Abstract
Background
Most patients with atrial fibrillation (AF) require rate control; however, the optimal target heart rate remains under debate. We aimed to assess rate control and subsequent outcomes among patients with permanent AF.
Methods and Results
We studied 2812 US outpatients with permanent AF in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation. Resting heart rate was measured longitudinally and used as a time-dependent covariate in multivariable Cox models of all-cause and cause-specific mortality during a median follow-up of 24 months. At baseline, 7.4% (n=207) had resting heart rate <60 beats per minute (bpm), 62% (n=1755) 60 to 79 bpm, 29% (n=817) 80 to 109 bpm, and 1.2% (n=33) ≥110 bpm. Groups did not differ by age, previous cerebrovascular disease, heart failure status, CHA2DS2-VASc scores, renal function, or left ventricular function. There were significant differences in race (P=0.001), sinus node dysfunction (P=0.004), and treatment with calcium-channel blockers (P=0.006) and anticoagulation (P=0.009). In analyses of continuous heart rates, lower heart rate ≤65 bpm was associated with higher all-cause mortality (adjusted hazard ratio [HR], 1.15 per 5-bpm decrease; 95% CI, 1.01 to 1.32; P=0.04). Similarly, increasing heart rate >65 bpm was associated with higher all-cause mortality (adjusted HR, 1.10 per 5-bpm increase; 95% CI, 1.05 to 1.15; P<0.0001). This relationship was consistent across endpoints and in a broader sensitivity analysis of permanent and nonpermanent AF patients.
Conclusions
Among patients with permanent AF, there is a J-shaped relationship between heart rate and mortality. These data support current guideline recommendations, and clinical trials are warranted to determine optimal rate control.
Clinical Trial Registration
URL: http://clinicaltrials.gov/. Unique identifier: NCT01165710.
Keywords: atrial fibrillation, heart rate, outcomes, rate control
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide and leads to significant morbidity and mortality.1,2 Several therapies are available for the maintenance of sinus rhythm in patients with AF; however, none has been definitively demonstrated to improve long-term survival.3,4 Therefore, many patients remain in chronic AF and are managed with a “rate control only” strategy5; such patients are often treated with medication or interventions to prevent excessive tachycardia, limit symptoms, and prevent the development of cardiomyopathy and/or heart failure. However, target heart rates are not well established and clinical trials to date have failed to demonstrate a benefit to strict heart rate control (<80 beats per minute [bpm]) relative to a more-lenient rate control strategy (ie, <110 bpm).6 Several shortcomings of these data have been cited, including lack of power and a lower than expected difference between treatment groups.7 Owing to these and other data, regional guidelines provide divergent recommendations regarding the optimal approach to rate control. The US guidelines recommend more-strict rate control (heart rate <80 bpm Class IIA recommendation, level of evidence B), whereas the European Society of Cardiology guidelines advocate lenient rate control (heart rate <110 Class IIA recommendation, level of evidence B).8,9
Patterns of heart rate control of patients with AF in routine clinical practice, as well as the association between heart rate and subsequent clinical outcomes, have not been well described. Using the nation’s largest prospective, outpatient clinical registry of AF patients, our study aimed to: (1) describe the patterns of heart rate control in US clinical practice; (2) describe the relationship between resting heart rate and AF symptom class; and (3) assess the relationship between resting heart rate control and clinical outcomes, including mortality.
Methods
We used data from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORIBT-AF), a prospective US registry of AF patients in the community, managed by primary care physicians, cardiologists, and/or electrophysiologists. A nationally representative sample of sites was recruited, with diversity by geography and practice type. Eligible patients were 18 years of age or older, with electrocardiographically documented AF that was not the result of a reversible cause. Sites enrolled consecutive patients that met the inclusion criteria without exclusions, and patients were expected to have clinical follow-up every 6 months for at least 2 years. The patients’ medical record served as the primary source of data, supplemented by the treating physician’s input and external medical records. All data were entered in a Web-based case report form, and site management and study coordination were performed by the Duke Clinical Research Institute. Data collection included sections on demographics, medical history, AF history (including symptoms), medical and interventional therapies, vital signs, laboratory and echocardiographic measures, and incident procedures and adverse events. Specifically, the patients’ resting heart rate in the clinic, their European Heart Rhythm Association (EHRA) symptom score (I to IV), and medicines (including anticoagulation, antiarrhythmic drugs, and rate control agents) were recorded at baseline and at each follow-up. Outcomes were collected at each follow-up. Additional information on the ORBIT-AF rationale and design has been reported previously.10
In order to assess the impact of resting heart rate in AF, the primary analysis cohort for the present study included only patients in ORBIT-AF with permanent AF at baseline. Patients without heart rate recorded at baseline were also excluded. In analyses of outcomes, patients without any follow-up visits were excluded.
The population was stratified by baseline resting heart rate: <60; 60 to 79; 80 to 109; and ≥110 bpm. Baseline characteristics, medical history, AF history, and medical therapies were compared among these groups. Using these data, the correlation between heart rate and EHRA score was assessed using all available visits for patients in the study population.
