Abstract
Background
Hypothyroidism is associated with an increased risk of coronary artery disease, beyond that which can be explained by its association with conventional cardiovascular risk factors. Coronary endothelial dysfunction precedes atherosclerosis, has been linked to adverse cardiovascular events, and may account for some of the increased risk in patients with hypothyroidism. The aim of this study was to determine whether there is an association between epicardial and microvascular coronary endothelial dysfunction and hypothyroidism.
Methods and Results
In 1388 patients (mean age 50.5 [12.3] years, 34% male) presenting with stable chest pain to Mayo Clinic, Rochester, MN for diagnostic coronary angiography, and who were found to have nonobstructive coronary artery disease (<40% stenosis), we invasively assessed coronary artery endothelial-dependent microvascular and epicardial function by evaluating changes in coronary blood flow (% Δ CBF Ach) and diameter (% Δ CAD Ach), respectively, in response to intracoronary infusions of acetylcholine. Patients were divided into 2 groups: hypothyroidism, defined as a documented history of hypothyroidism or a thyroid-stimulating hormone (TSH) >10.0 mU/mL, n=188, and euthyroidism, defined as an absence of a history of hypothyroidism in the clinical record and/or 0.3<TSH≤10.0 mU/mL, n=1200. Subjects with a history of hypothyroidism had a significantly lower % Δ CBF Ach (48.26 [80.66] versus 64.58 [128.30]) compared to patients with euthyroidism, while the % Δ CAD Ach did not vary significantly between groups. After adjusting for covariates, females with hypothyroidism still had a significantly lower % Δ CBF Ach (estimated difference in % Δ CBF Ach [SE]: −16.79 [8.18]).
Conclusions
Hypothyroidism in women is associated with microvascular endothelial dysfunction, even after adjusting for confounders, and may explain some of the increased risk of cardiovascular disease in these patients.
Keywords: atherosclerosis, cardiovascular, coronary artery disease, endothelial dysfunction, hypothyroidism, nonobstructive coronary artery disease
Overt hypothyroidism is associated with accelerated atherosclerosis and an increased risk of coronary artery disease (CAD).1–3 While some of these effects can be explained by a higher prevalence of hypertension and dyslipidemia in patients with hypothyroidism,4–7 not all individuals with hypothyroidism have abnormal blood pressure or lipid profiles.8 In addition, studies have shown an increased risk of all-cause mortality9 and cardiovascular events10 in patients with hypothyroidism, even after adjusting for the presence of conventional cardiovascular risk factors, implicating other factors in their elevated risk.
Endothelial dysfunction is characterized by an imbalance between vasodilator and vasoconstrictor activity in response to endothelial-dependent vasodilating agents such as acetylcholine.11–14 Coronary endothelial dysfunction may affect microvascular or epicardial vessels: Microvascular endothelial dysfunction can be identified as an attenuated increase or a decrease in coronary blood flow (CBF)15–19 while epicardial endothelial dysfunction can be identified as coronary artery vasoconstriction13,18,20,21 in response to intracoronary infusions of acetylcholine. Endothelial dysfunction has been shown to represent an early stage of atherosclerosis22,23 and is associated with coronary plaque progression24 and the presence of necrotic plaque,25 which is particularly vulnerable to rupture. Endothelial dysfunction is also independently associated with an increased risk of adverse cardiovascular events.26,27 Moreover, endothelial dysfunction has been associated with local macrophages and microchannels in early atherosclerosis28 as well as elevated local coronary29 and systemic30 levels of lipoprotein-associated phospholipase A2, features consistent with inflammation. As hypothyroidism is also believed to arise from inflammatory processes,31 concomitant coronary endothelial dysfunction could account for some of the increased risk observed in hypothyroid patients.
Previous studies that evaluated the prevalence of endothelial dysfunction in patients with hypothyroidism have been limited by a small sample size32–38 and the use of noninvasive techniques to assess endothelial dysfunction in the brachial artery32,33,35–40 or in the left anterior descending coronary artery,34 which are less direct than invasive methods. The aim of this study was to determine whether microvascular and epicardial endothelial dysfunction identified invasively are associated with hypothyroidism in a large cohort of patients presenting with nonobstructive CAD.
Methods
The following protocol was approved by the Mayo Clinic Institutional Review Board, and all patients provided written informed consent. Patients presenting with chest pain were referred by their physicians for diagnostic coronary angiography. Invasive endothelial functional testing was then undertaken in all patients with the exception of those with obstructive CAD (>40% diameter stenosis of any coronary artery determined at angiography); uncontrolled hypertension; left ventricular ejection fraction <50% or left ventricular hypertrophy determined on echocardiography or acute coronary syndrome.21,41
Assessment of Coronary Endothelial Function
Consecutive patients presented to the cardiac catheterization laboratory in the fasting state and all cardiovascular medications, including nitrates and calcium channel blockers, had been discontinued for at least 48 hours. Routine diagnostic coronary angiography was performed on all patients using standard clinical protocols. Angiograms were reviewed prior to the infusion of any pharmacological agent. In cases where the severity of stenosis was uncertain, online quantitative coronary angiography was used. Patients then underwent evaluation of coronary endothelial-dependent epicardial and microvascular function as previously described.18,20,21,41 In brief, following intravenous administration of 5000 to 7000 U of heparin, a Doppler guidewire (FloWire, Volcano Inc) 0.014 inches in diameter within a 3-F Slip-Cath Infusion Catheter (Cook Medical) was positioned into the midportion of the left anterior descending coronary artery, 2 to 3 mm distal to the tip of the infusion catheter. Acetylcholine was infused into the left anterior descending coronary artery at concentrations of 10−6, 10−5, and 10−4 mol/L (to achieve estimated coronary bed concentrations of 10−8, 10−7, and 10−6 mol/L, respectively) for 3 minutes at each concentration to assess coronary endothelial function.18,20,21,41 Infusions were performed using a Harvard pump to maintain infusion rates of <1% of the estimated CBF. Doppler measurements of mean peak velocity were performed after each infusion followed by repeat coronary angiography. Coronary artery diameter was measured at baseline and after the infusion with acetylcholine by an independent investigator blinded to Doppler velocity data using a previously described computer-based image analysis system.42,43 The percentage change in coronary artery diameter in response to intracoronary acetylcholine compared to baseline (% Δ CAD Ach) was then calculated as an index of epicardial endothelial function. CBF was also calculated using the following, as previously described18,20: CBF=π (mean peak velocity/2)(coronary artery diameter/2)2. The average maximal percentage increase in CBF in response to acetylcholine compared to CBF at baseline (% Δ CBF Ach) was calculated as an index of microvascular endothelial function. For quality control, all measurements were performed in the segment 5 mm distal to the tip of the Doppler guidewire and following each infusion, the diameter was measured in the same segment of the vessel.18,20,21,41
Hypothyroidism Status
Patients who had undergone endothelial functional assessment were retrospectively divided into 2 groups: hypothyroidism, defined as a history of hypothyroidism documented in the patient’s medical record or a thyroid-stimulating hormone (TSH) >10.0 mU/mL within 1 month of the patient’s endothelial functional assessment, and euthyroidism, defined as 0.3<TSH≤10.0 mU/mL within 1 month of the index procedure and/or no documented history of hypothyroidism in the medical record. Patients with a TSH <0.3 mU/mL or a documented history of hyperthyroidism were excluded from all analyses. All medical records were reviewed by a single investigator (J.S.) blinded to endothelial function data. After initial screening of medical records, patients categorized as hypothyroid had their clinical record reviewed again by a Consultant Endocrinologist (H.G.) to confirm the history of hypothyroidism, and in cases of discrepancy, the Consultant’s decision was accepted.
