Abstract
Background
Cardiac events after revascularization are equally attributable to recurrence at site of culprit lesions and development of nonculprit lesions. We evaluated the hypothesis that coronary computed tomography (CT) angiography and coronary artery calcium score (CACS) performed before revascularization predicts cardiac events after treatment.
Methods and Results
Among 2238 consecutive patients without known coronary artery disease who underwent coronary CT angiography and CACS, 359 patients underwent revascularization within 30 days after CT; in 337 of 359 (93.9%) follow-up clinical information was available. In addition to known cardiac risk factors, CT findings were evaluated as predictors of cardiac events after revascularization: CACS and the presence of CT-verified high-risk plaque (CT-HRP). Improvement of predictive accuracy by including CT findings was evaluated from a discrimination (Harrell’s C-statistics) standpoint. During the follow-up period (median: 673, interquartile range: 47 to 1529 days), a total of 98 cardiac events occurred. Cox proportional hazard model revealed that age, diabetes, triglyceride, CACS, and nonculprit CT-HRP were significant predictors of overall cardiac events. Although not statistically significant, discriminatory power was greater for the model with CACS (C-stat: 63.2%) and the model with both CACS and CT-HRP (65.8%) compared to the model including neither CACS nor CT-HRP (60.7%).
Conclusions
High CACS and the presence of nonculprit CT-HRP performed before revascularization are significant predictors of cardiac events after revascularization.
Keywords: coronary artery calcium score, coronary computed tomography angiography, coronary revascularization, computed tomography–high risk plaque, prognostic value
Cardiac events after revascularization are equally attributable to recurrence at the site of culprit lesions and to development of nonculprit lesions.1 Risk factors of cardiac events related to nonculprit lesions can be detected by virtual histology intravascular ultrasound (eg, plaque burden ≥70%, thin-cap fibroatheroma containing large necrotic cores, and a minimum lumen area ≤4 mm2).1 However, virtual histology intravascular ultrasound is a catheter-based technology and has risks of dissection and acute coronary syndrome,1 and thus is not suitable for coronary screening revascularization.
Coronary computed tomography angiography (CCTA) evaluates coronary artery disease (CAD) noninvasively.2–4 In addition to the evaluation of stenosis, CCTA identifies calcified lesions and characterizes plaque composition.5–10 Although a potential use of CT-derived plaque characterization for prognosis prediction has been investigated,8,9,11–14 to our knowledge there is no report focusing on the prediction ability of cardiac events after treatment by CT plaque characterization. Because CCTA performed before revascularization can evaluate all plaque, including nonculprit lesions, and in theory can serve as a predictor of cardiac events after treatment, the purpose of this study was to test the hypothesis that coronary artery calcium score (CACS) and high-risk lesions identified in a nonculprit lesion before revascularization by CCTA can predict cardiovascular events after revascularization.
Methods
Study Population
This study was approved by the ethics committee at the single recruitment institution, and written informed consent was obtained from subjects. Between September 30, 2008 and December 31, 2011, 2238 consecutive subjects without known CAD (ages: 35 to 74 years) underwent 64- or 320-detector row CCTA and CACS for suspected obstructive CAD. After excluding patients with poor image quality (n=21), 359 subjects were identified as those who underwent percutaneous coronary intervention as part of their standard clinical care within 30 days of CCTA. Among these 359 subjects, 337 (93.9%) had clinical follow-up data and thus formed the study cohort.
CT Acquisition
64-detector row CT protocol
Subjects with a heart rate >60 beats per minute received atenolol (25 mg) by mouth in the evening before the CT examination. Alternatively, heart rate control with a target of 60 beats per minute was achieved using 2 to 10-mg propranolol injected intravenously before data acquisition. The tube voltage for imaging (Aquilion 64; Toshiba Medical Systems Corporation, Tochigi-ken, Japan) was 120 kV. The tube current ranged from 400 to 600 mA; patients with a body mass index of 22 were imaged at 440 mA, and for each 2-point increase or decrease in body mass index, the tube current was incremented by 10 mA. For example, a patient with body mass index of 18 was imaged at 420 mA. The gantry rotation time (0.35 to 0.45 seconds) and beam pitch (0.125 to 0.26) were determined by the manufacturer-based algorithm to optimize image quality. Imaging was performed from caudal to cranial.
