Abstract
Background
Coronary artery disease and left ventricular hypertrophy are prevalent in the chronic kidney disease (CKD) and renal transplant (RT) population. Advances in cardiovascular magnetic resonance (CMR) with blood oxygen level–dependent (BOLD) technique provides capability to assess myocardial oxygenation as a measure of ischemia. We hypothesized that the myocardial oxygenation response to stress would be impaired in CKD and RT patients.
Methods and Results
Fifty-three subjects (23 subjects with CKD, 10 RT recipients, 10 hypertensive (HT) controls, and 10 normal controls without known coronary artery disease) underwent CMR scanning. All groups had cine and BOLD CMR at 3 T. The RT and HT groups also had late gadolinium CMR to assess infarction/replacement fibrosis. The CKD group underwent 2-dimensional echocardiography strain to assess fibrosis. Myocardial oxygenation was measured at rest and under stress with adenosine (140 μg/kg per minute) using BOLD signal intensity. A total of 2898 myocardial segments (1200 segments in CKD patients, 552 segments in RT, 480 segments in HT, and 666 segments in normal controls) were compared using linear mixed modeling. Diabetes mellitus (P=0.47) and hypertension (P=0.57) were similar between CKD, RT, and HT groups. The mean BOLD signal intensity change was significantly lower in the CKD and RT groups compared to HT controls and normal controls (−0.89±10.63% in CKD versus 5.66±7.87% in RT versus 15.54±9.58% in HT controls versus 16.19±11.11% in normal controls, P<0.0001). BOLD signal intensity change was associated with estimated glomerular filtration rate (β=0.16, 95% CI=0.10 to 0.22, P<0.0001). Left ventricular mass index and left ventricular septal wall diameter were similar between the CKD predialysis, RT, and HT groups. None of the CKD patients had impaired global longitudinal strain and none of the RT group had late gadolinium hyperenhancement.
Conclusions
Myocardial oxygenation response to stress is impaired in CKD patients and RT recipients without known coronary artery disease, and unlikely to be solely accounted for by the presence of diabetes mellitus, left ventricular hypertrophy, or myocardial scarring. The impaired myocardial oxygenation in CKD patients may be associated with declining renal function. Noncontrast BOLD CMR is a promising tool for detecting myocardial ischemia in the CKD population.
Keywords: blood oxygen level–dependent, chronic kidney disease, coronary artery disease, myocardial ischemia, myocardial oxygenation, renal transplant
Cardiovascular disease is the leading cause of mortality in patients with chronic kidney disease (CKD).1 CKD patients have a 10 to 20 times higher risk of cardiac death than the normal population, although the mechanism is uncertain.2 Furthermore, despite cardiovascular mortality being significantly reduced following renal transplantation,3 cardiovascular disease remains a major cause of mortality in post–renal transplant (RT) recipients.4 The majority of coronary artery disease (CAD) in CKD patients is asymptomatic and due to multivessel epicardial disease.5,6 These patients have significant cardiovascular risk factors ranging from atherosclerotic (hypercholesterolemia, diabetes, hypertension), myocardial (fluid overload), and metabolic (hyperphosphatemia, anemia, chronic inflammation). Left ventricular hypertrophy is also a frequent finding in the CKD population, with a prevalence of 75%.7
Current functional cardiac investigations are neither sensitive nor specific for ischemia in CKD patients.8,9 Blood oxygen level–dependent (BOLD) cardiovascular magnetic resonance (CMR) assesses myocardial tissue oxygenation, thus is potentially able to measure mismatches in myocardial oxygen demand and supply and indicate ischemia.10 It exploits the paramagnetic properties of deoxyhemoglobin as an endogenous contrast agent with increased deoxyhemoglobin content leading to signal reduction on T2-weighted images.11 The transition from diamagnetic oxyhemoglobin to paramagnetic deoxyhemoglobin induces a change in magnetic resonance signal intensity (SI) and thereby generates oxygen-dependent contrast.12 The BOLD technique has been validated in animal models13–21 and utilized in human studies in patients with angina, CAD, syndrome X, and hypertension.22–26
We utilized BOLD CMR to measure myocardial oxygenation response to stress in the CKD population as a measure of myocardial ischemia and searched for associations with renal function. We hypothesized that:
oxygenation would be impaired in the CKD population without known CAD, irrespective of the presence of diabetes mellitus and/or hypertension.
myocardial oxygenation would also be impaired in renal transplant recipients without known CAD.
Methods
Study Population
CKD patients with an estimated glomerular filtration rate (eGFR) <30 mL/min per 1.73 m2 or receiving regular dialysis were invited to participate at Flinders Medical Centre, a tertiary teaching hospital in South Australia, in 2012–2014. Ten RT recipients with reasonable renal function eGFR >45 mL/min per 1.73 m2 were prospectively recruited from our hospital transplant clinic. CKD and RT patients had the following inclusion criteria: no symptoms of cardiac disease, no established CAD (no history of myocardial infarction, angina, coronary artery stent or bypass surgery or angiographically documented significant CAD >70%, and no significant inducible myocardial ischemia on functional testing pretransplant), and no previous systolic heart failure. Ten people with a clinical diagnosis of hypertension (HT) and who were asymptomatic with no known CAD were prospectively recruited from the hospital’s Hypertension Clinic. Ten healthy volunteers without cardiovascular risk factors or symptoms served as normal controls.
The exclusion criteria for each group were standard contraindications to CMR (eg, severe claustrophobia, metallic implants) and contraindications to adenosine (second- or third-degree atrioventricular block, obstructive pulmonary disease, and dipyridamole use).
All participants gave written informed consent, and the study was approved by Southern Adelaide Clinical Human Research Ethics Committee.
Biochemistry
eGFR was calculated from serum creatinine using the CKD-Epidemiology Collaboration formula.27
CMR Protocol
All participants underwent scanning in a 3-T clinical MR scanner (Siemens, 3 T Trio, 4 channel Body Flex coil) and were instructed to refrain from caffeine 24 hours prior to the scan. All scans started with Half-Fourier single-shot turbo spin echo and Fast imaging with steady precession localizers. Cine images were acquired in vertical and horizontal long-axis, and 10 short-axis images covering the entire left ventricle, using a retrospective ECG gating steady-state free precession (SSFP) sequence (repetition time [TR] 3 ms, echo time [TE] 1.5 ms, flip angle 55°, 18 phases).28 For BOLD imaging, a single midventricular slice was acquired at mid-diastole using a T2-prepared ECG-gated SSFP sequence (TR 2.86 ms, TE 1.43 ms, T2 preparation time 40 ms, matrix 168×192, field of view 340×340 mm, slice thickness 8 mm, flip angle 44°), as previously described.23 Shimming and center frequency adjustments were performed as required before the oxygenation imaging to minimize off-resonance artifacts. A set of 6 BOLD images was acquired at rest during a single breath-hold over 6 heartbeats. Six stress BOLD images identical to the ones acquired at rest were acquired at peak adenosine stress (140 μg/kg per minute) 90 s after initiation for at least 3 minutes. Stress heart rate and blood pressure were obtained every minute of adenosine infusion. Each participant was questioned about the occurrence of adenosine effects: chest pain or tightness, shortness of breath, flushing, headache, and nausea.
