Abstract
Background
The safety of deferring revascularization based on fractional flow reserve (FFR) during acute coronary syndrome (ACS) is unclear. We evaluated the association of FFR and adverse cardiac events among patients with coronary lesions deferred revascularization based on FFR in the setting of ACS versus non-ACS.
Methods and Results
The study population (674 patients; 816 lesions) was divided into ACS (n=334) and non-ACS (n=340) groups based on the diagnosis when revascularization was deferred based on FFR values >0.80 between October 2002 and July 2010. The association and interaction between FFR and clinical outcomes was evaluated using Cox proportional hazards models within each group (mean follow-up of 4.5±2.1 years). Subsequent revascularization of a deferred lesion was classified as a deferred lesion intervention (DLI), whereas the composite of DLI or myocardial infarction (MI) attributed to a deferred lesion was designated as deferred lesion failure (DLF). In the non-ACS group, lower FFR values were not associated with any increase in adverse cardiac events. In the ACS group, every 0.01 decrease in FFR was associated with a significantly higher rate of cardiovascular death, MI, or DLI (hazard ratio [HR], 1.08; 95% confidence interval [CI], 1.03 to 1.12), MI or DLI (HR, 1.09; 95% CI: 1.04 to 1.14), DLF (HR, 1.12; 95% CI, 1.06 to 1.18), MI (HR, 1.07; 95% CI, 1.00 to 1.14), and DLI (HR, 1.12; 95% CI, 1.06 to 1.18).
Conclusion
Lower FFR values among ACS patients with coronary lesions deferred revascularization based on FFR are associated with a significantly higher rate of adverse cardiac events. This association was not observed in non-ACS patients.
Keywords: acute coronary syndrome, coronary disease, fractional flow reserve, revascularization, stenosis
Treatment of ischemic myocardium with percutaneous coronary intervention (PCI) in addition to optimal medical therapy reduces major adverse cardiac events.1 However, less than half of patients have a noninvasive ischemic evaluation before revascularization.2 Fractional flow reserve (FFR) can determine the hemodynamic significance of a coronary lesion by measuring the distal mean coronary and aortic pressures during maximal hyperemia.2 Previous studies conducted principally in stable coronary artery disease (CAD) patients have demonstrated that FFR-guided revascularization improves clinical outcomes, quality of life, and cost-efficiency.3–5 However, the reliability and safety of FFR assessment in the setting of acute coronary syndrome (ACS) is unclear.6 In ACS, microvascular dysfunction from myocardial injury can persist up to 6 months7 and can impair maximal hyperemia, leading to an overestimation of FFR.8 Observational studies and post-hoc analyses have suggested that FFR is reliable9–11 and deferral of lesions based on FFR is safe in ACS.12–16 A recent expert consensus statement concluded that FFR is a valid measure in ACS, including nonculprit lesions in the setting of ST-elevation myocardial infarction (STEMI).17 However, the statement acknowledged the effect of microvascular dysfunction on FFR in certain myocardial infarctions (MIs), as well as the potential for error in certain clinical situations.17 Thus, the prognostic significance of an FFR value may be more uncertain in ACS compared with stable CAD (ie, non-ACS).6 The goal of this study was to determine, in a large, real-world cohort, the predictive ability of FFR for patients with lesions deferred revascularization based on FFR in the setting of ACS and non-ACS.
Methods
Study Design
The study is a retrospective, single-center, observational study approved by the institutional review board. All patients provided written informed consent for the procedure.
Study Population
There were 1872 patients that underwent FFR assessment between October 2002 and July 2010 at our institution. From 2002 to 2008, an FFR value ≥0.75 was used to defer revascularization based on the DEFER (FFR to Determine Appropriateness of Angioplasty in Moderate Coronary Stenoses) study.3 In 2008, an FFR value >0.80 was used to defer lesions based on the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) trial.4 Of these 1872 patients, 742 patients with 906 coronary lesions were deferred revascularization based on FFR. Of the 742 patients, 21 patients or 24 lesions were excluded because of loss of follow-up after FFR assessment. Furthermore, 47 patients or 68 lesions were excluded because of FFR less than or equal to 0.80. Thus, the final population for this study consisted of 674 patients with 816 lesions that were deferred revascularization based on FFR >0.80 (Figure 1).
Figure 1.
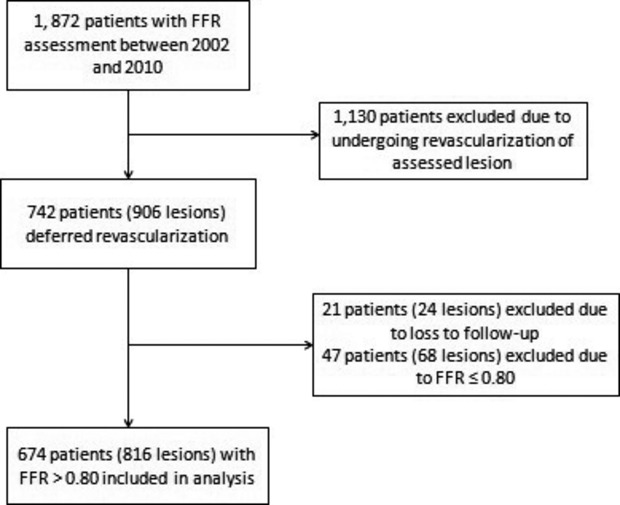
Study population. Flow diagram of the study population selection. FFR indicates fractional flow reserve.
