Abstract
Background
The latest guidelines do not make clear recommendations on the selection of antiplatelet therapies for long-term secondary prevention of stroke. We aimed to integrate the available evidence to create hierarchies of the comparative efficacy and safety of long-term antiplatelet therapies after ischemic stroke or transient ischemic attack.
Methods and Results
We performed a network meta-analysis of randomized controlled trials to compare 11 antiplatelet therapies in patients with ischemic stroke or transient ischemic attack. In December 2014, we searched Medline, Embase, and the Cochrane Library database for trials. The search identified 24 randomized controlled trials including a total of 85 667 patients with antiplatelet treatments for at least 1 year. Cilostazol significantly reduced stroke recurrence in comparison with aspirin (odds ratio 0.66, 95% credible interval 0.44 to 0.92) and dipyridamole (odds ratio 0.57, 95% credible interval 0.34 to 0.95), respectively. Cilostazol also significantly reduced intracranial hemorrhage compared with aspirin, clopidogrel, terutroban, ticlopidine, aspirin plus clopidogrel, and aspirin plus dipyridamole. Aspirin plus clopidogrel could not significantly reduce stroke recurrence compared with monotherapies but caused significantly more major bleeding than all monotherapies except terutroban. The pooled estimates did not change materially in the sensitivity analyses of the primary efficacy outcome.
Conclusions
Long-term monotherapy was a better choice than long-term dual therapy, and cilostazol had the best risk–benefit profile for long-term secondary prevention after stroke or transient ischemic attack. More randomized controlled trials in non–East Asian patients are needed to determine whether long-term use of cilostazol is the best option for the prevention of recurrent stroke.
Keywords: antiplatelet, network meta-analysis, stroke, transient ischemic attack
Stroke is the second most common cause of death and the third most common cause of disability worldwide.1,2 As stroke mortality has decreased over the past 2 decades, the absolute number of stroke survivors has increased and is huge.3 Given the high recurrence rate, secondary prevention of future stroke among these survivors plays a pivotal role in reducing disease burden.4 The use of antiplatelet agents is the standard treatment for patients with noncardioembolic ischemic stroke or transient ischemic attack (TIA).4 A number of randomized controlled trials (RCTs) have tested different antiplatelet mono- and dual therapies in secondary prevention after ischemic stroke or TIA5–28; however, comparisons of some antiplatelet therapies are currently lacking.
Several pairwise meta-analyses were performed previously to compare the efficacy of antiplatelet agents for the secondary prevention of stroke.29–31 These studies, however, could not generate clear hierarchies for the efficacy and safety of all available antiplatelet therapies because many antiplatelet treatments have not been compared head to head and because such analyses could not integrate all of the evidence from several comparators. Using a statistical technique called network meta-analysis, we were able to take advantage of both direct (head-to-head) and indirect evidence and formally compare all existing therapies.32,33
Two previous network meta-analyses were conducted to compare the efficacy of antiplatelet therapies among stroke or TIA patients34,35; however, neither provided hierarchies for the efficacy and safety of antiplatelet therapies. The earlier study compared only a small number of antiplatelet therapies.34 The later study identified 24 trials published before March 201235 but failed to incorporate a few major large-scale trials, such as the SPS3 trial,24 the JASAP study,17 and the study published by Fukuuchi et al.14 In addition, the second network meta-analysis did not restrict the duration of antiplatelet therapy.35 Because the American Heart Association/American Stroke Association (AHA/ASA) guidelines recommend that patients with ischemic stroke or TIA continuously receive antiplatelet treatment,4 we believe it is more important to evaluate overall recurrent stroke reduction and bleeding risk of long-term antiplatelet therapies in these patients. To achieve this goal, we performed the network meta-analysis of RCTs to compare the effectiveness and safety of long-term antiplatelet treatments among patients with ischemic stroke or TIA.
Methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.36 Ethics approval was not necessary for this study because only deidentified pooled data from individual studies were analyzed.
Data Sources and Search Strategy
A systematic literature search was conducted December 26, 2014, by searching Medline via Web of Science, Embase and Journals@Ovid Full Text via OvidSP, and the Cochrane Library database for trials. We limited our search to RCTs conducted in humans. Details of our search strategy are provided in Table S1. The search strategy in the current study was similar to those used in previous studies.37,38
Study Selection
RCTs were eligible for inclusion if they met the following criteria: Antiplatelet monotherapy versus monotherapy or dual versus monotherapy was tested in adult patients (aged ≥18 years) with ischemic stroke or TIA and had a treatment duration of at least 1 year. Because network meta-analysis requires a reasonably homogeneous sample,39,40 we did not include those RCTs assessing antiplatelet therapy (mostly aspirin) versus placebo because such studies had a wide range of daily doses (aspirin, from 75 to 1500 mg).41,42 Another reason is that the evaluation of antiplatelet therapy versus placebo becomes less important.
Initially, titles alone were reviewed for suitability. The abstracts of suitable titles were obtained and reviewed for suitability for full-text retrieval. Data were then extracted from suitable full-text reports. Additional appropriate reports were added when discovered by citation tracking.
Data Extraction and Quality Assessment
Data were independently extracted and assessed by 2 authors (F.Z. and B.Z.) using a predetermined data collection template. To resolve discrepancies about inclusion of studies and interpretation of data, a third investigator (W.X.) was consulted, and consensus was reached by discussion.
The primary efficacy outcome was stroke recurrence, including ischemic, hemorrhagic, and unknown stroke, and fatal and nonfatal stroke. The secondary efficacy outcome was the composite outcome of vascular events and all-cause or vascular mortality. The safety outcomes were intracranial hemorrhage and major bleeding. The definitions of the 4 outcomes in included trials are summarized in Table S2.
Study quality was independently assessed by 3 reviewers (F.Z., B.Z., and X.S.) who used the Cochrane Collaboration’s risk-of-bias method.43 Figure S1 shows the risk of bias of the included trials.
Data Synthesis and Analysis
Network meta-analysis combines direct and indirect evidence for all relative treatment effects and provides estimates with maximum statistical power.44–47 We fit the models within a Bayesian framework using WinBUGS software (version 1.4.3).48 The models, the WinBUGS codes, and R routines used in this study were open and could be found online.49 Convergence was assessed by running 3 Markov chains, and all results pertain to 100 000 Markov chain Monte Carlo cycles after a 10 000-simulation burn-in phase. Relative effect sizes were calculated as odds ratios (ORs) with corresponding 95% credible intervals. We assessed the fitness of our model using the deviance information criterion, a measure of model fitness that penalizes model complexity. If the tradeoff between model fitness and complexity favored the model with assumed consistency, this model was preferred (smaller deviance information criterion values correspond to more preferable values).50 As shown in Table S3, the assumption of consistency was supported for each outcome by a better tradeoff between model fitness and complexity (a smaller deviance information criterion value) when consistency was assumed rather than when it was not. We used the surface under the cumulative ranking curve, or SUCRA, probabilities to rank the antiplatelet therapies:47,51 SUCRA is a proportion expressed as the percentage of efficacy of an intervention on the outcome that would be ranked first without uncertainty, which equals 100% when the treatment is certain to be the best and 0% when it is certain to be the worst.47 The network results were assessed for consistency by comparing them with the results of pairwise meta-analyses. Furthermore, we estimated inconsistency as the difference between direct and indirect estimates (called the inconsistency factor) and the corresponding 95% confidence interval (CI) for inconsistency factor in each closed loop by using the R code ifplot.fun, which could be found online.52 Inconsistent loops are those that present inconsistency factors with 95% CIs incompatible with zero. Pairwise meta-analyses were performed by using STATA (version 11; Stata Corp) within a random-effects framework that takes study heterogeneity into account to generate the pooled OR and 95% CI. The percentage of variability across studies attributable to heterogeneity beyond chance was estimated using the I2 statistic.
We did sensitivity analyses on the primary efficacy outcome to explore whether the results of the present network meta-analysis were sensitive to certain restrictions on the data included. Those planned in advance were restricted to double-blind trials (n=18) and true randomization and allocation-concealed trials (n=16).