Between 60 and 80 mL of iodinated contrast (300 to 370-mg iodine/mL) (Iopamiron-370, Bayer, Osaka, Japan; Omnipaque 300 and 350; Daiichi-Sankyo Inc, Tokyo, Japan) was injected (Stellant Dual Flow, Nihon Medrad K.K., Osaka, Japan) via an antecubital vein using a 3-phase injection method: contrast alone (12 seconds), followed by an equal volume admixture of contrast and saline (9 seconds), and then saline only (2.5 seconds). The iodine load was weight based, and was timed using manual triggering when contrast arrived in the left ventricle.
The ECG gating acquisition strategy was divided into 4 patient groups depending on heart rate, with the patients with heart rate <60 beats per minute subjected to the least exposure and progressively increasing progressively for patients with faster heart rates.15
320-detector row CT protocol
Imaging using the 320-detector row CT paralleled the 64-detector row CT protocol, with the following differences: the first-generation16 320×0.5-mm scanner (Aquilion ONE, Toshiba Medical Systems Corporation) operated on the v4.51 software platform17; and the craniocaudual field of view was tailored to the smallest exposure (10, 12, 12.8, 14, or 16 cm) that encompassed the heart. The default tube voltage was 120 kV. The tube current was modulated according to the patient’s body habitus. Axial imaging had a gantry rotation time that ranged from 0.35 to 0.4 seconds per rotation. Based on prior experience with 64-detector row CCTA, the ECG gating acquisition strategy was divided into 5 heart rate groups.15 Contrast was injected via a 2-phase protocol: contrast medium alone for 10 seconds, followed by saline for 8 seconds. The contrast and saline injection rates were calculated as the individual patient’s mass in kilograms multiplied by 0.06 mL per second.
CACS protocol
CACS was performed by the following parameters: 120 kV, 150 mA, and 3-mm thickness. All data were evaluated on a dedicated workstation (Zio M900 or ZioStation, Ziosoft). A calcified lesion was defined as >3 contiguous pixels with a peak attenuation of at least 130 Hounsfield Units (HU). Regarding CACS, Agatston score was calculated.18 Patients were divided into 3 groups according to CACS as those with score <100, those with a score ranging from 100 to 400, and those with a score >400.
Image reconstruction
Half image reconstruction or segmental image reconstruction was performed in the slow filling phase and/or end-systole. Images used for interpretation were chosen by the attending cardiovascular imager and were based on minimizing motion artifact.
Plaque Interpretation by CT
For plaque detection, both cross-sectional and longitudinal curved multiplanar reformation images were analyzed. The coronary circulation was divided into 17 segments according to American Heart Association recommendations.19 Coronary artery segments with a diameter of >2 mm were evaluated. CCTA interpretation was evaluated by consensus of 2 experienced cardiovascular imagers and an experienced radiological technologist, all of whom were blinded to all clinical data. Nonculprit plaque was defined as plaque at >5 mm distant from stent position and/or at coronary vessels without culprit plaque.
Definition of High-Risk Plaque by CT
CT-verified high-risk plaque (CT-HRP) was defined as plaque with positive remodeling and low density. Positive remodeling was defined as a change in coronary diameter at the location of the plaque when compared to a reference segment with normal appearance (reference diameter). The remodeling index was defined as the lesion diameter divided by the reference diameter, and the measurements were made using both cross-sectional and longitudinal reconstructed images. The remodeling index was considered as positive remodeling when the diameter at the plaque site was at least 10% larger than the reference segment. Plaque density was computed for all noncalcified lesions plus lesions with spotty calcification. The attenuation was defined as the minimum HU among five 0.36×0.36-mm regions of interest. The lesion was defined as a low-density plaque when this minimum HU was <30.