Fractional Flow Reserve Assessment
FFR was performed according to standard techniques as detailed in a previous observational study using this study population.18 FFR was measured at maximal hyperemia after administering intracoronary (n=812 lesions) or intravenous adenosine (n=4 lesions), with the dose determined by the operator. FFR was not performed on lesions with less than Thrombolysis In Myocardial Infarction (TIMI) 3 flow.
Clinical Endpoints
Every patient included in the study was followed from the date of index stenting until March 12, 2013 by reviewing the medical records and/or telephone interview. All angiograms were reviewed independently by at least 2 authors. For patients with follow-up or hospitalizations outside our institution, the medical records, including angiograms, were obtained for review. The primary outcome was a composite of cardiovascular (CV) death, MI, or deferred lesion intervention (DLI). Secondary outcomes included a composite of CV death or MI and MI or DLI, as well as the individual endpoints of CV death, MI, MI-lesion, deferred lesion failure (DLF), and DLI. CV death and MI were defined by the Academic Research Consortium guidelines.19 DLI was defined as any percutaneous coronary intervention (PCI) performed within 5-mm proximal or distal to, or any coronary artery bypass graft (CABG) placed distal to, a lesion deferred revascularization based on the index FFR assessment.18 MI lesion was defined as any MI that was directly attributable to a lesion deferred revascularization at the index FFR. DLF was a composite endpoint of MI lesion or DLI.
Statistical Analysis
The study population was divided into ACS and non-ACS groups based on clinical diagnosis at the time of index FFR assessment. The ACS group included unstable angina (n=257), non-STEMI (NSTEMI; n=70), and STEMI (n=7) patients. Patient characteristics were compared between the ACS and non-ACS groups using the Student 2-sample t test for continuous variables and Fisher’s exact test for categorical variables. Non-normal and ordinal variables were reported as median (25th, 75th percentiles) and compared using the Kruskal–Wallis test. Lesion characteristics were compared using models developed from generalized estimating equation (GEE) methods to account for patients having multiple lesions (clustering). Logistic models were built for dichotomous lesion characteristics. Lesion characteristics that were continuous were evaluated by GEE methods using a normal probability distribution. Skewed continuous lesion variables were log-transformed preceding model development. For all GEE models, robust standard errors were used when comparing estimates.
Owing to the inherent risk for subsequent adverse cardiac events post-ACS that persist despite statistical adjustment, the clinical outcomes between ACS and non-ACS groups were not compared directly. Instead, the association between FFR and clinical outcomes was evaluated within each group using Cox proportional hazards modeling. A marginal Cox model was used to account for correlated data for patients with multiple lesions.20 Tests of interaction were performed to determine whether the FFR association with outcome was different between ACS and non-ACS groups. Furthermore, for the outcomes of a composite of CV death, MI or DLI, composite MI or DLI, and DLF, a multivariable analysis was performed adjusting for age, diabetes, smoking status, previous CAD (including PCI or CABG), congestive heart failure, chronic kidney disease, and myocardial jeopardy index.21 For the association between FFR and clinical outcomes as well as tests of interaction, a value of P<0.05 was considered statistically significant.22 All statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics of Study Population
Baseline patient and lesion characteristics of the study population (674 patients with 816 coronary lesions) are shown in Tables1 and 2. Approximately half of the study population was deferred revascularization post-FFR assessment in the setting of non-ACS (n=340 patients). The remaining patients (n=334) underwent FFR assessment during an ACS, where 77% of these patients presented with unstable angina, 21% with NSTEMI, and 2% during STEMI. The ACS group patients had a higher rate of congestive heart failure and were discharged more frequently with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) post-FFR assessment, compared to the non-ACS group. However, both groups were discharged on a similar median number of optimal medical therapy medications.23
Table 1.
