Abstract
Background
Diabetes is associated with coronary arteriolar endothelial dysfunction. We investigated the role of the small/intermediate (SKCa/IKCa) conductance of calcium-activated potassium channels in diabetes-related endothelial dysfunction.
Methods and Results
Coronary arterioles (80 to 150 μm in diameter) were dissected from discarded right atrial tissues of diabetic (glycosylated hemoglobin = 9.6±0.25) and nondiabetic patients (glycosylated hemoglobin 5.4±0.12) during coronary artery bypass graft surgery (n=8/group). In-vitro relaxation response of precontracted arterioles was examined in the presence of the selective SKCa/IKCa activator NS309 and other vasodilatory agents. The channel density and membrane potential of diabetic and nondiabetic endothelial cells was measured by using the whole cell patch-clamp technique. The protein expression and distribution of the SKCa/IKCa in the human myocardium and coronary arterioles was examined by Western blotting and immunohistochemistry. Our results indicate that diabetes significantly reduced the coronary arteriolar response to the SKCa/IKCa activator NS309 compared to the respective responses of nondiabetic vessels (P<0.05 versus nondiabetes). The relaxation response of diabetic arterioles to NS309 was prevented by denudation of endothelium (P=0.001 versus endothelium-intact). Diabetes significantly decreased endothelial SKCa/IKCa currents and hyperpolarization induced by the SKCa/IKCa activator NS309 as compared with that of nondiabetics. There were no significant differences in the expression and distribution of SKCa/IKCa proteins in the coronary microvessels.
Conclusions
Diabetes is associated with inactivation of endothelial SKCa/IKCa channels, which may contribute to endothelial dysfunction in diabetic patients.
Keywords: coronary microcirculation, coronary microvascular function, diabetes mellitus, potassium channels, vascular endothelial function
Cardiovascular disease is more prevalent in diabetic patients than nondiabetic patients, and is more often extensive and more rapidly progressive.1 Diabetes is associated with impairment in arterial and arteriolar endothelial function and vasomotor control as well as with increased morbidity and mortality in the patients with coronary artery diseases.2–4 Thus, maintaining coronary-arteriolar endothelial function can reduce the incidence of morbidity and mortality of diabetic patients. The mechanism behind the alteration in endothelial dysfunction in diabetic coronary microvasculature is incompletely understood, but may involve dysregulation of electrical signaling in the coronary arteriolar endothelium.5–8 This electrical signaling is mediated by small (SKCa) and intermediate (IKCa) conductance of calcium-activated potassium channels, which are largely responsible for coronary arteriolar relaxation mediated by endothelium-dependent hyperpolarizing factors.9,10 Interestingly, we recently found that the SKCa/IKCa channels are predominately present in the human coronary arteriolar endothelium, and inactivation of SKCa/IKCa may contribute to the ischemia/reperfusion (I/R) induced endothelial dysfunction in the human coronary arterioles of patients during cardiac surgery.11,12
However, few have studied the alterations of SKCa/IKCa activity and channel currents/densities, protein expression, and localization in the human coronary endothelium and vasculature in the setting of diabetes. Thus, the goal of this study is to elucidate the role of SKCa/IKCa in coronary-arteriolar endothelium dysfunction of diabetic patients. Our central hypothesis is that diabetes may be associated with alterations in the relaxation response of human coronary arterioles to SKCa/IKCa activator and dysregulation of SKCa/IKCa protein expression/localization, and reduction of SKCa/IKCa channel activity/current density of human coronary endothelial cells. Using isolated atrial/vessel tissues and endothelial cells harvested from diabetic and nondiabetic patients during cardiac surgery, we examined whether diabetes affects SKCa/IKCa activator-induced coronary arteriolar relaxation, SKCa/IKCa protein expression/distribution as measured by Western blotting and immuno-histochemistry, and studied whether diabetes alters SKCa/IKCa channel activity, current density, and SKCa/IKCa-mediated endothelium-hyperpolarization.