All RT and HT subjects (eGFR >45 mL/min per 1.73 m2) underwent additional late gadolinium imaging. Gadovist (Bayer Healthcare, Australia) 0.05 mmol/kg was injected, and after 6 minutes, images were acquired in the 3 long axes and in the short axis plane to obtain coverage of the entire left ventricle using a gated T1-weighted segmented inversion recovery turbo fast low-angle shot sequence (echo time, 4.8 ms; voxel size, 1.4×2.4×8 mm; flip angle, 20°). The inversion time was adjusted to achieve optimal nulling of noninfarcted myocardium, as previously described.29
Strain Echocardiography
Due to the risk of nephrogenic systemic fibrosis with gadolinium contrast in patients with severe renal dysfunction,30–32 gadolinium studies were not undertaken in the CKD cohort, who instead underwent speckle-tracking strain echocardiography study as a measure of myocardial fibrosis. Patients were imaged in the left lateral decubitus position with a 3.5-MHz transducer (Vivid E9 Ultrasound and M5S-D probe, General Electric Vingmed, Horten, Norway). Routine, 3-beat gray-scale images of the left ventricle were acquired from the apical 4-chamber, 2-chamber, and long-axis views. Frame rates in excess of 50 fps were maintained, and care was taken to ensure nonforeshortening of the left ventricle. Echocardiographic data were stored in a digital format for offline analysis, which was performed using commercially available software (EchoPac BT-11; GE-Vingmed, Horten, Norway). Left ventricular speckle-tracking strain was measured by manually demarcating the myocardial border with the region of interest optimized to ensure accurate tracking. All echocardiographic images were analyzed by a single observer (RP) with more than 15 years echocardiographic experience, who was independent and blinded to patients’ clinical and CMR data. Strain-versus-time curves were obtained for 18 segments from 3 apical views. Global longitudinal strain was measured from the average of 18 segments.
CMR Image Analysis
CMR analysis was performed with CMR42 Version 4.1 (Circle Cardiovascular Imaging Inc, Calgary, Canada). Left ventricular mass, and left and right ventricular volumes and functions were calculated using the 3-dimensional short-axis stack by tracing the endocardial and epicardial contours in end-diastole and end-systole, as previously described.28 Left ventricular mass, left and right ventricular end-diastolic volumes, and end-systolic volumes were indexed to body surface area. The septal and lateral wall diameters were measured in end-diastole at midventricular level from short-axis view.
BOLD analysis was performed as previously described.23 Myocardial SI was measured by the CMR software, after manually tracing the endocardial and epicardial contours. Each midventricular short-axis BOLD image was divided into 6 segments (anterior, anterolateral, inferolateral, inferior, inferoseptal, and anteroseptal) according to the American Heart Association 17-segment model.33 The mean myocardial SI within each segment was obtained, both at rest and stress, and corrected to variations in heart rate with the following equation previously described23:
with T1=1220 ms and β=0.59 (determined empirically for this sequence), SI0=the measured signal intensity, SI=signal intensity corrected to heart rate, and TR is the image-dependent time between acquisition of sections of k-space, governed by the heart rate (replaced by the RR interval).23
The SI change was calculated as:
A second operator blinded to the clinical data analyzed a subset of BOLD CMR scans to assess interobserver reproducibility.
Statistical Analysis
Statistical analysis was performed with STATA version 13.0. Normally distributed data are expressed as mean±SD and non-normally distributed data as median (interquartile range). Either ANOVA or a Kruskal–Wallis test was used to compare clinical characteristics between the study groups as appropriate. Chi-squared tests or Fisher’s exact tests were used for comparison of categorical variables. BOLD SI evaluation of coronary artery level data and association with eGFR was analyzed using linear mixed modeling with a random intercept used for each subject to account for the within-subject correlation present from measuring at 3 different artery sites.34 All analyses were adjusted for age and gender a priori, and also for other potential confounders where appropriate. Both unadjusted and adjusted linear mixed modeling was performed with adjustment for medication use (where significant in univariate analysis) and left ventricular mass a priori. Interobserver reproducibility of BOLD SI change was assessed by coefficient of variance. Statistical tests were 2-tailed and a P<0.05 was considered statistically significant.
Results
Subject Characteristics
Fifty-three subjects participated in the study: 23 CKD (11 [48%] dialysis and 12 [52%] predialysis), 10 RT, 10 HT controls, and 10 normal controls. Clinical characteristics are presented in Table 1. Age was similar between the CKD, RT, and HT groups (P=0.65). The presence of diabetes mellitus (P=0.47) and hypertension (P=0.57) were similar between the CKD, RT, and HT groups. After adjustment for multiple group comparisons using Bonferroni correction, the eGFR was lower in the CKD group compared to HT control (P<0.0001), lower in the RT group compared to HT control (P=0.001), lower in the CKD group compared to RT group (P<0.0001), and similar between the HT and the normal controls (P=1.00). The level of hemoglobin was lower in the CKD group compared to the HT control (P<0.0001) and lower in the CKD group compared to the RT group (P<0.0001), but similar between the RT and HT control (P=1.00). The HT control had a higher level of low-density lipoprotein compared to the CKD group (P=0.004) and compared to the RT group (P=0.007). The use of aspirin, angiotensin-converting enzyme inhibitor, β-blocker, and statin between the 4 groups was not statistically different. The use of angiotensin receptor blocker was lower in the CKD group compared to the HT group (P=0.017) but similar between the RT and HT group (P=0.36).
Table 1.