Baseline Patient-Level Characteristics
| Total Population (n=674) | ACS (n=334) | Non-ACS (n=340) | P Value | |
|---|---|---|---|---|
| Age, y | 64.5±11.1 | 63.8±11.9 | 65.3±10.2 | 0.07 |
| Male | 380 (56) | 180 (54) | 200 (59) | 0.21 |
| Medical history | ||||
| Diabetes mellitus | 248 (37) | 124 (37) | 124 (36) | 0.87 |
| Hypertension | 561 (83) | 278 (83) | 283 (83) | 1.00 |
| Hyperlipidemia | 547 (81) | 264 (79) | 283 (83) | 0.17 |
| Ever smoker | 344 (51) | 183 (55) | 161 (47) | 0.05 |
| History of CAD | 456 (68) | 232 (69) | 224 (66) | 0.32 |
| Previous PCI | 307 (46) | 165 (49) | 142 (42) | 0.05 |
| Previous CABG | 88 (13) | 44 (13) | 44 (13) | 1.00 |
| Peripheral arterial disease | 75 (11) | 39 (12) | 36 (11) | 0.71 |
| Chronic kidney disease | 73 (11) | 39 (12) | 34 (10) | 0.54 |
| Congestive heart failure | 146 (22) | 84 (25)* | 62 (18) | 0.03 |
| Creatinine level, mg/dL | 1.15±1.22 | 1.15±1.24 | 1.16±1.20 | 0.76 |
| Discharge medications after index FFR assessment | ||||
| OMT | ||||
| Aspirin | 643 (95) | 324 (97) | 319 (94) | 0.06 |
| ACEI/ARB | 436 (65) | 235 (70)* | 201 (59) | 0.003 |
| Beta-blocker | 516 (77) | 265 (79) | 251 (74) | 0.10 |
| Calcium-channel blocker | 205 (30) | 109 (33) | 96 (28) | 0.24 |
| Nitrates | 267 (40) | 140 (42) | 127 (37) | 0.24 |
| Statin | 543 (81) | 268 (80) | 275 (81) | 0.85 |
| Median number of OMT medications | 4 (3, 5) | 4 (3, 5) | 4 (3, 5) | 0.01 |
| Additional medications | ||||
| Clopidogrel | 335 (50) | 170 (51) | 165 (49) | 0.59 |
| Ranolazine | 11 (2) | 7 (2) | 4 (1) | 0.38 |
| Warfarin | 82 (12) | 45 (13) | 37 (11) | 0.35 |
Values are shown as absolute numbers (percentages), mean±SD, or median (1st, 3rd quartile). ACEI/ARB indicates angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CAD, coronary artery disease; FFR, fractional flow reserve; OMT, optimal medical therapy; PCI, percutaneous coronary intervention.
P<0.05 compared to non-ACS group. All remaining comparisons between the ACS and non-ACS groups were not statistically significant.
Table 2.
Baseline Lesion-Level and Procedural Characteristics
| Total Population (n=816) | ACS (n=411) | Non-ACS (n=405) | P Value | |
|---|---|---|---|---|
| Coronary vessel location | ||||
| Left main | 56 (7) | 29 (7) | 27 (7) | 0.82 |
| LAD | 344 (42) | 182 (44) | 162 (40) | 0.20 |
| Proximal | 110 (13) | 49 (12) | 61 (15) | 0.18 |
| Left circumflex | 208 (25) | 90 (22)* | 118 (29) | 0.02 |
| Proximal | 104 (13) | 37 (9)* | 67 (17) | 0.001 |
| RCA | 184 (23) | 96 (23) | 88 (22) | 0.58 |
| Proximal | 59 (7) | 29 (7) | 30 (7) | 0.85 |
| Bypass graft | 24 (3) | 14 (3) | 10 (2) | 0.43 |
| Multivessel CAD† | 430/674 (64) | 221/334 (66) | 209/340 (61) | 0.23 |
| Lesion characteristics | ||||
| Bifurcation lesion | 125 (15) | 48 (12)* | 77 (19) | 0.007 |
| Ostial lesion | 173 (21) | 102 (25)* | 71 (18) | 0.01 |
| Previous PCI of lesion | 75 (9) | 40 (10) | 35 (9) | 0.60 |
| Median myocardial jeopardy index | 6 (2, 6) | 4 (2, 6) | 6 (2, 6) | 0.03 |
| Maximum stenosis, % | 58±10 | 58±10 | 58±10 | 0.98 |
| Procedural characteristics | ||||
| Mean FFR value | 0.88±0.05 | 0.88±0.05 | 0.88±0.04 | 0.91 |
| IC adenosine | 812 (100) | 407 (99) | 405 (100) | 0.12 |
| Maximum IC dose, μg‡ | 120 (120, 180) | 120 (120, 180) | 120 (96, 180) | 0.58 |
| PCI of another lesion | 181/674 (27) | 97/334 (29) | 84/340 (25) | 0.22 |
Values are shown as absolute numbers (percentages), mean±SD, or median (1st, 3rd quartile). ACS indicates acute coronary syndrome; CAD, coronary artery disease; FFR, fractional flow reserve; IC, intracoronary; LAD, left anterior descending artery; PCI, percutaneous coronary intervention; RCA, right coronary artery.
P<0.05 compared to non-ACS group. All remaining comparisons between the ACS and non-ACS groups were not statistically significant.
Multivessel CAD was defined as 2 or more significant lesions (angiographic percent stenosis ≥50% at the time of FFR assessment.
Maximum IC dose was the highest dose of adenosine given to induce hyperemia during FFR assessment.