We then assessed the relationship between resting heart rate and subsequent clinical outcomes, after adjustment for known confounders. The primary outcome was all-cause mortality. Additional outcomes included: cause-specific death; cause-specific hospitalization; the composite of stroke or systemic embolism (SSE; adjudicated through primary source documentation review at the coordinating center) or major bleeding as defined by the International Society of Thrombosis and Haemostasis11; the composite of myocardial infarction (MI), coronary revascularization, or new-onset heart failure; and a composite of all adverse events (SSE, major bleeding, new heart failure, MI, coronary revascularization, hospitalization, or death). Tests for the interaction effect between baseline antiarrhythmic drug therapy and heart rate on outcomes were performed. The relationships between baseline beta-blocker (BB) use, baseline calcium-channel blocker use (nondihydropyridine, [ND-CCB]), and clinical outcomes were also assessed. The present analysis included all available follow-up out to 2 years. Sensitivity analyses measuring the association between heart rate and clinical outcomes in patients with all AF types were also performed.
Statistical Analyses
Univariate data across groups stratified by baseline heart rate are presented as percentages for categorical variables and medians (interquartile range; IQR) or means (SD) for continuous variables. Variables were compared using the chi-square test for categorical variables and continuous variables were compared using the Kruskal-Wallis test.
In order to describe the unadjusted association between heart rate and EHRA scores using data from every visit (including baseline and all follow-up assessments), we compared heart rates across symptom status as defined by EHRA scores (I: no symptoms, II: mild, III: severe, IV: disabling) using box plots. This included all visits for each patient, where both heart rate and EHRA score were recorded. We tested for a difference in heart rate across EHRA score using linear generalized estimating equations with heart rate as the response and EHRA a 4-level categorical variable, and a compound symmetry correlation structure to account for repeated measurements in each patient. Next, we evaluated the adjusted association between baseline heart rate and baseline EHRA score, we present a risk estimate (ie, odds ratio [OR]) and corresponding 95% confidence interval (CI) and P value from ordinal logistic regression. The regression model for EHRA score at baseline was developed based on risk factors from the candidate baseline characteristics (Tables S1 and S2) using backward selection, with an alpha for exclusion of 0.05. All continuous variables (including heart rate) were tested for linearity, and nonlinear relationships were accounted for using linear splines.
To describe the association between resting heart rate and clinical outcomes (listed above), we determined risk estimates (ie, hazard ratio [HR]) and corresponding 95% CIs and P values using Cox regression where longitudinally updated heart rate, as a continuous variable, is included as a time-dependent covariate, along with adjustment for baseline risk factors. Empirical standard errors were also used to account for correlation between patients at the same site. Adjustment risk factors were based on previously developed outcomes models in this population,12,13 which include all statistically significant covariates based on backward selection and α=0.05, selected from a large candidate list (Tables S1 and S2). The time-dependent heart rate covariate was tested for linearity, and nonlinear relationships were illustrated a priori using restricted cubic splines. This provided a flexible relationship that, in all cases, could be approximated by piece-wise linear splines, which were used to estimate HRs within appropriate ranges of heart rate (defined by the observed inflection point).
To assess the interaction between heart rate and the use of antiarrhythmic therapy for each outcome, we included one additional interaction term in the model. To determine the effect of rate control therapy on outcomes, propensity scores for ND-CCB versus BB use were generated using inclusive final covariates for 3 endpoints (all-cause death, cardiovascular death, noncardiovascular death). The outcome model was weighted using the inverse propensity score (IPW) for getting ND-CCB to minimize confounding and to incorporate ND-CCB (binary) therapy. The effectiveness of the IPW was evaluated using Cramer’s Phi (V) and R2. We calculated risk statistics (ie, HR, corresponding 95% CI, and P value) for ND-CCB versus BB by Cox regression with robust covariance.
Missing data among the baseline covariates used for multivariable adjustment (not heart rate) were handled with single imputation, using MCMC and regression methods in SAS. Missing data in these variables was <4% for all covariates, except left ventricular ejection fraction (LVEF; 10%), left atrial diameter (14%), serum creatinine (7%), and hematocrit (10%). Intermittent missing values in longitudinal heart rate were handled by a last value carried forward approach.
Several sensitivity analyses of the relationship between heart rate and clinical outcomes were performed. To test the durability of our findings, the above analysis was repeated in the overall ORBIT-AF population, including all types of AF. In response to peer review, we used linear regression modeling of baseline heart rate as an outcome to calculate the R2 for all baseline patient characteristics (assess factors associated with increased heart rate). Next, we calculated the variance of heart rate across different patients at the same point in time, and also for the same patient at different time points, using a mixed model for longitudinal heart rate.
The ORBIT-AF registry was approved by the Duke University Institutional Review Board (IRB), and all sites received IRB approval pursuant to local regulations. All patients provided written informed consent, and analyses of the aggregate, deidentified data were performed by the Duke Clinical Research Institute using SAS software (version 9.3; SAS Institute Inc., Cary, NC).