Results
Study Selection and Characteristics
Figure 1 shows the study selection process according to the PRISMA statement. The initial search and citation tracking identified 2678 publications. Fifty-five articles were reviewed by full text for details, and 31 of those were excluded. Finally, a total of 24 RCTs with 85 667 patients were included in the present network meta-analysis.5–28 Tables 1 and 2 summarize the characteristics of the 24 included trials. The following antiplatelet therapies were tested in the trials: cilostazol versus aspirin (3 trials with 3459 patients),9,11,15 clopidogrel versus aspirin (1 trial with 6431 patients),8 dipyridamole versus aspirin (1 trial with 3303 patients),13 sarpogrelate versus aspirin (1 trial with 1499 patients),23 terutroban versus aspirin (1 trial with 19 100 patients),21 ticlopidine versus aspirin (4 trials with 5488 patients),5,16,18,27 ticlopidine versus clopidogrel (1 trial with 1151 patients),14 triflusal versus aspirin (2 trials with 2536 patients),25,26 aspirin plus clopidogrel versus aspirin (2 trials with 7340 patients),10,24 aspirin plus clopidogrel versus clopidogrel (1 trial with 7599 patients),19 aspirin plus dipyridamole versus aspirin (5 trials with 8622 patients),6,7,12,13,17 aspirin plus dipyridamole versus clopidogrel (1 trial with 20 332 patients),22 aspirin plus dipyridamole versus dipyridamole (2 trials with 3490 patients),13,20 and aspirin plus ticlopidine versus ticlopidine (1 trial with 270 patients).28
Figure 1.
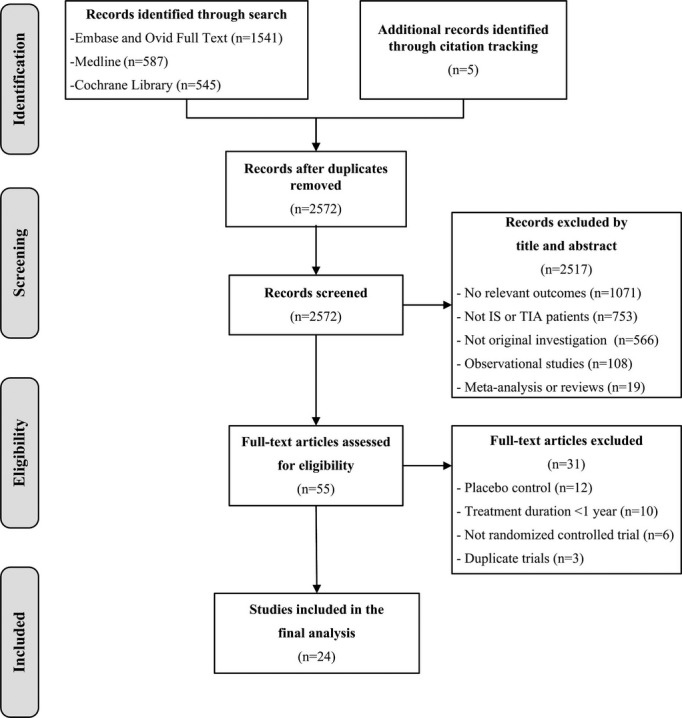
Study selection flow diagram adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. IS indicates ischemic stroke; TIA, transient ischemic attack.
Table 1.
Baseline Characteristics of Included Trials
| Trial | Country | Centers | Patients | Sample Size | Treatment Onset, Month | Treatment Duration, Month | Male, % | Mean Age, Y | Follow-Up, Mo | Lost to Follow-Up, % | Blinding |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AAASPS 20035 | US | 62 | IS | 1809 | 0.25 to 3 | 19 (mean) | 47 | 61 | 19 (mean) | 3.8 | Double-blind |
| ACCSG 19856 | US and Canada | 15 | TIA | 890 | <3 | 18 (median) | 67 | 63 | 25 (median) | 4.2 | Double-blind |
| AICLA 19837 | France | 4 | IS, TIA | 400 | <12 | 36 | 69 | 63 | 36 | 3.8 | Double-blind |
| CAPRIE 19968 | Worldwide | 384 | IS | 6431 | 0.25 to 6 | 20 (mean) | 64 | 65 | 23 (mean) | 0.7 | Double-blind |
| CASISP 20089 | China | 13 | IS | 719 | 1 to 6 | 12 to 18 | 69 | 60 | 12 (mean) | 1.3 | Double-blind |
| CHARISMA 201110 | Worldwide | 768 | IS, TIA | 4320 | <60 | 25 (median) | 63 | 65 | 25 (median) | 0.5 | Double-blind |
| CSPS2 201011 | Japan | 278 | IS | 2672 | <7 | 12 to 60 | 72 | 64 | 29 (mean) | 0.2 | Double-blind |
| ESPRIT 200612 | Worldwide | 79 | Minor IS, TIA | 2739 | <6 | 42 (mean) | 66 | 63 | 42 (mean) | 3.8 | Open |
| ESPS2 199613 | Europe | 59 | IS, TIA | 4953 | <3 | 24 | 58 | 67 | 24 | 0.6 | Double-blind |
| Fukuuchi 200714 | Japan | 129 | IS | 1151 | >0.25 | 52 | 73 | 65 | 52 | NS | Double-blind |
| Guo 200915 | China | 1 | IS | 68 | 1 to 6 | 12 | 35 | 61 | 12 | NS | NS |
| Hass 198916 | US and Canada | 56 | IS, TIA, RI | 3069 | <3 | 27 (mean) | 65 | 63 | 24 to 72 | 2.7 | Double-blind |
| JASAP 201117 | Japan | 157 | IS | 1294 | 0.25 to 6 | 15 (mean) | 72 | 66 | 24 | 0.2 | Double-blind |
| Li 200018 | China | 9 | IS, TIA | 329 | NS | 12 (mean) | 71 | 64 | 12 (mean) | 2.4 | NS |
| MATCH 200419 | Worldwide | 507 | IS, TIA | 7599 | <3 | 18 | 63 | 66 | 18 | 4.3 | Double-blind |
| Matias-Guiu 198720 | Spain | 1 | TIA | 186 | <12 | 21 (mean) | 77 | 55 | 21 (mean) | 4.5 | Open |
| PERFORM 201121 | Worldwide | 802 | IS, TIA | 19 100 | <3 | 28 (mean) | 63 | 67 | 28 (mean) | 0.3 | Double-blind |
| PRoFESS 200822 | Worldwide | 695 | IS | 20 332 | <3 | 30 (mean) | 64 | 66 | 30 (mean) | 0.6 | Double-blind |
| S-ACCESS 200823 | Japan | 14 | IS | 1499 | 0.25 to 6 | 19 (mean) | 72 | 65 | 19 (mean) | NS | Double-blind |
| SPS3 201224 | Worldwide | 82 | IS | 3020 | <6 | 41 (mean) | 63 | 63 | 41 (mean) | 2.0 | Double-blind |
| TACIP 200325 | Portugal and Spain | 43 | IS, TIA | 2107 | <6 | 12 to 36 | 66 | 65 | 30 (mean) | 2.1 | Double-blind |
| TAPIRSS 200426 | Argentina | 19 | IS, TIA | 429 | <6 | 19 (mean) | 68 | 65 | 19 (mean) | NS | Double-blind |
| Tohgi 198727 | Japan | 101 | TIA | 281 | <3 | 36 | NS | NS | 36 | NS | 12-mo double-blind, ≥24-mo open |
| TOPALS 200328 | Japan | 25 | IS, TIA | 270 | <6 | 19 (mean) | 65 | 67 | 19 (mean) | NS | NS |
IS indicates ischemic stroke; NS, not specified; RI, retinal ischemia; TIA, transient ischemic attack.
Table 2.