The left anterior descending artery (LAD) was the predominant vessel location (42%) for deferred lesions. Both groups had similar rates of multivessel CAD. The ACS group had more ostial lesions (25% vs. 18%) and fewer bifurcation lesions, compared to the non-ACS group. The mean FFR value for the study population was 0.88±0.05 and was not different between the ACS and non-ACS groups. Maximal hyperemia for FFR assessment was induced predominantly using intracoronary adenosine with similar maximum doses for both groups (120 μg). There were no significant differences between patients who were lost to follow-up and those who were included in the analysis except for the history of CAD (38% vs. 68%; P=0.008), proximal circumflex lesions (27% vs. 13%; P=0.044), FFR value (0.86 vs. 0.88; P=0.033), and adenosine dose (180 vs. 120; P=0.025).
Clinical Outcomes
Patients were followed for a mean of 4.5±2.1 years. Clinical outcomes after deferred revascularization based on FFR are shown in Table3. The rate of repeat catheterization was 45%. Subsequent MI in the lesion that was initially deferred (ie, MI lesion) accounted for 30% of the total MIs that occurred during follow-up.
Table 3.
Clinical Outcomes
| Total Population (n=674 Patients, 816 Lesions) | ACS (n=334 Patients, 411 Lesions) | Non-ACS (n=340 Patients, 405 Lesions) | P Value | |
|---|---|---|---|---|
| Cardiovascular death/MI/DLI | 226 (28) | 131 (32) | 95 (23) | 0.02 |
| Cardiovascular death/MI | 98 (15) | 65 (19) | 33 (10) | <0.001 |
| MI/DLI | 196 (24) | 109 (27) | 87 (21) | 0.14 |
| DLF | 148 (18) | 81 (20) | 67 (17) | 0.28 |
| Cardiovascular death | 31 (5) | 23 (7) | 8 (2) | 0.005 |
| MI | 73 (11) | 47 (14) | 26 (8) | 0.01 |
| MI lesion | 22 (3) | 14 (4) | 8 (2) | 0.20 |
| DLI | 144 (18) | 78 (19) | 66 (16) | 0.36 |
| Repeat catheterization | 303 (45) | 166 (50) | 137 (40) | 0.02 |
Parentheses values are expressed in percentage. ACS indicates acute coronary syndrome; DLF, deferred lesion failure; DLI, deferred lesion intervention; MI, myocardial infarction.
Association Between FFR Values and Outcomes for ACS and Non-ACS Groups
Table4 stratifies outcomes based on FFR value ranges and demonstrates a numerically higher absolute event rate for each composite outcome within the 0.81 to 0.85 and 0.86 to 0.90 ranges in the ACS group, compared to the non-ACS group. The associations between FFR values obtained during maximal hyperemia at the time of index FFR assessment and clinical outcome within each group assessed by marginal Cox proportional hazard modeling are shown in Table5. In the ACS group, lower FFR values were associated with a significantly higher rate of CV death, MI or DLI (hazard ratio [HR], 1.08 per 0.01 decrease; 95% confidence interval [CI], 1.03 to 1.12), MI or DLI (HR, 1.09; 95% CI, 1.04 to 1.14), DLF (HR, 1.12; 95% CI, 1.06 to 1.18), MI (HR, 1.07; 95% CI, 1.00 to 1.14), and DLI (HR, 1.12; 95% CI, 1.06 to 1.18). A trend toward a lower FFR value being associated with MI lesion in the ACS group was also observed (HR, 1.12; 95% CI, 0.996 to 1.26). The relationship between FFR and each of the clinical outcomes was found to differ significantly between ACS and non-ACS groups (P<0.05 for each). There was also a trend toward lower FFR being more likely associated with MI in the ACS group (P=0.05). In the non-ACS group, lower FFR values were not associated with an increase in the rate of any of the clinical outcomes. Using Cox proportional hazard modeling adjusting for covariates (Figure 2), lower FFR values remained an independent predictor in the ACS group, but not the non-ACS group of CV death, MI, or DLI (HR, 1.07; 95% CI, 1.02 to 1.11; interaction, P=0.08), MI or DLI (HR, 1.08; 95% CI, 1.03 to 1.13; interaction, P=0.026), and DLF (HR, 1.11; 95% CI, 1.05 to 1.17; interaction, P=0.005). A sensitivity analysis was performed including the 21 patients lost to follow-up and did not show any difference in adjusted HRs.
Table 4.
Outcomes Based on FFR Value
| FFR Value | CV Death/MI/DLI | MI/DLI | MI Lesion/DLI | |||
|---|---|---|---|---|---|---|
| ACS=No | ACS=Yes | ACS=No | ACS=Yes | ACS=No | ACS=Yes | |
| 0.81 to 0.85 | 36/133 (27) | 58/142 (41) | 32/133 (24) | 50/142 (35) | 26/133 (20) | 41/142 (29) |
| 0.86 to 0.90 | 27/155 (17) | 44/140 (31) | 25/155 (16) | 36/140 (26) | 17/155 (11) | 25/140 (18) |
| 0.91 to 0.95 | 28/94 (30) | 24/98 (24) | 27/94 (29) | 19/98 (19) | 22/94 (23) | 13/98 (13) |
| 0.96+ | 4/23 (17) | 5/31 (16) | 3/23 (13) | 4/31 (13) | 2/23 (9) | 2/31 (6) |
Parentheses values are expressed in percentage. ACS indicates acute coronary syndrome; CV, cardiovascular; DLI, deferred lesion intervention; FFR, fractional flow reserve; MI, myocardial infarction.
