Abstract
Background
Arterial luminal diameters are routinely used to assess for vascular disease. Although small diameters are typically considered pathological, arterial dilatation has also been associated with disease. We hypothesize that extreme arterial diameters are biomarkers of the risk of vascular events.
Methods and Results
Participants in the Northern Manhattan Study who had a time-of-flight magnetic resonance angiography were included in this analysis (N=1034). A global arterial Z-score, called the brain arterial remodeling (BAR) score, was obtained by averaging the measured diameters within each individual. Individuals with a BAR score <−2 SDs were considered to have the smallest diameters, individuals with a BAR score >−2 and <2 SDs had average diameters, and individuals with a BAR score >2 SDs had the largest diameters. All vascular events were recorded prospectively after the brain magnetic resonance imaging. Spline curves and incidence rates were used to test our hypothesis. The association of the BAR score with death (P=0.001), vascular death (P=0.02), any vascular event (P=0.05), and myocardial infarction (P=0.10) was U-shaped except for ischemic stroke (P=0.74). Consequently, incidence rates for death, vascular death, myocardial infarction, and any vascular event were higher in individuals with the largest diameters, whereas individuals with the smallest diameters had a higher incidence of death, vascular death, any vascular event, and ischemic stroke compared with individuals with average diameters.
Conclusions
The risk of death, vascular death, and any vascular event increased at both extremes of brain arterial diameters. The pathophysiology linking brain arterial remodeling to systemic vascular events needs further research.
Keywords: brain arterial remodeling, stroke, vascular death, vascular disease
Arterial diameters are routinely used by clinicians to assess vascular health. For example, focal luminal narrowing in the coronary arteries is considered an important determinant of future vascular events as well as of the need for intervention.1 Lumen reductions at the carotid bifurcation and focally within intracranial arteries, usually attributed to atherosclerosis, are routinely used to stratify stroke risk.2–4 Large arterial diameters, however, may also be associated with vascular events.5,6 Examples of dilatative arteriopathies include aortic aneurysm, intracerebral dolichoectasia, and coronary ectasias.6–8 Arterial dilatations are less well-understood entities than atherosclerosis and may have a broader array of causes.9 Although it can be argued that atherosclerosis may be the underlying pathology in cases of aortic aneurysm and coronary ectasia, dilatation of the aorta, the coronaries, or the brain arteries can also occur in the absence of pathologically defined atherosclerosis.10,11 Furthermore, recent evidence suggests that brain arteries do not undergo outward remodeling to the same extent that do coronary arteries, thus making atherosclerosis-driven dilatation a less-plausible mechanism for dilated intracranial arteries.12 Arterial dilatation, defined by lumen diameter, is thus a plausible and understudied regional and systemic biomarker of vascular disease.
We interpret heterogeneity in arterial diameters to be evidence of a spectrum and that the extremes of this spectrum represent maladaptive changes that are pathological. Because the cerebral circulation can undergo compensatory dilatation due to reduced diameters in collateral vessels of the circle of Willis,13 we postulate that brain arterial diameters can be used as a biomarker of vascular risk, particularly among individuals at the extremes of brain arterial remodeling (BAR). Therefore, we tested the hypothesis that BAR extremes are associated with a higher risk of vascular events in a multiethnic, population-based cohort in northern Manhattan, New York City, NY.
Methods
The Northern Manhattan Study (NOMAS) is a population-based sample of northern Manhattan that was started in 1993. Participants in the study were enrolled through random digit dialing of ≈25 000 households in a restricted geographic area as determined by the boundaries of the Hudson and East rivers and 120th Street of Manhattan. Participants were eligible if they had never had a stroke diagnosed, were older than age 40, and had resided in northern Manhattan for ≥3 months in a household with a telephone. From 2003 to 2008, a subsample of the NOMAS cohort was invited to undergo brain magnetic resonance imaging (MRI). To be eligible for the MRI substudy, participants had to be able to provide written consent for the brain MRI, have no contraindications to MRI, and be 50 years or older and stroke free at the time of study.14 This study was approved by the Columbia University Medical Center and University of Miami institutional review boards.
Demographic characteristics were obtained at the time of enrollment and at the time of MRI. Race and ethnicity were self-defined. Hypertension was defined by self-report diagnosis of hypertension, use of antihypertensive medications, or the presence of a blood pressure ≥140/90 mm Hg at the time of MRI. Blood pressure measurement was taken on arrival to the investigation site, after at least 10 minutes of rest and before the MRI procedure. Diabetes was defined as self-report diagnosis of diabetes, use of glucose-lowering medications, or a fasting glucose ≥126 mg/dL at the time of the MRI. Hypercholesterolemia was defined by self-report, use of cholesterol-lowering medications, or a total cholesterol level ≥240 mg/dL. To be considered a smoker in this analysis, the subject had to be a current smoker.
