Abstract
Background
Transcatheter aortic valve replacement (TAVR) is an effective alternative to surgical aortic valve replacement in patients at high surgical risk. However, there is little published literature on the exact causes of death.
Methods and Results
The PubMed database was systematically searched for studies reporting causes of death within and after 30 days following TAVR. Twenty-eight studies out of 3934 results retrieved were identified. In the overall analysis, 46.4% and 51.6% of deaths were related to noncardiovascular causes within and after the first 30 days, respectively. Within 30 days of TAVR, infection/sepsis (18.5%), heart failure (14.7%), and multiorgan failure (13.2%) were the top 3 causes of death. Beyond 30 days, infection/sepsis (14.3%), heart failure (14.1%), and sudden death (10.8%) were the most common causes. All possible subgroup analyses were made. No significant differences were seen for proportions of cardiovascular deaths except the comparison between moderate (mean STS score 4 to 8) and high (mean STS score >8) -risk patients after 30 days post-TAVR (56.0% versus 33.5%, P=0.005).
Conclusions
Cardiovascular and noncardiovascular causes of death are evenly balanced both in the perioperative period and at long-term follow-up after TAVR. Infection/sepsis and heart failure were the most frequent noncardiovascular and cardiovascular causes of death. This study highlights important areas of clinical focus that could further improve outcomes after TAVR.
Keywords: aortic stenosis, death, transcatheter valve replacement
For patients deemed inoperable or at high risk for surgical aortic valve replacement (SAVR), transcatheter aortic valve replacement (TAVR) has become a well-established treatment modality and remains a rapidly evolving technique.1 Although there are cumulative data suggesting comparable or even superior survival and symptomatic outcomes for patients who undergo TAVR versus medical palliation or the conventional SAVR,2–4 the frequency of mortality, especially late postprocedural mortality, remains high and their causes of death are often vaguely explained.
Although considerable attention has been afforded to procedural refinement and optimization of acute outcomes after TAVR, insufficient postprocedural management may also lead to late events. Thus, it is crucial to formally delineate the causes of death in patients undergoing TAVR with a view to identifying potentially preventable causes. Since a single study may lack the power to provide comprehensive and reliable conclusions,5,6 a systematic review and meta-analysis of all eligible studies was performed. In this study, we sought to identify and classify the causes of death within and after 30 days postprocedure.
Methods
Study Identification and Selection
A literature search of the PubMed online database was carried out to identify studies that reported the causes of death after TAVR on August 10, 2014 (Figure1). The search terms were as follows: (percutaneous OR transcatheter OR transfemoral OR transapical OR transsubclavian OR transaortic OR transaxillary) AND (aortic valve) AND (replacement OR implantation). Only articles in English were included. The inclusion criteria were: (1) studies that reported the specific causes of death after TAVR, (2) sufficient data (the number of patients who died because of 1 certain reason and the time interval after TAVR when they died) available, and (3) studies that at least covered both short-term (≤30 days) and long-term (>30 days) death events. Studies were excluded if 1 of the following existed: (1) causes of death were listed vaguely, (2) death events related to valve-in-valve procedures, (3) studies concerning death predictors instead of the actual causes of death, (4) published before 2002, or (5) studies were case reports, reviews, abstracts, guidelines, comments and conference presentations. If more than 1 study was published by the same authors using the same case series or overlapping case series, studies with the largest sample size were included except when different subgroup analysis could be done.
Figure 1.
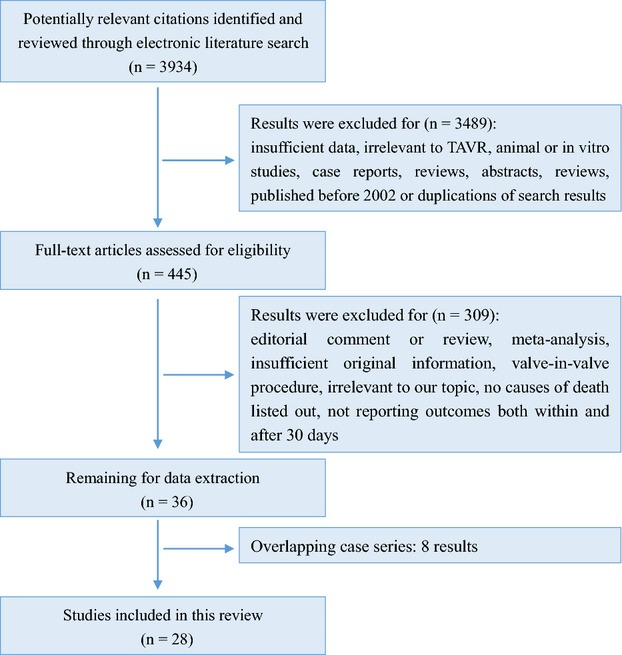
Summary of evidence search and selection. TAVR indicate Transcatheter aortic valve replacement.
Data Extraction and Quality Assessment
Two main reviewers (T.Y.X and Y.B.L) independently extracted the data and reached a consensus on all items. The following items were extracted from each study if available: first author’s name, publication year, study period, region, study design, single or multicenter study, TAVR case number, patients’ age, male proportion, New York Heart Association classification, ejection fraction, comorbidities, logistic European System for Cardiac Operative Risk Evaluation (EuroScore), the Society of Thoracic Surgeons (STS) score, chosen valve and access, and follow-up length. When conducting reclassification, cardiac death was defined as any death directly involving cardiac integrity and function (heart failure, acute myocardial infarction, sudden death/arrhythmia, and tamponade). Vascular complications and bleedings are regarded as procedure-related causes of death, and together with stroke, are grouped into cardiovascular causes of death in our study in accordance with Valve Academic Research Consortium (VARC) consensus document.7 Before reclassification of causes of death in included studies into different categories, all involved causes were discussed and double-checked by authors to reach the consensus on whether one certain cause of death belongs to a cardiovascular or noncardiovascular group. As for those already grouped into “other cardiovascular” or “other noncardiovascular” in original reports, we can only keep this classification even if certain causes may be separately listed in other studies. Those causes with only few case numbers were also grouped into “other” categories for the sake of clear illustrations.