Patients in the hypothyroidism group had their medical record evaluated further to determine whether they were prescribed thyroid replacement therapy (TRT) at the time of the procedure. As part of a subanalysis, patients from the hypothyroidism group who took TRT and had a TSH level documented within 1 month of the index procedure were divided according to whether they had adequate replacement on TRT, defined as 0.3 mU/mL<TSH≤10.0 mU/mL versus inadequate replacement on TRT, defined as TSH >10.0 mU/mL.44
In a separate final analysis, only patients with a TSH documented within 1 month of the index procedure were included, and patients were divided according to their TSH value only, regardless of past medical history, as follows: euthyroidism, 0.3 mU/mL<TSH≤4.5 mU/mL; subclinical hypothyroidism (SCH), 4.5 mU/mL<TSH≤10.0 mU/mL; and hypothyroidism, TSH >10.0 mU/mL.44
Other Patient Information
Data were collected on conventional cardiovascular risk factors including age, sex, hypertension, diabetes mellitus, hyperlipidemia, smoking, and body mass index (BMI). Hypertension was defined as a history of hypertension treated with antihypertensives; diabetes was defined as a history of diabetes treated with medication or insulin; and hyperlipidemia was defined as a history of total cholesterol levels of >240 mg/dL or treatment with lipid-lowering therapy. Information was also collected on a previous history of myocardial infarction (defined as a history of previous myocardial infarction documented in the patients’ clinical record or on patient self-report); other vasospasm disorders (defined as a history of Raynaud’s phenomenon or migraine headaches documented in the patients’ clinical record or on self-report); and other vascular diseases (defined as a history of peripheral vascular disease, stroke, or transient ischemic attack documented in the patient’s clinical record or on self -report). Blood samples for routine clinical laboratory tests (complete blood count, glucose, creatinine, and lipid profile) were drawn in all patients in the fasting state 48 hours before the procedure.
Statistical Analysis
Continuous variables are presented as a mean (SD) where data are normally distributed and as a median (quartile 1, quartile 3) for skewed data. Categorical variables are presented as frequencies (percentages). Differences between groups were analyzed using Student t test, Wilcoxon rank sign test, or Kruskal–Wallis test as appropriate for continuous variables. Correlation was assessed using Spearman’s correlation coefficient. Linear regression models were analyzed to estimate the partial association between hypothyroidism and adequate replacement on TRT with % Δ CAD Ach and % Δ CBF Ach. Risk factors from Table 1 known to be associated with hypothyroidism and coronary endothelial dysfunction were included, and no risk factor considered for the model had >10% missing data. P-values of <0.05 were accepted as significant. All statistical analyses were performed using JMP 9 software (SAS Institute, Inc, Cary, NC).
Table 1.
Summary of Baseline Characteristics for Patients With Hypothyroidism and Euthyroidism
| Clinical Variable | Hypothyroid, N=188 | Euthyroid, N=1200 | P Value |
|---|---|---|---|
| Age, y (SD) | 55.73 (11.25) | 49.64 (12.29) | <0.001* |
| Female, n (%) | 166 (88.30) | 742 (61.94) | <0.001* |
| BMI, kg/m2 (SD) | 30.15 (6.17) | 28.74 (6.17) | 0.004* |
| Hypertension, n (%) | 89 (47.59) | 513 (42.82) | 0.42 |
| Diabetes mellitus, n (%) | 28 (14.89) | 98 (8.16) | 0.007* |
| Hyperlipidemia, n (%) | 116 (61.70) | 646 (53.88) | 0.13 |
| History of MI, n (%) | 32 (17.02) | 177 (14.76) | 0.69 |
| History of vascular disease, n (%) | 17 (9.04) | 87 (7.25) | 0.01* |
| Smoking status, n (%) | 0.13 | ||
| Never smoked | 104 (55.32) | 605 (50.37) | |
| Former smoker | 70 (37.23) | 433 (36.05) | |
| Current smoker | 13 (6.91) | 152 (12.66) | |
| Total cholesterol, mg/dL (SD) | 195.24 (52.03) | 185.66 (42.27) | 0.02* |
| HDL-C, mg/dL (SD) | 57.55 (18.56) | 53.11 (17.23) | 0.003* |
| LDL-C, mg/dL (SD) | 107.91 (42.06) | 105.99 (36.27) | 0.57 |
| Triglycerides, mg/dL (SD) | 142.44 (88.40) | 132.51 (92.07) | 0.17 |
| Creatinine, mg/dL (SD) | 0.99 (0.44) | 0.99 (0.68) | 0.99 |
| Glucose, mg/dL (SD) | 101.04 (24.51) | 99.76 (25.29) | 0.52 |
| Aspirin, n (%) | 95 (50.53) | 578 (48.17) | 0.55 |
| ACE inhibitor, n (%) | 29 (15.43) | 171 (14.25) | 0.67 |
| β-Blocker, n (%) | 63 (33.51) | 335 (27.92) | 0.11 |
| Calcium channel blocker, n (%) | 58 (31.02) | 418 (34.83) | 0.50 |
| Lipid-lowering therapy, n (%) | 83 (44.15) | 422 (35.17) | 0.02* |
| Nitrates, n (%) | 65 (34.57) | 431 (35.92) | 0.72 |
| Thyroid replacement therapy, n (%) | 184 (97.87) | 0 (0.00) | <0.001* |
| Estrogen replacement therapy (in females only), n (%) | 54 (32.53) | 164 (22.10) | <0.001* |
| Corticosteroids, n (%) | 21 (11.17) | 81 (6.75) | 0.09 |
ACE indicates angiotensin-converting enzyme; BMI, body mass index; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction.