Longitudinal Follow-up and Vascular Outcomes
Participants in the NOMAS MRI substudy (N=1290) were screened annually with standardized telephone interviews and in-person visits if the participant screened positive to a predefined trigger question for in-person evaluation. The outcomes collected in this study included all death, vascular death, myocardial infarction (MI), any stroke, ischemic stroke, and any vascular event (a composite of vascular death, any stroke, or MI). A detailed description of the ascertainment procedures has been previously reported.15 Briefly, death and vascular death were adjudicated by NOMAS investigators with robust reliability measures as well as negligible loss to follow-up. The date and the causes of death were recorded. When the cause of death was MI, stroke, heart failure, pulmonary embolus, or cardiac arrhythmia, vascular death was attributed. Among participants with stroke, >70% were admitted to New York–Presbyterian Hospital/Columbia University Medical Center, where stroke evaluation was performed by a vascular neurologist unaware of the status of the patient in the study. Subsequently, 2 neurologists adjudicated the stroke subtype in each case independently and blinded to the baseline MRI. MI was defined by criteria from the Cardiac Arrhythmia Suppression Trial and the Lipid Research Clinics Coronary Primary Prevention Trial by a study cardiologist.16,17 We used incident MI only and excluded from the MI analyses those who had history of MI before the brain MRI. In the case of participants experiencing >1 vascular event (eg, stroke and MI), we used the first event for the combined vascular event outcome (35 of 152 participants with any vascular event had ≥2 vascular events).
Brain Arterial Diameters
Imaging was performed on a 1.5-T MRI system (Philips Medical Systems) at the Hatch Research Center using 3-dimensional time-of-flight magnetic resonance angiography (MRA) with the following parameters: field of view of 15 cm, 1 mm effective slice thickness, acquisition matrix interpolated to 256×228 matrix, flip angle of 25°, and repetition and echo times of 20 and 2.7 ms, respectively. Brain arterial diameters were obtained by using in-house software that performs automated tracking of the vessel centerline.18 The threshold for the 3-dimensional reconstruction was systematically set to the point of maximal pixel saturation of the arteries while avoiding any parenchymal pixel visualization (intraclass correlation for threshold=0.998). The maximum arterial diameter was measured bilaterally in the cavernous segment of each internal carotid artery, within the first 5-mm segment of each anterior, middle, and posterior cerebral artery (6 locations), in the more proximal aspect of each posterior communicating artery in respect to the internal carotid artery, in the more distal portion of the V4 segment of each vertebral artery, and in the first 5-mm segment of the basilar artery. Thus, each participant had a maximum of 13 blood vessels measured. One of 3 possible descriptions was recorded for each intracranial artery: visible in the 3-dimensional format reconstruction, not visible in the 3-dimensional format due to a very small size but visualized on the MRA axial source image (ie, hypoplastic) or not visible on either the 3-dimensional format or the MRA axial image (ie, absent artery).
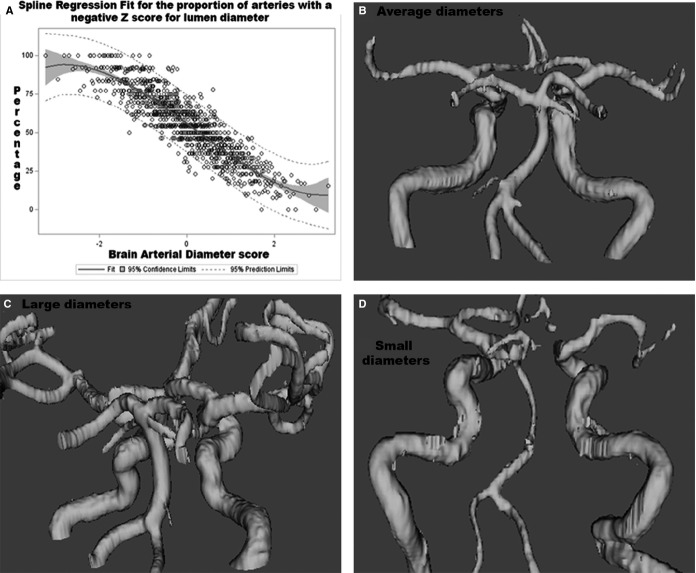
Because the normal ranges of diameters of each intracranial artery vary, we obtained the Z-score for each measured intracranial artery. A Z-score of −2 was attributed to hypoplastic arteries because its diameter was below the limit of our measurement (<10%). We then computed average Z-scores for each participant by adding all measured arteries and dividing by the total number of identified arteries. This average Z score, subsequently defined as the “BAR score” was normalized and used both continuously and categorized into 3 categories: (1) “individuals with the largest diameters” for participants with a BAR score ≥2 SDs, (2) “individuals with the smallest diameters” for participants with ≤−2 SDs, and “individuals with average diameters” for participants with a remodeling score between −2 and 2 SDs. The greater the number of arteries with negative scores within each individual, the more likely it was that the BAR score had a negative overall Z score, and vice versa (Figure 1).
Figure 1.