Risk Factors
Hypertension was defined as either systolic or diastolic blood pressure ≥140/90 mm Hg or the use of antihypertensive medications. Diabetes mellitus was defined as fasting blood sugar ≥126 mg/dL or postprandial blood sugar ≥200 mg/dL or hemoglobin A1c ≥6.5% (National Glycohemoglobin Standardization Program), or the use of medications. Dyslipidemia was defined as total cholesterol ≥220 mg/dL, low-density lipoprotein cholesterol ≥140 mg/dL, fasting triglycerides ≥150 mg/dL, high-density cholesterol <40 mg/dL, or the use of lipid-lowering medications. Smokers were defined as those patients who had smoked during the past 1 year from the time of CCTA acquisition.
Study Outcome
Follow-up clinical information was obtained from the review of medical records and/or human research committee approved telephone interviews by attending physicians. The study end point was cardiac events defined as cardiac death, nonfatal myocardial infarction, or hospitalization for unstable or progressive angina during the period from the time of the CT acquisition until December 31, 2012. Cardiac death was defined as death due to acute myocardial infarction, ventricular arrhythmias, refractory heart failure, or cardiogenic shock. Nonfatal myocardial infarction was defined based on the criteria of typical acute chest pain and persistent ST-segment elevation or positive cardiac enzymes.20,21 Hospitalization for unstable or progressive angina was defined based on the Braunwald unstable Angina Classification and the Canadian Cardiovascular Society Angina Classification.1
Statistical Analyses
Statistical analyses were performed using SPSS version 20 for Windows (SPSS Inc, Chicago, IL) and SAS version 9.4 (SAS Inc, Cary, NC). Continuous variables were expressed as the mean±SD and categorical variables were expressed as percentages. Cumulative event proportions were estimated using Kaplan–Meier methods and compared with the log-rank test for variables that were significant predictors for overall cardiac events in the univariate analysis using the Cox proportional hazards model. Cox proportional hazards modeling was used to determine the independent predictors for cardiac events. Significant predictors for cardiac events in the univariate analysis were entered into the multivariable model. Model-fit was tested by the likelihood-ratio test. P<0.05 was considered statistically significant in all instances.
In addition to estimating prognostic significance of each predictor by the coefficients (log hazard ratio) in the multivariable model, the improvement of predictive accuracy of the constructed model was evaluated from discrimination and reclassification standpoints. For discrimination, Harrell’s C-statistics were calculated from linear predictors (“X-beta”) of fitted multivariable models with and without CACS and nonculprit CT-HRP.22,23 Standard errors and P-values for the difference between the models were obtained by 500 bootstrap samples.
Results
Baseline Clinical Characteristics
Baseline clinical characteristics of all study patients are provided in Table 1. Median follow-up duration was 673 (interquartile range: 47 to 1529) days. All 337 subjects underwent revascularization without complication.
Table 1.
Baseline Clinical Characteristics of All Study Patients
| N | 337 |
| Age (y) | 64.2±7.9 |
| Gender (male/female) | 246/91 |
| Hypertension, % | 68.0 |
| Dyslipidemia, % | 85.5 |
| Diabetes, % | 36.2 |
| Current smoking, % | 30.3 |
| Body mass index | 24.5±3.1 |
| Coronary artery calcium score | 433.7±741.6 |
| Coronary artery calcium score (<100/100 to 400/>400) | 119/110/108 |
| Total cholesterol, mg/dL | 201.6±38.3 |
| Triglyceride, mg/dL | 163.2±105.3 |
| LDL cholesterol, mg/dL | 127.0±36.2 |
| HDL cholesterol, mg/dL | 48.5±12.3 |
| Culprit CT-HRP, % | 23.7 |
| Nonculprit CT-HRP, % | 9.5 |
| Statin, % | 57.0 |
| Observation period, days | 710.0±366.8 |
CT-HRP indicates computed tomography–verified high-risk plaque; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
At least 1 nonculprit CT-HRP was observed in 9.5% of patients. During the follow-up period, overall cardiac events occurred in 98 cases: death n=1, nonfatal myocardial infarction n=6, and hospitalization for unstable or progressive angina n=91. Cardiac events related to culprit lesion due to in-stent thrombosis and/or in-stent restenosis occurred in 24 cases.