Corresponds to P<0.05.
Results
Between January 1, 1993 and December 31, 2012, a total of 1591 endothelial functional assessment procedures were performed on 1552 consecutive unique patients. Of these, 1498 granted use of their records for research purposes in keeping with Minnesota statute. For each patient, only the first study date available was examined. Of these, 1439 had measurements available for both % Δ CBF Ach and % Δ CAD Ach. Thirty-six patients were excluded due to insufficient documentation in their medical record, and 15 were excluded due to a past medical history of hyperthyroidism or a TSH <0.3 mU/mL within 1 month of the index procedure, leaving 1388 patients in this study (Figure 1).
Figure 1.
Flow diagram demonstrating patient enrollment and study protocol. TRT indicates thyroid replacement therapy; TSH, thyroid-stimulating hormone.
Baseline Characteristics
One hundred eighty-eight (13.5%) patients had a history of hypothyroidism or a TSH >10.0 mU/mL, and 1200 were euthyroid. Table 1 summarizes their baseline characteristics. The hypothyroid group was significantly older; had a higher proportion of females; a higher BMI; a higher proportion of diabetics; and a higher frequency of a history of vascular disease compared with the euthyroid group. Other risk factors were not significantly different between groups. Patients with hypothyroidism also had a significantly higher total cholesterol and higher high-density lipoprotein-cholesterol (HDL) compared to the euthyroid group, while other blood laboratory results did not vary significantly between groups. A greater proportion of the hypothyroid group took lipid-lowering therapy and among females, a higher proportion of the hypothyroid group took estrogen replacement therapy compared to the euthyroid group. One hundred eighty-four patients (98%) of the hypothyroid group took TRT.
Clinical History of Hypothyroidism and Coronary Endothelial Function
Among all patients, the hypothyroid group had a significantly lower % Δ CBF Ach (mean [SD] %: 48.26 [80.66] versus 64.58 [128.30], P<0.05) compared to the euthyroid group (Figure 2A) while the % Δ CAD Ach did not vary significantly between groups (mean [SD] %: −11.91 [19.92] versus −13.94 [22.46], P=0.20) (Figure 2B). However, in a multivariate analysis adjusting for diabetes, hypertension, age, BMI, and total and HDL cholesterol, hypothyroidism was not significantly associated with the % Δ CBF Ach or % Δ CAD Ach in all patients (Figure 3A and 3B).
Figure 2.
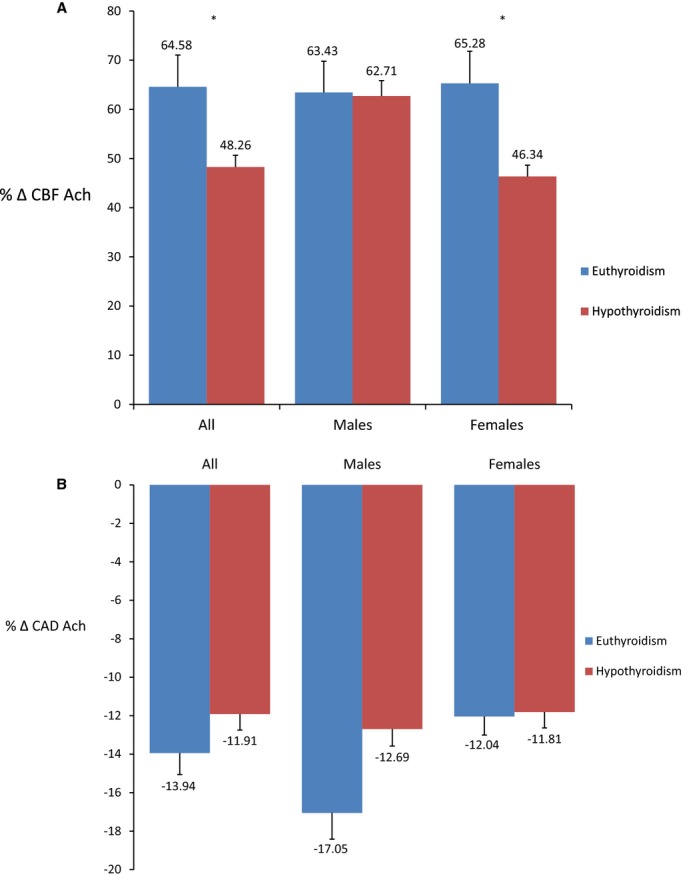
A, Average maximum percentage change in coronary blood flow in response to acetylcholine between hypothyroid and euthyroid patients. *Signifies P<0.05; T-bars represent standard errors. The percentage change in coronary blood flow in response to acetylcholine, as a metric of microvascular endothelial function, was significantly lower in patients with hypothyroidism compared to euthyroidism among all patients and females. B, Average maximum percentage change in coronary artery diameter in response to acetylcholine between hypothyroid and euthyroid patients. *Signifies P<0.05; T-bars represent standard errors. The percentage change in coronary artery diameter in response to acetylcholine, as a metric of epicardial endothelial function, was similar between patients with hypothyroidism and euthyroidism among all patients and after stratifying by sex. % Δ CAD Ach indicates percentage change in coronary artery diameter in response to acetylcholine; % Δ CBF Ach, percentage change in coronary blood flow in response to acetylcholine.
Figure 3.