A, The BAR score represents a construct that discloses in a single number the tendency of an individual to have small or large arteries. As the score gets smaller, the proportion of arteries with small lumina increases. In the opposite direction, as the BAR score increases, so does the proportion of arteries with larger diameters respective to other NOMAS participants. Those in the middle represent a combination of the above. B and C, Tridimensional reconstruction of the cerebral vasculature showing examples of individuals with average diameters (B), small diameters (C), and large diameters (D) defined categorically for this study. BAR indicates brain arterial remodeling; NOMAS, Northern Manhattan Study.
Statistical Analysis
Head size is the most important anthropomorphic predictor of brain arterial diameters, and sex is the most important predictor of head size.14 Consequently, we controlled for head size in all analyses. We carried out the data analysis in 4 steps. First, we investigated whether the demographic and vascular risk factors varied across the 3 remodeling groups. We assessed the strength of association with logistic regression proportional odds models using “individuals with average diameters” as the referent group. We then assessed the functional relationship between the BAR score and all death, vascular death, MI, ischemic stroke and any vascular event. We tested whether a linear model would fit the data better than a nonlinear model,19 and we used 2 procedures to assess goodness of fit. First, we used the Restricted Cubic Splines (RCS) macro in SAS, which computes the coefficient of the spline with a predetermined number of knots, to test the null hypothesis that a given relationship is linear using a χ2 distribution.20 A P value of <0.05 rejects the null hypothesis and confirms a nonlinear relationship between the outcome and exposure. Additionally, we assessed the change in −2 log score comparing the model with a linear assumption (degrees of freedom=1) compared with a spline curve with 3 knots (degrees of freedom=2). We decided to keep the model with the best fit when the significance of the −2 log differential was <0.10 in a χ2 distribution. Finally, we assessed the incidences rates and hazard ratios of outcome events with Cox proportional hazards regression with progressive adjustment for potentially confounding covariates. The statistical analysis was carried out with SAS software, version 9.3 (SAS Institute Inc).
Results
Sample Characteristics
Among the 1290 patients in the NOMAS MRI substudy, 1077 had brain MRA done, and 1034 were included in this analysis (43 were excluded due to motion artifact on MRI). The sample mean age was 70±9 years (60% were women; 66% were Hispanic, 18% were non-Hispanic blacks, and 16% were non-Hispanic whites). Hypertension was present in 75%, diabetes in 25%, hypercholesterolemia in 68%, and smoking in 16%. The participants were followed an mean of 8±2 years.
Cross-Sectional Associations Between the BAR Score and Demographic and Clinical Variables
The distribution of demographic and clinical variables according to BAR category is shown in Table 1. After multivariate analysis including demographic and clinical characteristics plus head size, individuals with the smallest diameters were more likely to be men (odds ratio [OR] 3.11, 95% CI 1.26 to 7.71) and non-Hispanic whites (OR 2.57, 95% 1.06 to 6.25) but less likely to have hypertension (OR 0.38, 95% 0.16 to 0.89), while individuals with the largest diameters were older (OR 1.05 per year, 95% CI 1.01 to 1.10) and more likely to be women (OR 15.19, 95% CI 3.67 to 62.97), to have hypercholesterolemia (OR 8.22, 95% 1.09 to 61.85), and to have a history of previous MI (OR 4.57, 95% 1.22 to 17.06).
Table 1.
Demographic and Clinical Characteristics of the Participants by Remodeling Category
| Individuals With the Smallest Diameters (BAR Score <−2 SDs) n=23 | Individuals With Average Diameters (BAR Score >−2 to 2 SDs) n=988 | Individuals With the Largest Diameters (BAR Score >2 SDs) n=23 | |
|---|---|---|---|
| Age (y), mean±SD | 72±9 | 70±9 | 72±9 |
| Male sex, % | 73 | 40 | 4 |
| Ethnicity, % | |||
| Non-Hispanic white | 45 | 16 | 2 |
| Non-Hispanic black | 20 | 17 | 20 |
| Hispanic | 35 | 67 | 78 |
| Hypertension, % | 61 | 75 | 82 |
| SBP, mm Hg | 135±17 | 136±17 | 140±23 |
| DBP, mm Hg | 79±9 | 78±9 | 79±11 |
| PP, mm Hg | 55±15 | 58±15 | 61±18 |
| Diabetes, % | 22 | 25 | 33 |
| Fasting glucose (mg/dL), mean±SD | 101±32 | 93±20 | 101±49 |
| Hypercholesterolemia, % | 67 | 68 | 78 |
| LDL (mg/dL), mean±SD | 184±34 | 194±39 | 194±43 |
| HDL (mg/dL), mean±SD | 56±17 | 53±17 | 51±13 |
| Triglycerides (mg/dL), mean±SD | 108±58 | 128±81 | 141±75 |
| Smoking (current), % | 20 | 17 | 8 |
| Prior MI, % | 8 | 3 | 10 |
| Atrial fibrillation, % | 4 | 2 | 0 |
BAR indicates brain arterial remodeling; DBP, diastolic blood pressure; SBP, systolic blood pressure; PP, pulse pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; MI, myocardial infarction.