Predictors of Cardiac Events
Time-to-event analysis showed that the probability of overall cardiac events increased significantly for diabetics (P=0.002) (Figure A – Panel ), CACS category (P=0.007) (Figure B – Panel ), and the presence of nonculprit CT-HRP (P=0.016) (Figure C – Panel ). Univariate analysis using Cox proportional hazards regression revealed that age, diabetes, CACS, triglyceride, and nonculprit CT-HRP were significant predictors of overall cardiac events (Table 2). As for subanalysis for only hard events (cardiac death and nonfatal myocardial infarction), although the small event number (n=7) made all CIs extremely wide, C-statistics and reclassification analysis also suggested that predictive accuracy for hard event could be improved by adding CACS and nonculprit CT-HRP (data are shown in Tables S1 through S5).
Figure 1.
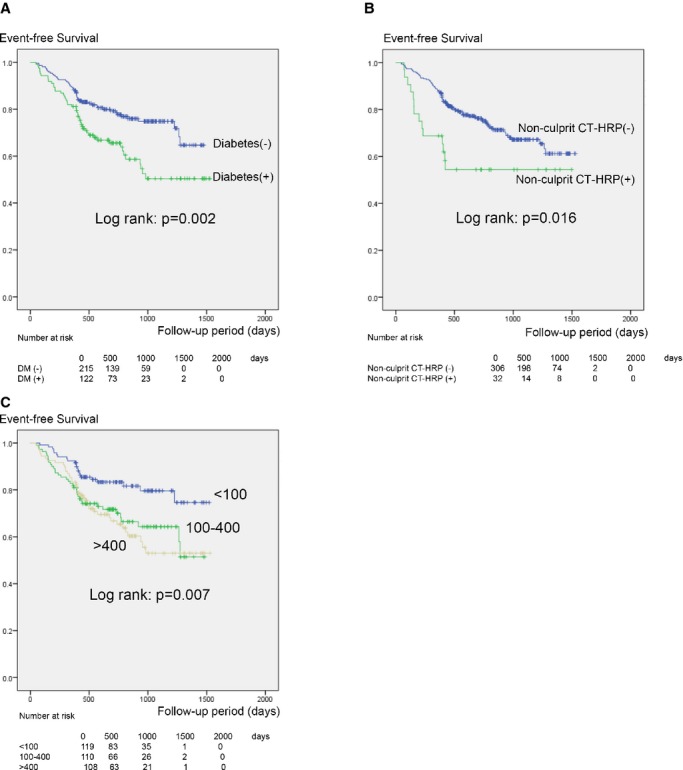
Kaplan–Meier analysis for cardiac event–free survival as stratified by diabetes (A), CACS (B), and nonculprit CT-HRP (C). CACS indicates coronary artery calcium score; CT-HRP, computed tomography–verified high-risk plaque; DM, diabetes mellitus.
Table 2.
Univariate Analysis Using Cox Proportional Hazards Regression to Identify the Significant Predictors of Cardiac Events
| Variables | HR | 95% CI | P Value |
|---|---|---|---|
| Age | 0.973 | 0.950 to 0.995 | P=0.019 |
| Male | 1.310 | 0.823 to 2.085 | P=0.26 |
| Hypertension | 0.840 | 0.556 to 1.267 | P=0.41 |
| Dyslipidemia | 0.914 | 0.520 to 1.606 | P=0.76 |
| Diabetes | 1.859 | 1.253 to 42.759 | P=0.002 |
| Current smoking | 1.339 | 0.885 to 2.026 | P=0.17 |
| Body mass index | 1.030 | 0.965 to 1.099 | P=0.38 |
| CACS <100 (reference) | 1.00 | 1.00 | NA |
| CACS 100 to 400 | 1.987 | 1.172 to 3.369 | P=0.011 |
| CACS >400 | 2.210 | 1.316 to 3.709 | P=0.003 |
| Total cholesterol | 1.005 | 1.000 to 1.010 | P=0.07 |
| Triglyceride | 1.003 | 1.001 to 1.004 | P=0.001 |
| LDL cholesterol | 1.000 | 0.995 to 1.006 | P=0.95 |
| HDL cholesterol | 0.987 | 0.971 to 1.003 | P=0.12 |
| Culprit CT-HRP | 0.805 | 0.494 to 1.313 | P=0.39 |
| Nonculprit CT-HRP | 1.985 | 1.129 to 3.490 | P=0.0178 |
| Statin | 1.000 | 0.672 to 1.489 | P=0.99 |
CACS indicates coronary artery calcium score; CT-HRP, computed tomography–verified high-risk plaque; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; NA, not available.