Methods
Human Subjects and Tissue Harvesting
Samples of discarded-right atrial appendage were harvested from clinically similar patients undergoing coronary artery bypass graft surgery before exposure of the heart to blood cardioplegia and cardiopulmonary bypass. All procedures were approved by the Institutional Review Board of Rhode Island Hospital, Alpert Medical School of Brown University, and informed consent was obtained from all enrolled patients as required by the Institutional Review Board.
Microvessel Reactivity
Coronary arterioles (80- to 150-μm internal diameters) were dissected from harvested right atrial appendage tissue samples during atrial cannulation before the onset of cardiopulmonary bypass. Microvessel studies were performed by in vitro organ bath video-microscopy as described previously.3,4,11,13 After a 60-minute stabilization period in the organ chamber, the microvessels were preconstricted with thromboxane A2 analog U46619 30% to 40% of the baseline diameter. After achievement of this constricted steady state, dose-dependent relaxation was measured in response to the application of the following vasodilators: the activator of intermediate and small conductance KCa channels (SKCa/IKCa), NS309, or the endothelium-dependent vasodilators ADP and substance P or the endothelium-independent vasodilator sodium nitroprusside. One or 3 interventions were performed on each vessel. The order of drug administration was random. In some cases, endothelial denudation was carried out by advancing a human hair into the lumen and gently abrading the luminal surface.
Cell Culture
Human coronary artery endothelial cells (HCAECs, passage 4) harvested from donors (patients) with and without diabetes (Lonza, Walkersville, MD) were cultured and grown in the EGMTM-2 Bullet Kit medium (Lonza) in a humidified incubator with 5% CO2 at 37°C according to the manufacturer’s protocols.
Patch-Clamp Recording of SKCa/IKCa Currents
Prior to the experiments, the cultured primary-HCAECs at passage 4 were washed twice with Ca2+-free DMEM, incubated with 0.05% trypsin, and 0.02% EDTA for 1 to 2 minutes. Perforated whole-cell voltage-clamp using Axopatch-200B amplifier (Molecular Devices, Foster City, USA) was employed for recording K+ currents. The bath solution contained (mmol/L): 140 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, 10 HEPES, and 30 glucose (pH 7.4; 22°C). The patch pipette had a resistance of 2–5 MΩ. The pipette solution contained (mmol/L): 110K-aspartate, 20 KCl, 1 MgCl2, 9 CaCl2, 10 HEPES, 8 NaCl, 0.01 niflumic acid and 10 BAPTA (1,2-bis[o-aminophenoxy]ethane-N,N,N’,N’-tetraacetic-acid) (pH 7.2, free Ca2+ 1 μmol/L). The cells were depolarized every 5 seconds from a holding potential of −50 mV to test potentials between −100 and +100 mV with a step of +20 mV for duration of 150 mV. Tracings were taken from a range of potentials (−100 to +100 mV) with the holding potential of −50 mV. The effects of the selective IKCa blocker TRAM34 or TRAM34 plus the SKCa blocker apamin on the basal K+ currents were measured in diabetic and nondiabetic endothelial cells. The effect of the selective SKCa/IKCa activator NS309 on the whole cell K+ currents was examined. Both TRAM34 and apamin were also applied for testing the specificity of NS309. The data were acquired and analyzed with p-CLAMP 6.0.4 (Axon Instruments Inc.).
HCAEC Membrane Potential Recording
Perforated whole-cell current-clamps were used to record membrane potentials (Axopatch-200B amplifier, Molecular Devices). Pipettes were filled with (in mmol/L): 120 potassium gluconate, 20 KCl, 5 NaCl, 5 HEPES, and 5 MgATP (pH 7.2). The extracellular bathing solution contained (in mmol/L) 140 NaCl, 5.4 KCl, 1 MgCl2, 10 HEPES, 1.8 CaCl2, and 5.5 glucose (pH 7.4). β-escin (50 μmol/L) is added to pipette solution. Pipette resistances of ≈5 MΩ were filled with internal solution. In current-clamp, voltages were low-pass filtered at 10 kHz and digitized at 20 kHz.