Results
The overall ORBIT-AF population included 10 132 patients from 176 US practices. We excluded 50 patients for missing baseline heart rate and 7270 patients for having nonpermanent AF. Patients with permanent AF who were excluded for other reasons were largely similar to patients included in the analysis. This resulted in a primary study cohort of 2812 patients with permanent AF, enrolled from June 2010 through August 2011. The median follow-up was 24 months (25th and 75th percentile: 18, 30 months) and included a total of 12 299 heart rate measurements for all patients throughout the study period. At baseline, 7.4% (n=207) had a heart rate <60 bpm; 62% (n=1755) 60 to 79 bpm; 29% (n=817) 80 to 109 bpm; and 1.2% (n=33) ≥110 bpm. Baseline characteristics of these groups are shown in Table1. Patients with heart rate <60 bpm were less likely to be female (32%; P=0.048), to be African American (3.4%; P=0.001), and had the lowest prevalence of sinus node dysfunction (10%; P=0.0048). There were no significant differences among the heart rate groups with respect to age (P=0.1), previous cerebrovascular disease (P=0.1), heart failure status (P=0.2), CHA2DS2-VASc scores (P=0.8), renal function (P=0.5),14 or left ventricular function (P=0.4).
Table 1.
Demographics, Past Medical History, and Laboratory Studies by Baseline Resting Heart Rate
| Heart Rate <60 bpm (n=207) | Heart Rate 60 to 79 bpm (n=1755) | Heart Rate 80 to 109 bpm (n=817) | Heart Rate ≥110 bpm (n=33) | P Value | |
|---|---|---|---|---|---|
| Age, y | 78 (71 to 83) | 78 (70 to 83) | 77 (70 to 82) | 77 (69 to 85) | 0.1 |
| Female | 32 | 38 | 42 | 42 | 0.048 |
| Race | 0.001 | ||||
| White | 88 | 88 | 89 | 67 | |
| Black or African American | 3.4 | 5.1 | 4.4 | 21 | |
| Hispanic | 7.7 | 5.4 | 4.8 | 12 | |
| Other | 0.97 | 1.6 | 1.7 | 0 | |
| Hypertension | 89 | 86 | 88 | 88 | 0.3 |
| Hyperlipidemia | 78 | 75 | 73 | 82 | 0.3 |
| Diabetes | 32 | 32 | 33 | 42 | 0.6 |
| History of CAD | 39 | 40 | 38 | 36 | 0.8 |
| Previous MI | 19 | 19 | 17 | 12 | 0.5 |
| Peripheral vascular disease | 15 | 17 | 15 | 21 | 0.6 |
| Previous stroke/TIA | 21 | 17 | 17 | 30 | 0.1 |
| Sinus node dysfunction | 10 | 20 | 17 | 15 | 0.004 |
| CHF | 0.2 | ||||
| No CHF | 60 | 59 | 57 | 55 | |
| NYHA Class I | 10 | 12 | 13 | 12 | |
| NYHA Class II | 24 | 19 | 21 | 12 | |
| NYHA Class III/IV | 5.8 | 10 | 9.1 | 21 | |
| CHADS2 risk score, mean (SD) | 2.7 (1.3) | 2.6 (1.3) | 2.6 (1.2) | 2.9 (1.5) | 0.5 |
| CHA2DS2-VASc risk score, mean (SD) | 4.3 (1.7) | 4.3 (1.7) | 4.4 (1.7) | 4.7 (2.2) | 0.8 |
| Calculated creatinine clearance* (mL/min per 1.73 m2) | 63.1 (46.7 to 89.1) | 65.3 (48 to 89.3) | 67.6 (48.7 to 91.5) | 61.1 (46.9 to 84.5) | 0.5 |
| Left ventricular EF | 0.4 | ||||
| Normal (≥50%) | 69 | 67 | 66 | 73 | |
| Mild dysfunction (40% to 50%) | 6.3 | 8 | 9.9 | 0 | |
| Moderate dysfunction (30% to 40%) | 7.7 | 10 | 9.2 | 12 | |
| Severe dysfunction (<30%) | 3.4 | 4.7 | 4.9 | 9.1 |
Values are presented as percentage or median (interquartile range), unless noted otherwise. P values were calculated across groups using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. bpm indicates beats per minute; CAD, coronary artery disease; CHF, congestive heart failure; EF, ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association heart failure class; TIA, transient ischemic attack.
As calculated by the Cockcroft-Gault formula.14
Previous and current therapies for AF, across these groups, are shown in Table2. Patients with lower resting heart rate (<60 bpm) were least likely to have received an antiarrhythmic drug previously (23%; P=0.01) and were more likely to be treated with ND-CCB (21%; P=0.001) and anticoagulation (90%; P=0.009) at baseline. Baseline use of antiarrhythmic drug (P=0.8) and digoxin (P=0.5) was balanced across groups.
Table 2.