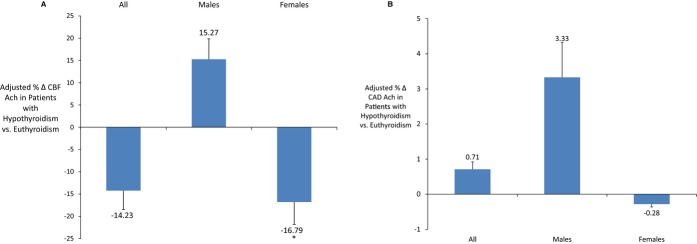
A, Multivariable adjusted average maximum-percentage change in coronary blood flow in response to acetylcholine in patients with hypothyroidism compared to euthyroidism. *Signifies P<0.05; T-bars represent standard errors. In males, estimates were adjusted for age, hypertension, diabetes mellitus, body mass index, and total and high-density lipoprotein-cholesterol. In females, estimates were adjusted for age, hypertension, diabetes mellitus, body mass index, and total and high-density lipoprotein-cholesterol and the use of estrogen replacement therapy. Females with hypothyroidism had a significantly lower percentage change in coronary blood flow in response to acetylcholine, a metric of microvascular endothelial function, compared to patients with euthyroidism even after adjusting for potential confounders. B, Multivariable adjusted average maximum-percentage change in coronary artery diameter in response to acetylcholine in patients with hypothyroidism compared to euthyroidism. T-bars represent standard errors. In males, estimates were adjusted for age, hypertension, diabetes mellitus, body mass index, and total and high-density lipoprotein-cholesterol. In females, estimates were adjusted for age, hypertension, diabetes mellitus, body mass index, and total and high-density lipoprotein-cholesterol and the use of estrogen replacement therapy. Patients with hypothyroidism had a similar percentage change in coronary artery diameter in response to acetylcholine, a metric of epicardial endothelial function, compared to patients with euthyroidism, even after stratifying by sex and adjusting for potential confounders. % Δ CAD Ach indicates percentage change in coronary artery diameter in response to acetylcholine; % Δ CBF Ach, percentage change in coronary blood flow in response to acetylcholine.
Among males, there was no significant difference in the % Δ CBF Ach or % Δ CAD Ach in the univariate analysis (Figure 2A and 2B) or after adjusting for potential confounders (Figure 3A and 3B). However, among females, the hypothyroid group had a significantly lower % Δ CBF Ach (mean [SD] %: 46.34 [79.85] versus 65.28 [101.89], P<0.05) compared to the euthyroid group (Figure 2A), but the % Δ CAD Ach did not vary significantly between groups (Figure 2B). When adjusting for diabetes, hypertension, age, BMI, total and HDL cholesterol, and the use of estrogen replacement therapy, hypothyroidism remained significantly associated with % Δ CBF Ach (estimate [SE] %: −16.79 [8.18], P<0.05) (Figure 3A) but not % Δ CAD Ach (Figure 3B), implying that females with hypothyroidism had worse microvascular endothelial function compared to females with euthyroidism, even after adjusting for covariates.
Adequate Replacement on TRT and Coronary Endothelial Function
Of the 188 patients with a history of hypothyroidism or TSH >10 mU/L, 184 were prescribed TRT at the time of the index procedure. Twenty-five of these patients did not have a TSH level documented within 1 month of the index procedure and were excluded, leaving 159 patients for this subanalysis (Figure 1). One hundred forty-nine patients (94%) with hypothyroidism had adequate replacement on TRT, defined as 0.3<TSH≤10.0 mU/mL, and 10 had inadequate replacement (TSH >10.0 mU/mL).44 There were no significant differences in the % Δ CAD Ach and % Δ CBF Ach between groups (Table 2). In a multivariate analysis adjusting for age, sex, diabetes, hypertension, BMI, and total and HDL cholesterol, adequate replacement on TRT was not significantly associated with the % Δ CAD Ach or % Δ CBF Ach (Table 3).
Table 2.
Average Maximum % Δ CAD Ach and % Δ CBF Ach in Hypothyroid Patients Taking Thyroid Replacement Therapy With Adequate Versus Inadequate Replacement
| All Subjects | Adequate Replacement N=149 | Inadequate Replacement N=10 | P Value |
|---|---|---|---|
| % Δ CAD Ach | −8.58 (−22.47, 0.24) | −9.76 (−20.71, 1.58) | 0.88 |
| % Δ CBF Ach | 29.61 (−11.74, 106.92) | −7.27 (−24.56, 178.59) | 0.60 |
% Δ CAD Ach indicates percentage change in coronary artery diameter in response to acetylcholine; % Δ CBF Ach, percentage change in coronary blood flow in response to acetylcholine.
Table 3.
Multivariate Analysis of the Association Between Adequate Replacement on TRT and Average Maximum % Δ CAD Ach and % Δ CBF in Subjects With Hypothyroidism
| All Subjects* | % Δ CAD Ach | P Value | % Δ CBF Ach | P Value |
|---|---|---|---|---|
| Inadequate vs adequate replacement on TRT (SD) | −1.02 (3.48) | 0.77 | −6.31 (15.72) | 0.69 |
% Δ CAD Ach indicates percentage change in coronary artery diameter in response to acetylcholine; % Δ CBF Ach, percentage change in coronary blood flow in response to acetylcholine; TRT, thyroid replacement therapy.
Adjusted for age, sex, body mass index, hypertension, diabetes mellitus, and total and high-density lipoprotein-cholesterol.
Thyroid-stimulating hormone as a Biomarker of Coronary Endothelial Function
Among the 1439 patients with measurements available for both % Δ CBF Ach and % Δ CAD Ach, 1175 had TSH levels documented within 1 month of the index procedure, of which 27 were excluded due to a TSH <0.3 mU/mL, leaving 1148 patients for this analysis (Figure 1). TSH did not correlate significantly with either the % Δ CAD Ach (r=0.028, 95% CI −0.030 to 0.086) or the % Δ CBF Ach (r=0.011 95% CI −0.047 to 0.069). Patients were divided into 3 groups according to their TSH level: euthyroid, 0.3<TSH≤4.5 mU/mL, n=1050; SCH, 4.5<TSH≤10.0 mU/mL, n=86 and hypothyroid, TSH >10 mU/mL, n=12. There were no significant differences in the % Δ CBF Ach or the % Δ CAD Ach (Table 4). After adjusting for age, sex, diabetes, hypertension, BMI, and total and HDL cholesterol, neither hypothyroidism nor SCH were significantly associated with % Δ CBF Ach or % Δ CAD Ach (data not shown).