BAR Score and Risk of Vascular Events
The relationship between the BAR score and the risk for vascular events varied with each outcome. A nonlinear relationship, specifically a U-shaped relationship, was confirmed for the BAR score with all death (P=0.001), vascular death (P=0.019), and any vascular events (0.05) (Figure 2, Table 2). For MI, the regression of the spline coefficient showed a trend for significance (P=0.098) and for improvement of goodness of fit (P=0.12). For ischemic stroke, a linear association fits the model better, with the highest risk of ischemic stroke observed in those with the lowest BAR score (ie, smallest average diameter). There were no statistical interactions between sex and the BAR score with the risk of the vascular events.
Figure 2.
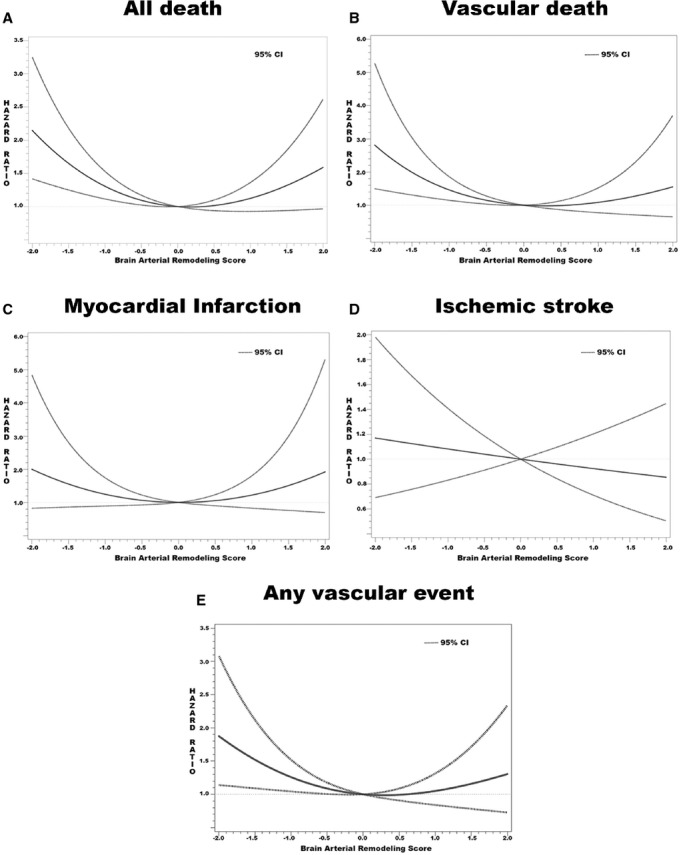
Graphic representation of the risk of vascular events by the brain arterial remodeling score. With the exception of ischemic stroke and myocardial infarction, the best-fitting model appear to show a U-shaped relationship indicating incremental risk as the score moved to each extreme.
Table 2.
Significance of the Spline Regression and Goodness of Fit of the Models Used to Test the Relationship Between the BAR Score and Vascular Events
| P Value for the Regression of the Linear B Coefficient | P Value for the Regression of the Spline B Coefficient | −2 log Differential (df) | P Value for −2 log Differential | |
|---|---|---|---|---|
| All death | 0.127 | 0.001 | 9.119 (1) | 0.002 |
| Vascular death | 0.059 | 0.019 | 4.731 (1) | 0.029 |
| Myocardial infarction | 0.795 | 0.098 | 2.416 (1) | 0.120 |
| Ischemic stroke | 0.559 | 0.744 | 0.100 (1) | 0.751 |
| Any vascular events | 0.162 | 0.050 | 3.788 (1) | 0.051 |
A P value for the regression of the spline B coefficient that is ≤0.05 suggests that the risk of vascular events is not linearly distributed. A P value for −2 log differential ≤0.050 implies improvement in the model fitness with a non-linear assumption of the risk. BAR indicates brain arterial remodeling.
Remodeling Category and Incidence Rates and Risk of Vascular Events
Individuals with the smallest diameters had higher rates of all death, vascular death, any vascular events, and ischemic stroke compared with individuals with average diameters but not of MI (Table 3). Individuals with the largest diameters also had a higher rate of all death, vascular death, MI, and any vascular event but not of ischemic stroke. After adjustment for demographic and vascular risk factors, individuals with the smallest diameters were at higher risk of all death, vascular death, MI, any vascular event, and ischemic stroke, but the risk was statistically significant only for all death (Table 4). Individuals with the largest diameters were also at a higher risk of death, vascular death, MI, and any vascular event, but only vascular death remained significant (Table 4). None of the individuals with the largest diameters had an ischemic stroke during follow-up.
Table 3.