Antiplatelet Treatments and Outcomes of Included Trials
| Trial | Treatment Groups and Dosages | Stroke Recurrence | Composite Outcome | Intracranial Hemorrhage | Major Bleeding | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 1 | Arm 2 | Arm 3 | Arm 1 | Arm 2 | Arm 3 | Arm 1 | Arm 2 | Arm 3 | Arm 1 | Arm 2 | Arm 3 | |
| AAASPS 20035 | Tic (250 mg BID) | Asp (325 mg BID) | — | 107/902 | 86/907 | — | 145/902 | 125/907 | — | NS | NS | — | 4/902 | 8/907 | — |
| ACCSG 19856 | Asp (325 mg QID)+Dip (75 mg QID) | Asp (325 mg QID) | — | 53/448 | 60/442 | — | 99/448 | 96/442 | — | 2/448 | 4/442 | — | 15/448 | 21/442 | — |
| AICLA 19837 | Asp (330 mg TID)+Dip (75 mg TID) | Asp (330 mg TID) | — | 18/202 | 17/198 | — | 28/202 | 30/198 | — | 1/202 | 2/198 | — | 3/202 | 3/198 | — |
| CAPRIE 19968 | Clop (75 mg OD) | Asp (325 mg OD) | — | 315/3233 | 338/3198 | — | 433/3233 | 461/3198 | — | NS | NS | — | NS | NS | — |
| CASISP 20089 | Cilo (100 mg BID) | Asp (100 mg OD) | — | 12/360 | 20/359 | — | 24/360 | 37/359 | — | 1/360 | 5/359 | — | 1/360 | 6/359 | — |
| CHARISMA 201110 | Asp (75 to 162 mg OD)+Clop (75 mg OD) | Asp (75 to 162 mg OD) | — | 105/2157 | 131/2163 | — | 174/2157 | 207/2163 | — | 13/2157 | 11/2163 | — | 92/2157 | 61/2163 | — |
| CSPS2 201011 | Cilo (100 mg BID) | Asp (81 mg OD) | — | 82/1337 | 119/1335 | — | 138/1337 | 186/1335 | — | 8/1337 | 27/1335 | — | 23/1337 | 57/1335 | — |
| ESPRIT 200612 | Asp (30 to 325 mg OD)+Dip (200 mg BID) | Asp (30 to 325 mg OD) | — | 108/1363 | 137/1376 | — | 198/1363 | 239/1376 | — | 12/1363 | 21/1376 | — | 35/1363 | 53/1376 | — |
| ESPS2 199613 | Asp (25 mg BID)+Dip (200 mg BID) | Dip (200 mg BID) | Asp (25 mg bid) | 157/1650 | 211/1654 | 206/1649 | 206/1650 | 271/1654 | 266/1649 | NS | NS | NS | 60/1650 | 24/1654 | 53/1649 |
| Fukuuchi 200714 | Clop (75 mg OD) | Tic (200 mg OD) | — | 17/573 | 15/578 | — | 25/573 | 24/578 | — | 4/573 | 1/578 | — | 5/573 | 1/578 | — |
| Guo 200915 | Cilo (100 mg BID) | Asp (100 mg OD) | — | 2/34 | 2/34 | — | 2/34 | 5/34 | — | 0/34 | 1/34 | — | 0/34 | 2/34 | — |
| Hass 198916 | Tic (250 mg BID) | Asp (650 mg BID) | — | 172/1529 | 212/1540 | — | 306/1529 | 349/1540 | — | 7/1529 | 7/1540 | — | 14/1529 | 28/1540 | — |
| JASAP 201117 | Asp (25 mg BID)+Dip (200 mg BID) | Asp (81 mg OD) | — | 57/655 | 39/639 | — | 69/655 | 58/639 | — | 13/655 | 13/639 | — | 26/655 | 23/639 | — |
| Li 200018 | Tic (250 mg OD) | Asp (50 mg OD) | — | 11/165 | 21/164 | — | 13/165 | 26/164 | — | 6/165 | 4/164 | — | 7/165 | 7/164 | — |
| MATCH 200419 | Asp (75 mg OD)+Clop (75 mg OD) | Clop (75 mg OD) | — | 339/3797 | 347/3802 | — | 552/3797 | 567/3802 | — | 40/3759 | 25/3781 | — | 73/3759 | 22/3781 | — |
| Matias-Guiu 198720 | Asp (50 mg OD)+Dip (100 mg TID) | Dip (100 mg QID) | — | 9/115 | 7/71 | — | 25/115 | 15/71 | — | NS | NS | — | NS | NS | — |
| PERFORM 201121 | Teru (30 mg OD) | Asp (100 mg OD) | — | 842/9556 | 828/9544 | — | 1530/9556 | 1485/9544 | — | 146/9479 | 121/9466 | — | 211/9479 | 210/9466 | — |
| PRoFESS 200822 | Asp (25 mg BID)+Dip (200 mg BID) | Clop (75 mg OD) | — | 916/10 181 | 898/10 151 | — | 1637/10 181 | 1630/10 151 | — | 147/10 181 | 103/10 151 | — | 419/10 181 | 365/10 151 | — |
| S-ACCESS 200823 | Sarp (100 mg TID) | Asp (81 mg OD) | — | 79/747 | 70/752 | — | 110/747 | 98/752 | — | 9/750 | 12/757 | — | NS | NS | — |
| SPS3 201224 | Asp (325 mg OD)+Clop (75 mg OD) | Asp (325 mg OD) | — | 125/1517 | 138/1503 | — | 239/1517 | 232/1503 | — | 22/1517 | 15/1503 | — | 105/1517 | 56/1503 | — |
| TACIP 200325 | Trif (600 mg OD) | Asp (325 mg OD) | — | 109/1055 | 112/1052 | — | 145/1055 | 141/1052 | — | 7/1055 | 11/1052 | — | 20/1055 | 42/1052 | — |
| TAPIRSS 200426 | Trif (600 mg OD) | Asp (325 mg OD) | — | 18/213 | 18/216 | — | 27/213 | 34/216 | — | 1/214 | 2/216 | — | 1/214 | 7/216 | — |
| Tohgi 198727 | Tic (200 mg OD) | Asp (500 mg BID) | — | 4/136 | 8/145 | — | 29/136 | 46/145 | — | 2/136 | 2/145 | — | NS | NS | — |
| TOPALS 200328 | Asp (81 mg OD)+Tic (100 mg OD) | Tic (200 mg OD) | — | 9/132 | 7/138 | — | 13/132 | 14/138 | — | 2/132 | 0/138 | — | NS | NS | — |
Asp indicates aspirin; Cilo, cilostazol; Clop, clopidogrel; Dip, dipyridamole; NS, not specified; Sarp, sarpogrelate; Teru, terutroban; Tic, ticlopidine; Trif, triflusal.
Network Meta-Analysis
The network of antiplatelet treatment comparisons for stroke recurrence is shown in Figure 2. The network meta-analysis results for stroke recurrence and intracranial hemorrhage are reported in Table 3. Cilostazol significantly reduced stroke recurrence compared with aspirin (OR 0.66, 95% credible interval 0.44 to 0.92) and dipyridamole (OR 0.57, 95% credible interval 0.34 to 0.95), respectively. Intracranial hemorrhage was also significantly reduced by cilostazol compared with aspirin, clopidogrel, terutroban, ticlopidine, aspirin plus clopidogrel, and aspirin plus dipyridamole.
Figure 2.
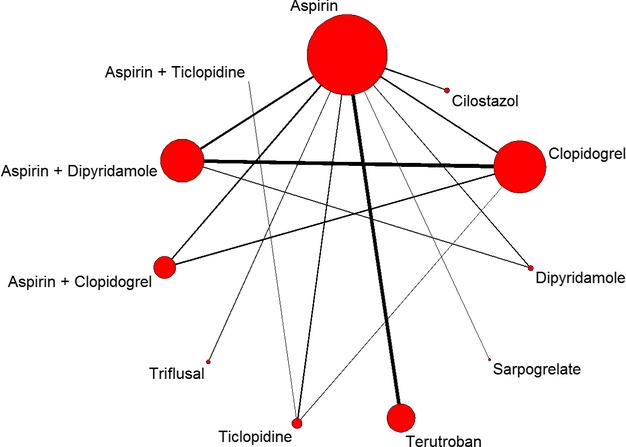
Network of treatment comparisons for the primary efficacy outcome. The size of the node corresponds to the total sample size of the treatment from all included trials. Directly comparable treatments are linked with a line, the thickness of which corresponds to the total sample size for assessing the comparison.
Table 3.