Table 4.
Average Maximum % Δ CAD Ach and Average Maximum % Δ CBF Ach Between Subjects Divided According to Serum TSH Levels
| TSH 0.3 to 4.5 mU/mL N=1050 | TSH 4.5 to 10 mU/mL N=86 | TSH >10 mU/mL N=12 | P Value | |
|---|---|---|---|---|
| TSH, mU/mL | 1.80 (1.20, 2.70) | 5.75 (5.00, 6.80) | 17.05 (12.75, 24.63) | <0.001* |
| % Δ CAD Ach | −10.58 (−25.42, 0.00) | −7.70 (−22.19, 00) | −6.15 (−16.16, 1.67) | 0.26 |
| % Δ CBF Ach | 40.28 (−7.45, 100.41) | 68.52 (6.28, 121.98) | 22.19 (−21.84, 189.20) | 0.16 |
Values in parenthesis are quartiles 1 and quartiles 3; median (Q1,Q3).
% Δ CAD Ach indicates percentage change in coronary artery diameter in response to acetylcholine; % Δ CBF Ach, percentage change in coronary blood flow in response to acetylcholine; TSH, thyroid-stimulating hormone.
signifies P<0.05
Discussion
Hypothyroidism Is Associated With Endothelial Dysfunction in Women
In the current study, we show that while the % Δ CAD Ach (an index of epicardial endothelial function) was not significantly different between patients with and without hypothyroidism, the % Δ CBF Ach (an index for microvascular endothelial function) was lower in patients with hypothyroidism, and remained lower in females after stratifying by sex. In fact, the % Δ CBF Ach in all patients and among females was <50%, a previously used threshold to identify microvascular endothelial dysfunction.21,41,45 While some of this may be explained by the concurrent association between hypothyroidism and endothelial dysfunction with conventional cardiovascular risk factors, females with hypothyroidism still had an impaired % Δ CBF ACh after adjusting for these variables. Thus, the current study suggests an independent link between hypothyroidism and the regulation of endothelial function.
By showing that hypothyroidism is associated with coronary endothelial dysfunction, our findings are consistent with the results of previous studies (Table 5) in which microvascular endothelial function was evaluated in the brachial artery using noninvasive measurements of forearm blood flow.36,38 However, the current study did not show a significant difference in epicardial (macrovascular) endothelial function between patients with hypothyroidism and euthyroidism. While some studies evaluating macrovascular endothelial function in the brachial artery showed that patients with hypothyroidism had lower flow-mediated dilatation compared to those with euthyroidism,32,33,37 this remains controversial as other studies have failed to show a difference between groups.35 The current study extends the observations of these previous studies into the coronary circulation and sheds new light onto the potential association between hypothyroidism and endothelial function by using invasive techniques to directly quantify % Δ CBF Ach and % Δ CAD Ach in, to our knowledge, the largest published cohort of hypothyroid patients evaluated for endothelial dysfunction. Moreover, dissociation between epicardial and microvascular endothelial function has been previously identified.46 For example, smokers without evidence of significant CAD have abnormal epicardial endothelial function and preserved microvascular endothelial function.47 Thus, there may be a different susceptibility of the microvascular and epicardial circulation to the effects of risk factors. Evidently, hypothyroidism preferentially affects the microvascular system, although it might subsequently progress to impair epicardial function. As to why various risk factors and specifically hypothyroidism have a predilection for the microvascular system compared to the epicardial system needs to be clarified.
Table 5.
Summary of Previous Studies Evaluating the Relationship Between Hypothyroidism and Endothelial Dysfunction
| Study | Study Design | Sample Size | Investigating Technique | Conclusion |
|---|---|---|---|---|
| Lekakis et al32 | Cross-sectional | 35 (mean age51.0 y, 94% female) | FMD of brachial artery using arm cuff and USS | FMD is lower in patients with hypothyroidism |
| Papaioannou et al33 | Prospective | 8 (mean age 48.9 y, 38% female) | FMD of brachial artery using arm cuff and USS | TRT improves FMD in patients with hypothyroidism |
| Biondi et al34 | Cross-sectional | 35 (mean age 34.8 y, 100% female) | CFR of LAD using transthoracic echo | CFR is lower in patients with SCH |
| Cabral et al35 | Cross-sectional | 42 (mean age 42.4 y, 100% female) | FMD of brachial artery using arm cuff and USS | FMD is not lower in patients with hypothyroidism |
| Dagre et al36 | Cross-sectional | 96 (mean age 42.0 y, 100% female) | FMD of brachial artery using arm cuff and USS | FBF is lower in patients with hypothyroidism At least 3 mo of well-controlled hypothyroidism on TRT improves FBF |
| Shavdatuashvili37 | Cross-sectional | 70 (age unknown, 100% female) | FMD of brachial artery using arm cuff and USS | FMD is lower in patients with hypothyroidism |
| Erbil et al38 | Prospective | 44 (mean age 46.9 y, 91% female) | FMD of brachial artery using arm cuff and USS | FMD is lower in patients with hypothyroidism FMD improves after 6 mo of TRT in patients with hypothyroidism |
| Razvi et al39 | Prospective | 100 (mean age 53.8 y, 81% female) | FMD of brachial artery using arm cuff and USS | At least 12 wks of TRT improves FMD in patients with SCH |
| Taddei et al40 | Cross-sectional | 42 (demographics not stated) | FMD of brachial artery using acetylcholine infusion and USS | FBF is lower in patients with hypothyroidism At least 6 mo of TRT improves FBF in patients with hypothyroidism |
CFR indicates coronary flow reserve, FBF, forearm blood blow; FMD, forearm-mediated dilatation; LAD, Left Anterior Descending coronary artery; SCH, subclinical hypothyroidism; TRT, thyroid replacement therapy; USS, ultrasound scan.