Prediction Accuracy of the Multivariable Models
Three multivariable models with or without CACS and CT-HRP showed a significant association with cardiac events (Table 3). Likelihood-ratio tests indicate that CACS increased model-fit over the age, diabetes, and triglyceride-only model (P=0.010) and that CT-HRP increased model-fit over the model with CACS (P=0.049). Although not statistically significant at the 5% level, C-statistics for the model with CACS (63.2%) and the model with both CACS and CT-HRP (65.8%) are greater than the model including neither CACS nor CT-HRP (60.7%).
Table 3.
Association and Prediction Measures From the 3 Candidate Cox Models for Cardiac Events
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Hazard Ratio (95% CI) | |||
| Age, per y | 0.982 (0.959, 1.006) | 0.977 (0.954, 1.001) | 0.973 (0.950, 0.997) |
| Diabetes (vs none) | 1.785 (1.197, 2.661) | 1.623 (1.079, 2.442) | 1.590 (1.059, 2.385) |
| Triglyceride, per 10 mg/dL | 1.020 (1.003, 1.037) | 1.018 (1.001, 1.035) | 1.017 (1.000, 1.033) |
| CACS 100 to 400 (vs <400) | 2.018 (1.180, 3.451) | 2.122 (1.236, 3.642) | |
| CACS >400 (vs <400) | 2.049 (1.203, 3.489) | 2.185 (1.285, 3.715) | |
| Nonculprit CT-HRP (vs none) | 2.202 (1.237, 3.918) | ||
| Harrell’s C, % (bootstrap SE)* | 60.67 (3.36) | 63.19 (2.83) | 65.81 (2.69) |
| Two-sided P for c-increment*† | 0.336 | 0.144 | |
| Minus-2 times log likelihood | 1048.6 | 1039.4 | 1033.3 |
| Likelihood ratio P† | 0.010 | 0.049 | |
CACS indicates coronary artery calcium score; CT-HRP, computed tomography–verified high-risk plaque; SE, standard error.
Based on 500 bootstrap samples.
Comparison between adjacent models using bootstrap SE (Models 1 vs 2 or Models 2 vs 3).
Reclassification tables of the model with CACS (Model 2) versus the model including neither CACS nor CT-HRP (Model 1), and the model with both CACS and CT-HRP (Model 3) versus the model with CACS (Model 2) using Net reclassification indices are shown in Tables S1 through S5.
Discussion
The presence of noncalcified plaque in a nonculprit lesion detected by CCTA in acute myocardial infarction patients has been reported as a predictor of cardiovascular events after treatment.24 To our knowledge, the current study is the first report to evaluate the prognostic value of CCTA performed before revascularization. Our results confirm our hypothesis that CACS and high-risk plaque in a nonculprit lesion on CCTA performed before revascularization predict cardiovascular events after revascularization. The findings had marginally statistically significant incremental prognostic value over clinical risk factors.