Results for Stroke Recurrence (Upper Diagonal Part) and Intracranial Hemorrhage (Lower Diagonal Part) From Network Meta-Analyses
| Treatment | Asp | Cilo | Clop | Dip | Sarp | Teru | Tic | Trif | Asp+Clop | Asp+Dip | Asp+Tic |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp | 1.00 | 0.66 (0.44 to 0.92)* | 0.89 (0.72 to 1.14) | 1.13 (0.80 to 1.54) | 1.19 (0.69 to 1.87) | 1.04 (0.72 to 1.44) | 0.87 (0.65 to 1.10) | 0.98 (0.68 to 1.37) | 0.85 (0.68 to 1.09) | 0.89 (0.74 to 1.06) | 1.45 (0.40 to 3.78) |
| Cilo | 0.27 (0.08 to 0.58)* | 1.00 | 1.39 (0.91 to 2.16) | 1.77 (1.06 to 2.94)* | 1.85 (0.97 to 3.12) | 1.62 (0.98 to 2.64) | 1.36 (0.85 to 2.15) | 1.53 (0.90 to 2.47) | 1.33 (0.86 to 2.05) | 1.38 (0.91 to 2.13) | 2.27 (0.59 to 6.48) |
| Clop | 0.74 (0.37 to 1.49) | 3.62 (1.07 to 10.60)* | 1.00 | 1.28 (0.85 to 1.79) | 1.35 (0.75 to 2.15) | 1.18 (0.75 to 1.73) | 0.99 (0.70 to 1.32) | 1.12 (0.70 to 1.65) | 0.97 (0.74 to 1.23) | 1.01 (0.77 to 1.27) | 1.65 (0.43 to 4.39) |
| Dip | — | — | — | 1.00 | 1.07 (0.59 to 1.80) | 0.94 (0.58 to 1.50) | 0.79 (0.50 to 1.16) | 0.89 (0.52 to 1.41) | 0.77 (0.52 to 1.12) | 0.80 (0.58 to 1.12) | 1.32 (0.33 to 3.56) |
| Sarp | 0.88 (0.23 to 2.32) | 4.44 (0.78 to 14.55) | 1.32 (0.27 to 3.90) | — | 1.00 | 0.93 (0.49 to 1.64) | 0.78 (0.43 to 1.28) | 0.88 (0.46 to 1.50) | 0.76 (0.42 to 1.29) | 0.79 (0.44 to 1.27) | 1.30 (0.31 to 3.68) |
| Teru | 1.27 (0.53 to 2.45) | 6.15 (1.55 to 16.85)* | 1.94 (0.56 to 4.37) | — | 2.06 (0.41 to 6.21) | 1.00 | 0.87 (0.53 to 1.28) | 0.98 (0.57 to 1.57) | 0.85 (0.56 to 1.24) | 0.88 (0.59 to 1.31) | 1.44 (0.35 to 3.83) |
| Tic | 0.96 (0.39 to 2.02) | 4.71 (1.09 to 14.72)* | 1.43 (0.46 to 3.34) | — | 1.60 (0.31 to 5.17) | 0.87 (0.28 to 2.19) | 1.00 | 1.14 (0.75 to 1.75) | 0.99 (0.71 to 1.37) | 1.03 (0.75 to 1.43) | 1.67 (0.47 to 4.38) |
| Trif | 0.68 (0.18 to 1.72) | 3.23 (0.59 to 10.75) | 1.02 (0.20 to 2.89) | — | 1.09 (0.15 to 3.94) | 0.65 (0.13 to 1.67) | 0.83 (0.16 to 2.62) | 1.00 | 0.90 (0.56 to 1.35) | 0.93 (0.63 to 1.34) | 1.53 (0.37 to 3.93) |
| Asp+Clop | 1.31 (0.67 to 2.46) | 6.45 (1.72 to 19.79)* | 1.91 (0.94 to 3.50) | — | 2.11 (0.48 to 6.08) | 1.18 (0.42 to 2.87) | 1.63 (0.52 to 3.90) | 2.72 (0.66 to 7.90) | 1.00 | 1.05 (0.78 to 1.40) | 1.73 (0.45 to 4.73) |
| Asp+Dip | 0.85 (0.46 to 1.37) | 4.17 (1.20 to 11.06)* | 1.25 (0.55 to 2.03) | — | 1.38 (0.32 to 3.81) | 0.77 (0.26 to 1.78) | 1.05 (0.34 to 2.30) | 1.74 (0.42 to 5.12) | 0.70 (0.29 to 1.32) | 1.00 | 1.65 (0.45 to 4.21) |
| Asp+Tic | — | — | — | — | — | — | — | — | — | — | 1.00 |
Each cell gives an odds ratio (OR) and 95% credible interval. In the upper diagonal part, the OR compares the column condition with the row condition, and in the lower diagonal part, this OR compares the row condition with the column condition. Asp indicates aspirin; Cilo, cilostazol; Clop, clopidogrel; Dip, dipyridamole; NS, not specified; Sarp, sarpogrelate; Teru, terutroban; Tic, ticlopidine; Trif, triflusal.
Significant results.
Similarly, cilostazol significantly reduced the composite outcome compared with aspirin (OR 0.68, 95% credible interval 0.48 to 0.93) and dipyridamole (OR 0.59, 95% credible interval 0.39 to 0.95) and reduced major bleeding compared with aspirin, clopidogrel, terutroban, aspirin plus clopidogrel, and aspirin plus dipyridamole (Table 4). In addition, dipyridamole, ticlopidine, and triflusal caused significantly less major bleeding than aspirin; terutroban caused significantly more major bleeding than dipyridamole and triflusal; aspirin plus clopidogrel caused significantly more major bleeding than aspirin, clopidogrel, dipyridamole, ticlopidine, triflusal, and aspirin plus dipyridamole; and aspirin plus dipyridamole caused significantly more major bleeding than dipyridamole and triflusal.
Table 4.
Results for Composite Outcome (Upper Diagonal Part) and Major Bleeding (Lower Diagonal Part), From Network Meta-Analyses
| Treatment | Asp | Cilo | Clop | Dip | Sarp | Teru | Tic | Trif | Asp+Clop | Asp+Dip | Asp+Tic |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp | 1.00 | 0.68 (0.48 to 0.93)* | 0.93 (0.77 to 1.16) | 1.11 (0.78 to 1.51) | 1.17 (0.72 to 1.77) | 1.04 (0.73 to 1.43) | 0.86 (0.67 to 1.06) | 0.96 (0.64 to 1.33) | 0.92 (0.73 to 1.18) | 0.88 (0.74 to 1.10) | 0.93 (0.33 to 2.15) |
| Cilo | 0.35 (0.15 to 0.62)* | 1.00 | 1.42 (0.95 to 2.10) | 1.68 (1.06 to 2.54)* | 1.79 (0.98 to 2.95) | 1.59 (0.95 to 2.48) | 1.32 (0.85 to 1.92) | 1.47 (0.86 to 2.33) | 1.40 (0.91 to 2.06) | 1.41 (0.97 to 2.04) | 1.43 (0.48 to 3.49) |
| Clop | 0.74 (0.43 to 1.15) | 2.50 (1.02 to 5.85)* | 1.00 | 1.20 (0.79 to 1.72) | 1.27 (0.75 to 2.05) | 1.13 (0.75 to 1.56) | 0.93 (0.67 to 1.20) | 1.04 (0.65 to 1.57) | 0.99 (0.75 to 1.30) | 1.00 (0.78 to 1.28) | 1.01 (0.35 to 2.34) |
| Dip | 0.40 (0.19 to 0.74)* | 1.30 (0.46 to 3.10) | 0.57 (0.24 to 1.12) | 1.00 | 1.09 (0.56 to 1.79) | 0.97 (0.57 to 1.56) | 0.80 (0.52 to 1.17) | 0.89 (0.53 to 1.37) | 0.86 (0.57 to 1.29) | 0.86 (0.62 to 1.17) | 0.87 (0.28 to 2.02) |
| Sarp | — | — | — | — | 1.00 | 0.94 (0.50 to 1.57) | 0.78 (0.44 to 1.22) | 0.87 (0.46 to 1.54) | 0.83 (0.49 to 1.34) | 0.83 (0.51 to 1.34) | 0.84 (0.26 to 2.06) |
| Teru | 1.04 (0.56 to 1.76) | 3.35 (1.35 to 7.55)* | 1.48 (0.66 to 2.90) | 2.89 (1.12 to 6.04)* | — | 1.00 | 0.85 (0.56 to 1.27) | 0.95 (0.56 to 1.49) | 0.91 (0.61 to 1.37) | 0.91 (0.63 to 1.36) | 0.92 (0.31 to 2.30) |
| Tic | 0.53 (0.29 to 0.89)* | 1.75 (0.61 to 3.69) | 0.76 (0.33 to 1.37) | 1.49 (0.56 to 2.92) | — | 0.56 (0.24 to 1.00) | 1.00 | 1.13 (0.72 to 1.67) | 1.08 (0.80 to 1.51) | 1.09 (0.83 to 1.49) | 1.08 (0.41 to 2.46) |
| Trif | 0.41 (0.18 to 0.74)* | 1.31 (0.45 to 2.92) | 0.59 (0.20 to 1.22) | 1.13 (0.37 to 2.53) | — | 0.42 (0.15 to 0.87)* | 0.83 (0.30 to 1.85) | 1.00 | 0.99 (0.63 to 1.56) | 0.99 (0.64 to 1.49) | 1.00 (0.35 to 2.39) |
| Asp+Clop | 1.93 (1.30 to 3.05)* | 6.32 (2.86 to 14.22)* | 2.71 (1.58 to 4.43)* | 5.37 (2.39 to 11.10)* | — | 2.02 (0.94 to 3.91) | 3.94 (1.83 to 7.81)* | 5.41 (2.33 to 11.28)* | 1.00 | 1.02 (0.75 to 1.37) | 1.03 (0.35 to 2.36) |
| Asp+Dip | 0.87 (0.62 to 1.19) | 2.86 (1.26 to 6.18)* | 1.23 (0.79 to 1.87) | 2.39 (1.21 to 4.44)* | — | 0.91 (0.47 to 1.65) | 1.78 (0.87 to 3.22) | 2.46 (1.09 to 5.34)* | 0.47 (0.27 to 0.76)* | 1.00 | 1.01 (0.37 to 2.35) |
| Asp+Tic | — | — | — | — | — | — | — | — | — | — | 1.00 |
Each cell gives an odds ratio (OR) and 95% credible interval. In the upper diagonal part, the OR compares the column condition with the row condition, and in the lower diagonal part, this OR compares the row condition with the column condition. Asp indicates aspirin; Cilo, cilostazol; Clop, clopidogrel; Dip, dipyridamole; NS, not specified; Sarp, sarpogrelate; Teru, terutroban; Tic, ticlopidine; Trif, triflusal.