After adjusting for confounders, we showed that hypothyroidism was associated with microvascular endothelial dysfunction in females. Indeed, women receiving treatment for hypothyroidism require higher doses of TRT in pregnancy48 and during exogenous estrogen therapy.49 Estrogen is believed to increase concentrations of thyroid-binding globulin, which binds thyroxine and in turn reduces levels of free thyroxine, limiting its effects on tissues. In contrast, androgens such as testosterone reduce the synthesis of thyroid-binding globulin, resulting in transiently increased levels of free thyroxine. Interestingly, androgen administration in hypothyroid women was shown to cause clinical manifestations of hyperthyroidism.50 Thus, baseline differences in hormone profile and the subsequent transient effects on free thyroxine levels may render females more vulnerable to the effects of thyroid status. This is further supported by the results of a community health survey in which serum TSH levels correlated with serum total cholesterol, low-density lipoprotein-cholesterol, and triglycerides levels more strongly in females compared with males.51
Clinical Profile of Hypothyroidism
Hypothyroidism is associated with accelerated atherosclerosis and an increased risk of CAD.1–3 A significant proportion of this can be explained by the independent association between hypothyroidism and conventional cardiovascular risk factors.4–7,52–54 Our findings support these associations, as hypothyroid patients in this study were significantly older, had a higher BMI, a higher prevalence of diabetes, and a higher total cholesterol level compared to the euthyroid group. Nevertheless, not all individuals with hypothyroidism have conventional risk factors,8 suggesting that other factors may be implicated in their increased risk. This is further supported by studies that have shown an increased risk of all-cause mortality9 and cardiovascular events10 in hypothyroid patients, even after adjusting for conventional risk factors. Coronary endothelial dysfunction may account for some of the increased risk in hypothyroid patients as it is an independent risk factor for atherosclerosis and cardiovascular events.26,27
The precise mechanisms by which hypothyroidism affects endothelial function are not entirely clear. Isoforms of the thyroid hormone receptor have been identified in human aortic vascular smooth muscle cells,55 suggesting that thyroid hormone may act directly on the vascular bed and influence vasomotion. Other studies have correlated hypothyroidism with the presence of anti–endothelial cell antibodies,56 which may be related to its autoimmune etiology in many patients. Endothelial dysfunction has also been linked to elevated local coronary29 and systemic30 levels of biomarkers such as lipoprotein-lipase A2 as well as the local presence of macrophages and microchannels, features consistent with inflammation.28 Previous studies have also shown that hypothyroidism is also associated with low-grade systemic inflammation, which may act as the potential trigger leading to endothelial dysfunction.57 However, the precise mechanisms involved require clarification.
Treating Hypothyroidism
In the current study, we did not observe that adequate replacement with TRT in hypothyroid patients was associated with better epicardial or microvascular endothelial function, even after adjusting for potential confounders. Other studies have shown an improvement in noninvasively measured flow-mediated dilatation in hypothyroid patients treated with thyroxine.33,39,40 In 2 of these studies, the sample size was very small (14 and 8 patients, respectively) while in the third, the observed difference in flow-mediated dilatation of 1.7% may not be clinically relevant. In an additional study, flow-mediated dilatation did not improve in hypothyroid patients until at least 6 months of treatment with TRT.38 Thus, the duration of treatment with TRT may be important, and while using TRT in hypothyroidism has been shown to result in favorable changes to cardiovascular risk factors,58 its precise role in modifying endothelial dysfunction requires further study. Thus, the current study supports the need for developing novel treatments for endothelial dysfunction directed at specific therapeutic targets.
Thyroid-stimulating hormone as a Biomarker
The current study demonstrated that TSH levels drawn within 1 month of the index procedure did not correlate significantly with the % Δ CBF Ach or the % Δ CAD Ach. In addition, when using TSH alone to categorize patients as euthyroid, SCH, or hypothyroid, no significant differences were observed in endothelial function in either microvascular or epicardial vessels. This may be a reflection of the fact that SCH has often not been shown to be a clinically important risk factor for cardiovascular disease.59,60 Nevertheless, the current study does not support the use of TSH as a biomarker to identify patients likely to have coronary endothelial dysfunction. This is in keeping with the consensus opinion that the routine screening of asymptomatic adults for thyroid dysfunction using TSH as a means of risk preventative health care is not recommended.60
Study Limitations
This study is limited by its retrospective and cross-sectional design. We were therefore unable to establish a causal relationship between hypothyroidism and endothelial dysfunction. Second, we did not prospectively follow patients with epicardial or microvascular coronary endothelial dysfunction for clinical events and so cannot comment on the clinical significance of these abnormalities. Third, we did not follow hypothyroid patients taking TRT to ascertain temporal changes in % Δ CBF Ach or % Δ CAD Ach. Fourth, some of our analyses may be limited by a small sample size. For example, the lack of finding a significant adjusted association between hypothyroidism and coronary endothelial dysfunction in the subset of men may be due to the small number of men with hypothyroidism included in the study. Similarly, the small number of hypothyroid patients with inadequate replacement on thyroid replacement therapy, as well as the small number of patients with TSH 4.5 to 10.0 mU/mL and TSH >10.0 mU/mL, may limit each of these analyses. Lastly, the current study is based on patients who were referred for coronary angiography to a tertiary referral center by an independent cardiologist and so constitute a unique population.
Conclusions
Hypothyroidism in women referred for coronary angiography is associated with microvascular endothelial dysfunction, even after adjusting for potential confounders, and may explain some of the increased risk of atherosclerotic cardiovascular disease in these patients. Adequate replacement with TRT in patients with hypothyroidism is not associated with better endothelial function, underscoring the need to develop novel therapies targeted at endothelial dysfunction.
Sources of Funding
This work was supported by the National Institute of Health (NIH Grants HL-92954 and AG-31750) and the Mayo Foundation.
Disclosures
None.