Clinical Characteristics
| CKD Subjects (n=23) | Renal Transplant Subjects (n=10) | Hypertensive Controls (n=10) | Normal Controls (n=10) | P Value* | ||
|---|---|---|---|---|---|---|
| Dialysis (n=11) | Predialysis (n=12) | |||||
| Age, y (mean±SD) | 65±12 | 54±14 | 59±7 | 55±11 | 45±10 | 0.02 |
| Male sex, n (%) | 6 (55) | 5 (42) | 6 (60) | 5 (50) | 4 (40) | 0.91 |
| BMI, kg/m² (mean±SD) | 27±4 | 26±6 | 27±3 | 33±3 | 22±2 | <0.001 |
| eGFR, mL/min per 1.73 m2—median (range) | 14 (8 to 18) | 72 (57 to 114) | 108 (57 to 144) | 110 (79 to 146) | <0.0001 | |
| Hemoglobin, g/L | 109±19 | 114±16 | 138±16 | 145±14 | — | <0.0001 |
| Cardiovascular risk factors, n (%) | ||||||
| Hypertension | 8 (73) | 8 (67) | 9 (90) | 10 (100) | 0 (0) | <0.001 |
| Diabetes mellitus | 5 (45) | 4 (33) | 1 (10) | 2 (20) | 0 (0) | 0.20 |
| Total cholesterol, mmol/L | 3.9±1.1 | 5.4±1.5 | 4.8±1.3 | 5.7±1.0 | — | 0.20 |
| Low-density lipoprotein, mmol/L | 2.0±0.7 | 2.7±0.6 | 2.1±1.2 | 3.6±0.9 | — | 0.002 |
| Triglyceride, mmol/L | 1.3±1.4 | 2.6±2.6 | 2.6±1.7 | 1.5±0.7 | — | 0.33 |
| Smoking history | 4 (36) | 4 (33) | 3 (30) | 3 (30) | 0 (0) | 0.20 |
| Cardiac medications, n (%) | ||||||
| Aspirin | 1 (9) | 0 (0) | 0 (0) | 2 (20) | 0 (0) | 0.21 |
| β-Blocker | 5 (45) | 2 (17) | 5 (50) | 2 (20) | 0 (0) | 0.05 |
| ACE inhibitor | 1 (9) | 2 (17) | 3 (30) | 2 (20) | 0 (0) | 0.45 |
| Angiotensin receptor blocker | 0 (0) | 2 (17) | 2 (20) | 5 (50) | 0 (0) | 0.01 |
| Calcium channel blocker | 3 (27) | 2 (17) | 3 (30) | 5 (50) | 0 (0) | 0.11 |
| Statin | 5 (45) | 2 (17) | 2 (20) | 2 (20) | 0 (0) | 0.16 |
Data are presented as n (%) or mean±SD. ACE indicates angiotensin-converting enzyme; ANOVA, analysis of variance; BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Assessed using ANOVA or Fisher’s exact test as appropriate.
The etiology of renal diseases in the CKD and RT groups were as follows: polycystic kidney disease (7 [30%] CKD, 6 [60%] RT), glomerulonephritis (3 [13%] CKD, 3 [30%] RT), diabetic nephropathy (4 [17%] CKD), medication related (2 [9%] CKD, 1 [10%] RT), Alport’s syndrome (1 [5%] CKD), previous nephrectomy (2 [9%] CKD), and unknown (4 [17%] CKD).
Assessment of Left Ventricular Mass, Volumes, and Function
The CMR results are summarized in Table 2. Left ventricular mass index was higher in the dialysis group, but similar between the predialysis, RT, and HT groups (P=0.40). Left ventricular septal (P=0.64) and lateral wall diameter (P=0.64) were similar between the CKD, RT, and HT groups.
Table 2.
Left Ventricular Mass, Septal and Lateral Wall Thickness, Ventricular Volumes, and Ejection Fraction
| CKD Subjects (n=23) | Renal Transplant Subjects (n=10) | Hypertensive Controls (n=10) | Normal Controls (n=10) | P Value* | ||
|---|---|---|---|---|---|---|
| Dialysis (n=11) | Predialysis (n=12) | |||||
| LV mass index, g/m² | 87±20 | 69±21 | 65±11 | 60±10 | 60±10 | 0.053 |
| LV septal wall thickness, cm | 1.2±0.3 | 1.1±0.3 | 1.2±0.2 | 1.1±0.3 | 1.1±0.3 | <0.001 |
| LV lateral wall thickness, cm | 1.0±0.2 | 0.9±0.3 | 0.9±0.2 | 0.9±0.2 | 0.9±0.2 | 0.30 |
| LV end diastolic volume index, mL/m2 | 74±17 | 71±26 | 71±19 | 70±13 | 67±13 | 0.91 |
| LV end systolic volume index, mL/m2 | 27±10 | 23±16 | 19±10 | 22±9 | 22±5 | 0.58 |
| LV stroke volume index, mL/m2 | 47±12 | 48±17 | 51±13 | 46±10 | 46±10 | 0.79 |
| LV ejection fraction, % | 64±9 | 68±13 | 73±9 | 69±8 | 68±6 | 0.29 |
| RV end diastolic volume index, mL/m2 | 69±13 | 73±31 | 71±8 | 71±18 | 70±14 | 0.99 |
| RV end systolic volume index, mL/m2 | 26±6 | 27±16 | 29±8 | 26±9 | 26±8 | 0.91 |
| RV stroke volume index, mL/m2 | 43±12 | 46±20 | 43±9 | 46±12 | 44±9 | 0.96 |
| RV ejection fraction, % | 61±11 | 63±11 | 61±13 | 63±6 | 64±7 | 0.94 |
All data are presented as mean±SD. ANOVA indicates analysis of variance; CKD, chronic kidney disease; LV, left ventricle; RV, right ventricle.
Assessed using ANOVA.
Assessment of Myocardial Oxygenation
A total of 2898 myocardial segments (1200 segments of CKD [648 segments of dialysis and 552 segments of predialysis], 552 segments of RT, 480 segments of HT, and 666 segments of normal controls) were compared using linear mixed modeling.
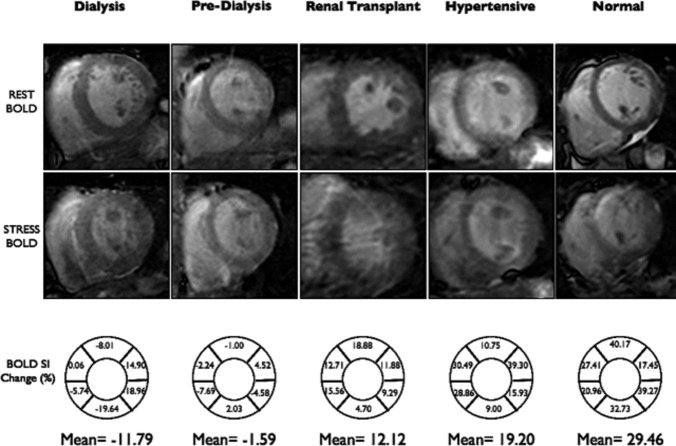
Global BOLD signal intensity (BOLD SI) change of the CKD (dialysis and predialysis), RT, HT, and normal controls is outlined in Figure 1. The mean global BOLD SI change was significantly lower in the CKD and RT groups compared to HT and normal controls (−0.89±10.63% in CKD [−3.46±12.83% in dialysis versus 1.44±7.62% in predialysis] versus 5.66±7.87% in RT versus 15.54±9.58% in HT controls, versus 16.19±11.11% in normal controls, P<0.0001). Mean BOLD SI change was significantly lower in CKD compared to HT controls (P<0.0001). Mean BOLD SI change was significantly lower in RT compared to HT controls (P=0.013). There was a trend of mean BOLD SI change to be lower in CKD compared to RT (P=0.068). The mean BOLD SI change was similar between HT and normal controls (P=0.89).
Figure 1.