Crude Incidence Rate Per 1000 Person-Year by Brain Arterial Remodeling Category
| All Death | Vascular Death | Myocardial Infarction | Ischemic Stroke | Any Vascular Event | |
|---|---|---|---|---|---|
| Incidence Rate (95% CI) | Incidence Rate (95% CI) | Incidence Rate (95% CI) | Incidence Rate (95% CI) | Incidence Rate (95% CI) | |
| Individuals with the smallest diameters | 121 (40 to 142) | 33 (12 to 87) | 8 (1 to 58) | 12 (3 to 46) | 49 (22 to 110) |
| Individuals with average diameters | 35 (30 to 40) | 11 (9 to 15) | 9 (6 to 11) | 7 (5 to 9) | 25 (21 to 30) |
| Individuals with the largest diameters | 102 (32 to 145) | 30 (9 to 92) | 10 (1 to 72) | 0 | 30 (9 to 92) |
Table 4.
Hazard Ratios by Remodeling Category
| Model 0 (HR, 95% CI) | Model 1 (HR, 95% CI) | Model 2 (HR, 95% CI) | |
|---|---|---|---|
| All death | |||
| Individuals with the smallest diameters | 2.68, 1.36 to 5.26 | 2.38, 1.20 to 4.70 | 2.11, 1.05 to 4.23 |
| Individuals with average diameters | Ref | Ref | Ref |
| Individuals with the average diameters | 1.82, 0.83 to 4.01 | 1.76, 0.79 to 3.96 | 1.79, 0.79 to 4.03 |
| Vascular death | |||
| Individuals with the smallest diameters | 2.98, 1.07 to 8.28 | 2.46, 0.87 to 6.89 | 2.11, 0.74 to 6.03 |
| Individuals with average diameters | Ref | Ref | Ref |
| Individuals with the largest diameters | 3.19, 0.97 to 10.55 | 3.58, 1.06 to 12.16 | 3.69, 1.09 to 12.47 |
| Myocardial infarction | |||
| Individuals with the smallest diameters | 1.14, 0.16 to 8.37 | 0.96, 0.13 to 7.14 | 1.10, 0.14 to 8.23 |
| Individuals with average diameters | Ref | Ref | Ref |
| Individuals with the largest diameters | 1.81, 0.25 to 13.26 | 1.97, 0.26 to 14.55 | 1.93, 0.26 to 14.24 |
| Ischemic stroke | |||
| Individuals with the smallest diameters | 1.63, 0.40 to 6.78 | 1.53, 0.36 to 6.40 | 1.57, 0.37 to 6.68 |
| Individuals with average diameters | Ref | Ref | Ref |
| Individuals with the largest diameters | * | * | * |
| Any vascular event | |||
| Individuals with the smallest diameters | 2.05, 0.90 to 4.70 | 1.65, 0.72 to 3.82 | 1.57, 0.68 to 3.66 |
| Individuals with average diameters | Ref | Ref | Ref |
| Individuals with the largest diameters | 2.05, 0.38 to 3.79 | 1.33, 0.42 to 4.20 | 1.35, 0.43 to 4.24 |
Model 0: Adjusted for head size only. Model 1: Model 0 plus age, sex, ethnicity. Model 2: Model 1 plus hypertension, diabetes, hypercholesterolemia and smoking. HR indicates hazard ratio; Ref, referent group.
There were zero ischemic strokes reported in this group.
Discussion
In this report, we confirmed our hypothesis that extremes of brain arterial diameters are associated with an increased number of vascular outcomes compared with individuals with less extreme forms of arterial remodeling. We documented a significant nonlinear U-shaped dose-effect curve using the BAR score as the main predictor for all death, vascular death, and any vascular event, suggesting that individuals in the extreme tails of the curve were at greater risk of all these outcomes. The incidence rates and hazard ratios for all death, vascular death, MI, and any vascular event were also greater in the extremes of the spectrum of arterial diameters, although the low numbers of events in each subgroup may have limited our power to confirm the significance of some of these associations. The only exception to our hypothesis was ischemic stroke, which occurred at a higher rate among participants with the smallest brain artery diameters. These results suggest to us that brain arterial diameters may be used as surrogates of systemic and cerebral vascular health and that a combination of genetic and environmental factors may influence the arterial diameters into the extreme pathological forms reported here.
