Adjusted event rate at 3 years, % (95% CI) | 49.6 (49.2 to 49.9) | 54.0 (53.7 to 54.3) |
| Adjusted HR (95% CI) for ICD vs no ICD | 0.88 (0.80 to 0.97) | |
| P value for HR | 0.013 | |
GWTG-HF indicates Get With The Guidelines–Heart Failure; HR, hazard ratio; ICD, implantable cardioverter-defibrillator.
Figure 1.
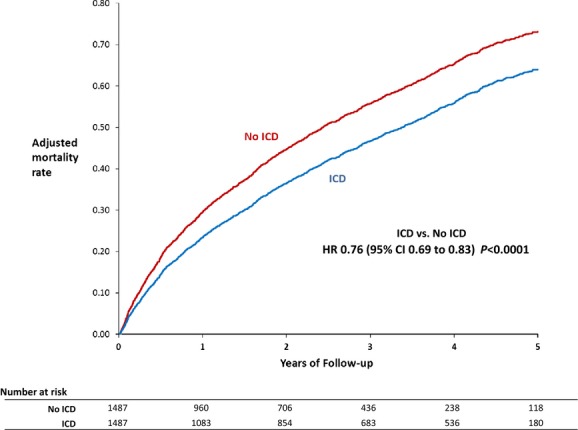
Mortality with and without ICDs (adjusted estimates derived from Cox model). HR indicates hazard ratio; ICD, implantable cardioverter-defibrillator.
Figure 2.
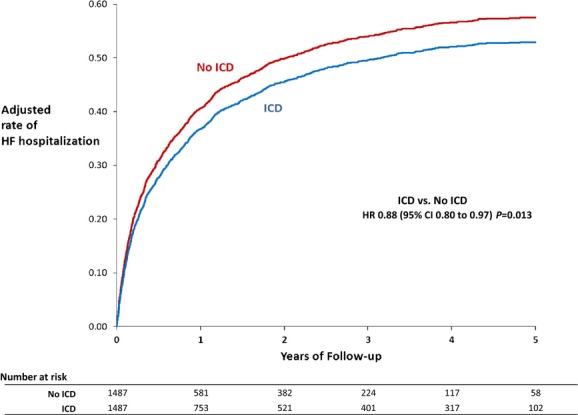
HF readmission with and without ICDs (adjusted estimates derived from Cox [Fine and Gray] model). HF indicates heart failure; HR, hazard ratio; ICD, implantable cardioverter-defibrillator.
Subgroup Analyses
In the first subgroup analysis examining comorbidity burden, patients with ≤3 comorbidities had lower unadjusted and adjusted absolute mortality rates compared with patients with >3 comorbidities, but both subgroups had similar adjusted HRs demonstrating 23% lower mortality for patients with an ICD compared with those without an ICD (P for interaction=0.95) (Table 3 and Figure 3). In the second subgroup analysis, patients with no HF hospitalization in the prior 6 months who received an ICD had lower observed and adjusted mortality rates compared with patients with at least 1 prior HF hospitalization. Among patients with no HF hospitalizations in the prior 6 months, patients with an ICD had 25% lower mortality compared with those without an ICD. For patients with at least 1 HF hospitalization in the prior 6 months, patients with an ICD had 31% lower mortality compared with those without an ICD (P for interaction=0.46) (Table 4 and Figure 4).
Table 3.