Immunoblot
Atrial tissues samples were solubilized in SDS-PAGE buffer. Total protein (40 μg) was fractionated on an 8% to 16% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corporation, Bedford, MA) as previously described.11,12 Membranes were incubated for 1 hour at room temperature with 1:200 dilutions of individual rabbit polyclonal primary antibodies to IKCa, or SKCa 2.3 (Alomone Labs Ltd, Jerusalem, Israel). The membranes were then incubated for 1 hour with horseradish peroxidase–conjugated secondary anti-Ig, washed 3 times in Tris saline buffer. Peroxidase activity was visualized with enhanced chemiluminescence (Thermo Scientific) and the images were captured with a digital camera system (G Box, Syngene, Cambridge, UK). The Western blot bands were quantified with densitometry using ImageJ software (National Institute of Health, Bethesda, MD). Specificities of the anti-IK-1, and anti-SK2.3 antibodies were demonstrated in the previous studies, respectively.11,12
Immunofluorescence Photomicroscopy
Atrial tissue sections from 5 patients were deparaffinized in xylene, rehydrated in graded ethanol and PBS solution, and antigen-unmasked with sodium citrate (10 mmol/L, pH=6.0) followed by PBS wash and blocking with 2% BSA in PBS at room temperature for 2 hours.11,12 After the PBS wash, overnight incubation with anti-SK2.3 and IK-1 (each used 1:200; Alomone) was performed at 4°C. Anti-mouse, smooth muscle actin (1:1000; Sigma, St. Louis, MO) was used to detect microvascular smooth muscle. Sections were then washed in PBS and incubated with the appropriate Alexa fluor secondary antibody and mounted using fluorescent mounting medium (Vector Labs, Burlingame, CA). Tissue was visualized using a Zeiss LSM510 confocal microscope system (Carl Zeiss MicroImaging, Inc, Thornwood, NY). Tissue labeling with secondary antibody alone or with normal rabbit IgG or serum in place of primary antibody served as a negative control.
Chemicals
ADP, substance P, sodium nitroprusside, NS309, TRAM34 and apamin were obtained from Sigma-Aldrich and dissolved in ultrapure distilled water on the day of the study.
Data Analysis
Data are presented as the mean and SEM. Microvessel responses are expressed as percent relaxation of the preconstricted diameter. Microvascular reactivity was analyzed using 2-way repeated-measures ANOVA with a post hoc Bonferroni test. Clinical and Western blot data were analyzed by Kruskal–Wallis tests followed with Dunn multiple comparison test (GraphPad Software, Inc, San Diego, CA). P<0.05 was considered significant.
Results
Patient Characteristics
Patient characteristics are listed in Table 1. All patients (n=16, 8/group) with preoperative hypertension were on antihypertensive medication (aspirin and/or β-blocker, and/or calcium channel blocker, and/or statins, and/or angiotensin-converting enzyme inhibitors). The preoperative hemoglobin A1C levels were 9.6±0.3 in diabetic patients and 5.4±0.12 in nondiabetic patients.
Table 1.
Patient Characteristics (n=8/Group)
| Patient Characteristics | ND | DM (Type 2) | P Values |
|---|---|---|---|
| Age, y* | 73±9.5 | 64±7.0 | 0.7 |
| Male/female, n | 5/3 | 6/2 | 0.96 |
| Patient blood glucose, mg/dL (pre-CPB)* | 101±13.0 | 258±77.0† | 0.001 |
| Preoperative insulin | 0 | 8 | 0.001 |
| HbA1c, %* | 5.4±0.12 | 9.6±0.3† | 0.001 |
| Hypertension, n | 8 | 8 | 1.0 |
| Atrial fibrillation, n | 0 | 0 | NS |
| Hypercholesterolemia, n | 8 | 8 | 1.0 |
| CABG only, n | 8 | 8 | 1.0 |
| Preoperative aspirin | 8 | 8 | 1.0 |
| Preoperative β-blocker | 5 | 6 | 0.82 |
| Preoperative CaCB | 4 | 5 | 0.79 |
| Preoperative ACEI | 3 | 4 | 0.75 |
| Preoperative statins | 5 | 6 | 0.82 |
Data expressed as mean±SEM. ACEI indicates angiotensin-converting enzyme inhibitor; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; ND, nondiabetics; NS, no significance.
vs ND.