Intuitively, it comes as no surprise that individuals with the smallest brain artery diameters have higher rates of all death, vascular death, MI, and ischemic stroke. After all, small peripheral arterial luminal diameters have been used traditionally as surrogates of arteriopathy, particularly of atherosclerosis.2,21–23 Small brain arterial diameters may also represent atherosclerosis, but intima thickening without atheroma (the defining characteristic of atherosclerosis) leading to luminal stenosis has also been reported.24,25 In fact, cerebral stenosis and cerebral atherosclerosis may have a differential association with stroke and MI, with cerebral stenosis being a better predictor of stroke, whereas atherosclerotic plaque is more frequent among those with prior MI.24 We do not have pathological correlates of our lumen-based definition of remodeling in NOMAS, but using a similar approach, we have reported that small lumina are strongly associated with atheromas, intima thickening, thicker vessel walls, and thinner media.26
In this same context, it is important to point out that the BAR score is a construct that reflects the average of brain arterial diameters in an individual. As can be seen in Figure 1A, among participants with a BAR score <−2 SDs, the majority of brain arteries will fall to the left of the normal distribution, reflecting an overall tendency to have a small diameter compared with others in this sample. This extreme phenotype is not the most common presentation of intracranial atherosclerosis (symptomatic or asymptomatic), which tends to occur more focally within the brain, typically accompanied with compensatory dilatations of other arteries through the circle of Willis.4,13,25 Because brain arteries are the last chronologically to show evidence of aging and intima thickening after the coronary, aorta, radial, and even skin arteries, the small average diameter phenotype observed in the brain may be a marker of advanced systemic arterial aging, although not necessarily atherosclerotic. This statement is supported by the fact that none of the individuals with the smallest average diameters had a stroke due to large intracranial atherosclerosis but rather cardioembolism (data not shown). Also, pathological studies that included samples from the coronary, aorta, carotid, and cerebral arteries in the same individual have shown little correlation between intracranial “raised lesions” (ie, atherosclerosis) with coronary, carotid, or aortic atherosclerosis or with radiographic evidence of coronary calcification.4,27 The burden of systemic atherosclerosis, defined by calcium scores of the coronary, aorta, and extracranial carotid arteries, are poor predictors of cerebrovascular events.28 The systemic correlates of vascular disease among individuals with the smallest average diameters may help improve our understanding of the reported risks and higher rates of death and vascular events.
Although less intuitive, dilated brain arteries appeared to be a biomarker of systemic but not cerebral atherosclerosis as evidenced by the higher rates and risks of vascular death, MI (a predominantly atherosclerotic disease), and any vascular events in this group. Further, the cross-sectional association of individuals with the largest average diameters with older age and hypercholesterolemia support a proatherosclerotic systemic phenotype. None of the individuals with the largest average diameters had an ischemic stroke during follow-up, suggesting that dilated brain arteries do not represent intracranial atherosclerosis, an argument advanced previously.9 However, substantial literature supports dilated brain arteries as markers of systemic atherosclerosis. For example, in this sample, we reported that in the setting of extracranial carotid atherosclerosis, there is a significant increase in the ipsilateral intracranial arterial diameters downstream from the carotid bifurcation.14 The observed compensatory dilatation was even greater with available collateral vessels through the circle of Willis. Individuals with evidence of intracranial dolichoectasia have a higher prevalence of MI and coronary artery disease, particularly if the dilated vessel is in the posterior circulation.14,29,30 This cross-sectional result fits well with the observation reported here that individuals with the largest average diameters had a higher prevalence of history of MI. Prospectively, the lumen diameter of the basilar artery is an independent predictor of vascular events and coronary events, but not of cerebrovascular events, thus supporting our claim that dilated brain diameters should be seen as biomarkers of systemic but not cerebral atherosclerosis, particularly in older individuals.6
The mechanisms underlying the association of systemic atherosclerosis with enlarged brain arteries are not yet described, but we hypothesize that they are different from other forms of dilatative arteriopathies seen among younger individuals, where diffuse dilatation of the brain arteries is more likely related to diseases that affect the structural strength of the wall, thus predisposing the arteries to dilate on exposure to otherwise normal arterial flow. Examples of these diseases include Marfan syndrome, Ehlers-Danlos syndrome, arterial tortuosity syndrome, Loeys-Dietz syndrome, and HIV infection.9
Our results provide evidence that the interconnection between the brain and systemic vasculatures needs to be better understood. Perhaps further incorporation of brain arterial phenotypes into ongoing epidemiological studies of stroke-free individuals who have genetic and biological biomarkers collected will reveal easily accessible and measurable biomarkers of these phenotypes. Genetic influences on these phenotypes are very likely, and identifying them should be considered a high-yield research area that may help the ongoing effort to personalize medicine, as recently demonstrated by the discovery of genetic determinants of height as a risk for coronary artery disease.31 This study has limitations. For example, although NOMAS is representative of northern Manhattan by virtue of its design, it is possible that the associations may not be reproduced in other populations with different ethnic compositions or age groups. The results here should also be interpreted with caution because some point estimates failed to reach statistical significance, although the small size of the extreme groups limited our power. We do not have repeated measures of brain diameters, a fact that weakens our claim that the diameters phenotypes represent remodeling phenotypes. It is implausible, however, that the subjects studied here were born with the reported diameters, which leaves remodeling as the most likely explanation for the observed differences in arterial caliber reported here. The lack of a more detailed characterization of the vascular risk factors after the brain MRI precludes any further testing on how vascular risk factors may play a role in mediating the BAR score. Furthermore, the lack of pathological confirmation prevents us from definitively ruling in or out a specific arterial wall phenotype, despite our attempts to refer to studies that have used pathology confirmation to support some of the arguments presented here.