Table 5.
Cox Proportional HR Per 0.01 Unit Decrease in FFR
| ACS HR (95% CI) | Non-ACS HR (95% CI) | Interaction P Value* | |
|---|---|---|---|
| Cardiovascular death/MI/DLI | 1.08 (1.03 to 1.12)† | 1.01 (0.96 to 1.06) | 0.04 |
| Cardiovascular death/MI | 1.05 (0.998 to 1.11) | 0.98 (0.92 to 1.05) | 0.14 |
| MI/DLI | 1.09 (1.04 to 1.14)† | 1.00 (0.95 to 1.05) | 0.01 |
| DLF | 1.12 (1.06 to 1.18)† | 1.00 (0.95 to 1.06) | 0.004 |
| Cardiovascular death | 1.04 (0.95 to 1.14) | 0.98 (0.82 to 1.17) | 0.57 |
| MI | 1.07 (1.00 to 1.14)† | 0.97 (0.90 to 1.04) | 0.05 |
| MI lesion | 1.12 (0.996 to 1.26) | 0.91 (0.79 to 1.04) | 0.02 |
| DLI | 1.12 (1.06 to 1.18)† | 1.01 (0.95 to 1.06) | 0.01 |
ACS indicates acute coronary syndrome; DLF, deferred lesion failure; DLI, deferred lesion intervention; FFR, fractional flow reserve; HR, hazard ratios; MI, myocardial infarction.
For comparing HRs between ACS and non-ACS groups.
P<0.05 for testing HR=1.
Figure 2.
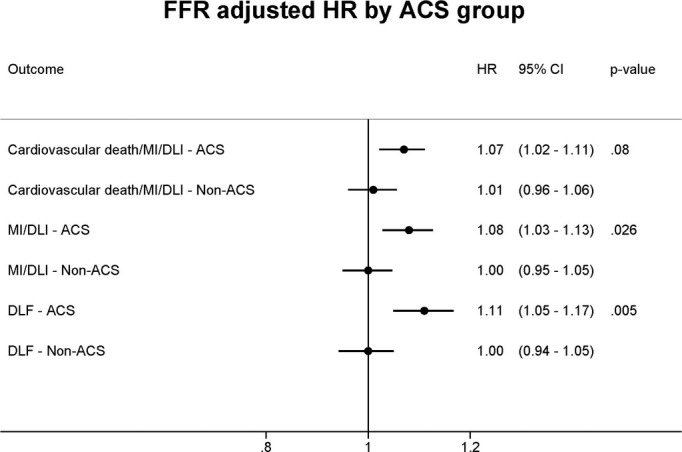
Adjusted hazard ratios for the association between FFR and clinical outcomes. A marginal Cox proportional hazard model was performed to adjust for age, diabetes, smoking status, previous coronary artery disease (including PCI or CABG), congestive heart failure, chronic kidney disease, and myocardial jeopardy index. ACS indicates acute coronary syndrome; CABG, coronary artery bypass graft; DLF, deferred lesion failure; DLI, deferred lesion intervention; FFR, fractional flow reserve; HR, hazard ratios; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Discussion
This study demonstrates that, for patients with coronary lesions deferred revascularization in the setting of ACS based on non-significant FFR values (ie, FFR >0.80), lower FFR values were associated with a significantly higher rate of adverse cardiac events. After adjustment, lower FFR values remained an independent predictor of CV death, MI, or DLI, CV death or MI, and DLF among ACS patients. The association between lower FFR values and adverse clinical outcomes was not observed for any of the composite or individual endpoints among non-ACS patients deferred revascularization based on FFR. One third of the subsequent MIs that occurred during follow-up were attributable to the lesion initially deferred based on FFR assessment. Our study provides new evidence that lesions with lower FFR values deferred in the setting of ACS are at higher risk for subsequent adverse cardiac events.