Microvascular Reactivity
There was no significant difference in the baseline diameter of the microvessels among the 2 groups (nondiabetic: 119±8.5 μm, diabetic: 122±7.4 μm). The degrees of precontraction by the thromboxane A2 analog U46619 were 33±3% in the nondiabetic group, and 35±4% in the diabetic group. The baseline microvascular responses to the endothelium-dependent vasodilators ADP, substance P, and the endothelium-independent vasodilator sodium nitroprusside of arterioles from diabetic patients were significantly decreased as compared to the respective response from nondiabetic patients (P<0.05, Figure 1). Importantly, diabetes significantly reduced the arteriolar response to the SKCa/IKCa opener NS309 compared to the respective responses of nondiabetic vessels (Figure 2A). In addition, the relaxation response of diabetic arterioles to NS309 was prevented by denudation of endothelium, suggesting that NS309-induced response is endothelium dependent in the diabetic arterioles (Figure 2B).
Figure 1.
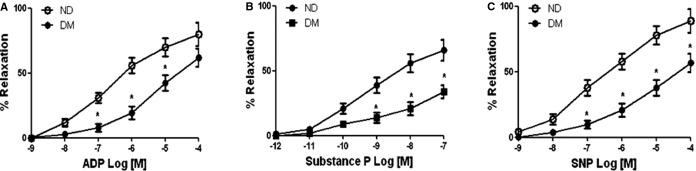
Decreased relaxation responses of diabetic (DM) coronary arterioles to the dose-dependent, endothelium-dependent vasodilators ADP (A), substance P (B), and the endothelium-independent vasodilator SNP (C); n=8/group. *P<0.001. ND indicates nondiabetics; SNP, sodium nitroprusside.
Figure 2.
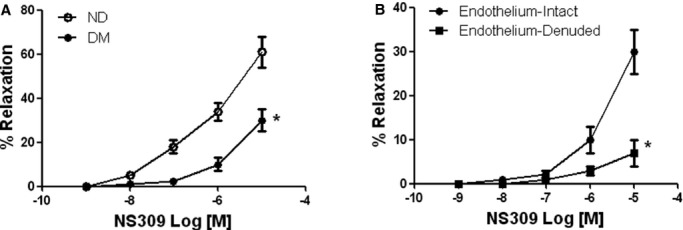
A, In-vitro coronary arteriolar response to selective SKCa/IKCa activator NS309 from diabetic (DM) and ND patients. Decreased relaxation responses of diabetic coronary arterioles to the selective SKCa/IKCa activator NS309. *P<0.05 vs ND; n=8/group. B, Denudation of endothelium prevented diabetic arteriolar response to NS309, *P<0.05 vs endothelium-intact, n=5/group. ND indicates nondiabetics; SKCa/IKCa, small/intermediate conductance of calcium-activated potassium channels.
Effects of Diabetes on SKCa/IKCa Channel Currents
Using the primary-cultured HCAECs (passage 4) harvested from diabetic and nondiabetic patients, we have successfully recorded SKCa/IKCa currents in the HCAECs by whole cell patch-clamp (Figures3 and 4, n=4/group). In the nondiabetic endothelial cells, the whole cell K+ currents at base level (control) were significantly inhibited by addition of the selective IKCa blocker TRAM34 (10 μmol/L). Inclusion of the selective SKCa blocker apamin (100 nmol/L) further reduces the whole cell K+ currents (Figure 3A, 3B and 3C). In contrast, diabetes significantly reduced TRAM34-sensitive and apamin-sensitive K+ currents (P=0.002, Figure 3D; and P=0.02 Figure 3E), respectively. The basal-whole-cell-K+ currents were also reduced in the diabetic endothelial cells as compared with the nondiabetic endothelial cells (Figure 3A, P<0.05)
Figure 3.