Significant results.
Table 5 shows the mean values of SUCRA probabilities that provided the hierarchies for the efficacy and safety of the 11 antiplatelet therapies. In particular, cilostazol displayed the best risk–benefit profile, with SUCRA probabilities of 0.9343, 0.9252, 0.9718, and 0.8850 for reducing stroke recurrence, composite outcome, intracranial hemorrhage, and major bleeding, respectively. Figures S2 through S5 show the ranking probability of each treatment for outcomes.
Table 5.
The SUCRA Probabilities of Antiplatelet Therapies on Efficacy and Safety Outcomes
| Treatment | Stroke Recurrence | Composite Outcome | Intracranial Hemorrhage | Major Bleeding | ||||
|---|---|---|---|---|---|---|---|---|
| SUCRA | Rank* | SUCRA | Rank | SUCRA | Rank | SUCRA | Rank | |
| Aspirin | 0.3551 | 7 | 0.3465 | 8 | 0.3361 | 7 | 0.2136 | 8 |
| Cilostazol | 0.9343 | 1 | 0.9252 | 1 | 0.9718 | 1 | 0.8850 | 1 |
| Clopidogrel | 0.6252 | 5 | 0.5380 | 6 | 0.6487 | 3 | 0.4950 | 5 |
| Dipyridamole | 0.2250 | 11 | 0.2446 | 10 | — | — | 0.8221 | 2 |
| Sarpogrelate | 0.2433 | 10 | 0.2223 | 11 | 0.5538 | 4 | — | — |
| Terutroban | 0.3547 | 8 | 0.3234 | 9 | 0.2025 | 8 | 0.2451 | 7 |
| Ticlopidine | 0.6510 | 3 | 0.6854 | 2 | 0.4496 | 6 | 0.6740 | 4 |
| Triflusal | 0.4551 | 6 | 0.4811 | 7 | 0.6794 | 2 | 0.8096 | 3 |
| Aspirin plus clopidogrel | 0.7033 | 2 | 0.5741 | 4 | 0.1650 | 9 | 0.0064 | 9 |
| Aspirin plus dipyridamole | 0.6321 | 4 | 0.5657 | 5 | 0.4931 | 5 | 0.3492 | 6 |
| Aspirin plus ticlopidine | 0.3208 | 9 | 0.5937 | 3 | — | — | — | — |
SUCRA indicates surface under the cumulative ranking curve.
Ranking SUCRA probabilities in order as the best treatment, the second best, the third best, and so on, among the antiplatelet therapies.
No inconsistent loop was identified in the analyses of inconsistency factor (Figure S6).
Pairwise Meta-Analysis
We examined pairwise comparisons of all interventions with available head-to-head data. The results are presented in Figures 3 through 6. In general, the results obtained from pairwise meta-analysis closely matched those of the network meta-analysis. Stroke recurrence, composite efficacy outcome, intracranial hemorrhage, and major bleeding were all significantly reduced by cilostazol versus aspirin. Among the 22 pairwise meta-analyses, each of which included at least 2 trials (Figures 3 through 6), significant heterogeneity was identified in 2 pairwise meta-analyses. One of the 2 pairwise meta-analyses compared ticlopidine with aspirin for preventing stroke recurrence (including 4 trials, I2=69.9, P=0.019) (Figure 3), and the other compared ticlopidine with aspirin for composite outcome (including 4 trials, I2=73.1, P=0.011) (Figure 4). There was no evidence of heterogeneity across trials in the remaining 20 pairwise meta-analyses.
Figure 3.
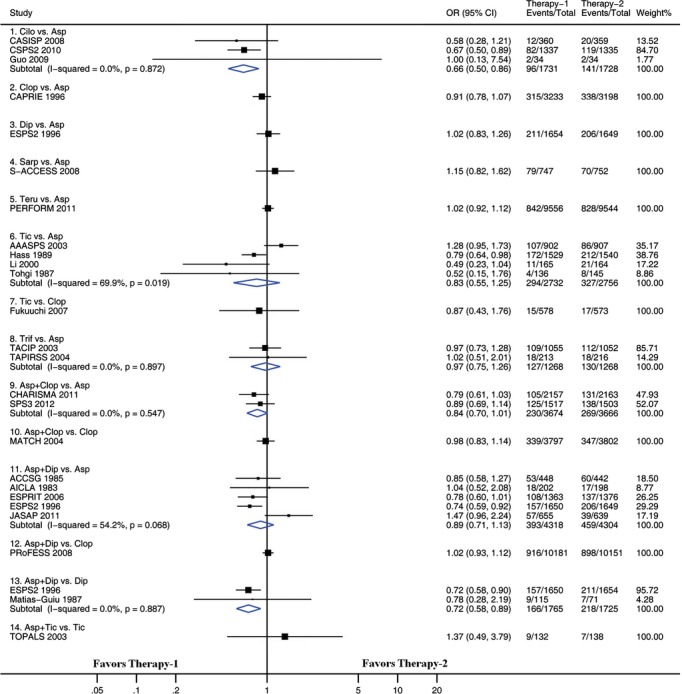
Pairwise meta-analyses comparing antiplatelet therapies on the composite outcome. Squares represent point estimates for effect size expressed as an odds ratio, with the size proportional to the inverse variance of the estimate. Diamonds represent pooled estimates. Lines represent 95% CIs. Asp indicates aspirin; CI, confidence interval; Cilo, cilostazol; Clop, clopidogrel; Dip, dipyridamole; OR, odds ratio; Sarp, sarpogrelate; Teru, terutroban; Tic, ticlopidine; Trif, triflusal.
Figure 6.
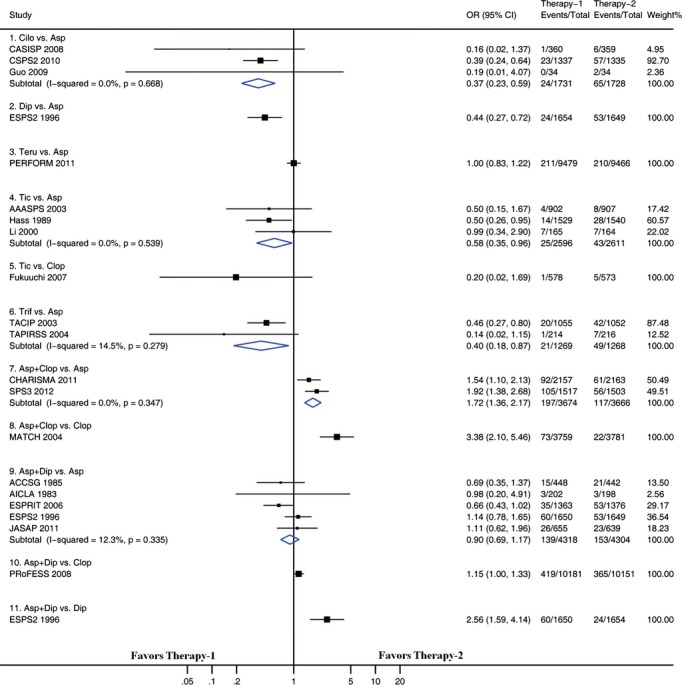
Pairwise meta-analyses comparing antiplatelet therapies on stroke recurrence. Squares represent point estimates for effect size expressed as an odds ratio, with the size proportional to the inverse variance of the estimate. Diamonds represent pooled estimates. Lines represent 95% CIs. Asp indicates aspirin; CI, confidence interval; Cilo, cilostazol; Clop, clopidogrel; Dip, dipyridamole; OR, odds ratio; Sarp, sarpogrelate; Teru, terutroban; Tic, ticlopidine; Trif, triflusal.