References
- Vanhaelst L, Neve P, Chailly P, Bastenie PA. Coronary-artery disease in hypothyroidism. Observations in clinical myxoedema. Lancet. 1967;2:800–802. doi: 10.1016/s0140-6736(67)92235-0. [DOI] [PubMed] [Google Scholar]
- Steinberg AD. Myxedema and coronary artery disease—a comparative autopsy study. Ann Intern Med. 1968;68:338–344. doi: 10.7326/0003-4819-68-2-338. [DOI] [PubMed] [Google Scholar]
- Auer J, Berent R, Weber T, Lassnig E, Eber B. Thyroid function is associated with presence and severity of coronary atherosclerosis. Clin Cardiol. 2003;26:569–573. doi: 10.1002/clc.4960261205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. Hypothyroidism and atherosclerotic heart disease: pathogenesis, medical management, and the role of coronary artery bypass surgery. Endocr Rev. 1985;6:432–440. doi: 10.1210/edrv-6-3-432. [DOI] [PubMed] [Google Scholar]
- Martinez-Triguero ML, Hernandez-Mijares A, Nguyen TT, Munoz ML, Pena H, Morillas C, Lorente D, Lluch I, Molina E. Effect of thyroid hormone replacement on lipoprotein(a), lipids, and apolipoproteins in subjects with hypothyroidism. Mayo Clin Proc. 1998;73:837–841. doi: 10.4065/73.9.837. [DOI] [PubMed] [Google Scholar]
- O’Brien T, Katz K, Hodge D, Nguyen TT, Kottke BA, Hay ID. The effect of the treatment of hypothyroidism and hyperthyroidism on plasma lipids and apolipoproteins AI, AII and E. Clin Endocrinol (Oxf) 1997;46:17–20. doi: 10.1046/j.1365-2265.1997.d01-1753.x. [DOI] [PubMed] [Google Scholar]
- Saito I, Saruta T. Hypertension in thyroid disorders. Endocrinol Metab Clin North Am. 1994;23:379–386. [PubMed] [Google Scholar]
- Streeten DH, Anderson GH, Jr, Howland T, Chiang R, Smulyan H. Effects of thyroid function on blood pressure. Recognition of hypothyroid hypertension. Hypertension. 1988;11:78–83. doi: 10.1161/01.hyp.11.1.78. [DOI] [PubMed] [Google Scholar]
- McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a PreCIS database study. Thyroid. 2011;21:837–843. doi: 10.1089/thy.2010.0298. [DOI] [PubMed] [Google Scholar]
- Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–843. doi: 10.1016/0735-1097(94)90627-0. [DOI] [PubMed] [Google Scholar]
- Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- Drexler H, Zeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by l-arginine. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- Egashira K, Inou T, Hirooka Y, Yamada A, Maruoka Y, Kai H, Sugimachi M, Suzuki S, Takeshita A. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. 1993;91:29–37. doi: 10.1172/JCI116183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira K, Inou T, Hirooka Y, Yamada A, Urabe Y, Takeshita A. Evidence of impaired endothelium-dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms. N Engl J Med. 1993;328:1659–1664. doi: 10.1056/NEJM199306103282302. [DOI] [PubMed] [Google Scholar]
- Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- Quyyumi AA, Cannon RO, III, Panza JA, Diodati JG, Epstein SE. Endothelial dysfunction in patients with chest pain and normal coronary arteries. Circulation. 1992;86:1864–1871. doi: 10.1161/01.cir.86.6.1864. [DOI] [PubMed] [Google Scholar]
- Hasdai D, Cannan CR, Mathew V, Holmes DR, Jr, Lerman A. Evaluation of patients with minimally obstructive coronary artery disease and angina. Int J Cardiol. 1996;53:203–208. doi: 10.1016/0167-5273(95)02548-0. [DOI] [PubMed] [Google Scholar]
- Hasdai D, Holmes DR, Jr, Higano ST, Burnett JC, Jr, Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc. 1998;73:1133–1140. doi: 10.4065/73.12.1133. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Armstrong ML, Marcus ML, Piegors DJ, Mark AL. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res. 1984;54:711–718. doi: 10.1161/01.res.54.6.711. [DOI] [PubMed] [Google Scholar]
- Lopez JA, Armstrong ML, Piegors DJ, Heistad DD. Effect of early and advanced atherosclerosis on vascular responses to serotonin, thromboxane A2, and ADP. Circulation. 1989;79:698–705. doi: 10.1161/01.cir.79.3.698. [DOI] [PubMed] [Google Scholar]
- Yoon MH, Reriani M, Mario G, Rihal C, Gulati R, Lennon R, Tilford JM, Lerman LO, Lerman A. Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int J Cardiol. 2013;168:1316–1321. doi: 10.1016/j.ijcard.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S, Bae JH, Rihal CS, Prasad A, Barsness GW, Lennon RJ, Holmes DR, Jr, Lerman A. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525–1530. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- Reriani MK, Flammer AJ, Jama A, Lerman LO, Lerman A. Novel functional risk factors for the prediction of cardiovascular events in vulnerable patients following acute coronary syndrome. Circ J. 2012;76:778–783. doi: 10.1253/circj.cj-12-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BJ, Matsuo Y, Aoki T, Kwon TG, Prasad A, Gulati R, Lennon RJ, Lerman LO, Lerman A. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:2473–2477. doi: 10.1161/ATVBAHA.114.304445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- Yang EH, McConnell JP, Lennon RJ, Barsness GW, Pumper G, Hartman SJ, Rihal CS, Lerman LO, Lerman A. Lipoprotein-associated phospholipase A2 is an independent marker for coronary endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2006;26:106–111. doi: 10.1161/01.ATV.0000191655.87296.ab. [DOI] [PubMed] [Google Scholar]
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348:2646–2655. doi: 10.1056/NEJMra021194. [DOI] [PubMed] [Google Scholar]
- Lekakis J, Papamichael C, Alevizaki M, Piperingos G, Marafelia P, Mantzos J, Stamatelopoulos S, Koutras DA. Flow-mediated, endothelium-dependent vasodilation is impaired in subjects with hypothyroidism, borderline hypothyroidism, and high-normal serum thyrotropin (TSH) values. Thyroid. 1997;7:411–414. doi: 10.1089/thy.1997.7.411. [DOI] [PubMed] [Google Scholar]