In the DEFER and FAME trials, when compared with angiographic guidance, FFR-guided selection of lesions for the deferral or performance of PCI improved clinical outcomes, although the majority of patients included in those trials had stable CAD.3,4 FFR can improve the diagnostic efficiency over angiography alone in the setting of ACS,24 especially in the presence of multivessel CAD. However, the reliability of FFR assessment of lesions during ACS has been controversial.6 FFR assessment is performed by inducing maximal hyperemia to determine the physiological significance of a coronary stenosis.25 Compared with stable CAD, ACS is a dynamic process with the potential for lesion instability and microvascular dysfunction that has been found in patients with MI to involve both infarct-related and non-infarct-related myocardial segments, and that persists for a poorly defined period, possibly up to 6 months.7 Microvascular dysfunction has also been demonstrated in patients with unstable angina, where vasoconstriction and increased distal microvascular resistance reduces coronary blood flow.26 Theoretically, when contrasted with intact microvascular function, an inability to induce maximal coronary hyperemia could lead to a smaller gradient and a higher FFR value for a given stenosis.8,27 Beyond microvascular dysfunction, lower oxygen consumption in an infarcted territory, abnormal vasomotion of the epicardial and resistance vessels distal to a coronary thrombosis, and/or obstruction of the microvasculature, could limit myocardial blood flow and affect FFR assessment.9 Therefore, there are multiple potential mechanisms that could adversely influence the reliability of FFR in the setting of ACS. Given these considerations and the fact that the decision to revascularize is largely based on an accepted ischemic threshold of 0.80,17 it appears possible that a nonsignificant FFR value that is close to the threshold in a patient with ACS could underestimate lesion physiological significance and potential for causing ischemia.4,5 Not inconsistent with our data, a recent meta-analysis suggested that FFR has been shown to have a continuous and independent relationship with clinical outcomes, with the suggestion that the FFR threshold for composite major adverse CV events in ACS patients may exceed the current threshold of 0.80.28
Several studies have previously examined the reliability of FFR for lesion assessment in the setting of ACS.9–11 A study of 57 patients who suffered an MI ≥6 days before FFR assessment determined that an FFR <0.75 had an 82% sensitivity and 87% specificity for detecting inducible ischemia determined by stress single-photon emission computed tomography (SPECT) imaging.9 Despite concluding that an FFR value of 0.75 was valid 6 days after acute MI, the investigators cautioned that the results should not be extrapolated to FFR measurements obtained during the acute phase of an MI.9 A prospective study performed SPECT, FFR, and PCI of the infarct-related artery 3.7±1.3 days after an MI (73% STEMI; 27% NSTEMI) in 48 patients and then repeated SPECT imaging 11 weeks later to assess for reversibility after revascularization.10 The sensitivity and specificity of an FFR value ≤0.75 to accurately identify reversible ischemia on SPECT were 88% and 50%, respectively.10 The findings of these studies provided information regarding FFR post-ACS, but they may not be generalizable to our study, given that FFR measurements in the ACS group in our study were obtained during the acute phase of a coronary event.
To examine the reproducibility of an FFR value measured acutely to one measured after recovery from ACS, a prospective study measured FFR in 112 nonculprit lesions in AMI patients (≈75% STEMI) at the time of clinical presentation and 35±4 days later.11 Only 2 patients with an FFR value >0.80 at the time of their MI had an FFR value <0.75 at follow-up.11 Given that microvascular dysfunction may persist for an unknown interval and possibly up to 6 months after an MI,7 the follow-up time between FFR measurements in that study may not have been sufficient for full recovery and restoration of microcirculatory function.
Observational studies and post-hoc analyses of randomized trials have suggested that FFR-guided revascularization is safe in ACS.12–16 In FAME, 33% of patients (n=328) were studied in the setting of ACS (unstable angina or NSTEMI), where 178 and 150 patients were randomized to angiographic- or FFR-guided PCI, respectively.12 The benefit of FFR-guided PCI, compared with angiography alone, appeared similar for the ACS and stable CAD (n=677) groups.12 Despite a higher rate of adverse cardiac events in ACS patients, compared to stable CAD patients, none of the MIs during follow-up reportedly occurred in the index lesion deferred PCI based on FFR.4,12 Nevertheless, it is notable that the number of ACS patients in FAME undergoing FFR-guided revascularization was relatively small with shorter follow-up, which, as acknowledged by the investigators, may have limited the power of the study to show differences among subgroups.
Several additional smaller observational studies have assessed the clinical outcomes for lesions that were deferred revascularization in the setting of ACS.13–16 Three of these studies, including a total of less than 250 ACS patients, concluded that there were similar outcomes for patients deferred PCI based on FFR in the setting of ACS or non-ACS.14,15 A more recent study of 162 patients deferred PCI based on an FFR ≥0.75 in the setting of ACS (60%) and non-ACS (40%) demonstrated that patients with an FFR value of 0.75 to 0.85 and microvascular dysfunction (measured by an abnormal corrected thrombolysis in myocardial infarction frame count [CTFC]) had a significantly higher rate of adverse cardiac events, compared with patients with an FFR value >0.85 and normal CTFC.16 Similar to our study, lower FFR values in their analysis were an independent predictor of later adverse cardiac events (HR, 1.09; 95% CI, 1.01 to 1.19; P=0.03).16
Previous studies assessing the clinical safety and reliability of FFR for deferring revascularization among patients with ACS were limited by small sample sizes13–16 and by lesion assessment by FFR beyond the acute phase of ACS.12 Our study represents one of the largest studies on the clinical outcomes of lesions deferred PCI based on FFR, where half of the study population underwent assessment during ACS. Compared with previous studies,12–16 our study has a significantly higher rate of adverse cardiac events, which may be secondary to the nonselected, real-world study population and the longer follow-up (mean 4.5±2.1 years). Our study population also included patients with multivessel disease (64%) and left main lesions (7%), many of which were excluded from other studies.12,13 The recent prospective randomized Fractional flow reserve versus Angiography in guiding Management to Optimize oUtcomeS in NSTEMI (FAMOUS-NSTEMI) trial demonstrated that routine FFR measurement is feasible in NSTEMI patients, but was not powered to assess for differences in clinical outcomes compared to angiographic-guided care.6 Further prospective evidence is needed to fully elucidate the reliability, reproducibility, and safety of FFR measurements in ACS.