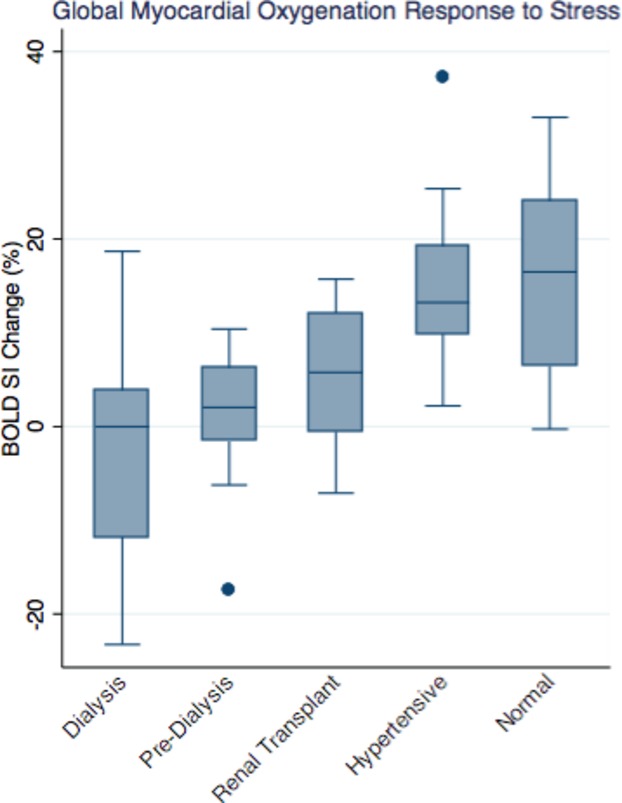
Distribution of myocardial oxygenation BOLD SI change of the chronic kidney disease (dialysis, predialysis), RT, hypertensive, and normal controls groups (−3.46±12.83% in dialysis vs 1.44±7.62% in predialysis vs 5.66±7.87% in RT vs 15.54±9.58% in HT vs 16.19±11.11% in normal controls, P<0.0001). BOLD indicates blood oxygen level–dependent; HT, hypertensive; RT, renal transplant; SI, signal intensity.
Regional BOLD SI change in the left anterior descending, left circumflex (LCx), and right coronary artery territories were significantly lower in CKD and RT compared to HT and normal controls (Figure 2) (BOLD SI Change in left anterior descending territory: −1.70±14.00% in CKD [−3.95±14.48% in dialysis versus 0.34±13.85% in predialysis] versus 6.40±7.90% in RT versus 16.67±7.60% in HT controls, versus 17.40±11.54% in normal controls, P<0.001; BOLD SI change in left circumflex territory: 2.19±14.41% in CKD [−3.60±14.69% in dialysis versus 7.45±12.54% in predialysis] versus 4.86±8.59% in RT versus 13.26±15.49% in HT controls, versus 16.52±14.76% in normal controls, P=0.018; BOLD SI change in right coronary artery territory: −3.17±10.66% in CKD [−2.83±12.77% in dialysis versus −3.48±8.97% in predialysis] versus 5.73±12.95% in RT versus 16.71±12.09% in HT controls, versus 14.64±12.30% in normal controls, P<0.001).
Figure 2.
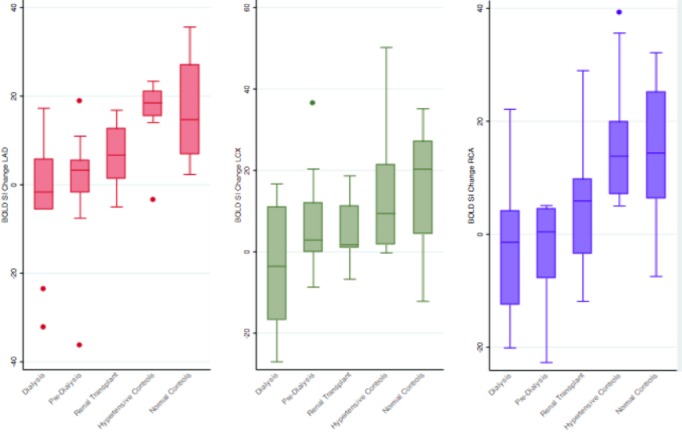
Myocardial oxygenation BOLD SI change of the CKD (dialysis, predialysis), RT, hypertensive, and normal controls groups within each of the 3 coronary artery territories. BOLD indicates blood oxygen level–dependent; CKD, chronic kidney disease; LAD, left anterior descending; LCx, left circumflex; RCA, right coronary artery; RT, renal transplant; SI, signal intensity.
The reproducibility of BOLD SI measurements was good, with a coefficient of variation of 1.2% for rest, 2.4% for stress images, and 3.0% for BOLD SI change measurements. Multivariate analysis revealed that only the patient group remained an independent predictor of BOLD SI change. Figure 3 shows examples of rest and stress BOLD images representative of each group.
Figure 3.
Examples of myocardial oxygenation (BOLD) images at rest and during stress of the CKD (dialysis, predialysis), RT, hypertensive, and normal controls groups. BOLD SI change (%)=(SI stress−SI rest)/SI rest×100%. SI rest and SI stress were automatically calculated by CMR software, and corrected to RR interval. BOLD indicates blood oxygen level–dependent; CKD, chronic kidney disease; CMR, cardiovascular magnetic resonance; eGFR, estimated glomerular filtration rate; LAD, left anterior descending; LCx, left circumflex; RCA, right coronary artery; RT, renal transplant; SI, signal intensity.
Association Between Myocardial Oxygenation and Renal Function
BOLD SI change was associated with eGFR (β=0.16, 95% CI=0.10 to 0.22, P<0.0001) in all groups combined (Figure 4). There was a significant positive correlation between BOLD SI change and eGFR in the CKD group alone (r=0.27, P=0.03) (Figure 5). In the RT group alone, however, there was no significant correlation between BOLD SI change and eGFR (r=−0.09, P=0.65).
Figure 4.