Figure 4.
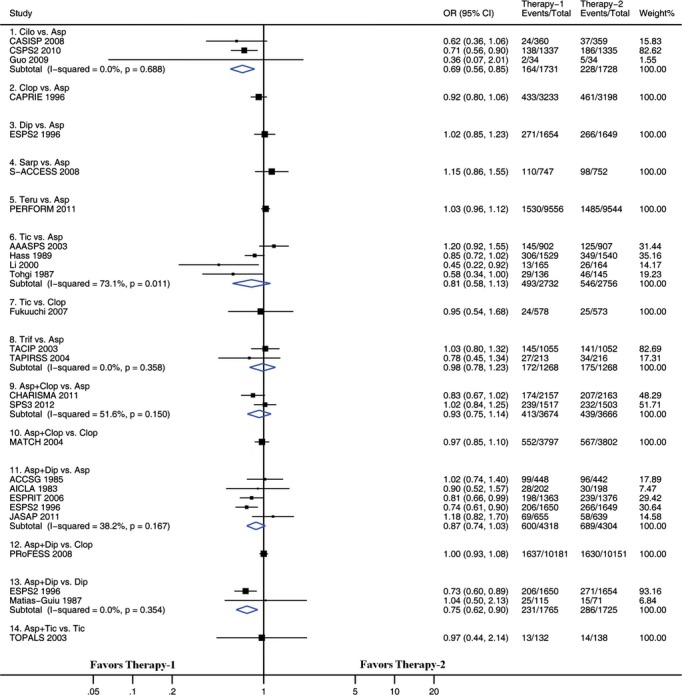
Pairwise meta-analyses comparing antiplatelet therapies on intracranial hemorrhage. Squares represent point estimates for effect size expressed as an odds ratio, with the size proportional to the inverse variance of the estimate. Diamonds represent pooled estimates. Lines represent 95% CIs. Asp indicates aspirin; CI, confidence interval; Cilo, cilostazol; Clop, clopidogrel; Dip, dipyridamole; OR, odds ratio; Sarp, sarpogrelate; Teru, terutroban; Tic, ticlopidine; Trif, triflusal.
Figure 5.
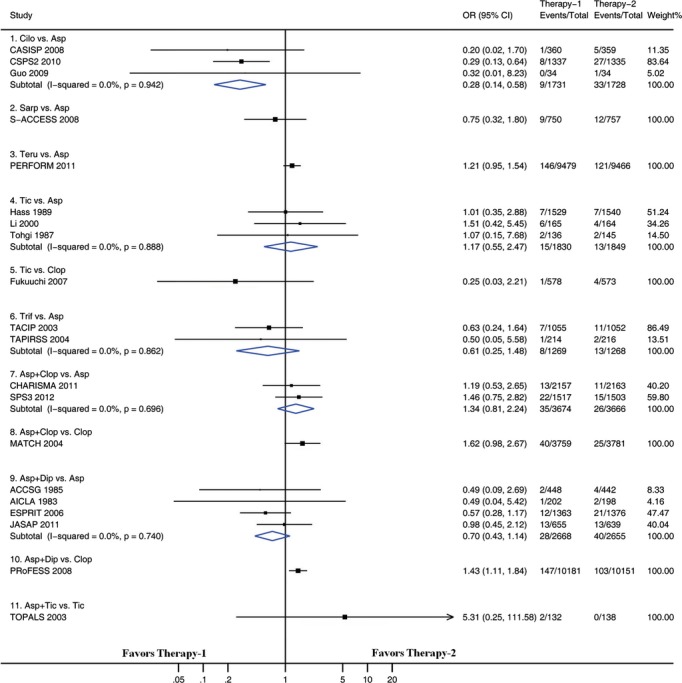
Pairwise meta-analyses comparing antiplatelet therapies on major bleeding. Squares represent point estimates for effect size expressed as an odds ratio, with the size proportional to the inverse variance of the estimate. Diamonds represent pooled estimates. Lines represent 95% CIs. Asp indicates aspirin; CI, confidence interval; Cilo, cilostazol; Clop, clopidogrel; Dip, dipyridamole; OR, odds ratio; Sarp, sarpogrelate; Teru, terutroban; Tic, ticlopidine; Trif, triflusal.
Sensitivity Analysis
The pooled risk estimates did not change substantially in the sensitivity analyses on the primary efficacy outcome from both network meta-analyses and pairwise meta-analyses. Tables S4 and S5 and Figures S7 and S8 show the full results of the sensitivity analysis.
Discussion
Our network meta-analysis provided evidence-based hierarchies for the efficacy and safety of long-term antiplatelet mono- and dual therapies among patients with ischemic stroke or TIA. It overcame the major limitation of conventional pairwise meta-analyses by combining direct and indirect evidence of relative treatments in the analysis. Results from this study indicated that when compared with antiplatelet monotherapy, dual therapy was not associated with a reduction in stroke recurrence and composite outcome but rather with a significant increase in the risk of major bleeding, especially aspirin plus clopidogrel. In addition, our results showed that cilostazol displayed the best risk–benefit profile among the 11 antiplatelet treatments.
The effects of dual therapy in short- and long-term prevention of recurrent stroke might be different. A recent meta-analysis that combined the results from 14 RCTs reported that dual therapy was more effective than monotherapy in reducing the risk of early recurrent stroke in patients with an index stroke in the previous 3 days.37 The latest AHA/ASA guidelines also recommend that the combination of aspirin and clopidogrel might be considered for initiation within 24 hours of a minor ischemic stroke or TIA.4 For long-term secondary prevention, however, the combination of aspirin and clopidogrel is not recommended by the AHA/ASA guidelines for routine long-term secondary prevention of stroke due to high risk of bleeding,4 which is consistent with our results. Moreover, a recent pairwise meta-analysis based on 7 RCTs that involved 39 574 patients with ischemic stroke or TIA reported that antiplatelet dual therapy lasting >1 year is not associated with a greater reduction in overall recurrent stroke risk than monotherapy, and that finding also supported our results.29 As far as we know, this network meta-analysis is the first to evaluate the efficacy and safety of long-term antiplatelet therapies after ischemic stroke or TIA and provides the most robust evidence in support of long-term monotherapy as a better choice than long-term dual therapy.
The present study indicated that cilostazol had the best risk–benefit profile among 11 antiplatelet therapies and supported cilostazol as a possible therapeutic option to recommend for secondary prevention of stroke. In the CASISP trial, which included 720 Chinese patients with ischemic stroke within the previous 1 to 6 months, cilostazol reduced the rate of recurrent stroke compared with aspirin (hazard ratio 0.62, 95% CI 0.30 to 1.26), although the benefit was not significant.9 The rate of any hemorrhagic event was also lower in the cilostazol group than in the aspirin group.9 The CSPS 2 study in 2757 Japanese patients is another trial conducted in an East Asian population to compare the efficacy and safety of cilostazol and aspirin in patients with ischemic stroke.11 This trial found that cilostazol significantly reduced the recurrence rate of stroke compared with aspirin (hazard ratio 0.74, 95% CI 0.56 to 0.98) and that major bleeding events occurred in fewer patients on cilostazol than on aspirin (hazard ratio 0.46, 95% CI 0.30 to 0.71).11 On the basis of this evidence, cilostazol has been approved by the China Food and Drug Administration for treatment of noncardioembolic ischemic stroke (license number H10960014), and the latest Chinese guidelines for secondary prevention of stroke recommends cilostazol (100 mg BID) as an alternative to aspirin.53 Similarly, cilostazol is used in Japan for secondary prevention of stroke and is included in the Japanese guidelines for the treatment of ischemic stroke.54 Cilostazol is not licensed in the United States for ischemic stroke or TIA treatment because the efficacy and safety of cilostazol have not been tested in non–East Asian patients. Generalizing the effect of cilostazol to other groups can be challenging because the risk of both ischemic and hemorrhagic stroke is higher in the East Asian population compared with other populations. Further trials in non–East Asian patients are needed to confirm whether cilostazol is effective and safe as a monotherapy for long-term secondary prevention after ischemic stroke or TIA. In addition, cost-effectiveness studies are also required to explore whether long-term use of cilostazol is cost-effective compared with other monotherapies.