Study quality was assessed using the Cross-Sectional/Prevalence Study Quality Assessment Form or Newcastle-Ottawa Quality Assessment Scale (NOS).8,9
Statistical Analysis
Results are expressed as counts and percentages for categorical variables. DerSimonian and Laird’s random-effects model was used to pool the estimates of proportions of cardiovascular deaths in each duration and subgroup by Comprehensive Meta Analysis Software Version 3. Differences in the proportions of cardiovascular deaths were compared with the χ2 test by using SPSS software 19.0. Statistical significance was set at P<0.05 (2-tailed). Pie charts and bar charts were used to illustrate our results.
Results
Studies Selection and Characteristics
A total of 3934 results were identified after an initial search from the PubMed database. After careful review of abstracts, 3489 results were excluded. After reading full texts of the remaining 445 results, 36 were deemed suitable for data extraction; 8 studies were excluded due to overlapping case series, leaving 28 results for further study10–37 (Figure1). Follow-up time ranges from 6 months to (3.8±2) years with acceptable follow-up rate. A summary of included studies is presented in Table1. Baseline characteristics are provided in Table S1.
Table 1.
Summary of Included Studies
| Study Information | Procedure Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Study Period | Region | Design | TAVR (n) | Edwards, n (%) | Medtronic, n (%) | TF, n (%) | TA, n (%) | Follow-Up | STS Score (%) |
| Martinez, GJ10 | 2009.6 to 2013.7 | Australia | Single center | 100 | 98 (98) | 2 (2) | 68 (68) | 32 (32) | Mean 17 months | — |
| Stabile, E11* | 2010.4 to 2011.4 | Italy | Single center | 60 | 120 (100) | 0 | — | 6 months | 10.4±6.8/9.7±5.1 | |
| Omer, S12 | 2011.12 to 2012.12 | US | Single center | 19 | 19 (100) | 0 | 19 (100) | 0 | Mean 8.8±3.9 months | 8.8±10.7 |
| Noble, S13 | 2008.8 to 2012.11 | Switzerland | Single center | 23 | 1 (4.3) | 19 (82.6) | 21 (91.3) | — | Mean 408±294 days | 8.7±2.9 |
| Walther, T14 | 2009.9 to 2010.8 | European | PREVAIL transapical study | 150 | 150 (100) | 0 | 0 | 150 (100) | 1 year | 7.5±4.4 |
| Latib, A15 | 2007.11 to 2011.2 | Italy | Single center | 111 | 70 (63) | 41 (37) | 111 (100) | 0 | 1 year | 4.57±2.28 |
| Yamamoto, M16 | 2007.12 to 2011.6 | Japan | Single center | 26 | 2 (7.7) | 24 (92.3) | 24 (92.3) | 0 | 6 months | 13.4±7.2 |
| Wendler, O17 | 2007.11 to 2009.12 | European | SOURCE | 1387 | 1387 (100) | 0 | 0 | 1387 (100) | 2 years | — |
| Wendler, O18 | 2009.12 to 2011.2 | Mixed | Multi centers | 120 | 120 (100) | 0 | — | — | 6.8±4.0 | |
| Kempfert, J19 | 2009.11 to 2010.8 | Germany | Single center | 40 | 0 | 0 | 0 | 40 (100) | 6 months | 9.0±4.7 |
| Van Mieghem, NM20 | 2005.11 to 2011.12 | Netherlands | Single center | 237 | 12 (5) | 222 (94) | 228 (96) | 3 (1) | Median 13 months | — |
| Doss, M21 | 2005.1 to 2008.12 | Germany | Single center | 100 | 100 (100) | 0 | 0 | 100 (100) | 3.8±2 years | 16±3 |
| Ducrocq, G22† | 2006.10 to 2010.6 | France | Single center | 201 | 171 (85) | 30 (15) | 131 (65) | 61 (30) | Mean 7±9 months | — |
| Ussia, GP23 | 2007.6 to 2008.8 | Italy | Italian CoreValve registry | 181 | 0 | 181 (100) | 172 (95) | 0 | 3 years | 11.4±9.9 |
| D’Onofrio, A24 | 2008.4 to 2010.11 | Italy | I-TA | 504 | 504 (100) | 0 | 0 | 504 (100) | Mean 9.2±6.5 months | 11±4 |
| Bosmans, JM25 | Until April 2010 | Belgium | Belgian TAVR Registry | 328 | 187 (57) | 141 (43) | 232 (71) | 88 (27) | 1 year | — |
| Hernández-Antolín, RA26 | 2007.5 to 2010.4 | Spain | Single center | 76 | 50 (66) | 26 (34) | 76 (100) | 0 | 367±266 days for ES; 172±159 days for MCV | 6.34±1.8 |
| Johansson, M27 | 2008.1 to 2009.11 | Sweden | Single center | 40 | 40 (100) | 0 | 10 (25) | 30 (70) | Mean 10±8 months | — |
| Lefèvre, T28 | 2007.4 to 2008.1 | European | European PARTNER | 130 | 130 (100) | 0 | 61 (47) | 69 (53) | 1 year | 11.6±6.5 |
| Leon, MB29‡ | 2007.5 to 2009.3 | Mixed | PARTNER | 179 | 179 (100) | 0 | 179 (100) | 0 | 1 year | 11.2±5.8 |
| Drews, T30 | 2008.4 to 2010.1 | Germany | Single center | 158 | 198 (100) | 0 | 0 | 198 (100) | — | 21±16.3/29±18.1 |
| Attias, D31† | 2006.10 to 2009.6 | France | Single center | 83 | 72 (87) | 11 (13) | 83 (100) | 0 | Median 9 months | 15±8 |
| Ye, J32 | 2005.10 to 2009.2 | Canada | Single center | 71 | 71 (100) | 0 | 0 | 71 (100) | 3 years | 12.1±7.7 |
| Guinot, PG33† | 2006.10 to 2009.2 | France | Single center | 90 | — | 62 (69) | 28 (31) | — | 15 (11 to 23) | |
| Avanzas, P34 | 2007.12 to 2009.7 | Spain | Multi centers | 108 | 0 | 108 (100) | 103 (95) | 0 | Average 7.6 months | — |
| Kapadia, SR35 | 2006.2 to 2007.3 | US | Single center | 18 | — | 278±128 days | 11.4±7.5 | |||
| Walther, T36 | 2006.2 to 2008.3 | Germany | Single center | 25 | 25 (100) | 0 | 0 | 25 (100) | Mean 351 days | 17 (6 to 43) |
| Webb, JG37 | — | Canada | Single center | 50 | 50 (100) | 0 | 50 (100) | 0 | Median 359 days | — |
I-TA indicates Italian registry of transapical aortic valve implantation; MCV indicates Medtronic CoreValve; STS, Society of Thoracic Surgeons; TA-TAVR, transapical TAVR; TAVR, transcatheter aortic valve replacement; TF-TAVR, transfemoral TAVR.