Atrial Fibrillation History and Management by Baseline Resting Heart Rate
| Heart Rate <60 bpm (n=207) | Heart Rate 60 to 79 bpm (n=1755) | Heart Rate 80 to 109 bpm (n=817) | Heart Rate ≥110 bpm (n=33) | P Value | |
|---|---|---|---|---|---|
| Previous AF management | |||||
| Previous cardioversion | 23 | 28 | 30 | 24 | 0.2 |
| Previous antiarrhythmic drug therapy | 23 | 33 | 36 | 33 | 0.01 |
| Catheter ablation of AF | 0 | 2.9 | 2.8 | 3.0 | 0.1 |
| AV node/His bundle ablation | 0.5 | 3.9 | 3.2 | 3.0 | 0.08 |
| Medical therapies at baseline | |||||
| Diuretic | 64 | 59 | 61 | 67 | 0.3 |
| Aldosterone antagonist | 9.2 | 8.1 | 7.7 | 9.1 | 0.9 |
| Beta-blockers | 72 | 71 | 66 | 64 | 0.06 |
| ACE-I or ARB | 35 | 30 | 32 | 33 | 0.3 |
| Calcium channel blockers | 14 | 16 | 21 | 18 | 0.006 |
| Nondihydropyridine | 21 | 14 | 11 | 18 | 0.001 |
| Dihydropyridine | 7.7 | 8.6 | 6.9 | 12 | 0.4 |
| Digoxin | 33 | 34 | 30 | 36 | 0.5 |
| Antiarrhythmic drug therapy | 7.7 | 8.6 | 6.9 | 12 | 0.4 |
| Amiodarone | 4.8 | 3.7 | 3.6 | 3.0 | 0.8 |
| Sotalol | 0.5 | 1.1 | 0.5 | 0 | 0.3 |
| Oral anticoagulation | 90 | 88 | 84 | 91 | 0.009 |
| Warfarin | 88 | 85 | 81 | 82 | 0.02 |
| Dabigatran | 1.9 | 3.3 | 2.9 | 9.1 | 0.2 |
Values are presented as percentage. P values were calculated across groups using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. ACE-I indicates angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; AV, atrioventricular; bpm, beats per minute.
Heart Rate and Symptoms
Unadjusted assessment of heart rate and EHRA class included 12 299 visits for all patients documenting resting heart rate and EHRA score: 2701 patients at baseline; 2402 at 6 months; 2153 at 12 months; 1957 at 18 months; 1792 at 24 months; 960 at 30 months; 310 at 36 months; and 24 recorded at early study termination. There were 6797 measurements correlating with no symptoms (EHRA class I), 4414 with mild symptoms (class II), 1000 with severe symptoms (class III), and 88 with disabling symptoms (class IV). There was a significant association between increasing heart rate and worse concomitant EHRA symptom class across visits (Figure1; P<0.0001). In adjusted analyses, the relationship between baseline-only resting heart rate and concomitant, baseline EHRA symptom class was found to be linear, and increasing heart rate at baseline was significantly associated with more-severe baseline EHRA symptom class (adjusted OR, 1.04 per 5 bpm increase; 95% CI, 1.01 to 1.08; P=0.007; see Table S3).
Figure 1.
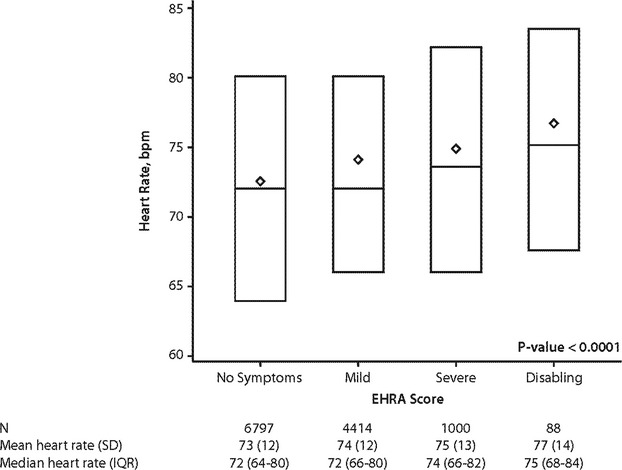
Distribution of 12 299 observations of resting heart rate versus concomitant EHRA symptom score in 2812 patients with permanent AF. owing to multiple follow-up visits, individual patients may contribute multiple observations of heart rate and EHRA score. Diamonds represent the means; horizontal lights reflect median and interquartile ranges. The P value is derived by testing for the overall significance of EHRA score levels from the correlated errors model, which yielded a coefficient of 1.11 for mild EHRA (vs. no symptoms), 2.06 for severe EHRA (vs no symptoms), and 2.36 for disabling EHRA (vs. no symptoms). AF indicates atrial fibrillation; bpm, beats per minute; EHRA, European Heart Rhythm Association; IQR, interquartile range.