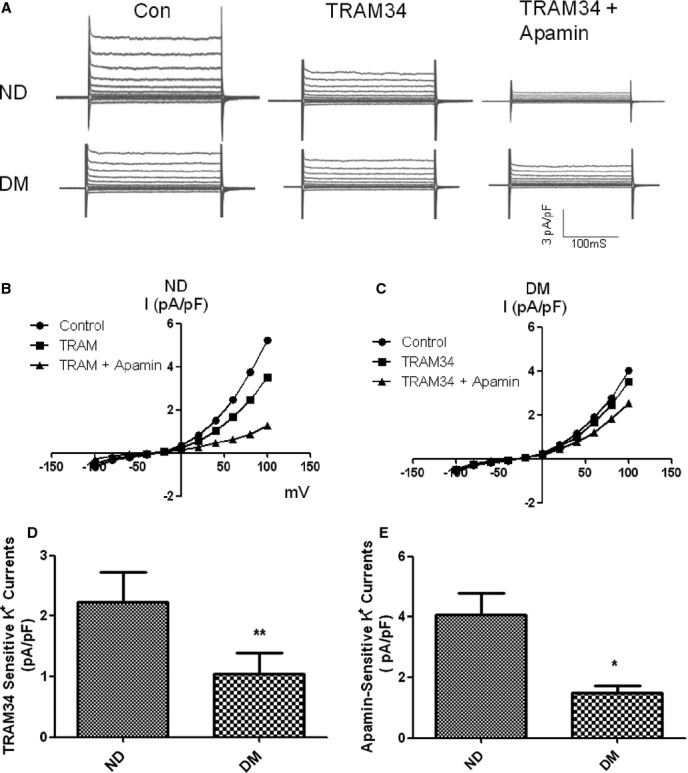
Effects of the selective IKCa blocker TRAM34 and the selective SKCa blocker apamin on the basal (control) whole-cell K+ currents in primary-cultured HCAECs of ND and diabetics (DM). Currents were elicited by 20-mV step pulses between −100 and +100 mV from a holding potential of 50 mV with 400 nmol/L free calcium pipette solution. Representative current traces (A) were obtained under control condition (Con) and after cumulative application of TRAM34 (10 μmol/L) and apamin (100 nmol/L). The cells displayed a linear-like I-V relationship (B and C). The mean data of TRAM34-sensitive- and apamin-sensitive K+ currents at 100-mV pulse are summarized in the bar graphs (D and E); n=4/group, **P=0.002 vs ND; *P=0.02 vs ND. HCAECs indicates human coronary arterial endothelial cells; IKCa, intermediate conductance of calcium-activated potassium channels; ND, nondiabetics; SKCa, small conductance of calcium-activated potassium channels.
Figure 4.
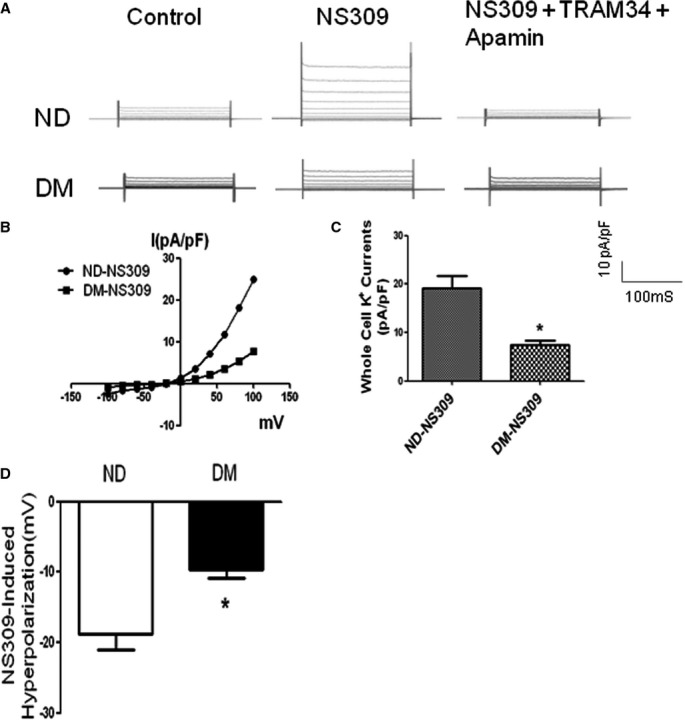
The effects of selective SKCa/IKCa activator NS309 on whole-cell K+ currents of ND and diabetic (DM) HCAECs with and without the presence of selective IK blockers TRAM and selective SKCa blocker apamin. A, Representative current traces were obtained under control condition, after NS309 (10 μmol/L) treatment, and after TRAM34 (10 μmol/L) plus apamin (100 nmol/L). B, I-V plot curve showing diabetes shifted the curve to the right. C, Bar graph showing that the amplitude of the SKCa/IKCa currents in response to the SKCa/IKCa activator NS309 was significantly reduced in the diabetic (DM) HCAECs compared to ND (at 100-mV pulse); n=4/group, *P=0.02 vs ND-NS309. D, Bar graph showing the amplitude of NS309-induced HCAEC hyperpolarization; *P<0.05 vs ND. HCAECs indicates human coronary arterial endothelial cells; IKCa, intermediate conductance of calcium-activated potassium channels; ND, nondiabetics; SKCa, small conductance of calcium-activated potassium channels.