Two previous network meta-analyses have been conducted to compare the effect of antiplatelet therapies after ischemic stroke or TIA34,35; however, neither provided hierarchies for the efficacy and safety of antiplatelet therapies. In one of the studies,34 Thijs et al found that the combination of aspirin and dipyridamole was more effective than aspirin, ticlopidine, and clopidogrel in the prevention of serious vascular events; this finding was not consistent with our analysis. We consider the main reason to be that 13 of the 24 trials identified by Thijs et al did not meet the inclusion criteria for our study because of placebo control or treatment duration <1 year. In addition, the network meta-analysis by Thijs et al excluded trials assessing triflusal, cilostazol, and sarpogrelate and did not report safety data.34 In the other study,35 Malloy et al reported that more overall hemorrhagic events seemed to occur with the combination of aspirin and clopidogrel than with other treatments, and that finding supported our results. Nevertheless, they found that aspirin plus dipyridamole was more protective than aspirin alone, which was not consistent with our results. Similarly, we consider the main reason to be that 9 of the 24 trials identified by Malloy et al did not meet the inclusion criteria for our study.
The main strength of our study is the inclusion of 24 RCTs with 85 667 patients, thus it is the largest evaluation of long-term antiplatelet therapies for stroke recurrence to date. Furthermore, the network meta-analysis based on a Bayesian model makes indirect comparison among multiple treatments available, especially when there are few trials for direct comparison between different antiplatelet therapies. Consequently, this study can provide evidence-based hierarchies for the long-term efficacy and safety of all available antiplatelet therapies among patients with ischemic stroke or TIA.
This study also has some limitations. First, the full-text articles reviewed were limited to English- and Chinese-language studies, and that can introduce selection bias. A relevant article in French identified from the literature was not included in this study.55 Nonetheless, we believe that the possibility of selection bias is reduced by the relatively large number of studies available in English and Chinese. In addition, previous studies demonstrated that excluding studies published in languages other than English generally has little effect on summary effect estimates.56,57 Second, not all included trials reported the results of intracranial hemorrhage or major bleeding, thus some comparisons between antiplatelet therapies for safety outcomes were not available. Third, all comparisons involving aspirin plus ticlopidine are tenuous, given that only 1 small trial was included in this study, and that may affect the stability of relevant results. Finally, most pairs for comparison included only 1 trial, and cilostazol versus aspirin has not been tested in non–East Asian patients, which might undermine the strength of our results to affect clinical practice.
In conclusion, based on this network meta-analysis, we suggested that long-term monotherapy was a better choice than long-term dual therapy and that cilostazol had the best risk–benefit profile for long-term secondary prevention after stroke or TIA. More high-quality trials in non–East Asian patients are needed to determine whether long-term use of cilostazol is the best option for the prevention of recurrent stroke.
Sources of Funding
This study was funded by the National Natural Science Foundation of China (contract no. 81302503). The funder provider had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Disclosures
None.
Supporting Information
Table S1. Search strategy
Table S2. Definitions of the 4 outcomes in included trials
Table S3. Values of deviance information criterion from consistency and inconsistency models
Table S4. Sensitivity analysis on the primary efficacy outcome restricted to 18 double-blind trials, results from a network meta-analysis
Table S5. Sensitivity analysis on the primary efficacy outcome restricted to 16 true randomization and allocation concealed trials, results from a network meta-analysis
Figure S1. Risk of bias assessment.
Figure S2. Cumulative probability and probability of surface under the cumulative ranking curve (SUCRA) for stroke recurrence.
Figure S3. Cumulative probability and probability of surface under the cumulative ranking curve (SUCRA) for the composite outcome.
Figure S4. Cumulative probability and probability of surface under the cumulative ranking curve (SUCRA) for intracranial hemorrhage.
Figure S5. Cumulative probability and probability of surface under the cumulative ranking curve (SUCRA) for major bleeding.
Figure S6. Inconsistent loops in efficacy and safety outcomes, results from inconsistency factor analyses.
Figure S7. Sensitivity analysis on the primary efficacy outcome restricted to 18 double-blind trials, results from pairwise meta-analyses.
Figure S8. Sensitivity analysis on the primary efficacy outcome restricted to 16 true randomization and allocation-concealed trials, results from pairwise meta-analyses.
References
- Global Burden of Diseases, Injuries, and Risk Factors Study 2010 Experts Group. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Diseases, Injuries, and Risk Factors Study 2010 Experts Group. Disability-adjusted life years (DALYS) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Richardson D, Kelly M, Ruland S, Hung E, Harris Y, Kittner S, Leurgans S. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial. JAMA. 2003;289:2947–2957. doi: 10.1001/jama.289.22.2947. [DOI] [PubMed] [Google Scholar]
- The American-Canadian Co-Operative Study Group. Persantine aspirin trial in cerebral ischemia. Part II: endpoint results. Stroke. 1985;16:406–415. doi: 10.1161/01.str.16.3.406. [DOI] [PubMed] [Google Scholar]
- Bousser MG, Eschwege E, Haguenau M, Lefaucconnier JM, Thibult N, Touboul D, Touboul PJ. “AICLA” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke. 1983;14:5–14. doi: 10.1161/01.str.14.1.5. [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z, Li Z, Zhang W, Ding M, Gao X, Fan D, Zeng J, Wong K, Lu C, Xiao J, Yao C. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol. 2008;7:494–499. doi: 10.1016/S1474-4422(08)70094-2. [DOI] [PubMed] [Google Scholar]
- Hankey GJ, Johnston SC, Easton JD, Hacke W, Mas JL, Brennan D, Mak KH, Bhatt DL, Fox KA, Topol EJ. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3–9. doi: 10.1111/j.1747-4949.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, Kitagawa Y, Kusuoka H, Nishimaru K, Tsushima M, Koretsune Y, Sawada T, Hamada C. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9:959–968. doi: 10.1016/S1474-4422(10)70198-8. [DOI] [PubMed] [Google Scholar]
- Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–1673. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- Fukuuchi Y, Tohgi H, Okudera T, Ikeda Y, Miyanaga Y, Uchiyama S, Hirano M, Shinohara Y, Matsumoto M, Yamaguchi T. A randomized, double-blind study comparing the safety and efficacy of clopidogrel versus ticlopidine in Japanese patients with noncardioembolic cerebral infarction. Cerebrovasc Dis. 2008;25:40–49. doi: 10.1159/000111498. [DOI] [PubMed] [Google Scholar]
- Guo JJ, Xu E, Lin QY, Zeng GL, Xie HF. Effect of cilostazol on cerebral arteries in secondary prevention of ischemic stroke. Neurosci Bull. 2009;25:383–390. doi: 10.1007/s12264-009-6192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass WK, Easton JD, Adams HP, Jr, Pryse-Phillips W, Molony BA, Anderson S, Kamm B. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med. 1989;321:501–507. doi: 10.1056/NEJM198908243210804. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Ikeda Y, Urano Y, Horie Y, Yamaguchi T. The Japanese aggrenox (extended-release dipyridamole plus aspirin) stroke prevention versus aspirin programme (JASAP) study: a randomized, double-blind, controlled trial. Cerebrovasc Dis. 2011;31:601–613. doi: 10.1159/000327035. [DOI] [PubMed] [Google Scholar]
- Li Y, Li D, Wang L, Yu P, Han E, Han D, Ma R, Zhu S, Hu Z, Zhi H, Huang Y, Liu T, Xu L, Liu S, Yuan R. A prospective randomized controlled study on ticlopidine with aspirin for the prevention of ischemic cerebral stroke. J Clin Neurol. 2000;13:146–148. [Google Scholar]
- Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- Matias-Guiu J, Davalos A, Pico M, Monasterio J, Vilaseca J, Codina A. Low-dose acetylsalicylic acid (ASA) plus dipyridamole versus dipyridamole alone in the prevention of stroke in patients with reversible ischemic attacks. Acta Neurol Scand. 1987;76:413–421. doi: 10.1111/j.1600-0404.1987.tb03596.x. [DOI] [PubMed] [Google Scholar]