Clinical Event Outcomes
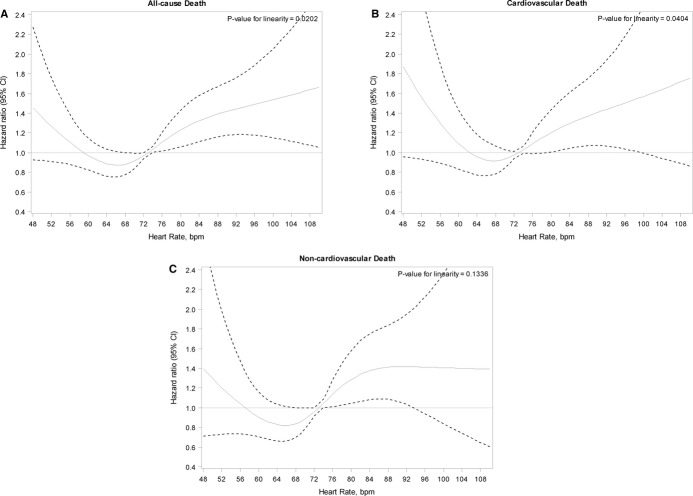
Overall event rates, as well as unadjusted and adjusted hazards for clinical events, are shown in Table3. Unadjusted outcomes demonstrated a J-shaped relationship between resting time-dependent heart rate and all-cause mortality (unadjusted HR per 5-bpm decrease in heart rate ≤65 bpm, 1.10; 95% CI, 0.96 to 1.25; unadjusted HR per 5-bpm increase in heart rate >65 bpm, 1.07; 95% CI, 1.03 to 1.12). In multivariable analysis using heart rate as a continuous, time-dependent covariate, the relationship between heart rate and cause-specific mortality remained nonlinear, with an inflection point at 65 bpm. Thus, linear splines were used in multivariable models of endpoints that included mortality. The adjusted HRs of these splines, with 95% CIs, are shown in Figure2A through 2C. Decreasing heart rate ≤65 bpm was associated with increasing all-cause mortality (adjusted HR, 1.15 per 5-bpm increase; 95% CI, 1.01 to 1.32; P=0.04), and increasing heart rate >65 bpm was associated with worse all-cause mortality (adjusted HR, 1.10 per 5-bpm increase; 95% CI, 1.05 to 1.15; P<0.0001).
Table 3.
Unadjusted and Adjusted Association Between Increasing Heart Rate and Clinical Outcomes
| Endpoint | Crude Event Rates | Unadjusted HR (95% CI) Per 5-bpm Change in Heart Rate | Adjusted HR (95% CI) Per 5-bpm Change in Heart Rate | Adjusted P Value |
|---|---|---|---|---|
| All-cause death | 377 (14%) | |||
| Heart rate ≤65 bpm per 5-bpm decrease | 1.10 (0.96, 1.25) | 1.15 (1.01, 1.32) | 0.04 | |
| Heart rate >65 bpm per 5-bpm increase | 1.07 (1.03, 1.12) | 1.10 (1.05, 1.15) | <0.0001 | |
| Cardiovascular death | 167 (6.2%) | |||
| Heart rate ≤65 bpm per 5-bpm decrease | 1.17 (0.98, 1.41) | 1.26 (1.04, 1.53) | 0.02 | |
| Heart rate >65 bpm per 5-bpm increase | 1.07 (1.00, 1.13) | 1.09 (1.02, 1.17) | 0.01 | |
| Noncardiovascular death | 172 (6.4%) | |||
| Heart rate ≤65 bpm per 5-bpm decrease | 1.10 (0.89, 1.35) | 1.13 (0.92, 1.39) | 0.2 | |
| Heart rate >65 bpm per 5-bpm increase | 1.07 (1.00, 1.14) | 1.02 (1.01, 1.03) | 0.003 | |
| All-cause hospitalization | 1388 (51%) | |||
| Heart rate | 1.01 (0.99, 1.03) | 1.00 (0.98, 1.03) | 0.7 | |
| Cardiovascular hospitalization | 726 (27%) | |||
| Heart rate | 1.01 (0.98, 1.04) | 1.00 (0.97, 1.04) | 0.8 | |
| Bleeding hospitalization | 219 (8.1%) | 0.8 | ||
| Heart rate | 1.01 (0.98, 1.04) | 1.01 (0.95, 1.07) | ||
| Other hospitalization | 880 (33%) | |||
| Heart rate | 1.01 (0.95, 1.07) | 1.01 (0.97, 1.04) | 0.7 | |
| SSE or major bleeding | 297 (11%) | |||
| Heart rate | 1.02 (0.97, 1.08) | 1.02 (0.97, 1.08) | 0.4 | |
| MI, revascularization, new-onset heart failure | 208 (7.7%) | |||
| Heart rate | 1.05 (1.00, 1.11) | 1.05 (0.99, 1.11) | 0.08 | |
| SSE, major bleeding, new heart failure, MI, revascularization, all-cause hospitalization, all-cause death | 1491 (55%) | |||
| Heart rate ≤65 bpm per 5-bpm decrease | 1.07 (1.00, 1.16) | 1.10 (1.02, 1.19) | 0.01 | |
| Heart rate >65 bpm per 5-bpm increase | 1.03 (1.00, 1.06) | 1.03 (1.00, 1.06) | 0.04 |
Denominators may differ owing to competing risks. Details of the adjustment covariates for each outcome are provided in Table S2. bpm indicates beats per minute; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; SSE, stroke or systemic embolism.