Treatment of diabetic and nondiabetic cells with the selective SKCa/IKCa opener NS309 increased the total K+ currents of culture HCAECs from patients with or without diabetes (Figure 4A through 4C). Importantly, the amplitude of the increased K+ currents by NS309 was significantly reduced in the diabetic endothelial cells as compared with that of nondiabetics (P=0.02, Figure 4A through 4C, n=4/group). Treatment of the endothelial cells with TRAM34 and apamin abolished NS309-induced K+ currents of HCAEC in the 2 groups (Figure 4A through 4C).
Effects of Diabetes on HCAEC Membrane Potential
Administration of NS309 induced hyperpolarization of HCAEC in both diabetics and nondiabetics. However, diabetes significantly decreased the amplitude of NS309-induced hyperpolarization of HCAEC compared with nondiabetes (P<0.05, Figure 4D).
Effect of Diabetes on Levels of SKCa/IKCa Polypeptides
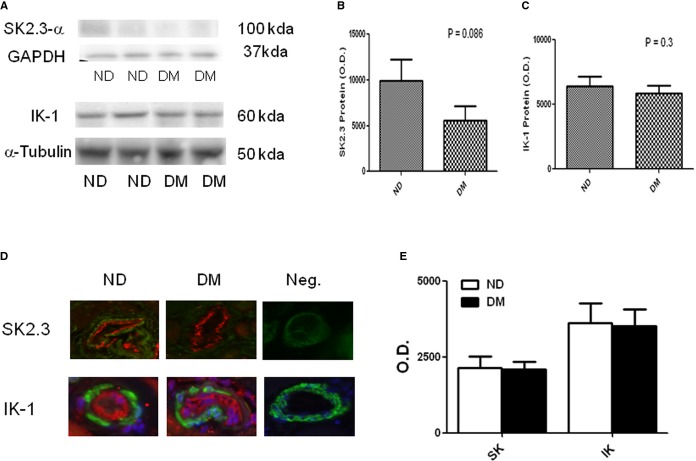
There was dramatic decrease in SK2.3 protein levels in the diabetic myocardium, but no statistical significance (P=0.086, Figure 5A and 5B) as compared with nondiabetics. There were slight, but insignificant decreases in IKCa protein levels in the diabetic myocardium versus nondiabetics (P=0.3, n=5/group, Figure 5A and 5C).
Figure 5.
A, Top left, Representative immunoblots of human atrial tissue samples from diabetic (DM) and nondiabetic (ND) patients. Lanes 1 to 4 loaded with 40 μg protein were developed for SK2.3-α and IK-1 polypeptides. B, Top middle and (C) top right, Densitometric evaluation of immunoblot band intensity shows no significant differences in the levels of SK2.3 (P=0.086 vs ND) and IK-1 (P=0.3) polypeptides between ND and DM groups. D, Bottom left, Immunolocalization of KCa channel polypeptides in human coronary microvessels. Vessels were co-stained for smooth muscle α–actin (green) and either SK2.3 or IK-1. Matched negative controls are displayed at the end of each row of primary antibody staining as indicated, n=6/group. E, Densitometric analysis of immunofluorescence intensities shows unaltered levels of SK2.3 or IK-1 in the coronary vessels between ND and DM groups. O.D. indicates optical density.