Randomized by single or double antiplatelet therapy after the procedure.
These 3 studies shared an overlapping case series. The study by Ducrocq, G et al was used for overall analysis. The study by Attias, D et al was used for TF-TAVR subgroup analysis. The study by Guinot, P et al was used for TA-TAVR subgroup analysis.
Inoperable patients were randomly assigned to standard therapy (including balloon aortic valvuloplasty) or TF-TAVR.
Quality Assessment
The quality of eligible cohort studies was assessed using the Newcastle-Ottawa Quality Assessment Scale scale, while quality of single-arm studies was evaluated by the Cross-Sectional/Prevalence Study Quality. Overall quality of these included studies was good (evaluation forms for each study were not shown).
Time Interval and Causes of Death
Within 30 days, 8.4% (265/3155) of patients died, 46.4% (123/265) of which were deemed noncardiovascular. Noncardiovascular causes accounted for 51.6% (282/546) of 546 deaths after 30 days. As for individual causes, within 30 days, infection/sepsis accounted for 18.5% of deaths, followed by heart failure and multiorgan failure, accounting for 14.7% and 13.2% of deaths, respectively. Beyond 30 days, infection/sepsis each accounted for 14.3% of deaths, followed by heart failure and sudden death as the reported cause of 14.1% and 10.8% of deaths, respectively (Figures3 through 4). Because of the broad-spectrum character of noncardiovascular causes, detailed modes of “infection/sepsis” and “other noncardiovascular causes” are provided in Table2 separately from figures for the sake of clear illustrations and better understanding.
Figure 3.
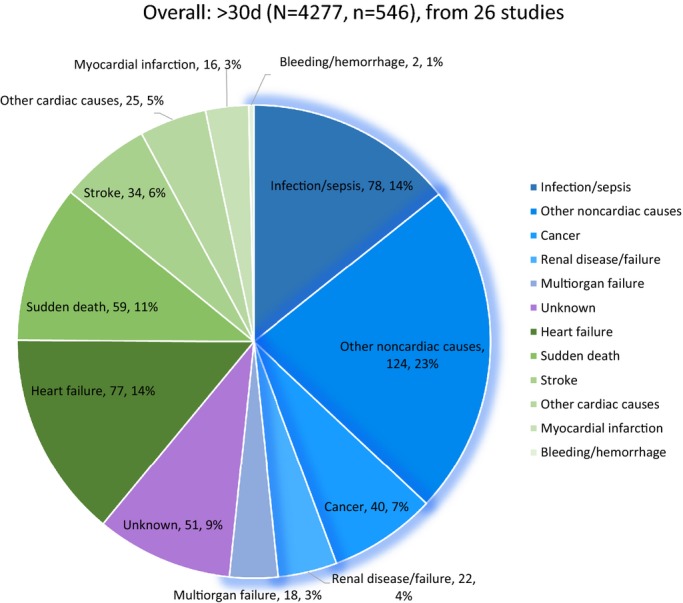
Overall analysis of causes of death after the first 30 days following transcatheter aortic valve replacement (TAVR). Figure 2 and 3 show causes of death post-TAVR per time interval, with total patients included (N), deaths (n), and the blue parts standing for noncardiovascular causes and the green parts for cardiac/procedure-related causes.
Figure 4.
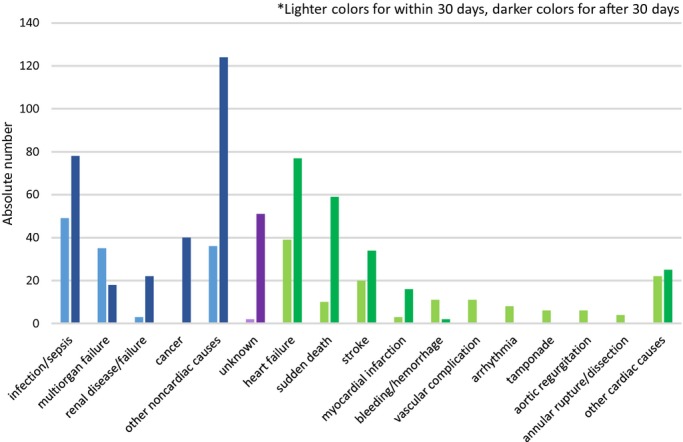
Overall analysis in both durations, within 30 days and after 30 days of the valve procedure.
Table 2.
Detailed Modes of Noncardiovascular Deaths in Overall Analysis
| Category | Time Interval | |
|---|---|---|
| ≤30 Days | >30 Days | |
| Infection/sepsis | Pneumonia | |
| Urologic infection | ||
| Other noncardiovascular causes | Acute adrenal insufficiency | Peripheral vascular disease |
| Gastrointestinal event | Intestinal ileus | |
| Psychosis | Trauma | |
| Acute liver failure | Cachexia | |
| Ischemic bowel | Cirrhosis | |
| Frailty/natural causes | ||
| Abdominal aortic aneurysm | ||
| Accident | ||
| Bone fracture | ||
| Hypercapnic encephalopathy | ||
| Acute pancreatitis | ||
Figure 2.