- Bousser MG, Amarenco P, Chamorro A, Fisher M, Ford I, Fox KM, Hennerici MG, Mattle HP, Rothwell PM, de Cordoue A, Fratacci MD. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet. 2011;377:2013–2022. doi: 10.1016/S0140-6736(11)60600-4. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vandermaelen C, Voigt T, Weber M, Yoon BW. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara Y, Nishimaru K, Sawada T, Terashi A, Handa S, Hirai S, Hayashi K, Tohgi H, Fukuuchi Y, Uchiyama S, Yamaguchi T, Kobayashi S, Kondo K, Otomo E, Gotoh F. Sarpogrelate-aspirin comparative clinical study for efficacy and safety in secondary prevention of cerebral infarction (S-ACCESS): a randomized, double-blind, aspirin-controlled trial. Stroke. 2008;39:1827–1833. doi: 10.1161/STROKEAHA.107.505131. [DOI] [PubMed] [Google Scholar]
- Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias-Guiu J, Ferro JM, Alvarez-Sabin J, Torres F, Jimenez MD, Lago A, Melo T. Comparison of triflusal and aspirin for prevention of vascular events in patients after cerebral infarction: the TACIP study: a randomized, double-blind, multicenter trial. Stroke. 2003;34:840–848. doi: 10.1161/01.STR.0000063141.24491.50. [DOI] [PubMed] [Google Scholar]
- Culebras A, Rotta-Escalante R, Vila J, Dominguez R, Abiusi G, Famulari A, Rey R, Bauso-Tosselli L, Gori H, Ferrari J, Reich E. Triflusal vs aspirin for prevention of cerebral infarction: a randomized stroke study. Neurology. 2004;62:1073–1080. doi: 10.1212/01.wnl.0000113757.34662.aa. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Murakami M. The effect of ticlopidine on TIA compared with aspirin-a double-blind, twelve-month and open 24-month follow-up study. Jpn J Med. 1987;26:117–119. [Google Scholar]
- Ito E, Takahashi A, Yamamoto H, Kuzuhara S, Uchiyama S, Nakajima M. Ticlopidine alone versus ticlopidine plus aspirin for preventing recurrent stroke. Intern Med. 2003;42:793–799. doi: 10.2169/internalmedicine.42.793. [DOI] [PubMed] [Google Scholar]
- Lee M, Saver JL, Hong KS, Rao NM, Wu YL, Ovbiagele B. Risk-benefit profile of long-term dual- versus single-antiplatelet therapy among patients with ischemic stroke: a systematic review and meta-analysis. Ann Intern Med. 2013;159:463–470. doi: 10.7326/0003-4819-159-7-201310010-00006. [DOI] [PubMed] [Google Scholar]
- Qian Y, Bi Q. Systematic study of cilostazol on secondary stroke prevention: a meta-analysis. Eur J Med Res. 2013;18:53. doi: 10.1186/2047-783X-18-53. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li X, Zhou G, Zhou X, Zhou S. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92–96. doi: 10.1016/j.jns.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313–2324. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]
- Thijs V, Lemmens R, Fieuws S. Network meta-analysis: simultaneous meta-analysis of common antiplatelet regimens after transient ischaemic attack or stroke. Eur Heart J. 2008;29:1086–1092. doi: 10.1093/eurheartj/ehn106. [DOI] [PubMed] [Google Scholar]
- Malloy RJ, Kanaan AO, Silva MA, Donovan JL. Evaluation of antiplatelet agents for secondary prevention of stroke using mixed treatment comparison meta-analysis. Clin Ther. 2013;35:1490–1500.e1497. doi: 10.1016/j.clinthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KS, Wang Y, Leng X, Mao C, Tang J, Bath PM, Markus HS, Gorelick PB, Liu L, Lin W. Early dual versus mono antiplatelet therapy for acute non-cardioembolic ischemic stroke or transient ischemic attack: an updated systematic review and meta-analysis. Circulation. 2013;128:1656–1666. doi: 10.1161/CIRCULATIONAHA.113.003187. [DOI] [PubMed] [Google Scholar]
- Geeganage CM, Diener HC, Algra A, Chen C, Topol EJ, Dengler R, Markus HS, Bath MW, Bath PM. Dual or mono antiplatelet therapy for patients with acute ischemic stroke or transient ischemic attack: systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:1058–1066. doi: 10.1161/STROKEAHA.111.637686. [DOI] [PubMed] [Google Scholar]
- Caldwell DM, Gibb DM, Ades AE. Validity of indirect comparisons in meta-analysis. Lancet. 2007;369:270. doi: 10.1016/S0140-6736(07)60138-X. author reply 271. [DOI] [PubMed] [Google Scholar]
- Song F, Xiong T, Parekh-Bhurke S, Loke YK, Sutton AJ, Eastwood AJ, Holland R, Chen YF, Glenny AM, Deeks JJ, Altman DG. Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ. 2011;343:d4909. doi: 10.1136/bmj.d4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Salt Collaborative Group. Swedish aspirin low-dose trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet. 1991;338:1345–1349. [PubMed] [Google Scholar]
- Reuther R, Dorndorf W, Loew D. The treatment of transitory ischemic attacks with acetylsalicylic acid: results of a double-blind-study (author’s transl) MMW Munch Med Wochenschr. 1980;122:795–798. [PubMed] [Google Scholar]
- Higgins JP, Green S. Chapter 8: assessing risk of bias in included studies. In: Higgins JP, Altman DG, Sterne JA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Atrium, Southern Gate, Chichester, England: John Wiley & Sons; 2011. pp. 8.1–8.53. [Google Scholar]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17:279–301. doi: 10.1177/0962280207080643. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med. 1996;15:2733–2749. doi: 10.1002/(SICI)1097-0258(19961230)15:24<2733::AID-SIM562>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- The Biostatistics Unit, Medical Research Council, Cambridge Institute of Public Health. Winbugs 1.4.3. Available at: http://www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-winbugs. Accessed October 11, 2014.
- Salanti G, Mavridis D, Nikolakopoulou A, Gianiatsi M. Multiple-treatments meta-analysis of a network of interventions. Available at: http://www.mtm.uoi.gr. Accessed October 11, 2014.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- Salanti G, Mavridis D, Nikolakopoulou A, Gianiatsi M. Presenting the results from an MTM analysis. Available at: http://www.mtm.uoi.gr/index.php/how-to-do-an-mtm/10-how-to-do-an-mtm/20-results. Accessed October 11, 2014.
- Salanti G, Mavridis D, Nikolakopoulou A, Gianiatsi M. Evaluate inconsistency in a network of trials. Available at: http://www.mtm.uoi.gr/index.php/how-to-do-an-mtm/10-how-to-do-an-mtm/18-inconsistency. Accessed October 11 2014.
- Chinese Society of Neurology. Chinese guidelines for the secondary prevention of stroke in patients with ischemic stroke and transient ischemic attack 2014. Chin J Neurol. 2015;48:258–273. [Google Scholar]
- Shinohara Y, Yamaguchi T. Outline of the Japanese guidelines for the management of stroke 2004 and subsequent revision. Int J Stroke. 2008;3:55–62. doi: 10.1111/j.1747-4949.2008.00178.x. [DOI] [PubMed] [Google Scholar]
- Guiraud-Chaumeil B, Rascol A, David J, Boneu B, Clanet M, Bierme R. Prevention of recurrences of cerebral ischemic vascular accidents by platelet antiaggregants. Results of a 3-year controlled therapeutic trial. Rev Neurol (Paris) 1982;138:367–385. [PubMed] [Google Scholar]
- Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31:115–123. doi: 10.1093/ije/31.1.115. [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Klassen TP, Schulz KF, Berlin JA, Jadad AR, Liberati A. What contributions do languages other than english make on the results of meta-analyses? J Clin Epidemiol. 2000;53:964–972. doi: 10.1016/s0895-4356(00)00188-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategy
Table S2. Definitions of the 4 outcomes in included trials
Table S3. Values of deviance information criterion from consistency and inconsistency models
Table S4. Sensitivity analysis on the primary efficacy outcome restricted to 18 double-blind trials, results from a network meta-analysis
Table S5. Sensitivity analysis on the primary efficacy outcome restricted to 16 true randomization and allocation concealed trials, results from a network meta-analysis
Figure S1. Risk of bias assessment.
Figure S2. Cumulative probability and probability of surface under the cumulative ranking curve (SUCRA) for stroke recurrence.
Figure S3. Cumulative probability and probability of surface under the cumulative ranking curve (SUCRA) for the composite outcome.
Figure S4. Cumulative probability and probability of surface under the cumulative ranking curve (SUCRA) for intracranial hemorrhage.
Figure S5. Cumulative probability and probability of surface under the cumulative ranking curve (SUCRA) for major bleeding.
Figure S6. Inconsistent loops in efficacy and safety outcomes, results from inconsistency factor analyses.
Figure S7. Sensitivity analysis on the primary efficacy outcome restricted to 18 double-blind trials, results from pairwise meta-analyses.
Figure S8. Sensitivity analysis on the primary efficacy outcome restricted to 16 true randomization and allocation-concealed trials, results from pairwise meta-analyses.