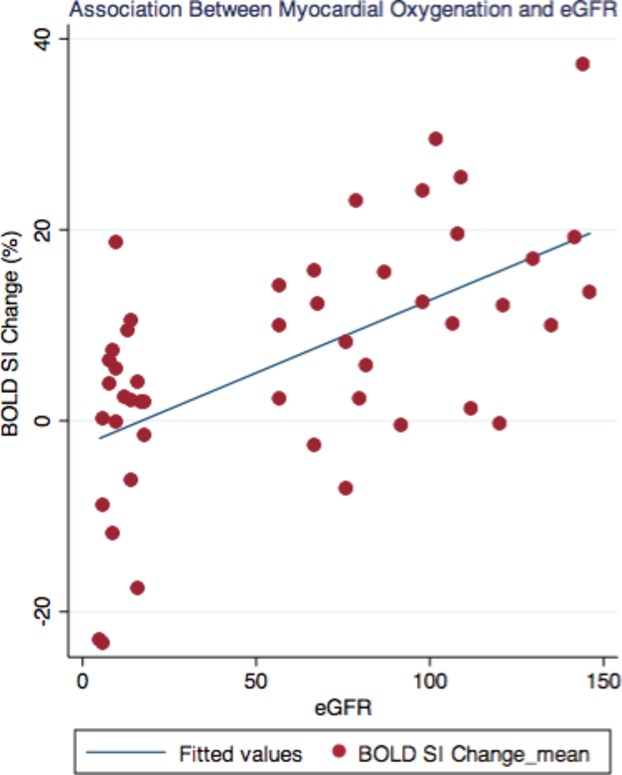
Association of myocardial oxygenation BOLD SI change of the chronic kidney disease, renal transplant, hypertensive, and normal controls groups (β=0.16, 95% CI=0.10 to 0.22, P<0.0001). BOLD indicates blood oxygen level–dependent; SI, signal intensity.
Figure 5.
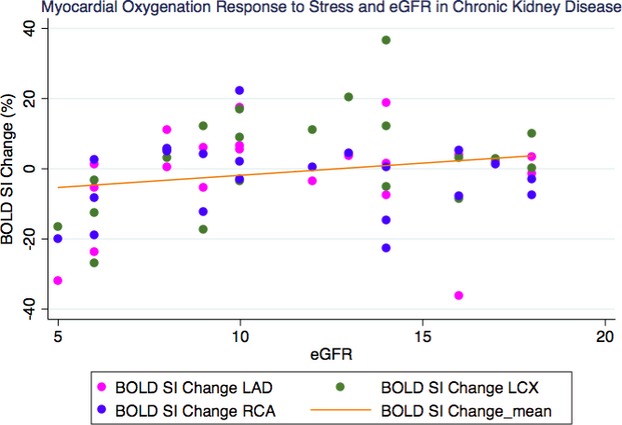
Correlation of myocardial oxygenation BOLD SI change and renal function of the chronic kidney disease group (r=0.27, P=0.03). BOLD indicates blood oxygen level–dependent; eGFR, estimated glomerular filtration rate; SI, signal intensity.
In the CKD and RT groups, there was no significant correlation between BOLD SI change and the level of hemoglobin (r=0.19, P=0.13) and (r=0.05, P=0.83), respectively.
Assessment of Myocardial Fibrosis
Global longitudinal strain in the CKD group was −18.10±1.74 versus in the normal volunteers −18.3±2.16, P=0.82, which was within normal limits.
None of the RT subjects had late-gadolinium hyperenhancement. Two HT subjects had midwall hyperenhancement typical of hypertensive heart disease.
Discussion
The principal result from this study is that myocardial oxygenation response to stress is impaired in the CKD patients and renal transplant recipients without known CAD, independent of the presence of diabetes mellitus, left ventricular hypertrophy, and myocardial scar. Furthermore, the impairment in the stress BOLD signal is associated with the degree of renal dysfunction. To the best of our knowledge, this is the first study to utilize BOLD CMR in CKD and RT patients.
BOLD CMR Technique
A major strength of this study is that we directly assessed the imbalance between myocardial oxygen demand and supply characteristic of ischemia noninvasively using nongadolinium contrast BOLD CMR technique at 3 T BOLD CMR utilizes the fact that deoxygenated hemoglobin in blood can act as an intrinsic contrast agent, changing proton signals in a fashion that can be imaged to reflect the level of blood oxygenation. Increases in O2 saturation increase the BOLD imaging signal (T2), whereas decreases diminish it. As recently reviewed, the BOLD CMR technique has been extensively validated in preclinical and clinical studies at both 1.5 and 3 T using a variety of sequences.35 The SSFP sequence used in this study was initially validated in the canine heart at 1.5 T by Fieno et al.20 We used the higher field strength (3 T) given the limited signal-to-noise ratio between normal and deoxygenated myocardial regions at 1.5 T.21 The BOLD measurement reproducibility in this study compares favorably with the BOLD SI measurement reproducibility demonstrated by Karamitsos et al, who used the same SSFP sequence at 3 T.23
Myocardial Oxygenation, Epicardial and Microvascular CAD
BOLD CMR technique can identify not only epicardial coronary artery stenosis, but also potentially coronary microvascular dysfunction. BOLD CMR has moderate accuracy in detecting significant CAD.24,36 Myocardial oxygenation response to stress has been recognized to be impaired in patients with significant epicardial CAD.23,24 The normal BOLD SI index threshold is defined in recent CAD versus normal control studies (using the same SSFP T2 prep sequence as the current study) as 2.64% (sensitivity 92% and specificity 72% for coronary stenosis >50%) by Arnold et al,12 3.74% (sensitivity 67% and specificity 88% for coronary stenosis >50%) by Karamitsos et al,23 and 1.2% (sensitivity 88% and specificity 47% for coronary stenosis >75%) by Friedrich et al25 Walcher et al, using T2-prepared SSFP, evaluated the diagnostic accuracy of BOLD CMR compared to invasive fractional flow reserve (FFR), for measuring hemodynamically significant coronary artery lesion.37 They found that relative BOLD signal intensity increase was significantly lower in myocardial segments supplied by coronary arteries with an FFR ≤0.8 compared with segments with an FFR >0.8 (1.1±0.2 versus 1.5±0.2; P<0.0001).37 The BOLD CMR sensitivity and specificity compared to FFR ≤0.8 were 88.2% and 89.5%, respectively.37
We demonstrated significant blunted myocardial oxygenation response to stress in the CKD patients with negative mean BOLD SI change values, which could imply significant coronary artery stenosis, either epicardial, microvascular, or both. Luu et al demonstrated that coronary territories with FFR <0.80 had negative-value mean BOLD SI change, and FFR <0.54 predicted a complete lack of vasodilator-induced oxygenation increase with sensitivity of 71% and specificity of 75%.38 Karamitsos et al also demonstrated negative BOLD SI change values in patients with significant coronary artery stenoses.23
Microvascular CAD can be present in the presence or absence of coexistent epicardial CAD. We have previously demonstrated an intermediate BOLD SI change in the myocardial segments subtended by nonstenosed coronary arteries, compared to segments subtended by stenosed coronary arteries and coronary arteries of normal volunteers. Microvascular disease could also be present in our population in the absence of significant CAD. CKD patients have a high prevalence of left ventricular hypertrophy.7 Microvascular disease is well known in left ventricular hypertrophy,39 and may explain myocardial ischemia in the absence of obstructive coronary disease. In addition, Mahmod et al showed reduced mean BOLD SI change during stress in patients with aortic stenosis without significant epicardial coronary artery stenosis, suggestive of microvascular disease.40 Blunted myocardial oxygenation response to stress was also demonstrated in patients with hypertrophic cardiomyopathy.41 Given our CKD cohort were asymptomatic, with no known significant epicardial coronary disease, severe microvascular disease might well explain the impaired myocardial oxygenation response in all coronary artery territories.