Immunolocalization of Microvessel SKCa/IKCa Polypeptides
Immunofluorescent staining of coronary microvessels from diabetic and nondiabetic patients displayed a strong signal in the endothelium (stained in red) for IKCa-1 and SKCa2.3 (Figure 5D and 5E). IKCa-1 subunits localized to the microvascular smooth muscle and less abundantly to smooth muscles (stained in green). There were no significant changes in distribution of SKCa2.3 and IKCa-1 between diabetes and nondiabetes. Negative controls documented low-level background fluorescence.
Discussion
Diabetes-induced changes in endothelial SKCa/IKCa function have been studied in detail in diabetic rodents.6–8 For instance, impaired endothelium-dependent hyperpolarizing factors-dilator response has been demonstrated in various vascular beds, particularly in small-sized arteries in different diabetic animal models. The present study further indicates that the dose-dependent/endothelium-dependent relaxation response to the selective SKCa/IKCa activator NS309 was significantly decreased in the diabetic coronary arterioles as compared with that of case-matched nondiabetics. This novel finding suggests that diabetes may be associated with downregulation of SKCa/IKCa channels in the human coronary microvasculature.
SKCa/IKCa in endothelial cells modulate vascular diameter through regulation of their membrane potential and blockade of SKCa/IKCa channels, causing a significant endothelial membrane potential depolarization.14–16 In the animal models, diabetes reduced endothelial SKCa/IKCa channel activity and channel current densities and endothelial-dependent hyperpolarization.6–8 In the present study, we further investigated the effects of diabetes on SKCa/IKCa channel activity/current densities and membrane potential in human coronary endothelial cells. Our results demonstrate that diabetes is associated with significant reduction of SKCa/IKCa channel activity, current densities, and NS309-induced hyperpolarization of human coronary endothelial cells. Clearly, the reduced amount of endothelial SKCa/IKCa current density and endothelial hyperpolarization could be responsible for the reduced NS309-induced endothelium-dependent dilation observed in the diabetic vessels.
There are 3 types of KCa channels: BKCa, IKCa, and SKCa. The BKCa channels are predominately present in smooth muscle cells, whereas SKCa/IKCa channels are found in abundance in endothelial cells.9–11 IKCa is also classified as SK4 of the SKCa family due to its similarity to the other SKCa (SK1-SK3).17 Several animal studies have shown that diabetes is associated with alterations of protein/mRNA expression of endothelial SKCa/IKCa channels, which underlies the impaired endothelium-dependent hyperpolarizing factors-dilator response in the rodents.6–8 To detect SKCa/IKCa protein expression and localization in the human vasculature in patients with diabetes, we performed immunoblot and immunohistochemistry to quantify and localize SKCa/IKCa channel polypeptides. We found that SKCa/IKCa polypeptides were detected by immunoblot of extracts from harvested human atrial tissue samples. Immunostaining further showed that SKCa/IKCa channels were predominately present at coronary arteriolar endothelial cells. However, diabetes did not alter expression/distribution of SKCa/IKCa polypeptides, suggesting that diabetes may induce posttranslational modification (gating or trafficking) of SKCa/IKCa channels rather than the steady-state levels of their protein.
There are several limitations of the current study that deserve mention. First, this work should be considered a pilot study, considering the relatively small number of patients and samples. Due to the small sample size/low power, some of the statistical comparisons failed to reach significance. Another limitation is the heterogeneity of the patients; even though they were reasonably well matched, there were differences in medications and the incidence of coexisting illnesses that may affect the findings. This is a limitation of all studies dealing with patients. Third, the effects of insulin therapy might have also contributed to the microvascular function in patients with chronic, poorly controlled diabetes. Finally, due to technical difficulties, we were unable to harvest and culture coronary arteriolar endothelial cells from a small piece of atrial tissue samples. For those experiments shown in Figure 3 and Figure 4, we instead used human coronary endothelial cells (primary-cultured HCAECs, passage 4), which were derived from large coronary arteries and were typically from donors with or without diabetes.