Study Limitations
This is a retrospective, observational study conducted at a large, single urban tertiary referral center. The possibility of reporting bias and the absence of an independent clinical events committee are potential limitations. A small number of patients (n=21) were excluded from the study population because of loss to follow-up. The results may not be generalizable to different populations and hospitals of different sizes and/or level of acuity. In the ACS group, culprit versus nonculprit lesions were distinguished based on the operator’s judgment and this determination was therefore subjective, similar to common clinical practice. However, FFR was not performed on any lesion with less than TIMI 3 flow. Moreover, there may be unmeasured confounders or selection bias that contributed to differences in clinical outcomes between the ACS and non-ACS groups. Although FFR has been well validated as a measure of ischemia, it may have limited relevance for predicting the stability of a plaque. The study cannot determine the safety of deferring lesions based on FFR versus angiographic-guidance in the setting of ACS. Although our study demonstrated an association between lower FFR values and worse clinical outcomes in ACS patients, the clinical benefits of FFR-guided PCI over angiography alone, as demonstrated in FAME,12 may still persist in an ACS population.
Conclusions
For patients with intermediate severity coronary lesions that are deferred revascularization in the setting of ACS based on FFR >0.80, lower FFR values are associated with a significantly higher rate of adverse cardiac events. The association between lower FFR values and adverse clinical outcomes was not observed among deferred lesions in non-ACS patients. Further study is needed to determine the reliability and safety of deferring lesions with relatively lower FFR values in the setting of ACS based on the currently accepted FFR threshold validated in predominantly non-ACS patients.
Disclosures
Dr Zajarias is a steering committee member of the Partner 2 trial. Dr Lasala is on a Boston Scientific Advisory Board. Dr Singh is a consultant for Abbott Vascular, Boston Scientific, and Volcano Corp and is on the speakers’ bureaus of, or receives honoraria from, The Medicines Company, Medtronic Vascular, Volcano Corp, and St. Jude Medical Corp. Dr Bach receives research grants from Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, and Schering-Plough and is a consultant (clinical event committee activity only) for Eli Lilly, Novo Nordisk, and Pfizer.
References
- Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE Investigators C. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahn JM, Kang SJ. Paradigm shift to functional angioplasty: new insights for fractional flow reserve- and intravascular ultrasound-guided percutaneous coronary intervention. Circulation. 2011;124:951–957. doi: 10.1161/CIRCULATIONAHA.110.012344. [DOI] [PubMed] [Google Scholar]
- Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bär F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van’t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B Investigators FS. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (fractional flow reserve versus angiography for multivessel evaluation) study. J Am Coll Cardiol. 2010;56:177–184. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrøm T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF Investigators FT. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- Layland J, Oldroyd KG, Curzen N, Sood A, Balachandran K, Das R, Junejo S, Ahmed N, Lee M, Shaukat A, O’Donnell A, Nam J, Briggs A, Henderson R, McConnachie A, Berry C. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J. 2015;36:100–111. doi: 10.1093/eurheartj/ehu338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction. N Engl J Med. 1994;331:222–227. doi: 10.1056/NEJM199407283310402. [DOI] [PubMed] [Google Scholar]
- Claeys MJ, Bosmans JM, Hendrix J, Vrints CJ. Reliability of fractional flow reserve measurements in patients with associated microvascular dysfunction: importance of flow on translesional pressure gradient. Catheter Cardiovasc Interv. 2001;54:427–434. doi: 10.1002/ccd.2005. [DOI] [PubMed] [Google Scholar]
- De Bruyne B, Pijls NH, Bartunek J, Kulecki K, Bech JW, De Winter H, Van Crombrugge P, Heyndrickx GR, Wijns W. Fractional flow reserve in patients with prior myocardial infarction. Circulation. 2001;104:157–162. doi: 10.1161/01.cir.104.2.157. [DOI] [PubMed] [Google Scholar]