Our results are consistent with the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study that used virtual histology intravascular ultrasound; nonculprit lesions with vulnerable plaque predicted future cardiovascular events after revascularization.1 CCTA is not only an established diagnostic modality for CAD, but also a useful tool for treatment planning.25–28
A growing literature supports the concept that high-risk plaque detected by CCTA coincides with vulnerable plaque on virtual histology intravascular ultrasound or optical coherence tomography.29,7,30–32 Moreover, previous investigations indicate a prognostic value of CT-derived plaque volume for future coronary events; several studies reported a correlation between larger plaque volume detected by CCTA with a higher rate of acute coronary syndrome.14,24 Regarding the high-risk plaque (positive remodeling and low attenuation) identified by CCTA, 22% of patients developed acute coronary syndrome within 2 years, while only 0.5% of remaining patients presented with an acute coronary syndrome.8 This paper also showed that positive plaque remodeling and/or low plaque attenuation was an independent predictor of acute coronary syndrome in a clinical study with 27±10 months follow-up (hazard ratio 22.8; 95% CI 6.9 to 75.2; P<0.001).8 In line with this literature, the current results plus implementation of reliable plaque analysis software33 support a greater clinical use of CCTA plaque characterization.
Although CACS is a known risk factor for cardiovascular events,34–36 the current data demonstrate that CACS remains a predictor of future cardiovascular events even after appropriate revascularization. It may be that CACS reflects an overall arteriosclerosis burden that includes nonculprit lesions that induce post-treatment events. Our model highest predictive ability (Model 3, Table 3) thus includes both “quality of instability” of coronary arteriosclerosis represented by nonculprit CT-HRP and “overall burden” of coronary arteriosclerosis represented by CACS.
Diabetes and triglyceride level were significant predictors as well. On the other hand, low-density lipoprotein cholesterol level, a well-known risk factor of cardiac event, was not a predictor. One possible explanation is that low-density lipoprotein cholesterol used in the prediction model was recorded at the time of the CT scan. The low-density lipoprotein cholesterol is usually strictly controlled by statin, especially in secondary prevention. In our population, almost 60% of patients took statin (Table 1). Although antiplatelet agents are reported as a predictor of cardiac events,37 we did not include this in the analysis as all patients took an antiplatelet agent. Based on our results, diabetes may require earlier intervention and more strict management after revascularization. Regarding triglyceride, improvement of lifestyle and development of therapies are warranted.
We acknowledge several study limitations. First, this is a retrospective single-center study. A significant selection bias could be introduced. Second, our data were collected from a single Japanese medical center, and the overall Japanese prevalence of CAD is relatively low. Our results should be confirmed by external validation. Third, while the number is <10%, we had subjects who were excluded because of incomplete follow-up data. Fourth, although the discrimination for cardiac event related to nonculprit lesion or not was evaluated, we could not confirm that when nonculprit CT-HRP had been detected by pretreatment CCTA, it was a lesion responsible for cardiovascular events after treatment. Fifth, most of the events were hospitalization for unstable angina. While the small hard-event number (death and nonfatal myocardial infarction) made all CIs extremely wide, point estimates lay in the same direction as those from the original end point analysis. Moreover, prediction of hospitalization for unstable angina that included both nonculprit- and culprit-related events would be important from the standpoint of secondary prevention.
Conclusions
High CACS and nonculprit high-risk plaque on CCTA performed before revascularization are significant predictors of cardiac events after revascularization.
Disclosures
Dr Rybicki has a research agreement with Toshiba Medical Systems Corporation that is unrelated to this project. Dr Daida has received lecture fees that is unrelated to this project from AstraZeneca, MSD, Kowa Pharmaceutical Company, Sanofi-Aventis, GlaxoSmithKline, Shionogi & Co., DaiichiSankyo Company, Takeda Pharmaceutical Co., Mitsubishi Tanabe Pharma Corp., Pfizer Co., Astellas Pharma Inc. and research funds from Takeda Pharmaceutical Co., Bristol-Myers Squibb Company, Nippon Boehringer Ingelheim Co., Astellas Pharma Inc., Novartis Pharma, MSD, Sanofi-Aventis, Otsuka Pharmaceutical Co., Dainippon Sumitomo Pharma Co., Pfizer Co., Kowa Pharmaceutical Company, Shionogi & Co., AstraZeneca, Teijin Limited, Morinaga Milk Industry Co.
Supporting Information
Table S1.Association and prediction measures from the 3 candidate Cox models for “hard” cardiac events (death and myocardial infarction).