Myocardial Oxygenation Response to Stress and Myocardial Fibrosis
Detection of scarred and viable myocardium using BOLD CMR was studied by Egred et al.42 They showed reduced BOLD SI change in scarred myocardial segments.42 Late gadolinium enhancement to assess myocardial scarring and fibrosis was not performed in the CKD cohort due to the risk of nephrogenic systemic fibrosis. Echocardiographic strain imaging enables a comprehensive assessment of myocardial function with the ability to differentiate between active and passive movement of myocardial segments, longitudinal myocardial shortening, and dyssynchrony that are not visually assessable.43 The accuracy of 2-dimensional speckle-tracking echocardiography has been compared with delayed enhancement cardiac magnetic resonance imaging where a strain value of −15% identified myocardial fibrosis with sensitivity of 83% and specificity of 93%.44 Our CKD subjects did not exhibit impaired global longitudinal strain assessed by 2-dimensional speckle-tracking echocardiography and none of the RT subjects had late gadolinium hyperenhancement, suggesting the impaired BOLD SI change in our cohort was not related to replacement myocardial fibrosis or scar.
Myocardial Oxygenation and CKD
Our study suggests that impaired myocardial oxygenation response to stress may be associated with declining renal function. The severity of renal dysfunction has been shown to have a strong association with the incidence of cardiovascular events.45 Further analysis of our CKD patients showed that the degree of renal impairment (ie, lower eGFR) was correlated with more impaired myocardial oxygenation response to stress. The impaired myocardial oxygenation in RT recipients, however, was not associated with the residual renal function, perhaps suggesting that there may be other mechanism(s), such as immunosuppression-induced microvascular dysfunction, that may cause the impaired BOLD signal. The impaired oxygen supply and demand in our CKD and RT cohort was not significantly associated with the degree of anemia.
Clinical Implications
CAD in the CKD patients is often multivessel and causes silent or asymptomatic myocardial ischemia.5,46 Myocardial ischemia can be caused by both epicardial or microvascular CAD. Our CKD and RT cohorts demonstrate impaired myocardial oxygenation in all coronary artery territories, suggesting multivessel microvascular CAD, which has been shown to be present in the CKD population.47 It may explain the reduced accuracy of stress echocardiography in detecting ischemia in CKD patients.9 Stress echocardiography technique detects inducible myocardial ischemia based on detection of wall-motion abnormalities, and thus would detect significant epicardial CAD, not microvascular disease.8 Microvascular CAD has been shown to be associated with reduced survival.48 Thus, our study may lead to better management of multivessel microvascular CAD and cardiac risk factors in the CKD and post-transplant population.
Study Limitations
Our study has some limitations. The sample size was small, consistent with being a pilot study; therefore, the findings need to be confirmed in a larger patient population. However, we applied strict inclusion and exclusion criteria and made every possible effort to include CKD patients who were reasonably well clinically and without previous CAD,systolic heart failure, significant valvular stenosis, or significant conduction disorder. The dialysis group was older. It is very difficult to find relatively young patients who are dialysis dependent since the median age of dialysis patients at our institution is 75. However, the predialysis CKD group with impaired myocardial oxygenation had a similar mean age to the controls. Secondly, the CKD patients are contraindicated to have gadolinium contrast due to the risk of nephrogenic systemic fibrosis; therefore, absolute quantification of stress perfusion CMR and late gadolinium enhancement cannot be performed. Noncontrast T1 mapping, which would have been useful for assessing diffuse fibrosis, unfortunately was not available in our center at time of the study. Co-registration of images was not performed, but there was careful alignment between the rest and stress images using well-recognized landmarks in the heart (eg, anterior and posterior insertion point of the right ventricle into the intraventricular septum). While elastic changes related to stress may not be completely accounted for by this method, we feel that this is unlikely to have resulted in major errors. Recently, myocardial oxygenation response to breathing maneuvers49 and controlled vasodilatory carbon dioxide delivery through hypercapnia50 have been described, and may be able to offer a more comfortable option of BOLD CMR in CKD patients; however, further research is indicated. BOLD techniques are constantly evolving. The new approach of cardiac phase-resolved BOLD potentially may enable assessment of myocardial ischemia completely at rest.51
Conclusions
Our study suggests that the myocardial oxygenation response to stress is impaired in CKD patients and RT recipients, and is unlikely to be accounted for by the presence of diabetes mellitus, left ventricular hypertrophy, or myocardial scarring. These patients did not have known epicardial CAD, and therefore it is likely microvascular disease. The impaired myocardial oxygenation response to stress may be associated with the degree of renal function. There was no significant correlation between BOLD SI change and the level of hemoglobin. This study demonstrates a new technique for examining myocardial oxygenation response to stress in patients with CKD or who have received a RT. Furthermore, it suggests that multivessel microvascular coronary disease may be highly prevalent in this population who have a high prevalence of cardiac mortality and morbidity.
Acknowledgments
Author Contributions: Parnham recruited the patients, undertook data collection, analysis, interpretation of the results, and drafted the manuscript. Grover wrote the initial study application, helped analyze the data, and made critical changes to the manuscript. Perry analyzed the strain echo data. Woodman provided statistical analysis and made critical review of the manuscript. De Pasquale and Bangalore made critical review of the manuscript. Gleadle and Selvanayagam conceived the original study design, made an interpretation of the data, and critically reviewed the manuscript.
Disclosures
None.