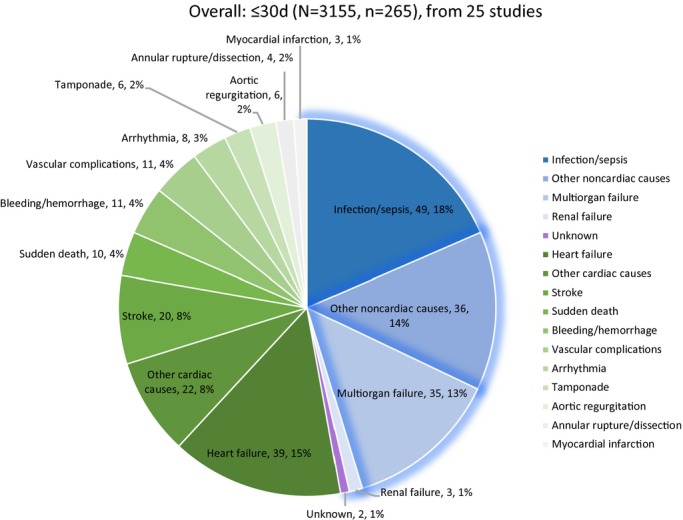
Overall analysis of causes of death within the first 30 days following transcatheter aortic valve replacement.
Subgroup Analysis by Access
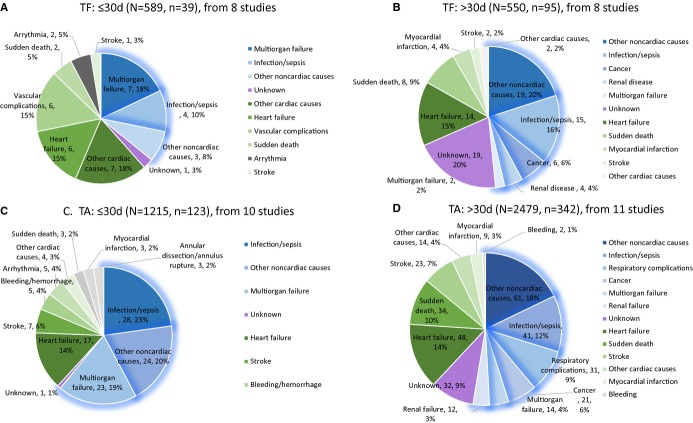
Causes of death by transfemoral TAVR (TF-TAVR)12,15,26–29,31,37 and transapical TAVR (TA-TAVR)14,17,19,21,24,27,28,30,32,33,36 were individually delineated (Figure5 with absolute numbers). For the TF-TAVR group, 6.6% (39/589) and 17.3% (95/550) of patients died within and beyond 30 days, which were 10.1% (123/1215) and 13.8% (342/2479) for TA-TAVR. In general, multiorgan failure, heart failure, and vascular complications were the 3 leading causes of death for TF-TAVR within 30 days (17.9%, 15.4%, and 15.4%, respectively); beyond 30 days, infection/sepsis, heart failure, and sudden death accounted for 15.8%, 14.7%, and 8.4% of deaths, respectively. Within 30 days of TA-TAVR, infection/sepsis, multiorgan failure, and heart failure accounted for 22.8%, 18.7%, and 13.8% of deaths, while beyond 30 days, heart failure, infection/sepsis, and sudden death accounted for 14.0%, 12.0%, and 9.9% deaths. The pooled estimate of proportions of cardiovascular deaths in the TF-TAVR subgroup were 61.5% (95% CI: 44.4% to 76.2%) within 30 days post-TAVR and 35.7% (95% CI: 23.4% to 50.3%) after 30 days. In the TA-TAVR subgroup, the pooled estimate of proportions of cardiovascular deaths were 44.7% (95% CI: 31.2% to 59.0%) and 39.6% (95% CI: 28.1% to 52.4%), respectively. No significant differences were found between the 2 subgroups in terms of the proportion of cardiovascular deaths (P=0.067 within 30 days; P=0.514 after 30 days) (Figure S1).
Figure 5.
Subgroup analysis by access. TA indicates transapical; TF, transfemoral.
Subgroup Analysis by Types of Prosthesis
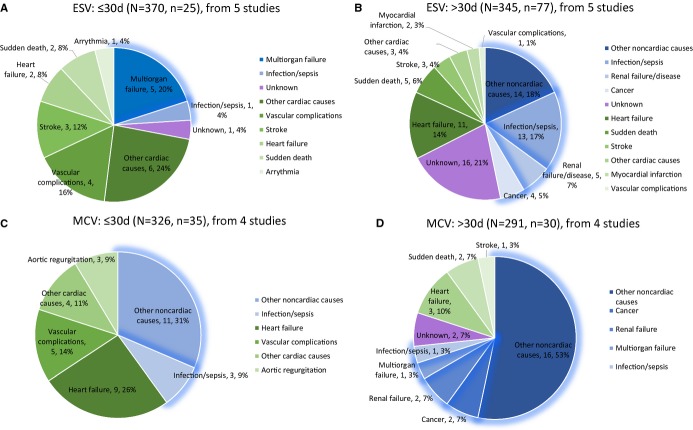
Two most-used valve types, balloon expandable Edwards valve (Edwards Lifesciences, Irvine, CA) and the self-expanding CoreValve system (Medtronic, Inc, Minneapolis, MN), were included in this subgroup analysis (Figure6 with absolute numbers). Since the CoreValve system cannot be delivered transapically, only TF-TAVR studies were included in this subgroup analysis.12,23,26,29,31,34,37 For the Edwards SAPIEN Valve group, 6.8% (25/370) and 22.3% (77/345) of patients died within and beyond 30 days, which were 10.7% (35/326) and 10.3% (30/291) for Medtronic CoreValve. Overall, multiorgan failure, vascular complications, and stroke with 20.0%, 16.0%, and 12.0% of deaths were the 3 individual and specific leading causes of death for TAVR using only Edwards SAPIEN Valve within 30 days, while infection/sepsis, heart failure, and renal disease took the lead after 30 days (16.9%, 14.3%, and 6.5% of deaths, respectively). For Medtronic CoreValve, heart failure, vascular complications, infection/sepsis, and aortic regurgitation accounted for 25.7%, 14.3%, 8.6%, and 8.6% of deaths within 30 days, while heart failure was the most common cause after 30 days (10.0% of deaths). The pooled estimates of proportions of cardiovascular deaths in the Edwards SAPIEN Valve subgroup were 66.8% (95% CI: 45.7% to 82.7%) within 30 days post-TAVR and 40.2% (95% CI: 18.7% to 66.2%) after 30 days. In the Medtronic CoreValve subgroup, the pooled estimates of proportions of cardiovascular deaths were 57.5% (95% CI: 36.6% to 76.0%) and 27.6% (95% CI: 14.4% to 46.2%), respectively. No significant differences were found between the 2 subgroups in terms of the proportion of cardiovascular deaths (P=0.394 within 30 days; P=0.189 after 30 days) (Figure S2).