Mortality Risk for Patients With and Without ICDs by Comorbidity Burden
| Patients With ≤3 Comorbidities | Patients With >3 Comorbidities | |||
|---|---|---|---|---|
| ICD (Registry) | No ICD (GWTG-HF) | ICD (Registry) | No ICD (GWTG-HF) | |
| n | 1202 | 978 | 283 | 278 |
| Follow-up duration among survivors, y | ||||
| Median | 4.5 | 3.0 | 4.5 | 2.8 |
| 25th, 75th percentiles | 2.6, 5.1 | 2.0, 4.4 | 3.3, 4.9 | 1.0, 4.6 |
| Minimum, maximum | 0.014, 6.0 | 0.019, 6.7 | 0.049, 5.9 | 0.115, 6.0 |
| Total deaths | 677 | 566 | 198 | 200 |
| Mortality rate (KM) at 1 year, % (95% CI) | 20.9 (18.7 to 23.3) | 28.5 (25.8 to 31.5) | 29.8 (24.8 to 35.6) | 35.1 (29.7 to 41.1) |
| Mortality rate (KM) at 3 years, % (95% CI) | 43.9 (41.0 to 46.9) | 53.5 (50.2 to 56.9) | 57.6 (51.8 to 63.6) | 65.4 (59.4 to 71.4) |
| Mortality rate at 3 years for 1-year survivors, % (95% CI) | 29.1 (26.2 to 32.3) | 34.9 (31.2 to 39.0) | 39.6 (32.9 to 47.1) | 46.8 (39.2 to 55.0) |
| Adjusted mortality rate at 1 year, % (95% CI) | 21.7 (21.4 to 22.1) | 27.2 (26.8 to 27.6) | 29.8 (29.2 to 30.5) | 36.8 (36.0 to 37.6) |
| Adjusted mortality rate at 3 years, % (95% CI) | 44.3 (43.7 to 44.8) | 52.8 (52.2 to 53.4) | 57.2 (56.2 to 58.1) | 66.4 (65.5 to 67.4) |
| Adjusted HR (95% CI) for ICD vs no ICD | 0.77 (0.69 to 0.87) | 0.77 (0.64 to 0.93) | ||
| P value for HR | <0.0001 | 0.0070 | ||
| P value for interaction of subgroup with ICD | 0.95 | |||
GWTG-HF indicates Get With The Guidelines–Heart Failure; HR, hazard ratio; ICD, implantable cardioverter-defibrillator; KM, Kaplan–Meier.
Figure 3.
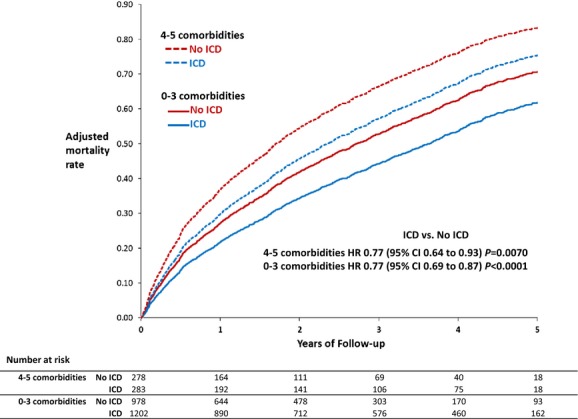
Mortality with and without ICDs in comorbidity subgroups (adjusted estimates derived from Cox model). HR indicates hazard ratio; ICD, implantable cardioverter-defibrillator.
Table 4.
Mortality Risk for Patients With and Without ICDs by Prior HFH
| HFH in Past 6 Months | No HFH in Past 6 Months | |||
|---|---|---|---|---|
| ICD (ICD Registry) | No ICD (GWTG-HF) | ICD (ICD Registry) | No ICD (GWTG-HF) | |
| n | 781 | 352 | 702 | 553 |
| Follow-up duration among survivors, y | ||||
| Median | 4.5 | 2.9 | 4.5 | 3.1 |
| 25th, 75th percentiles | 2.6, 5.1 | 1.2, 3.9 | 3.0, 5.0 | 2.0, 4.7 |
| Minimum, maximum | 0.014, 5.9 | 0.052, 6.7 | 0.016, 6.0 | 0.019, 6.7 |
| Total deaths | 489 | 237 | 385 | 324 |
| Mortality rate (KM) at 1 year, % (95% CI) | 27.9 (24.9 to 31.3) | 37.5 (32.6 to 42.9) | 16.9 (14.2 to 19.9) | 25.6 (22.1 to 29.6) |
| Mortality rate (KM) at 3 years, % (95% CI) | 51.5 (47.9 to 55.2) | 61.9 (56.5 to 67.4) | 41.2 (37.5 to 45.1) | 53.5 (49.1 to 58.1) |
| Mortality rate at 3 years for 1-year survivors, % (95% CI) | 32.7 (28.8 to 37.0) | 39.1 (32.4 to 46.6) | 29.3 (25.6 to 33.4) | 37.5 (32.6 to 42.9) |
| Adjusted mortality rate at 1 year, % (95% CI) | 26.4 (25.9 to 27.0) | 33.5 (32.8 to 34.1) | 20.3 (19.9 to 20.8) | 26.1 (25.6 to 26.6) |
| Adjusted mortality rate at 3 years, % (95% CI) | 51.9 (51.1 to 52.7) | 61.7 (60.9 to 62.6) | 42.1 (41.3 to 42.8) | 51.3 (50.5 to 52.1) |
| Adjusted HR (95% CI) for ICD vs no ICD | 0.69 (0.58 to 0.82) | 0.75 (0.65 to 0.86) | ||
| P value for HR | <0.0001 | <0.0001 | ||
| P value for interaction of subgroup with ICD | 0.46 | |||
GWTG-HF indicates Get With The Guidelines–Heart Failure; HFH, heart failure hospitalization; HR, hazard ratios; ICD, implantable cardioverter-defibrillator; KM, Kaplan–Meier.