References
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg A. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75:1291–1295. doi: 10.1097/01.TP.0000061602.03327.E2. [DOI] [PubMed] [Google Scholar]
- Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;82:603–611. doi: 10.1097/01.tp.0000235527.81917.fe. [DOI] [PubMed] [Google Scholar]
- Ohtake T, Kobayashi S, Moriya H, Negishi K, Okamoto K, Maesato K, Saito S. High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: an angiographic examination. J Am Soc Nephrol. 2005;16:1141–1148. doi: 10.1681/ASN.2004090765. [DOI] [PubMed] [Google Scholar]
- Charytan D, Kuntz RE, Mauri L, DeFilippi C. Distribution of coronary artery disease and relation to mortality in asymptomatic hemodialysis patients. Am J Kidney Dis. 2007;49:409–416. doi: 10.1053/j.ajkd.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–S23. [PubMed] [Google Scholar]
- Parnham S, Gleadle J, De Pasquale C, Selvanayagam J. Myocardial ischemia assessment in chronic kidney disease: challenges and pitfalls. Front Cardiovasc Med. 2014;1:1–5. doi: 10.3389/fcvm.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LW, Fahim MA, Hayen A, Mitchell RL, Lord SW, Baines LA, Lord S, Craig JC, Webster AC. Cardiac testing for coronary artery disease in potential kidney transplant recipients: a systematic review of test accuracy studies. Am J Kidney Dis. 2011;57:476–487. doi: 10.1053/j.ajkd.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Parnham S, Selvanayagam J. Myocardial blood oxygenation assessment: ready for clinical prime time? Curr Cardiovasc Imaging Rep. 2013:1–3. [Google Scholar]
- Manka R, Paetsch I, Schnackenburg B, Gebker R, Fleck E, Jahnke C. BOLD cardiovascular magnetic resonance at 3.0 tesla in myocardial ischemia. J Cardiovasc Magn Reson. 2010;12:54. doi: 10.1186/1532-429X-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JR, Karamitsos TD, Bhamra-Ariza P, Francis JM, Searle N, Robson MD, Howells RK, Choudhury RP, Rimoldi OE, Camici PG, Banning AP, Neubauer S, Jerosch-Herold M, Selvanayagam JB. Myocardial oxygenation in coronary artery disease: insights from blood oxygen level-dependent magnetic resonance imaging at 3 tesla. J Am Coll Cardiol. 2012;59:1954–1964. doi: 10.1016/j.jacc.2012.01.055. [DOI] [PubMed] [Google Scholar]
- Zheng J, Wang J, Nolte M, Li D, Gropler RJ, Woodard PK. Dynamic estimation of the myocardial oxygen extraction ratio during dipyridamole stress by MRI: a preliminary study in canines. Magn Reson Med. 2004;51:718–726. doi: 10.1002/mrm.20025. [DOI] [PubMed] [Google Scholar]
- Vohringer M, Flewitt JA, Green JD, Dharmakumar R, Wang J, Jr, Tyberg JV, Friedrich MG. Oxygenation-sensitive CMR for assessing vasodilator-induced changes of myocardial oxygenation. J Cardiovasc Magn Reson. 2010;12:20. doi: 10.1186/1532-429X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCommis KS, Zhang H, Goldstein TA, Misselwitz B, Abendschein DR, Gropler RJ, Zheng J. Myocardial blood volume is associated with myocardial oxygen consumption: an experimental study with cardiac magnetic resonance in a canine model. JACC Cardiovasc Imaging. 2009;2:1313–1320. doi: 10.1016/j.jcmg.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCommis KS, Goldstein TA, Abendschein DR, Herrero P, Misselwitz B, Gropler RJ, Zheng J. Quantification of regional myocardial oxygenation by magnetic resonance imaging: validation with positron emission tomography. Circ Cardiovasc Imaging. 2010;3:41–46. doi: 10.1161/CIRCIMAGING.109.897546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder SB, Holmes AA, McVeigh ER, Forder JR. Simultaneous noninvasive determination of regional myocardial perfusion and oxygen content in rabbits: toward direct measurement of myocardial oxygen consumption at MR imaging. Radiology. 1999;212:739–747. doi: 10.1148/radiology.212.3.r99se27739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SM, Fieno DS, Schirf BE, Bi X, Huang J, Omary RA, Li D. T2-prepared steady-state free precession blood oxygen level-dependent MR imaging of myocardial perfusion in a dog stenosis model. Radiology. 2005;236:503–509. doi: 10.1148/radiol.2362040149. [DOI] [PubMed] [Google Scholar]
- Foltz WD, Huang H, Fort S, Wright GA. Vasodilator response assessment in porcine myocardium with magnetic resonance relaxometry. Circulation. 2002;106:2714–2719. doi: 10.1161/01.cir.0000039475.66067.dc. [DOI] [PubMed] [Google Scholar]
- Fieno DS, Shea SM, Li Y, Harris KR, Finn JP, Li D. Myocardial perfusion imaging based on the blood oxygen level-dependent effect using T2-prepared steady-state free-precession magnetic resonance imaging. Circulation. 2004;110:1284–1290. doi: 10.1161/01.CIR.0000140673.13057.34. [DOI] [PubMed] [Google Scholar]
- Dharmakumar R, Arumana JM, Tang R, Harris K, Zhang Z, Li D. Assessment of regional myocardial oxygenation changes in the presence of coronary artery stenosis with balanced SSFP imaging at 3.0 T: theory and experimental evaluation in canines. J Magn Reson Imaging. 2008;27:1037–1045. doi: 10.1002/jmri.21345. [DOI] [PubMed] [Google Scholar]
- Karamitsos TD, Arnold JR, Pegg TJ, Francis JM, Birks J, Jerosch-Herold M, Neubauer S, Selvanayagam JB. Patients with syndrome X have normal transmural myocardial perfusion and oxygenation: a 3-T cardiovascular magnetic resonance imaging study. Circ Cardiovasc Imaging. 2012;5:194–200. doi: 10.1161/CIRCIMAGING.111.969667. [DOI] [PubMed] [Google Scholar]
- Karamitsos TD, Leccisotti L, Arnold JR, Recio-Mayoral A, Bhamra-Ariza P, Howells RK, Searle N, Robson MD, Rimoldi OE, Camici PG, Neubauer S, Selvanayagam JB. Relationship between regional myocardial oxygenation and perfusion in patients with coronary artery disease: insights from cardiovascular magnetic resonance and positron emission tomography. Circ Cardiovasc Imaging. 2010;3:32–40. doi: 10.1161/CIRCIMAGING.109.860148. [DOI] [PubMed] [Google Scholar]
- Wacker CM, Hartlep AW, Pfleger S, Schad LR, Ertl G, Bauer WR. Susceptibility-sensitive magnetic resonance imaging detects human myocardium supplied by a stenotic coronary artery without a contrast agent. J Am Coll Cardiol. 2003;41:834–840. doi: 10.1016/s0735-1097(02)02931-5. [DOI] [PubMed] [Google Scholar]
- Friedrich MG, Niendorf T, Schulz-Menger J, Gross CM, Dietz R. Blood oxygen level-dependent magnetic resonance imaging in patients with stress-induced angina. Circulation. 2003;108:2219–2223. doi: 10.1161/01.CIR.0000095271.08248.EA. [DOI] [PubMed] [Google Scholar]