Figure 6.
Subgroup analysis by types of prosthesis. ESV indicates Edwards SAPIEN valve; MCV, Medtronic CoreValve.
Subgroup Analysis by STS Score
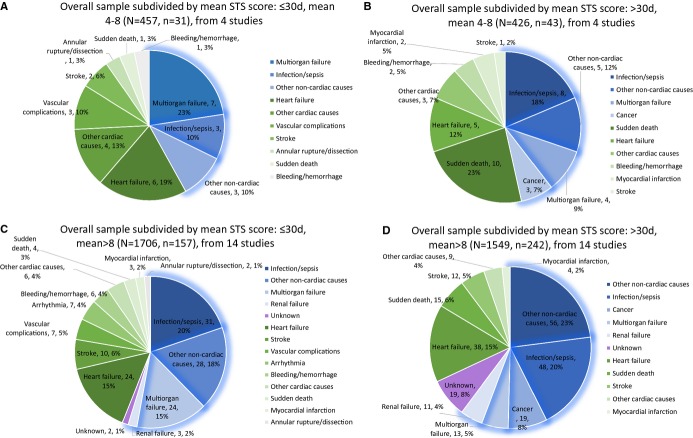
According to mean STS score, studies conducted in patients with a mean STS score between 4 and 8 (regarded as moderate risk) and with mean score larger than 8 (regarded as high risk) were divided into 2 subgroups (Figure7 with absolute numbers). For moderate-risk patients, 6.8% (31/457) and 10.1% (43/426) died within and beyond 30 days, which were 9.2% (157/1706) and 15.6% (242/1549) for high-risk patients. Multiorgan failure and heart failure accounted for 22.6% and 19.4% of deaths in the moderate-risk group within the first 30 days, while infection/sepsis, heart failure, and multiorgan failure took the lead in the high-risk group (19.7%, 15.3%, and 15.3%, respectively). After 30 days, sudden death, infection/sepsis, and heart failure (23.3%, 18.6%, and 11.6% of deaths) were the top 3 killers for moderate-risk patients; on the other hand, infection/sepsis, heart failure, and cancer (19.8%, 15.7%, and 7.9% of deaths) were the 3 leading causes of death for high-risk patients. Comparison for the proportion of cardiovascular deaths saw a significant difference in 2 subgroups after 30 days post-TAVR (moderate risk versus high risk: 55.5% [95% CI: 37.3% to 72.3%] versus 45.6% [95% CI: 32.6% to 59.1%], P=0.360, within 30 days; 56.0% [95% CI: 40.6% to 70.4%] versus 33.5% [95% CI: 22.1% to 47.3%], P=0.005, after 30 days) (Figure S3).
Figure 7.
Subgroup analysis by mean Society of Thoracic Surgeons (STS) score.
Comparisons Between TAVR and Other Treatment Choices
Studies that reported causes of death both in patients who underwent TAVR and those who underwent other treatment choices were used for further comparison. In 3 single-center studies that compared outcomes after TAVR and SAVR in a nonrandomized fashion,15,35,38 TAVR saw a trend of increased proportion of noncardiovascular causes of death while SAVR had a decreased trend within and after 30 days following the procedure, although the difference is not significant. In cohort B of The Placement of Aortic Transcatheter Valves (PARTNER) trial29 inoperable patients were randomly assigned to either TAVR or standard therapy (including balloon aortic valvuloplasty). Again, a decreased trend was seen for noncardiovascular causes of death in the traditional treatment group but not in the TAVR group (Figure S4).
Discussion
Aortic stenosis is the most common valvular disease in the world, with a 3-year survival rate less than 30% after symptom onset with medical therapy.39 Although SAVR is the established therapy for aortic stenosis, over 30% patients are considered at high or prohibitive risk for this procedure40 and TAVR has evolved as a promising alternative.41 Certainly, there have been an increasing number of large multicenter studies and numerous single-center small studies trying to identify mortality after TAVR. However, when managing a postprocedural patient, predicted mortality alone is far from enough. A delineation of the causes of death after TAVR is fundamental to the optimization of acute and late outcomes. The present study afforded important insights in this regard.
We saw noncardiovascular causes of death as important modes of death, particularly beyond 30 days: infection/sepsis, heart failure, and multiorgan failure were the top 3 individual and specific causes of death within the first 30 days and infection/sepsis, heart failure, and sudden death beyond 30 days, leaving infection/sepsis and heart failure being the biggest killers in noncardiovascular and cardiovascular causes of death, respectively. This is in line with prior works in this area.17,20 Although TAVR is minimally invasive, patients usually have predisposing factors for infection including age; poor pulmonary, renal, and immune function; diabetes; and need for ventilation and central venous access and monitoring.42 Also, as high-efficiency particulate air-filtered laminar airflow is absent in most catheterization labs, this may increase the risk for procedure-related infection.43 Thus, the use of broad-spectrum antibiotics for prophylaxis beforehand merits further investigation. It is accepted that aortic stenosis leads to an adaptive hypertrophic response of the myocardium, which allows for maintenance of cardiac output but may lead to progressive myocardial failure. Monrad et al44 identified the remodeling of left ventricular volume, mass, and shape as a process that may proceed up to almost a decade after correction of the valvular abnormality. Thus, the long-standing left ventricular hypertrophy, increased myocardial oxygen demand, and increased left ventricular end-diastolic pressure may play an important role in cardiac deaths seen.
Previous studies demonstrated that after an early phase of high risk of cardiovascular deaths, risk of cardiovascular death was substantially reduced in patients receiving SAVR.45 Non-valve-related cardiac failure and malignancy were reported as the most common certified cardiac and noncardiac causes of death in octogenarians involved in the UK Heart Valve Registry.46 In our following comparison between TAVR and SAVR or standard therapy, decreased trends were seen for noncardiovascular causes of death within and after 30 days following SAVR and standard therapy, the proportion of which increased following TAVR. Yet, because of current selection criteria, patients undergoing TAVR tend to have more comorbidities and may end up dying from those noncardiovascular comorbidities. Nonetheless, TAVR still proves itself to be a reliable alternative with similar mortality and mode of death even when the referred patients are deemed to be at high risk.