Table S2. Reclassification tables by predicted 1000-day “hard” cardiac risks from the Cox models.
Table S3. Reclassification tables by predicted 1000-day “hard” cardiac risks from the Cox models.
Table S4. Reclassification tables by predicted 1000-day cardiac risks from the Cox models.
Table S5. Reclassification tables by predicted 1000-day cardiac risks from the Cox models.
References
- Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC, de Vos AM, Pugliese F, Rensing B, Jukema JW, Bax JJ, Prokop M, Doevendans PA, Hunink MG, Krestin GP, de Feyter PJ. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–2144. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S, Brady TJ. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47:1655–1662. doi: 10.1016/j.jacc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Kitagawa T, Yamamoto H, Ohhashi N, Okimoto T, Horiguchi J, Hirai N, Ito K, Kohno N. Comprehensive evaluation of noncalcified coronary plaque characteristics detected using 64-slice computed tomography in patients with proven or suspected coronary artery disease. Am Heart J. 2007;154:1191–1198. doi: 10.1016/j.ahj.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- Otsuka K, Fukuda S, Tanaka A, Nakanishi K, Taguchi H, Yoshikawa J, Shimada K, Yoshiyama M. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc Imaging. 2013;6:448–457. doi: 10.1016/j.jcmg.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Kondo T, Narula J. Evaluation of plaque morphology in coronary computed tomographic angiography. Cardiol Clin. 2012;30:69–75. doi: 10.1016/j.ccl.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Dedic A, Kurata A, Lubbers M, Meijboom WB, van Dalen B, Snelder S, Korbee R, Moelker A, Ouhlous M, van Domburg R, de Feijter PJ, Nieman K. Prognostic implications of non-culprit plaques in acute coronary syndrome: non-invasive assessment with coronary CT angiography. Eur Heart J Cardiovasc Imaging. 2014;15:1231–1237. doi: 10.1093/ehjci/jeu111. [DOI] [PubMed] [Google Scholar]
- Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U, Ferencik M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64:684–692. doi: 10.1016/j.jacc.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;1:390–402. doi: 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- Versteylen MO, Kietselaer BL, Dagnelie PC, Joosen IA, Dedic A, Raaijmakers RH, Wildberger JE, Nieman K, Crijns HJ, Niessen WJ, Daemen MJ, Hofstra L. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J Am Coll Cardiol. 2013;61:2296–2305. doi: 10.1016/j.jacc.2013.02.065. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Matsutani H, Kondo T, Sano T, Kumamaru K, Takase T, Rybicki FJ. Image quality and radiation dose stratified by patient heart rate for 64- and 320-detector row coronary CT angiography. Am J Roentgenol. 2013;200:765–770. doi: 10.2214/AJR.12.9037. [DOI] [PubMed] [Google Scholar]
- Rybicki FJ, Otero HJ, Steigner ML, Vorobiof G, Nallamshetty L, Mitsouras D, Ersoy H, Mather RT, Judy PF, Cai T, Coyner K, Schultz K, Whitmore AG, Di Carli MF. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- Bedayat A, Rybicki FJ, Kumamaru K, Powers SL, Signorelli J, Steigner ML, Steveson C, Soga S, Adams K, Mitsouras D, Clouse M, Mather RT. Reduced exposure using asymmetric cone beam processing for wide area detector cardiac CT. Int J Cardiovasc Imaging. 2012;28:381–388. doi: 10.1007/s10554-011-9814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP Society of cardiovascular Computed Tomography. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr American College of Cardiology; American Heart Association; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:671–719. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Antman EM, Beasley JW, Califf RM, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, III, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Smith SC, Jr American College of Cardiology; American Heart Association. Committee on the Management of Patients With Unstable Angina. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction–summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–1374. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Lee KL, Mark DB. Tutorial in biostatistics: multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- Kristensen TS, Kofoed KF, Kühl JT, Nielsen WB, Nielsen MB, Kelbæk H. Prognostic implications of nonobstructive coronary plaques in patients with non-ST-segment elevation myocardial infarction: a multidetector computed tomography study. J Am Coll Cardiol. 2011;58:502–509. doi: 10.1016/j.jacc.2011.01.058. [DOI] [PubMed] [Google Scholar]