Figure 4.
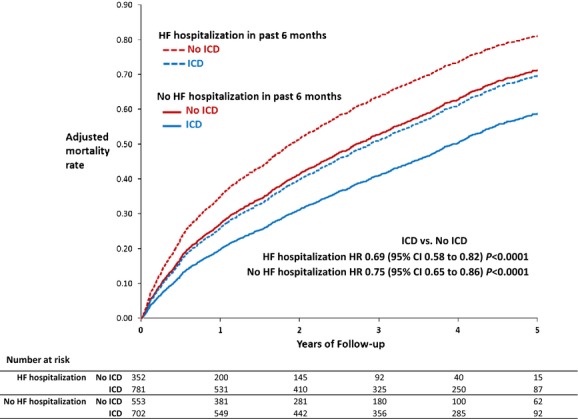
Mortality with and without ICDs in prior HF hospitalization subgroups (adjusted estimates derived from Cox model). HF indicates heart failure; HR, hazard ratio; ICD, implantable cardioverter-defibrillator.
Discussion
This analysis used the largest ICD registry in the United States and the largest national HF registry to assess outcomes of patients receiving a primary-prevention ICD in clinical practice. It showed that among older patients with HF, those who received ICDs were associated with lower mortality than those not receiving ICDs. In subgroup analyses, there was no statistical heterogeneity in associated mortality benefits with ICD use by the extent of comorbid conditions or prior HF hospitalization.
Although landmark clinical trials demonstrated the efficacy of ICD therapy for primary prevention of sudden cardiac death, the results from these trials may not be generalizable to HF patients in clinical practice. A prior study by our group found that patients who received ICDs in clinical practice were considerably older and had more comorbidities than patients in the landmark primary-prevention ICD trials.19 A prior retrospective study compared older patients with and without ICDs in clinical practice using the OPTIMIZE-HF registry and GWTG-HF and found lower mortality among patients receiving an ICD compared with those who did not (adjusted HR 0.71; 95% CI 0.56 to 0.91).20 Our findings confirmed and extended these results by studying the largest repository of primary-prevention ICDs in the United States, examining all-cause and HF readmissions, and analyzing important subgroups in a more contemporary cohort.
Patients in our analysis were much more medically complex than those seen in clinical trials, but interestingly, patients receiving ICDs in our study had more comorbidities than patients not receiving ICDs. The adjusted mortality rates in our real-world population were higher than those seen in the landmark clinical trials (ICD group: adjusted mortality rate 46.7% at 3 years versus 14.2% at 20 months in MADIT II and 22% at 45.5 months in SCD-HeFT; non-ICD group: 55.8% versus 19.8% MADIT II and 29% SCD-HeFT). These higher mortality rates are consistent with prior studies and risk-prediction models demonstrating higher mortality rates among older HF patients receiving ICD therapy5–7; however, despite these event rates, the survival difference between the ICD and non-ICD groups demonstrated in our analysis was comparable to those seen in MADIT II and SCD-HeFT (adjusted HR 0.76 versus 0.69 in MADIT II and 0.77 in SCD-HeFT).
Our analysis also examined readmission outcomes. We found that ICDs were not associated with any increase or decrease in all-cause readmission but were associated with a modestly lower risk of HF readmission. The lower risk of HF readmission among patients with an ICD could be due to their “healthier” status in ways we could not capture and adjust for in our analysis. This lower risk of HF hospitalization may also be because these patients, due to regular device follow-ups, have more contact with health care professionals who can detect early signs and symptoms of HF and abnormal HF diagnostic parameters on ICD interrogations that may prompt them to treat patients early and avoid HF hospitalizations. In addition, decisions to implant an ICD may reflect better quality of care in general.