Figure 2.
Relationship between time-dependent resting heart rate and clinical outcome among 2812 patients with permanent AF. Adjusted hazard ratios (with 95% CIs) of increasing heart rate (using the mean heart rate of 73 bpm as the referent) for (A) all-cause mortality, (B) cardiovascular death, and (C) noncardiovascular death. Details of the adjustment covariates for each outcome are provided in Table S2. AF indicates atrial fibrillation; bpm, beats per minute; CI, confidence interval.
Linear splines were also derived for the composite endpoint of all adverse events (SSE, major bleeding, new heart failure, MI, revascularization, all-cause hospitalization, and all-cause death). Heart rates below and above 65 bpm were associated with worse outcomes (adjusted HR, 1.10 per 5-bpm decrease ≤65 bpm; 95% CI, 1.02 to 1.19; adjusted HR, 1.03 per 5-bpm increase >65 bpm; 95% CI, 1.00 to 1.06).
Medical Therapies
Overall 227 patients (8%) were receiving antiarrhythmic therapy at baseline, most commonly amiodarone (n=105; 3.7%). Interaction testing was performed between heart rate and baseline antiarrhythmic therapy for each of the clinical outcomes (SSE, major bleeding, new heart failure, MI, revascularization, cause-specific hospitalization, and cause-specific death). A significant interaction (Pinteraction<0.05) was identified only for the endpoint of noncardiovascular death. There was a significant association between increased heart rate and noncardiovascular death among patients not on an antiarrhythmic drug at baseline (n=2496; 92%; adjusted HR, 1.08 per 5-bpm increase; 95% CI, 1.02 to 1.15; P=0.01), but not for patients receiving an antiarrhythmic drug at baseline (n=215; 8%; adjusted HR, 0.75 per 5-bpm increase; 95% CI, 0.52 to 1.07; P=0.1, Pinteraction=0.02).
Propensity score modeling to assess different associations between ND-CCB and BB therapy and outcomes included 117 patients treated with ND-CCB and 1638 patients with BB (n=1755). We excluded 143 patients because of nonoverlapping propensity scores. Cox regression models did not demonstrate significant differences in outcomes between groups: adjusted HR for ND-CCB (versus BB) for all-cause death, 0.99 (95% CI, 0.46 to 2.14; P=1.0); cardiovascular death, 0.97 (95% CI, 0.23 to 3.11; P=0.8); and noncardiovascular death, 1.29 (95% CI, 0.49 to 3.37; P=0.6).
Sensitivity Analyses
In sensitivity analyses of patients with any type of AF (n=9648), including nonpermanent forms of AF, the associations between heart rate and clinical outcomes were consistent (Tables S3 and S4). Additional, exploratory sensitivity analyses were performed assessing the factors associated with baseline heart rate, heart rate change over time, and the association between baseline-only heart rate and subsequent clinical outcomes. Other patient characteristics were minimally associated with baseline heart rate (R2=0.03), and heart rate varied nearly twice as much within individuals over time as it did between patients (variance 95 within individuals vs. 54 between individuals, in a mixed model for longitudinal heart rate). Without updating heart rate as a time-dependent covariate, there were not significant associations between heart rate and clinical outcomes, unadjusted or adjusted (Tables S5 and S6).
Discussion
There is insufficient evidence to guide heart rate targets in patients with AF; however, these data provide several insights into current practice and outcomes associated with different heart rates in AF. In this nation-wide community cohort of patients with permanent AF, nearly all patients had resting heart rates <110 bpm (99%) and the majority (70%) were <80 bpm. However, we found that increasing heart rate above 65 bpm was associated with worse symptom class and lower survival rates, even after adjusting for baseline clinical factors. Last, medical therapies appeared to have little impact on the relationship between heart rate and mortality.
Our results are not completely consistent with the US guidelines applicable during the study period (2011), which designated strict heart rate control (<80 bpm) as a Class III recommendation (harm exceeds benefit)15 This recommendation was based mainly on a single trial,6 whereas previous studies had demonstrated adverse hemodynamic consequences of prolonged, uncontrolled ventricular rates.16 Our data suggest that patients in community practice routinely (70%) achieved more-stringent rate control (below 80) and that the associated outcomes were more favorable so long as heart rate was 65 or greater. Clinicians may have been reluctant to employ a modified guideline recommendation based upon a single study, given the previous accumulated clinical evidence.