- Nakazawa G, Tanabe K, Onuma Y, Yachi S, Aoki J, Yamamoto H, Higashikuni Y, Yagishita A, Nakajima H, Hara K. Efficacy of culprit plaque assessment by 64-slice multidetector computed tomography to predict transient no-reflow phenomenon during percutaneous coronary intervention. Am Heart J. 2008;155:1150–1157. doi: 10.1016/j.ahj.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Uetani T, Amano T, Kunimura A, Kumagai S, Ando H, Yokoi K, Yoshida T, Kato B, Kato M, Marui N, Nanki M, Matsubara T, Ishii H, Izawa H, Murohara T. The association between plaque characterization by CT angiography and post-procedural myocardial infarction in patients with elective stent implantation. JACC Cardiovasc Imaging. 2010;3:19–28. doi: 10.1016/j.jcmg.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Kodama T, Kondo T, Oida A, Fujimoto S, Narula J. Computed tomographic angiography-verified plaque characteristics and slow-flow phenomenon during percutaneous coronary intervention. JACC Cardiovasc Interv. 2012;5:636–643. doi: 10.1016/j.jcin.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Watabe H, Sato A, Akiyama D, Kakefuda Y, Adachi T, Ojima E, Hoshi T, Murakoshi N, Ishizu T, Seo Y, Aonuma K. Impact of coronary plaque composition on cardiac troponin elevation after percutaneous coronary intervention in stable angina pectoris: a computed tomography analysis. J Am Coll Cardiol. 2012;59:1881–1888. doi: 10.1016/j.jacc.2012.01.051. [DOI] [PubMed] [Google Scholar]
- Motoyama S, Kondo T, Anno H, Sugiura A, Ito Y, Mori K, Ishii J, Sato T, Inoue K, Sarai M, Hishida H, Narula J. Atherosclerotic plaque characterization by 0.5-mm-slice multislice computed tomographic imaging. Circ J. 2007;71:363–366. doi: 10.1253/circj.71.363. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Tanaka A, Kitabata H, Tsujioka H, Kataiwa H, Komukai K, Tanimoto T, Takemoto K, Takarada S, Kubo T, Hirata K, Nakamura N, Mizukoshi M, Imanishi T, Akasaka T. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging. 2009;2:1412–1419. doi: 10.1016/j.jcmg.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Ito T, Terashima M, Kaneda H, Nasu K, Matsuo H, Ehara M, Kinoshita Y, Kimura M, Tanaka N, Habara M, Katoh O, Suzuki T. Comparison of in vivo assessment of vulnerable plaque by 64-slice multislice computed tomography versus optical coherence tomography. Am J Cardiol. 2011;107:1270–1277. doi: 10.1016/j.amjcard.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Obaid DR, Calvert PA, Gopalan D, Parker RA, Hoole SP, West NE, Goddard M, Rudd JH, Bennett MR. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging. 2013;6:655–664. doi: 10.1161/CIRCIMAGING.112.000250. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Kondo T, Kodama T, Fujisawa Y, Groarke J, Kumamaru KK, Takamura K, Matsunaga E, Miyauchi K, Daida H, Rybicki FJ. A novel method for non-invasive plaque morphology analysis by coronary computed tomography angiography. Int J Cardiovasc Imaging. 2014;30:1373–1382. doi: 10.1007/s10554-014-0461-5. [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2001;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.Association and prediction measures from the 3 candidate Cox models for “hard” cardiac events (death and myocardial infarction).
Table S2. Reclassification tables by predicted 1000-day “hard” cardiac risks from the Cox models.
Table S3. Reclassification tables by predicted 1000-day “hard” cardiac risks from the Cox models.
Table S4. Reclassification tables by predicted 1000-day cardiac risks from the Cox models.
Table S5. Reclassification tables by predicted 1000-day cardiac risks from the Cox models.