Our subgroup analysis examining the association between ICD benefit and comorbidities found that patients with ≤3 comorbidities had lower unadjusted and adjusted mortality rates compared with patients with >3 comorbidities, but both subgroups had similar adjusted HRs demonstrating 23% lower mortality in patients with an ICD compared with those without an ICD. Similarly, in both patients with no and with at least 1 prior HF hospitalization, ICDs were associated with better survival. A recent study using the ICD Registry linked with claims data suggested that ICDs may not offer benefit in many actual patients who have a high HF burden, as measured by the number of prior HF hospitalizations8; however, that study compared mortality only among ICD recipients rather than those with and without ICDs. Our analysis used a comparator group of patients who were eligible but who did not receive ICDs and found that ICDs were still associated with improved survival despite a high mortality rate.
Taken together, our results confirm prior concerns that older HF patients are more medically complex and have higher mortality than those seen in the landmark clinical trials; however, our results suggest that ICD use is associated with improved survival without an associated increase in all-cause readmission. Our results can be used to counsel older HF patients about their outcomes and disease process and to help with shared decision making about whether or not to pursue a an ICD for primary prevention.
This study has several limitations. First, this retrospective observational study could still have residual confounding that could affect the association between ICDs and outcomes. Although our analysis included patients from 2006 to 2007, ICDs and the manner in which they are used do not appear to have changed appreciably over time.21,22 Second, the use of 2 registries with slightly different data definitions may have increased the likelihood of confounding; however, we used only variables with similar definitions and applied propensity score matching and Cox proportional hazards modeling with adjustment to minimize confounding. The data were derived from clinical registries linked with fee-for-service Medicare claims, so the results may not be generalizable to Medicare beneficiaries in managed care and to non-Medicare patients. Generalizability is further limited by our use of exclusion criteria and propensity score matching to create comparable groups and increase internal validity. Third, patients in these clinical registries may represent the best possible care in clinical practice rather than the true representation of real-world care because hospitals generally participate in GWTG-HF for quality-improvement purposes. We were unable to measure medication doses and adherence, severity of illness, NYHA functional class, quality of life, ICD device discharges, and socioeconomic issues that may have affected whether or not patients received an ICD. We were unable to determine when or why some patients did not receive an ICD. Although we did not have data on NYHA class in GWTG-HF, those patients who were admitted for HF had either class II or III HF symptoms because we excluded patients who had class IV HF symptoms listed as a reason for not implanting an ICD. We also adjusted for all variables available in both data sets that could be surrogates for HF severity, such as LVEF, systolic blood pressure, and prior atrial arrhythmias. Consequently, significant differences in symptomatic HF between the 2 populations are unlikely. Although ICD shocks or the threat of such shocks may affect patients’ behavior and medication adherence and, as such, would have been good to examine and potentially adjust for, data on ICD shocks are not available. Finally, in our analysis of comorbidities, we did not assign different weights to different comorbidities because there is no well-accepted scoring system for accomplishing that, and data on the severity of each comorbidity were not available. We also did not consider all comorbidities that could be associated with outcomes because we had to limit our list to comorbidities that are common to the ICD Registry and GWTG-HF.
Conclusions
Using the ICD Registry and GWTG-HF, we found that ICDs for primary prevention are associated with a lower risk of mortality without an associated higher risk in all-cause readmissions. Lower mortality rates associated with an ICD were observed despite the high mortality rates in the treated and untreated groups and regardless of the comorbidity burden or the occurrence of prior HF hospitalizations.