In the following subgroup analyses, no significant differences were seen for proportions of cardiovascular deaths except the comparison between moderate and high-risk patients after 30 days post-TAVR. Bearing different performing characteristics and risk potentials, TF and TA as access choices at least showed evenly distributed modes of death, which may be helpful when stratifying patients to certain treatment options, as was the case for Edwards SAPIEN Valve and Medtronic CoreValve as valve choices. On the other hand, in current practice, patient selection is often based on the evaluation of surgical risk. STS score or the EuroSCORE I/II are now frequently used for risk stratification. Hemmann et al47 have identified STS score as the strongest predictor of long-term survival following TAVR. Thus, we used the mean value of each study’s STS score as a risk stratification indicator. The finding of a higher proportion of cardiovascular deaths in moderate-risk patients after 30 days may be explained by the increasing deaths from more comorbidities in high-risk patients. There are certain limitations that need to be stressed. First, because of the huge variation of the number of causes of death, we have made some grouping such as putting accidental death and bone fracture into the “noncardiovascular causes” category. Also, although the VARC-2 criteria were used, further distinguishing of some possible procedure-related deaths was difficult because the lack of original information could cause overestimation of the proportion of noncardiovascular deaths, especially within 30 days after TAVR. Thus, this reclassification may inevitably lead to some bias. Second, heterogeneity should not be forgotten since our study did include large multicenter registries and also single-center studies with relatively small samples. Moreover, heterogeneity from different inclusion criteria in different centers also exists. Third, our results were related to time; however, for those results in the period after 30 days following TAVR, the death toll will be altered if follow-up time changes. Thus, there is a limitation for our included studies without a uniform time to end follow-up. Fourth, we did not exclude studies whose sample size was small. Considering the learning curve, heterogeneity in performing skills does exist across different centers.
Conclusions
The presented data provide important insights into the causes of early and late deaths after TAVR. Many of these causes are potentially preventable and merit specific attention to areas including infection prophylaxis and heart failure optimization. We believe that bearing all the most possible adverse events in mind and with the assistance of future technological advances, cardiologists will further improve patients’ outcomes after TAVR.
Sources of Funding
The work was funded by a grant from the National Natural Science Foundation of China (grant numbers: 81370219 and 81400267, Beijing, China).
Disclosures
Jilaihawi declares that he is a consultant for Edwards Lifesciences, St. Jude Medical, and Venus Medtech. The other authors declare no competing interests.
Supporting Information
Table S1. Patients’ baseline characteristics of enrolled studies.
Figure S1. Forest plot showing the individual and pooled event rates for different accesses.
Figure S2. Forest plot showing the individual and pooled event rates for different valve choices.
Figure S3. Forest plot showing the individual and pooled event rates for different risk score.
Figure S4. Available comparisons between TAVI and other treatment choices.
References
- Webb JG, Wood DA. Current status of transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;60:483–492. doi: 10.1016/j.jacc.2012.01.071. [DOI] [PubMed] [Google Scholar]
- Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Jr, Kleiman NS. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen LM, Wang MN, Chen XJ, Li N, De Huang Y, Chen M. The G894t, T-786c and 4b/a polymorphisms in Enos gene and cancer risk: a meta-analysis. J Evid Based Med. 2014;7:263–269. doi: 10.1111/jebm.12126. [DOI] [PubMed] [Google Scholar]
- Li CC, Wang YQ, Li YP, Li XL. High-intensity focused ultrasound for treatment of pancreatic cancer: a systematic review. J Evid Based Med. 2014;7:270–281. doi: 10.1111/jebm.12128. [DOI] [PubMed] [Google Scholar]
- Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G-A. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- Cui X, Li Y, Liu J, He S, Liu M. Aortic arch atheroma and the risk of stroke: a meta-analysis. J Evid Based Med. 2014;7:185–191. doi: 10.1111/jebm.12113. [DOI] [PubMed] [Google Scholar]
- Martinez GJ, Seco M, Jaijee SK, Adams MR, Cartwright BL, Forrest P, Celermajer DS, Vallely MP, Wilson MK, Ng MK. Introduction of an interdisciplinary heart team-based transcatheter aortic valve implantation program: short and mid-term outcomes. Intern Med J. 2014;44:876–883. doi: 10.1111/imj.12514. [DOI] [PubMed] [Google Scholar]
- Stabile E, Pucciarelli A, Cota L, Sorropago G, Tesorio T, Salemme L, Popusoi G, Ambrosini V, Cioppa A, Agrusta M, Catapano D, Moscariello C, Trimarco B, Esposito G, Rubino P. SAT-TAVI (single antiplatelet therapy for TAVI) study: a pilot randomized study comparing double to single antiplatelet therapy for transcatheter aortic valve implantation. Int J Cardiol. 2014;174:624–627. doi: 10.1016/j.ijcard.2014.04.170. [DOI] [PubMed] [Google Scholar]
- Omer S, Kar B, Cornwell LD, Blaustein A, Levine GN, Ali N, Jneid H, Paniagua D, Atluri PV, Bechara CF, Kougias P, Ruma M, Preventza O, Bozkurt B, Carabello BA, Bakaeen FG. Early experience of a transcatheter aortic valve program at a Veterans Affairs facility. JAMA Surg. 2013;148:1087–1093. doi: 10.1001/jamasurg.2013.3743. [DOI] [PubMed] [Google Scholar]