- Samady H, Lepper W, Powers ER, Wei K, Ragosta M, Bishop GG, Sarembock IJ, Gimple L, Watson DD, Beller GA, Barringhaus KG. Fractional flow reserve of infarct-related arteries identifies reversible defects on noninvasive myocardial perfusion imaging early after myocardial infarction. J Am Coll Cardiol. 2006;47:2187–2193. doi: 10.1016/j.jacc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, Barbato E, Hamilos M, Mangiacapra F, Heyndrickx GR, Wijns W, Pijls NH, De Bruyne B. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274–1281. doi: 10.1016/j.jcin.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Sels JW, Tonino PA, Siebert U, Fearon WF, Van’t Veer M, De Bruyne B, Pijls NH. Fractional flow reserve in unstable angina and non-st-segment elevation myocardial infarction experience from the FAME (fractional flow reserve versus angiography for multivessel evaluation) study. JACC Cardiovasc Interv. 2011;4:1183–1189. doi: 10.1016/j.jcin.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Leesar MA, Abdul-Baki T, Akkus NI, Sharma A, Kannan T, Bolli R. Use of fractional flow reserve versus stress perfusion scintigraphy after unstable angina. Effect on duration of hospitalization, cost, procedural characteristics, and clinical outcome. J Am Coll Cardiol. 2003;41:1115–1121. doi: 10.1016/s0735-1097(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Potvin JM, Rodés-Cabau J, Bertrand OF, Gleeton O, Nguyen CN, Barbeau G, Proulx G, De Larochelliére R, Dery JP, Batalla N, Dana A, Facta A, Roy L. Usefulness of fractional flow reserve measurements to defer revascularization in patients with stable or unstable angina pectoris, non-ST -elevation and ST-elevation acute myocardial infarction, or atypical chest pain. Am J Cardiol. 2006;98:289–297. doi: 10.1016/j.amjcard.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Fischer JJ, Wang XQ, Samady H, Sarembock IJ, Powers ER, Gimple LW, Ragosta M. Outcome of patients with acute coronary syndromes and moderate coronary lesions undergoing deferral of revascularization based on fractional flow reserve assessment. Catheter Cardiovasc Interv. 2006;68:544–548. doi: 10.1002/ccd.20748. [DOI] [PubMed] [Google Scholar]
- Esen AM, Acar G, Esen O, Emiroglu Y, Akcakoyun M, Pala S, Karapinar H, Kargin R, Barutcu I, Turkmen M. The prognostic value of combined fractional flow reserve and TIMI frame count measurements in patients with stable angina pectoris and acute coronary syndrome. J Interv Cardiol. 2010;23:421–428. doi: 10.1111/j.1540-8183.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- Lotfi A, Jeremias A, Fearon WF, Feldman MD, Mehran R, Messenger JC, Grines CL, Dean LS, Kern MJ, Klein LW. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography: a consensus statement of the Society of Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2014;83:509–518. doi: 10.1002/ccd.25222. [DOI] [PubMed] [Google Scholar]
- Depta JP, Patel JS, Novak E, Masrani SK, Raymer D, Facey G, Patel Y, Zajarias A, Lasala JM, Singh J, Bach RG, Kurz HI. Outcomes of coronary stenoses deferred revascularization for borderline versus nonborderline fractional flow reserve values. Am J Cardiol. 2014;113:1788–1793. doi: 10.1016/j.amjcard.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research C. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, editors. Survival Analysis: State of the Art. Dordrecht, Netherlands: Kluwer Academic Publishers; 1992. pp. 237–247. [Google Scholar]
- Califf RM, Phillips HR, III, Hindman MC, Mark DB, Lee KL, Behar VS, Johnson RA, Pryor DB, Rosati RA, Wagner GS, Harrell FE. Prognostic value of a coronary artery Jeopardy score. J Am Coll Cardiol. 1985;5:1055–1063. doi: 10.1016/s0735-1097(85)80005-x. [DOI] [PubMed] [Google Scholar]
- Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd ed. Hoboken, N.J: John Wiley & Sons; 2003. [Google Scholar]
- Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS Group CTR. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- Carrick D, Behan M, Foo F, Christie J, Hillis WS, Norrie J, Oldroyd KG, Berry C. Usefulness of fractional flow reserve to improve diagnostic efficiency in patients with non-ST elevation myocardial infarction. Am J Cardiol. 2013;111:45–50. doi: 10.1016/j.amjcard.2012.08.046. [DOI] [PubMed] [Google Scholar]
- Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- Marzilli M, Sambuceti G, Fedele S, L’Abbate A. Coronary microcirculatory vasoconstriction during ischemia in patients with unstable angina. J Am Coll Cardiol. 2000;35:327–334. doi: 10.1016/s0735-1097(99)00554-9. [DOI] [PubMed] [Google Scholar]
- Cuculi F, DeMaria GL, Meier P, Dall’Armellina E, de Caterina AR, Channon KM, Prendergast BD, Choudhury RC, Forfar JC, Kharbanda RK, Banning AP. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1894–1904. doi: 10.1016/j.jacc.2014.07.987. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Tόth GG, Lai D, Zhu H, Açar G, Agostini P, Appelman Y, Arslan F, Barbato E, Chen SL, Di Serafino L, Dόminguez-Franco AJ, DuPouy P, Esen AM, Esen OB, Hamilos M, Iwasaki K, Jensen LO, Jiménez-Navarro MF, Katritsis DG, Kocaman SA, Koo BK, Lόpez-Palop R, Lorin JD, Miller LH, Muller O, Nam CW, Oud N, Puymirat E, Rieber J, Rioufol G, Rodés-Cabau J, Sedlis SP, Takeishi Y, Tonino PA, Van Belle E, Verna E, Werner GS, Fearon WF, Pijls NH, De Bruyne B, Gould KL. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1651–1654. doi: 10.1016/j.jacc.2014.07.973. [DOI] [PubMed] [Google Scholar]