Sources of Funding
This analysis was funded by a grant (1R01-HL093071-01A1) from the National Heart, Lung, and Blood Institute. Dr Khazanie was supported, in part, by grant T32HL069749 from the National Heart, Lung, and Blood Institute. The National Heart, Lung, and Blood Institute had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Disclosures
Dr Fonarow: Research AHRQ, NIH; Consultant: Novartis, Medtronic, Gambro, Bayer. Dr Bhatt: Advisory Board: Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: AHA GWTG Steering Committee; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor, Harvard Heart Letter), Clinical trial steering committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Population Health Research Institute, HMP Communications (Editor, Journal of Invasive Cardiology); Slack Publications (Editor, Cardiology Today’s Intervention), WebMD (CME steering committees); Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda. Dr Masoudi: Grant and contract funding from the American College of Cardiology and the Oklahoma Foundation for Medical Quality. Dr Anstrom: research support from AstraZeneca, Eli Lilly & Company, and Medtronic; Consultant for Abbott Vascular, AstraZeneca, Bristol Meyer Squibb, Pfizer, Ikaria. Dr Heidenreich: Research support from Medtronic. Dr Curtis: research grants from GlaxoSmithKline and Johnson & Johnson. Dr Hernandez: consultant for AstraZeneca, Bristol-Myers Squibb, Corthera, Cytokinetics, and Johnson & Johnson; grants from Amylin, Bristol-Myers Squibb, Johnson & Johnson, and Portola. Dr Peterson: Research Grant support from Eli Lilly & Company, Janssen Pharmaceuticals, Inc, and the American Heart Association; Consultant/Advisory Board support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen Pharmaceuticals, Inc, Pfizer, and Genentech Inc. No other authors have disclosures. The manuscript was reviewed and approved by the American College of Cardiology/NCDR ICD Registry Research and Publications Committee and by the American Heart Association/GWTG Science Committee.
References
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, Maggioni AP, Anand I, Poole-Wilson PA, Fishbein DP, Johnson G, Anderson J, Mark DB, Bardy GH. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60:1647–1655. doi: 10.1016/j.jacc.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Stevenson LW, Stewart GC, Seeger JD, Williams L, Jalbert JJ, Setoguchi S. Impact of baseline heart failure burden on post-implantable cardioverter-defibrillator mortality among Medicare beneficiaries. J Am Coll Cardiol. 2013;61:2142–2150. doi: 10.1016/j.jacc.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhruva SS, Redberg RF. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med. 2008;168:136–140. doi: 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- Hammill SC, Kremers MS, Stevenson LW, Heidenreich PA, Lang CM, Curtis JP, Wang Y, Berul CI, Kadish AH, Al-Khatib SM, Pina IL, Walsh MN, Mirro MJ, Lindsay BD, Reynolds MR, Pontzer K, Blum L, Masoudi F, Rumsfeld J, Brindis RG. Review of the registry’s fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7:1340–1345. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA. The National Cardiovascular Data Registry (NCDR) data quality brief: the NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Fonarow GC, Abraham WT, Albert NM, Gattis WA, Gheorghiade M, Greenberg B, O’Connor CM, Yancy CW, Young J. Organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA, III, Ferguson TB, Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Creager MA, Demets D, Ettinger SM, Guyton RA, Hochman JS, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- Al-Khatib SM, Hellkamp A, Bardy GH, Hammill S, Hall WJ, Mark DB, Anstrom KJ, Curtis J, Al-Khalidi H, Curtis LH, Heidenreich P, Peterson ED, Sanders G, Clapp-Channing N, Lee KL, Moss AJ. Survival of patients receiving a primary prevention implantable cardioverter-defibrillator in clinical practice vs clinical trials. JAMA. 2013;309:55–62. doi: 10.1001/jama.2012.157182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AF, Fonarow GC, Hammill BG, Al-Khatib SM, Yancy CW, O’Connor CM, Schulman KA, Peterson ED, Curtis LH. Clinical effectiveness of implantable cardioverter-defibrillators among Medicare beneficiaries with heart failure. Circ Heart Fail. 2010;3:7–13. doi: 10.1161/CIRCHEARTFAILURE.109.884395. [DOI] [PubMed] [Google Scholar]
- Hernandez AF, Fonarow GC, Liang L, Al-Khatib SM, Curtis LH, LaBresh KA, Yancy C, Albert NM, Peterson ED GWTG Steering Committee and Hospitals. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- Al-Khatib SM, Hellkamp A, Hernandez AF, Fonarow GC, Thomas KL, Al-Khalidi HR, Heidenreich PA, Hammill S, Yancy C, Peterson ED. Trends in use of implantable cardioverter defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–1101. doi: 10.1161/CIRCULATIONAHA.111.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]