- Noble S, Frangos E, Samaras N, Ellenberger C, Frangos C, Cikirikcioglu M, Bendjelid K, Frei A, Myers P, Licker M, Roffi M. Transcatheter aortic valve implantation in nonagenarians: effective and safe. Eur J Intern Med. 2013;24:750–755. doi: 10.1016/j.ejim.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Walther T, Thielmann M, Kempfert J, Schroefel H, Wimmer-Greinecker G, Treede H, Wahlers T, Wendler O. One-year multicentre outcomes of transapical aortic valve implantation using the SAPIEN XT valve: the PREVAIL transapical study. Eur J Cardiothorac Surg. 2013;43:986–992. doi: 10.1093/ejcts/ezs589. [DOI] [PubMed] [Google Scholar]
- Latib A, Maisano F, Bertoldi L, Giacomini A, Shannon J, Cioni M, Ielasi A, Figini F, Tagaki K, Franco A, Covello RD, Grimaldi A, Spagnolo P, Buchannan GL, Carlino M, Chieffo A, Montorfano M, Alfieri O, Colombo A. Transcatheter vs surgical aortic valve replacement in intermediate-surgical-risk patients with aortic stenosis: a propensity score-matched case-control study. Am Heart J. 2012;164:910–917. doi: 10.1016/j.ahj.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Meguro K, Mouillet G, Bergoend E, Monin JL, Lim P, Dubois-Rande JL, Teiger E. Comparison of effectiveness and safety of transcatheter aortic valve implantation in patients aged >/=90 years versus <90 years. Am J Cardiol. 2012;110:1156–1163. doi: 10.1016/j.amjcard.2012.05.058. [DOI] [PubMed] [Google Scholar]
- Wendler O, Walther T, Schroefel H, Lange R, Treede H, Fusari M, Rubino P, Thomas M. Transapical aortic valve implantation: mid-term outcome from the SOURCE registry. Eur J Cardiothorac Surg. 2013;43:505–511. doi: 10.1093/ejcts/ezs297. discussion 511-502. [DOI] [PubMed] [Google Scholar]
- Wendler O, Thielmann M, Schroefel H, Rastan A, Treede H, Wahlers T, Eichinger W, Walther T. Worldwide experience with the 29-mm Edwards SAPIEN XT transcatheter heart valve in patients with large aortic annulus. Eur J Cardiothorac Surg. 2013;43:371–377. doi: 10.1093/ejcts/ezs203. [DOI] [PubMed] [Google Scholar]
- Kempfert J, Treede H, Rastan AJ, Schonburg M, Thielmann M, Sorg S, Mohr FW, Walther T. Transapical aortic valve implantation using a new self-expandable bioprosthesis (ACURATE TA): 6-month outcomes. Eur J Cardiothorac Surg. 2013;43:52–56. doi: 10.1093/ejcts/ezs139. discussion 57. [DOI] [PubMed] [Google Scholar]
- Van Mieghem NM, van der Boon RM, Nuis RJ, Schultz C, van Geuns RJ, Serruys PW, Kappetein AP, van Domburg RT, de Jaegere PP. Cause of death after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2014;83:E277–E282. doi: 10.1002/ccd.24597. [DOI] [PubMed] [Google Scholar]
- Doss M, Buhr EB, Martens S, Moritz A, Zierer A. Transcatheter-based aortic valve implantations at midterm: what happened to our initial patients? Ann Thorac Surg. 2012;94:1400–1406. doi: 10.1016/j.athoracsur.2012.05.051. [DOI] [PubMed] [Google Scholar]
- Ducrocq G, Al-Attar N, Himbert D, Messika-Zeitoun D, Iung B, Descoutures F, Nataf P, Vahanian A. Early and mid-term outcomes in patients undergoing transcatheter aortic valve implantation after previous coronary artery bypass grafting. Eur J Cardiothorac Surg. 2012;41:499–504. doi: 10.1093/ejcts/ezr041. [DOI] [PubMed] [Google Scholar]
- Ussia GP, Barbanti M, Petronio AS, Tarantini G, Ettori F, Colombo A, Violini R, Ramondo A, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A, Poli A, De Carlo M, Napodano M, Fiorina C, De Marco F, Antoniucci D, de Cillis E, Capodanno D, Tamburino C. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J. 2012;33:969–976. doi: 10.1093/eurheartj/ehr491. [DOI] [PubMed] [Google Scholar]
- D’Onofrio A, Rubino P, Fusari M, Salvador L, Musumeci F, Rinaldi M, Vitali EO, Glauber M, Di Bartolomeo R, Alfieri OR, Polesel E, Aiello M, Casabona R, Livi U, Grossi C, Cassese M, Pappalardo A, Gherli T, Stefanelli G, Faggian GG, Gerosa G. Clinical and hemodynamic outcomes of “all-comers” undergoing transapical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation (I-TA) J Thorac Cardiovasc Surg. 2011;142:768–775. doi: 10.1016/j.jtcvs.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Bosmans JM, Kefer J, De Bruyne B, Herijgers P, Dubois C, Legrand V, Verheye S, Rodrigus I. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg. 2011;12:762–767. doi: 10.1510/icvts.2010.253773. [DOI] [PubMed] [Google Scholar]
- Hernandez-Antolin RA, Garcia E, Sandoval S, Almeria C, Cuadrado A, Serrano J, de Obeso E, Del Valle R, Banuelos C, Alfonso F, Guerrero F, Heredia J, Martin Benitez JC, Garcia-Rubira JC, Rodriguez E, Macaya C. Findings of a mixed transfemoral aortic valve implantation program using Edwards and CoreValve devices. Rev Esp Cardiol (Engl Ed) 2011;64:35–42. doi: 10.1016/j.recesp.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjogren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg. 2011;91:57–63. doi: 10.1016/j.athoracsur.2010.07.072. [DOI] [PubMed] [Google Scholar]
- Lefevre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schachinger V, De Bruyne B, Eltchaninoff H, Thielmann M, Himbert D, Romano M, Serruys P, Wimmer-Greinecker G. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–157. doi: 10.1093/eurheartj/ehq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- Drews T, Pasic M, Buz S, Unbehaun A, Dreysse S, Kukucka M, Mladenow A, Hetzer R. Transapical aortic valve implantation after previous heart surgery. Eur J Cardiothorac Surg. 2011;39:625–630. doi: 10.1016/j.ejcts.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Attias D, Himbert D, Ducrocq G, Detaint D, Al-Attar N, Iung B, Francis F, Maury JM, Brochet E, Enguerrand D, Nataf P, Vahanian A. Immediate and mid-term results of transfemoral aortic valve implantation using either the Edwards Sapien transcatheter heart valve or the Medtronic CoreValve System in high-risk patients with aortic stenosis. Arch Cardiovasc Dis. 2010;103:236–245. doi: 10.1016/j.acvd.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Ye J, Cheung A, Lichtenstein SV, Nietlispach F, Albugami S, Masson JB, Thompson CR, Munt B, Moss R, Carere RG, Jamieson WR, Webb JG. Transapical transcatheter aortic valve implantation: follow-up to 3 years. J Thorac Cardiovasc Surg. 2010;139:1107–1113. doi: 10.1016/j.jtcvs.2009.10.056. 1113.e1101. [DOI] [PubMed] [Google Scholar]