Consistently, across endpoints and patient populations, increasing heart rate >65 bpm was associated with worse outcomes, including all-cause and cause-specific mortality, as well as adverse cardiovascular events. These findings contrast with the results of previous studies comparing “strict” versus “lenient” heart rate control.6,17,18 The suggestion that strict rate control is unnecessary is predominantly based on the RACE II trial, which randomized patients to each approach. Yet, several shortcomings of that trial have been noted.7 Principally, RACE II was a noninferiority trial that was underpowered to detect a benefit of strict rate control: statistically, the trial could not exclude even a 4.6% absolute risk reduction in cardiovascular death, heart failure hospitalization, or stroke at 3 years with a strict rate control strategy (in a binary comparison).6,19 Additionally, follow-up heart rates in the 2 groups were closer than the targets suggested (mean 85 bpm for the <110 group vs. 76 bpm for the <80 group). Last, the relationship between heart rate and clinical outcomes, particularly mortality, could not be assessed. In contrast, the present analysis includes a significantly larger sample, and despite relatively low heart rates in this population, our data demonstrate a significant association between increased heart rate (>65 bpm, as a continuous covariate) and adverse outcome.
Importantly, outcomes did not differ among pharmacological strategies. Higher heart rates were associated with increased all-cause mortality in patients with or without antiarrhythmic therapy. Though there was a significant interaction between antiarrhythmic use and heart rate for the endpoint of cardiovascular death, the sample was relatively small (8%) and the CIs were wide (95% CI, 0.52 to 1.07). Additionally, no difference in outcomes was observed in inverse propensity score models comparing ND-CCBs to BBs. Whereas these are observational data and do not yield definitive conclusions, the findings support the hypothesis that choice of medical therapy may be a secondary consideration to the primary achievement of optimal resting heart rate.
Given the divergent treatment guidelines for rate control, our findings have several important clinical implications. Though we cannot definitively identify providers’ target heart rates in our study, the association between increasing heart rate and adverse outcomes suggests that strict rate control may be associated with superior outcomes. Additional adequately powered, randomized, superiority studies to identify optimal rate control strategies are warranted, particularly given that there are more than 33 million individuals with AF across the world. Furthermore, there may be a floor effect, below which lowering heart rate is no longer beneficial (and may be harmful). Whereas physicians in practice appear to be comfortable with lower resting heart rates than previous guidelines dictated, identifying the optimal threshold, and the optimal therapies to achieve that threshold, will require further investigation.
Limitations
This study is based on data from a prospective, observational registry. Neither the extent of heart rate control nor the medical therapies were randomized, and thus residual and unmeasured confounding may exist. Additionally, a small proportion of patients were lost to follow-up. Though outcomes models included time-dependent covariates for heart rate, interim events or modifications to treatment were not included. There may have been other influences in outcome that were not captured by the multivariable models. Additionally, few patients in this sample had heart rates ≥110 bpm; therefore, conclusions regarding the increasing risk of very high heart rates are limited by power. However, the relative paucity of heart rates over 110 bpm may have led to underestimation of the impact of tachycardia on survival and other outcomes. In-depth analyses comparing and contrasting different rate control therapies were limited owing to smaller comparator subgroups. Last, there also may be differences between patients enrolled in ORBIT-AF and the broader AF population.
Conclusions
Patients with permanent AF in US community practice maintain relatively low resting heart rates, and increased heart rates are associated with worse symptom class. Moreover, there is a J-shaped relationship between heart rate and mortality for patients with AF, and this is a consistent finding across endpoints. These data support current American College of Cardiology/American Heart Association guideline recommendations for strict rate control. Clinical trials to determine optimal rate control are warranted.
Sources of Funding
The ORBIT-AF registry is sponsored by Janssen Scientific Affairs, LLC (Raritan, NJ). Dr. Steinberg was funded by NIH T-32 training Grant No. 5 T32 HL 7101-38.
Disclosures
Fonarow reports modest consultant/advisory board support from Ortho McNeil. Gersh reports modest DSMB/advisory board support from Medtronic, Baxter Healthcare Corporation, InspireMD, Cardiovascular Research Foundation, PPD Development, LP, Boston Scientific, and St. Jude. E.H. reports: modest honoraria support form Boehringer Ingelheim and Bayer; modest consultant/advisory board to Johnson & Johnson, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, and Ortho-McNeil-Janssen. P.R.K. reports modest consultant/advisory board support from Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, Portola, Merck, Sanofi, and Daiichi Sankyo. Mahaffey reports research grants from AmGen, Daiichi, Johnson & Johnson, Medtronic, St. Jude, and Tenax; modest consulting from the American College of Cardiology, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Elsevier, Epson, Forest, Medtronic, Mt. Sinai, Myokardia, Omithera, Portola, Purdue Pharma, Spring Publishing, Vindico, and WebMD; significant consulting from AstraZeneca, Cubist, GlaxoSmithKline, Johnson & Johnson, Merck, and The Medicines Company, and modest equity interest in BioPrint Fitness. G.N. reports research grants from Wyeth, Reliant, Medtronic, Boston Scientific, Sanofi-Aventis, and Boehringer Ingelheim and consultancies to Wyeth, Reliant, Medtronic, Boston Scientific, Sanofi-Aventis, Boehringer Ingelheim, Xention, Pfizer, Novartis, GlaxoSmithKline, and St. Jude Medical; P.C. reports significant employment with Janssen Pharmaceuticals, Inc. Peterson reports: significant research grant support from Eli Lilly & Company, Janssen Pharmaceuticals, Inc, and the American Heart Association; modest consultant/advisory board support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen Pharmaceuticals, Inc, Pfizer, and Genentech Inc. Piccini reports: significant research grant support from Johnson & Johnson/Janssen Pharmaceuticals; significant other research support from Boston Scientific Corporation, Johnson & Johnson Pharmaceutical Research & Development; modest consultant/advisory board support from Medtronic, Inc; and significant consultant/advisory board support from Johnson & Johnson/Janssen Pharmaceuticals.