- Papaioannou GI, Lagasse M, Mather JF, Thompson PD. Treating hypothyroidism improves endothelial function. Metabolism. 2004;53:278–279. doi: 10.1016/j.metabol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Biondi B, Galderisi M, Pagano L, Sidiropulos M, Pulcrano M, D’Errico A, Ippolito S, Rossi A, de Divitiis O, Lombardi G. Endothelial-mediated coronary flow reserve in patients with mild thyroid hormone deficiency. Eur J Endocrinol. 2009;161:323–329. doi: 10.1530/EJE-09-0196. [DOI] [PubMed] [Google Scholar]
- Cabral MD, Teixeira PF, Silva NA, Morais FF, Soares DV, Salles E, Henriques JM, Leite SP, Montenegro CA, Vaisman M. Normal flow-mediated vasodilatation of the brachial artery and carotid artery intima-media thickness in subclinical hypothyroidism. Braz J Med Biol Res. 2009;42:426–432. doi: 10.1590/s0100-879x2009000500005. [DOI] [PubMed] [Google Scholar]
- Dagre AG, Lekakis JP, Protogerou AD, Douridas GN, Papaioannou TG, Tryfonopoulos DJ, Papamichael CM, Alevizaki M. Abnormal endothelial function in female patients with hypothyroidism and borderline thyroid function. Int J Cardiol. 2007;114:332–338. doi: 10.1016/j.ijcard.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Shavdatuashvili T. Lipoprotein profile and endothelial function in patients with subclinical and overt hypothyroidism. Georgian Med News. 2005;129:57–60. [PubMed] [Google Scholar]
- Erbil Y, Ozbey N, Giris M, Salmaslioglu A, Ozarmagan S, Tezelman S. Effects of thyroxine replacement on lipid profile and endothelial function after thyroidectomy. Br J Surg. 2007;94:1485–1490. doi: 10.1002/bjs.5915. [DOI] [PubMed] [Google Scholar]
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of l-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:1715–1723. doi: 10.1210/jc.2006-1869. [DOI] [PubMed] [Google Scholar]
- Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L, Salvetti A, Ferrannini E, Monzani F. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab. 2003;88:3731–3737. doi: 10.1210/jc.2003-030039. [DOI] [PubMed] [Google Scholar]
- Widmer RJ, Flammer AJ, Herrmann J, Rodriguez-Porcel M, Wan J, Cohen P, Lerman LO, Lerman A. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol. 2013;304:H393–H397. doi: 10.1152/ajpheart.00765.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Britson PJ, Chu A, Holmes DR, Jr, Bresnahan JF, Schwartz RS. Validation of a new UNIX-based quantitative coronary angiographic system for the measurement of coronary artery lesions. Cathet Cardiovasc Diagn. 1997;40:66–74. doi: 10.1002/(sici)1097-0304(199701)40:1<66::aid-ccd12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bove AA, Holmes DR, Jr, Owen RM, Bresnahan JF, Reeder GS, Smith HC, Vlietstra RE. Estimation of the effects of angioplasty on coronary stenosis using quantitative video angiography. Cathet Cardiovasc Diagn. 1985;11:5–16. doi: 10.1002/ccd.1810110103. [DOI] [PubMed] [Google Scholar]
- Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (women’s ischemia syndrome evaluation) study. JACC Cardiovasc Interv. 2012;5:646–653. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler KJ, Galili O, Rodriguez-Porcel M, Krier JD, Lerman LO, Lerman A. Dietary reversal of experimental hypercholesterolemia improves endothelial dysfunction of epicardial arteries but not of small coronary vessels in pigs. Atherosclerosis. 2006;188:301–308. doi: 10.1016/j.atherosclerosis.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, Lerman LO, Lerman A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–2627. doi: 10.1161/CIRCULATIONAHA.106.641654. [DOI] [PubMed] [Google Scholar]
- Mandel SJ, Larsen PR, Seely EW, Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–96. doi: 10.1056/NEJM199007123230204. [DOI] [PubMed] [Google Scholar]
- Arafah BM. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N Engl J Med. 2001;344:1743–1749. doi: 10.1056/NEJM200106073442302. [DOI] [PubMed] [Google Scholar]
- Arafah BM. Decreased levothyroxine requirement in women with hypothyroidism during androgen therapy for breast cancer. Ann Intern Med. 1994;121:247–251. doi: 10.7326/0003-4819-121-4-199408150-00002. [DOI] [PubMed] [Google Scholar]
- Walsh JP, Bremner AP, Bulsara MK, O’Leary P, Leedman PJ, Feddema P, Michelangeli V. Thyroid dysfunction and serum lipids: a community-based study. Clin Endocrinol (Oxf) 2005;63:670–675. doi: 10.1111/j.1365-2265.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int J Clin Pract. 2010;64:1130–1139. doi: 10.1111/j.1742-1241.2010.02376.x. [DOI] [PubMed] [Google Scholar]
- Tabatabaie V, Surks MI. The aging thyroid. Curr Opin Endocrinol Diabetes Obes. 2013;20:455–459. doi: 10.1097/01.med.0000433055.99570.52. [DOI] [PubMed] [Google Scholar]
- Knudsen N, Laurberg P, Rasmussen LB, Bulow I, Perrild H, Ovesen L, Jorgensen T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- Mizuma H, Murakami M, Mori M. Thyroid hormone activation in human vascular smooth muscle cells: expression of type II iodothyronine deiodinase. Circ Res. 2001;88:313–318. doi: 10.1161/01.res.88.3.313. [DOI] [PubMed] [Google Scholar]
- Wangel AG, Kontiainen S, Melamies L, Weber T. Hypothyroidism and anti-endothelial cell antibodies. APMIS. 1993;101:91–94. doi: 10.1111/j.1699-0463.1993.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L, Ferrannini E, Salvetti A, Monzani F. Low-grade systemic inflammation causes endothelial dysfunction in patients with Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2006;91:5076–5082. doi: 10.1210/jc.2006-1075. [DOI] [PubMed] [Google Scholar]
- Scherer T, Wolf P, Winhofer Y, Duan H, Einwallner E, Gessl A, Luger A, Trattnig S, Hoffmann M, Niessner A, Baumgartner-Parzer S, Krssak M, Krebs M. Levothyroxine replacement in hypothyroid humans reduces myocardial lipid load and improves cardiac function. J Clin Endocrinol Metab. 2014;99:E2341–E2346. doi: 10.1210/jc.2014-2112. [DOI] [PubMed] [Google Scholar]
- Chu JW, Crapo LM. The treatment of subclinical hypothyroidism is seldom necessary. J Clin Endocrinol Metab. 2001;86:4591–4599. doi: 10.1210/jcem.86.10.7961. [DOI] [PubMed] [Google Scholar]
- Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab. 2005;90:581–585. doi: 10.1210/jc.2004-1231. discussion 586–587. [DOI] [PubMed] [Google Scholar]