- Guinot PG, Depoix JP, Etchegoyen L, Benbara A, Provenchere S, Dilly MP, Philip I, Enguerand D, Ibrahim H, Vahanian A, Himbert D, Al-Attar N, Nataf P, Desmonts JM, Montravers P, Longrois D. Anesthesia and perioperative management of patients undergoing transcatheter aortic valve implantation: analysis of 90 consecutive patients with focus on perioperative complications. J Cardiothorac Vasc Anesth. 2010;24:752–761. doi: 10.1053/j.jvca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Avanzas P, Munoz-Garcia AJ, Segura J, Pan M, Alonso-Briales JH, Lozano I, Moris C, de Lezo JS, Hernandez-Garcia JM. Percutaneous implantation of the CoreValve((R)) self-expanding aortic valve prosthesis in patients with severe aortic stenosis: early experience in Spain. Rev Esp Cardiol (Engl Ed) 2010;63:141–148. doi: 10.1016/s1885-5857(10)70031-1. [DOI] [PubMed] [Google Scholar]
- Kapadia SR, Goel SS, Svensson L, Roselli E, Savage RM, Wallace L, Sola S, Schoenhagen P, Shishehbor MH, Christofferson R, Halley C, Rodriguez LL, Stewart W, Kalahasti V, Tuzcu EM. Characterization and outcome of patients with severe symptomatic aortic stenosis referred for percutaneous aortic valve replacement. J Thorac Cardiovasc Surg. 2009;137:1430–1435. doi: 10.1016/j.jtcvs.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Walther T, Falk V, Borger MA, Kempfert J, Ender J, Linke A, Schuler G, Mohr FW. Transapical aortic valve implantation in patients requiring redo surgery. Eur J Cardiothorac Surg. 2009;36:231–234. doi: 10.1016/j.ejcts.2009.02.016. discussion 234-235. [DOI] [PubMed] [Google Scholar]
- Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, Sinhal A, Carere RG, Munt B, Ricci D, Ye J, Cheung A, Lichtenstein SV. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116:755–763. doi: 10.1161/CIRCULATIONAHA.107.698258. [DOI] [PubMed] [Google Scholar]
- Zierer A, Wimmer-Greinecker G, Martens S, Moritz A, Doss M. Is transapical aortic valve implantation really less invasive than minimally invasive aortic valve replacement? J Thorac Cardiovasc Surg. 2009;138:1067–1072. doi: 10.1016/j.jtcvs.2009.04.057. [DOI] [PubMed] [Google Scholar]
- Spaccarotella C, Mongiardo A, Indolfi C. Pathophysiology of aortic stenosis and approach to treatment with percutaneous valve implantation. Circ J. 2010;75:11–19. doi: 10.1253/circj.cj-10-1105. [DOI] [PubMed] [Google Scholar]
- Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P, Gohlke-Bärwolf C, Boersma E, Ravaud P, Vahanian A. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Tuzcu EM, Krishnaswamy A, Schoenhagen P, Stewart WJ, Svensson LG, Kapadia SR. Transcatheter aortic valve replacement: current perspectives and future implications. Heart. 2015;101:169–177. doi: 10.1136/heartjnl-2014-306254. [DOI] [PubMed] [Google Scholar]
- Falcone M, Russo A, Mancone M, Carriero G, Mazzesi G, Miraldi F, Pennacchi M, Pugliese F, Tritapepe L, Vullo V. Early, intermediate and late infectious complications after transcatheter or surgical aortic-valve replacement: a prospective cohort study. Clin Microbiol Infect. 2013;20:758–763. doi: 10.1111/1469-0691.12470. [DOI] [PubMed] [Google Scholar]
- Onsea K, Agostoni P, Voskuil M, Samim M, Stella P. Infective complications after transcatheter aortic valve implantation: results from a single centre. Neth Heart J. 2012;20:360–364. doi: 10.1007/s12471-012-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monrad ES, Hess O, Murakami T, Nonogi H, Corin W, Krayenbuehl H. Time course of regression of left ventricular hypertrophy after aortic valve replacement. Circulation. 1988;77:1345–1355. doi: 10.1161/01.cir.77.6.1345. [DOI] [PubMed] [Google Scholar]
- Svensson LG, Blackstone EH, Rajeswaran J, Brozzi N, Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Tuzcu EM, Webb JG, Kapadia S, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Thourani VH, Pichard AD, Bavaria JE, Herrmann HC, Williams MR, Babaliaros V, Genereux P, Akin JJ. Comprehensive analysis of mortality among patients undergoing TAVR: results of the PARTNER trial. J Am Coll Cardiol. 2014;64:158–168. doi: 10.1016/j.jacc.2013.08.1666. [DOI] [PubMed] [Google Scholar]
- Asimakopoulos G, Edwards M-B, Taylor KM. Aortic valve replacement in patients 80 years of age and older survival and cause of death based on 1100 cases: collective results from the UK heart valve registry. Circulation. 1997;96:3403–3408. doi: 10.1161/01.cir.96.10.3403. [DOI] [PubMed] [Google Scholar]
- Hemmann K, Sirotina M, De Rosa S, Ehrlich JR, Fox H, Weber J, Moritz A, Zeiher AM, Hofmann I, Schächinger V. The STS score is the strongest predictor of long-term survival following transcatheter aortic valve implantation, whereas access route (transapical versus transfemoral) has no predictive value beyond the periprocedural phase. Interact Cardiovasc Thorac Surg. 2013;17:359–364. doi: 10.1093/icvts/ivt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patients’ baseline characteristics of enrolled studies.
Figure S1. Forest plot showing the individual and pooled event rates for different accesses.
Figure S2. Forest plot showing the individual and pooled event rates for different valve choices.
Figure S3. Forest plot showing the individual and pooled event rates for different risk score.
Figure S4. Available comparisons between TAVI and other treatment choices.