Supporting Information
Table S1. Candidate covariates.
Table S2. Adjustment covariates for multivariable models of each endpoint.
Table S3. Adjusted association between baseline heart rate and baseline EHRA symptom class.
Table S4. Adjusted association between increasing heart rate and outcomes for patients with all AF types.
Table S5. Unadjusted association between baseline-only heart rate and outcomes.
Table S6. Unadjusted association between baseline-only heart rate and outcomes.
References
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study G. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- Steinberg BA, Holmes DN, Ezekowitz MD, Fonarow GC, Kowey PR, Mahaffey KW, Naccarelli G, Reiffel J, Chang P, Peterson ED, Piccini JP. Rate versus rhythm control for management of atrial fibrillation in clinical practice: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) registry. Am Heart J. 2013;165:622–629. doi: 10.1016/j.ahj.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- Dorian P. Rate control in atrial fibrillation. N Engl J Med. 2010;362:1439–1441. doi: 10.1056/NEJMe1002301. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P Guidelines ESCCfP. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW Members AATF. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J. 2011;162:606–612.e601. doi: 10.1016/j.ahj.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- Piccini JP, Kim S, Steinberg BA, Holmes D, Ansell J, Fonarow G, Gersh B, Hylek E, Kowey PR, Mahaffey KW, Thomas L, Chang P, Peterson E. Quality of care, symptoms, and 1 year outcomes for women vs men with atrial fibrillation: primary results from the ORBIT-AF registry. J Am Coll Cardiol. 2013:751–758. Abstract. [Google Scholar]
- Steinberg BA, Kim S, Piccini JP, Fonarow GC, Lopes RD, Thomas L, Ezekowitz MD, Ansell J, Kowey P, Singer DE, Gersh B, Mahaffey KW, Hylek E, Go AS, Chang P, Peterson ED Investigators O-A, Patients. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721–728. doi: 10.1161/CIRCULATIONAHA.113.002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA, III, Page RL, Ezekowitz MD, Slotwiner DJ, Jackman WM, Stevenson WG, Tracy CM, Fuster V, Ryden LE, Cannom DS, Le Heuzey JY, Crijns HJ, Olsson SB, Prystowsky EN, Halperin JL, Tamargo JL, Kay GN, Jacobs AK, Anderson JL, Albert N, Hochman JS, Buller CE, Kushner FG, Creager MA, Ohman EM, Ettinger SM, Guyton RA, Tarkington LG, Yancy CW. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104–123. [Google Scholar]
- Packer DL, Bardy GH, Worley SJ, Smith MS, Cobb FR, Coleman RE, Gallagher JJ, German LD. Tachycardia-induced cardiomyopathy: a reversible form of left ventricular dysfunction. Am J Cardiol. 1986;57:563–570. doi: 10.1016/0002-9149(86)90836-2. [DOI] [PubMed] [Google Scholar]
- Smit MD, Crijns HJGM, Tijssen JGP, Hillege HL, Alings M, Tuininga YS, Groenveld HF, Van den Berg MP, Van Veldhuisen DJ, Van Gelder IC RACE II Investigators. Effect of lenient versus strict rate control on cardiac remodeling in patients with atrial fibrillation: data of the RACE II (rate control efficacy in permanent atrial fibrillation II) study. J Am Coll Cardiol. 2011;58:942–949. doi: 10.1016/j.jacc.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Van Gelder IC, Wyse DG, Chandler ML, Cooper HA, Olshansky B, Hagens VE, Crijns HJ Race, Investigators A. Does intensity of rate-control influence outcome in atrial fibrillation? An analysis of pooled data from the RACE and AFFIRM studies. Europace. 2006;8:935–942. doi: 10.1093/europace/eul106. [DOI] [PubMed] [Google Scholar]
- Van Gelder IC, Van Veldhuisen DJ, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Bosker HA, Cornel JH, Kamp O, Veeger NJ, Volbeda M, Rienstra M, Ranchor AV, TenVergert EM, Van den Berg MP. Rate control efficacy in permanent atrial fibrillation: a comparison between lenient versus strict rate control in patients with and without heart failure. Background, aims, and design of RACE II. Am Heart J. 2006;152:420–426. doi: 10.1016/j.ahj.2006.02.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Candidate covariates.
Table S2. Adjustment covariates for multivariable models of each endpoint.
Table S3. Adjusted association between baseline heart rate and baseline EHRA symptom class.
Table S4. Adjusted association between increasing heart rate and outcomes for patients with all AF types.
Table S5. Unadjusted association between baseline-only heart rate and outcomes.
Table S6. Unadjusted association between baseline-only heart rate and outcomes.