- Beache GM, Herzka DA, Boxerman JL, Post WS, Gupta SN, Faranesh AZ, Solaiyappan M, Bottomley PA, Weiss JL, Shapiro EP, Hill MN. Attenuated myocardial vasodilator response in patients with hypertensive hypertrophy revealed by oxygenation-dependent magnetic resonance imaging. Circulation. 2001;104:1214–1217. doi: 10.1161/hc3601.096307. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghome KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgam BR, Levey AS. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamitsos TD, Hudsmith LE, Selvanayagam JB, Neubauer S, Francis JM. Operator induced variability in left ventricular measurements with cardiovascular magnetic resonance is improved after training. J Cardiovasc Magn Reson. 2007;9:777–783. doi: 10.1080/10976640701545073. [DOI] [PubMed] [Google Scholar]
- Cheng AS, Robson MD, Neubauer S, Selvanayagam JB. Irreversible myocardial injury: assessment with cardiovascular delayed-enhancement MR imaging and comparison of 1.5 and 3.0 T–initial experience. Radiology. 2007;242:735–742. doi: 10.1148/radiol.2423060299. [DOI] [PubMed] [Google Scholar]
- Saxena SK, Sharma M, Patel M, Oreopoulos D. Nephrogenic systemic fibrosis: an emerging entity. Int Urol Nephrol. 2008;40:715–724. doi: 10.1007/s11255-008-9361-8. [DOI] [PubMed] [Google Scholar]
- Rydahl C, Thomsen HS, Marckmann P. High prevalence of nephrogenic systemic fibrosis in chronic renal failure patients exposed to gadodiamide, a gadolinium-containing magnetic resonance contrast agent. Invest Radiol. 2008;43:141–144. doi: 10.1097/RLI.0b013e31815a3407. [DOI] [PubMed] [Google Scholar]
- Othersen JB, Maize JC, Woolson RF, Budisavljevic MN. Nephrogenic systemic fibrosis after exposure to gadolinium in patients with renal failure. Nephrol Dial Transplant. 2007;22:3179–3185. doi: 10.1093/ndt/gfm584. [DOI] [PubMed] [Google Scholar]
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS American Heart Association Writing Group on Myocardial S, Registration for Cardiac I. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- Selvanayagam JB, Jerosch-Herold M, Porto I, Sheridan D, Cheng AS, Petersen SE, Searle N, Channon K, Banning A, Neubauer S. Resting myocardial blood flow is impaired in hibernating myocardium: a magnetic resonance study of quantitative perfusion assessment. Circulation. 2005;112:3289–3296. doi: 10.1161/CIRCULATIONAHA.105.549170. [DOI] [PubMed] [Google Scholar]
- Friedrich MG, Karamitsos TD. Oxygenation-sensitive cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:43. doi: 10.1186/1532-429X-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker CM, Bock M, Hartlep AW, Bauer WR, van Kaick G, Pfleger S, Erti G, Schad LR. BOLD-MRI in ten patients with coronary artery disease: evidence for imaging of capillary recruitment in myocardium supplied by the stenotic artery. MAGMA. 1999;8:48–54. doi: 10.1007/BF02590635. [DOI] [PubMed] [Google Scholar]
- Walcher T, Manzke R, Hombach V, Rottbauer W, Wohrle J, Bernhardt P. Myocardial perfusion reserve assessed by T2-prepared steady-state free precession blood oxygen level-dependent magnetic resonance imaging in comparison to fractional flow reserve. Circ Cardiovasc Imaging. 2012;5:580–586. doi: 10.1161/CIRCIMAGING.111.971507. [DOI] [PubMed] [Google Scholar]
- Luu JM, Friedrich MG, Harker J, Dwyer N, Guensch D, Mikami Y, Faris P, Hare JL. Relationship of vasodilator-induced changes in myocardial oxygenation with the severity of coronary artery stenosis: a study using oxygenation-sensitive cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2014;15:1358–1367. doi: 10.1093/ehjci/jeu138. [DOI] [PubMed] [Google Scholar]
- Marcus ML, Harrison DG, Chilian WM, Koyanagi S, Inou T, Tomanek RJ, Martins JB, Eastham CL, Hiratzka LF. Alterations in the coronary circulation in hypertrophied ventricles. Circulation. 1987;75:I19–I25. [PubMed] [Google Scholar]
- Mahmod M, Francis JM, Pal N, Lewis A, Dass S, De Silva R. Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. J Cardiovasc Magn Reson. 2014;16:29. doi: 10.1186/1532-429X-16-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamitsos TD, Dass S, Suttie J, Sever E, Birks J, Holloway CJ, Robson MD, Jerosch-Herold M, Watkins H, Neubauer S. Blunted myocardial oxygenation response during vasodilator stress in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;61:1169–1176. doi: 10.1016/j.jacc.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egred M, Al-Mohammad A, Waiter GD, Redpath TW, Semple SK, Norton M, Welch A, Walton S. Detection of scarred and viable myocardium using a new magnetic resonance imaging technique: blood oxygen level dependent (BOLD) MRI. Heart. 2003;89:738–744. doi: 10.1136/heart.89.7.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattilo G, Lamari A, Zito C, Carerj S, Marte F, Patane S. 2-Dimensional strain echocardiography and early detection of myocardial ischemia. Int J Cardiol. 2010;145:e6–e8. doi: 10.1016/j.ijcard.2008.12.100. [DOI] [PubMed] [Google Scholar]
- Gjesdal O, Hopp E, Vartdal T, Lunde K, Helle-Valle T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Global longitudinal strain measured by two-dimensional speckle tracking echocardiography is closely related to myocardial infarct size in chronic ischaemic heart disease. Clin Sci (Lond) 2007;113:287–296. doi: 10.1042/CS20070066. [DOI] [PubMed] [Google Scholar]
- Bello AK, Hemmelgarn B, Lloyd A, James MT, Manns BJ, Klarenbach S, Tonelli M. Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol. 2011;6:1418–1426. doi: 10.2215/CJN.09741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase H, Tsunoda T, Tanaka Y, Takahashi Y, Imamura Y, Ishikawa H, Inishi Y, Joki N. Risk factors for de novo acute cardiac events in patients initiating hemodialysis with no previous cardiac symptom. Kidney Int. 2006;70:1142–1148. doi: 10.1038/sj.ki.5001726. [DOI] [PubMed] [Google Scholar]
- Charytan DM, Shelbert HR, Di Carli MF. Coronary microvascular function in early chronic kidney disease. Circ Cardiovasc Imaging. 2010;3:663–671. doi: 10.1161/CIRCIMAGING.110.957761. [DOI] [PubMed] [Google Scholar]
- Lin T, Rechenmacher S, Rasool S, Varadarajan P, Pai RG. Reduced survival in patients with “coronary microvascular disease”. Int J Angiol. 2012;21:89–94. doi: 10.1055/s-0032-1315799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Guensch DP, Friedrich MG. Response of myocardial oxygenation to breathing manoeuvres and adenosine infusion. Eur Heart J Cardiovasc Imaging. 2015;16:395–401. doi: 10.1093/ehjci/jeu202. [DOI] [PubMed] [Google Scholar]
- Guensch DP, Fischer K, Flewitt JA, Friedrich MG. Impact of intermittent apnea on myocardial tissue oxygenation–a study using oxygenation-sensitive cardiovascular magnetic resonance. PLoS One. 2013;8:e53282. doi: 10.1371/journal.pone.0053282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu C, Morisi R, Boschetto D, Dharmakumar R, Tsaftaris SA. Synthetic generation of myocardial blood-oxygen-level-dependent MRI time series via structural sparse decomposition modeling. IEEE Trans Med Imaging. 2014;33:1422–1433. doi: 10.1109/TMI.2014.2313000. [DOI] [PMC free article] [PubMed] [Google Scholar]