In summary, we report here that the extremes of brain arterial diameters are biomarkers that confer vascular risk, as individuals with the smallest brain artery diameters had twice the risk of all death and vascular death compared with those with more average arterial diameters, whereas those with the largest diameters had a 3 times greater risk of vascular death and twice the risk of MI, compared with average diameters. We see these results as evidence of nonlinear relationship between the systemic and cerebral vasculatures that should be further studied.
Sources of Funding
This work was supported by National Institutes of Health grants R37 NS029993 (Drs Sacco and Elkind) and K24 NS 062737-03 (Dr Rundek).
Disclosures
Drs Gutierrez, Bagci, Sacco, Wright, and Rundek disclosures: None. Dr Alperin is a shareholder in alperin noninvasive diagnostics, Inc. Dr Elkind receives compensation for providing consultative services for Biogen IDEC, Biotelemetry/Cardionet, BMS-Pfizer Partnership, Boehringer-Ingelheim, Daiichi-Sankyo, and Janssen Pharmaceuticals; receives research support from diaDexus, Inc, and the NIH/NINDS; has given expert legal opinions on behalf of Merck/Organon (NuvaRing® and stroke litigation); and serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. He receives royalties from UpToDate for chapters related to stroke.
References
- Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, Kligfield PD, Krumholz HM, Kwong RYK, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Smith SC, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:e354–e471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG Warfarin-Aspirin Symptomatic Intracranial Disease Trial I. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- Brott TG, Hobson RW, II, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberg LA, McGarry PA, Moossy J, Strong JP, Tejada C, Loken AC. Severity of atherosclerosis in cerebral arteries, coronary arteries, and aortas. Ann N Y Acad Sci. 1968;149:956–973. doi: 10.1111/j.1749-6632.1968.tb53849.x. [DOI] [PubMed] [Google Scholar]
- Eigenbrodt ML, Evans GW, Rose KM, Bursac Z, Tracy RE, Mehta JL, Couper DJ. Bilateral common carotid artery ultrasound for prediction of incident strokes using intima-media thickness and external diameter: an observational study. Cardiovasc Ultrasound. 2013;11:22. doi: 10.1186/1476-7120-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Sakaguchi M, Miwa K, Okazaki S, Furukado S, Yagita Y, Mochizuki H, Kitagawa K. Basilar artery diameter is an independent predictor of incident cardiovascular events. Arterioscler Thromb Vasc Biol. 2013;33:2240–2244. doi: 10.1161/ATVBAHA.113.301467. [DOI] [PubMed] [Google Scholar]
- Antoniadis AP, Chatzizisis YS, Giannoglou GD. Pathogenetic mechanisms of coronary ectasia. Int J Cardiol. 2008;130:335–343. doi: 10.1016/j.ijcard.2008.05.071. [DOI] [PubMed] [Google Scholar]
- McMillan WD, Tamarina NA, Cipollone M, Johnson DA, Parker MA, Pearce WH. Size matters: the relationship between MMP-9 expression and aortic diameter. Circulation. 1997;96:2228–2232. doi: 10.1161/01.cir.96.7.2228. [DOI] [PubMed] [Google Scholar]
- Gutierrez J. Dolichoectasia and the risk of stroke and vascular disease: a critical appraisal. Curr Cardiol Rep. 2014;16:525. doi: 10.1007/s11886-014-0525-0. [DOI] [PubMed] [Google Scholar]
- Maleszewski JJ, Miller DV, Lu J, Dietz HC, Halushka MK. Histopathologic findings in ascending aortas from individuals with Loeys-Dietz Syndrome (LDS) Am J Surg Pathol. 2009;33:194–201. doi: 10.1097/PAS.0b013e31817f3661. [DOI] [PubMed] [Google Scholar]
- Hampole CV, Philip F, Shafii A, Pettersson G, Anesi GL, Patel JB, Menon V. Spontaneous coronary artery dissection in Ehlers-Danlos syndrome. Ann Thorac Surg. 2011;92:1883–1884. doi: 10.1016/j.athoracsur.2011.03.136. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Goldman J, Honig LS, Elkind MSV, Morgello S, Marshall RS. Determinants of cerebrovascular remodeling: do large brain arteries accommodate stenosis? Atherosclerosis. 2014;235:371–379. doi: 10.1016/j.atherosclerosis.2014.05.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Sultan S, Bagci A, Rundek T, Alperin N, Elkind MS, Sacco RL, Wright CB. Circle of Willis configuration as a determinant of intracranial dolichoectasia. Cerebrovasc Dis. 2013;36:446–453. doi: 10.1159/000356347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Elkind MS, Gomez-Schneider M, DeRosa JT, Cheung K, Bagci A, Alperin N, Sacco RL, Wright CB, Rundek T. Compensatory intracranial arterial dilatation in extracranial carotid atherosclerosis: the Northern Manhattan Study. Int J Stroke. 