Obviously, using human coronary arterioles and endothelial cells harvested from nondiabetic and diabetic patients undergoing cardiac surgery provides plenty of advantages over animal conduit arteries and endothelial cells, since the majority of animal diabetic models are performed on young, otherwise healthy animals in the absence of any comorbid illnesses, which on the whole are not adequately representative of the clinical scenario. Thus, the current investigation of SKCa/IKCa regulation by using human heart tissues, isolated coronary arterioles, and primary cultured HCAECs is a crucial step between “bench and bedside,” although obtaining human heart/vessel tissues and cells is not easy. Obviously, this translational study improves our understanding of the effects of diabetes on endothelial SKCa/IKCa channels and may lead to a novel therapy for preserving endothelial function of coronary arteries and arterioles for diabetic patients with coronary heart diseases.
In conclusion, the present study demonstrates that in human coronary arterioles, diabetes inhibits the SKCa/IKCa activator-mediated endothelium-dependent relaxation with significant reduction of endothelial SKCa/IKCa currents. Inactivation of endothelial SKCa/IKCa channels may contribute to coronary endothelial dysfunction in diabetic patients.
Acknowledgments
We would like to thank all nurses, physician assistants, and perfusionists at cardiac- surgery-operation rooms in Lifespan Hospitals for collecting tissue samples and the data of patient characteristics. We would also like to thank the nurses and physician assistants at Division of Cardiac Surgery, Lifespan Hospitals for collecting patient consent forms.
Sources of Funding
This research project was supported by Rhode Island Foundation RIF-20123834 (Feng), NIGMS/NIH grant (pilot project) 1P20GM103652 (Feng), AHA-Grant-in-Aid 15GRNT25710105 (Feng), and supported in part by the National Heart, Lung, and Blood Institute HL-46716 (Sellke).
Disclosures
None.
References
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Liu YH, Chu LM, Singh AK, Dobrilovic N, Fingleton JG, Clements R, Bianchi C, Sellke FW. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation. 2012;126:S73–S80. doi: 10.1161/CIRCULATIONAHA.111.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Chu LM, Dobrilovic N, Liu YH, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. Surgery. 2012;152:282–289. doi: 10.1016/j.surg.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham MP, Johnson IT, Weston AH. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in type II diabetic ZDF rats. Br J Pharmacol. 2006;148:434–441. doi: 10.1038/sj.bjp.0706748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston AH, Absi M, Harno E, Geraghty AR, Ward DT, Ruat M, Dodd RH, Dauban P, Edwards G. The expression and function of Ca(2+)-sensing receptors in rat mesenteric artery; comparative studies using a model of type II diabetes. Br J Pharmacol. 2008;154:652–662. doi: 10.1038/bjp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Hashem M, Wiehler WB, Lau W, Martin J, Reid J, Triggle C. Endothelial dysfunction in the streptozotocin-induced diabetic apoE-deficient mouse. Br J Pharmacol. 2005;146:1110–1118. doi: 10.1038/sj.bjp.0706417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses—relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Liu YH, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V, Nishimura KK, Alper SL, Sellke FW. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegia arrest. Circulation. 2008;118(suppl I):S46–S51. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YH, Sellke EW, Feng J, Clements RT, Sodha NR, Khabbaz K, Senthilnathan V, Alper SL, Sellke FW. Calcium-activated potassium channels contribute to human skeletal muscle microvascular endothelial dysfunction related to cardiopulmonary bypass. Surgery. 2008;144:239–244. doi: 10.1016/j.surg.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellke FW, Shafique T, Schoen FJ, Weintraub RM. Impaired endothelium-dependent coronary microvascular relaxation after cold potassium cardioplegia and reperfusion. Ann Thorac Surg. 1993;55:724–728. [PubMed] [Google Scholar]
- Yang Q, Huang JH, Man YB, Yao XQ, He GW. Use of intermediate/small conductance calcium-activated potassium-channel activator for endothelial protection. J Thorac Cardiovasc Surg. 2011;141:501–510. doi: 10.1016/j.jtcvs.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Rusko J, Tanzi F, van Breemen C, Adams DJ. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;5:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]