2015;10:843–848. doi: 10.1111/ijs.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden-Albala B, Roberts ET, Bazil C, Moon Y, Elkind MSV, Rundek T, Paik MC, Sacco RL. Daytime sleepiness and risk of stroke and vascular disease: findings from the Northern Manhattan Study (NOMAS) Circ Cardiovasc Qual Outcomes. 2012;5:500–507. doi: 10.1161/CIRCOUTCOMES.111.963801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DW. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, Abolafia JM, Lippel K, Levy RI. Lipoprotein(a) levels and risk of coronary heart disease in men. The Lipid Research Clinics Coronary Primary Prevention Trial. JAMA. 1994;271:999–1003. doi: 10.1001/jama.1994.03510370051031. [DOI] [PubMed] [Google Scholar]
- Jiang H, Alperin N. A new automatic skeletonization algorithm for 3D vascular volumes. Conf Proc IEEE Eng Med Biol Soc. 2004;2:1565–1568. doi: 10.1109/IEMBS.2004.1403477. [DOI] [PubMed] [Google Scholar]
- Fang J, Austin PC, Tu JV. Test for linearity between continuous confounder and binary outcome first, run a multivariate regression analysis second. SAS Glob Forum [serial online] 2009 ; Available at: http://support.sas.com/resources/papers/proceedings09/252-2009.pdf. Accessed August 04, 2015. [Google Scholar]
- Heinzl H, Kaider A. 1996. Manual for the SAS-Macro RCS (Version 2. 0): Technical Report KB 1-96. Universitat Wien, Institut fur Medizinische Computerwissenschaften Abteilung fur Klinische Biometrie. [Google Scholar]
- Virmani R. The Vulnerable Atherosclerotic Plaque: Strategies for Diagnosis and Management. Malden, MA: Blackwell Futura; 2007. [Google Scholar]
- Pasterkamp G, Schoneveld AH, van Wolferen W, Hillen B, Clarijs RJ, Haudenschild CC, Borst C. The impact of atherosclerotic arterial remodeling on percentage of luminal stenosis varies widely within the arterial system. A postmortem study. Arterioscler Thromb Vasc Biol. 1997;17:3057–3063. doi: 10.1161/01.atv.17.11.3057. [DOI] [PubMed] [Google Scholar]
- Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Benesch CG, Sila CA, Jovin TG, Romano JG, Cloft HJ. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39:1142–1147. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Elkind MS, Virmani R, Goldman J, Honig L, Morgello S, Marshall RS. A pathological perspective on the natural history of cerebral atherosclerosis. Int J Stroke. 2015 doi: 10.1111/ijs.12496. ; doi: 10.1111/ijs.12496. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Elkind MS, Goldman J, Honig L, Morgello S, Marshall R. Abstract T P419: pathological arterial wall correlates of lumen-based remodeling: results from the Brain Arterial Remodeling Study. Stroke. 2015;46:ATP419. [Google Scholar]
- Worm-Petersen J, Pakkenberg H. Atherosclerosis of cerebral arteries, pathological and clinical correlations. J Gerontol. 1968;23:445–449. doi: 10.1093/geronj/23.4.445. [DOI] [PubMed] [Google Scholar]
- Elias-Smale SE, Wieberdink RG, Odink AE, Hofman A, Hunink MGM, Koudstaal PJ, Krestin GP, Breteler MMB, van der Lugt A, Witteman JCM. Burden of atherosclerosis improves the prediction of coronary heart disease but not cerebrovascular events: the Rotterdam Study. Eur Heart J. 2011;32:2050–2058. doi: 10.1093/eurheartj/ehr125. [DOI] [PubMed] [Google Scholar]
- Ubogu EE, Zaidat OO. Vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography and risk of stroke and death: a cohort study. J Neurol Neurosurg Psychiatry. 2004;75:22–26. [PMC free article] [PubMed] [Google Scholar]
- Pico F, Labreuche J, Touboul PJ, Amarenco P. Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology. 2003;61:1736–1742. doi: 10.1212/01.wnl.0000103168.14885.a8. [DOI] [PubMed] [Google Scholar]
- Nelson CP, Hamby SE, Saleheen D, Hopewell JC, Zeng L, Assimes TL, Kanoni S, Willenborg C, Burgess S, Amouyel P, Anand S, Blankenberg S, Boehm BO, Clarke RJ, Collins R, Dedoussis G, Farrall M, Franks PW, Groop L, Hall AS, Hamsten A, Hengstenberg C, Hovingh GK, Ingelsson E, Kathiresan S, Kee F, Konig IR, Kooner J, Lehtimaki T, Marz W, McPherson R, Metspalu A, Nieminen MS, O’Donnell CJ, Palmer CN, Peters A, Perola M, Reilly MP, Ripatti S, Roberts R, Salomaa V, Shah SH, Schreiber S, Siegbahn A, Thorsteinsdottir U, Veronesi G, Wareham N, Willer CJ, Zalloua PA, Erdmann J, Deloukas P, Watkins H, Schunkert H, Danesh J, Thompson JR, Samani NJ Consortium CACD. Genetically determined height and coronary artery disease. N Engl J Med. 2015;372:1608–1618. doi: 10.1056/NEJMoa1404881. [DOI] [PMC free article] [PubMed] [Google Scholar]