Abstract
Background
Catheter ablation is increasingly used for rhythm control in symptomatic atrial fibrillation (AF), but the demographic characteristics of patients undergoing this procedure are unclear.
Methods and Results
We used data on all admissions at nonfederal acute care hospitals in California, Florida, and New York to identify patients discharged with a primary diagnosis of AF between 2006 and 2011. Our primary outcome was readmission for catheter ablation of AF, identified using validated International Classification of Diseases, Ninth Revision, Clinical Modification procedure codes. Cox regression models were used to assess relationships between demographic characteristics and catheter ablation, adjusting for Elixhauser comorbidities. We identified 397 612 eligible patients. Of these, 16 717 (4.20%, 95% CI 0.41 to 0.43) underwent ablation. These patients were significantly younger, more often male, more often white, and more often privately insured, with higher household incomes and lower rates of medical comorbidity. In Cox regression models, the likelihood of ablation was lower in women than men (hazard ratio [HR] 0.83; 95% CI 0.80 to 0.86) despite higher rates of AF-related rehospitalization (HR 1.23; 95% CI 1.21 to 1.24). Compared to whites, the likelihood of ablation was lower in Hispanics (HR 0.60; 95% CI 0.56 to 0.64) and blacks (HR 0.68; 95% CI 0.64 to 0.73), even though blacks had only a slightly lower likelihood of AF-related rehospitalization (HR 0.97; 95% CI 0.94 to 0.99) and a higher likelihood of all-cause hospitalization (HR 1.38; 95% CI 1.37 to 1.39). Essentially the same pattern existed in Hispanics.
Conclusions
We found differences in use of catheter ablation for symptomatic AF according to sex and race despite adjustment for available data on demographic characteristics and medical comorbidities.
Keywords: ablation, arrhythmia, catheter ablation, sex, women
Atrial fibrillation (AF) imposes a large public health burden due to its high prevalence and its associated risk of serious complications.1 AF that results in a rapid ventricular rate can cause hemodynamic instability and disabling symptoms. While heart rate can be controlled with medications alone, pharmacologic therapy does not suffice or causes significant side effects in many patients. In these cases, catheter ablation is strongly recommended for maintaining normal sinus rhythm.2 Catheter ablation has been shown to significantly improve quality of life in symptomatic AF patients.3 Randomized controlled trials are currently ongoing to determine whether catheter ablation impacts the morbidity and mortality of AF.4
Despite this recommendation, little is known about which patients receive this therapy. Nationwide utilization of catheter ablation for AF has doubled over the past decade,5 but it is unclear whether this has occurred uniformly, or whether it has been driven by particular types of patients. In the United States, marked demographic differences exist in the utilization of other specialized procedures similar to catheter ablation, as well as treatment for cardiac disease. Black patients are substantially less likely to receive deep brain stimulators for Parkinson disease than nonblack patients, even after controlling for comorbidities and other socioeconomic factors such as insurance type.6 Female congestive heart failure patients are less likely to be treated by a heart failure specialist than men with the same illness.7 In a recent, large study of Medicare beneficiaries, we found differences in AF treatment according to race and sex, including pharmacologic and nonpharmacologic modalities.8 To investigate whether similar disparities exist in the use of catheter ablation for symptomatic AF in patients across multiple insurance types, we compared the demographic characteristics of patients of who did or did not undergo catheter ablation after presentation to the hospital with AF.
Methods
Study Design
This was a retrospective cohort study conducted using de-identified administrative patient claims data from emergency department (ED) visits and acute care hospitalizations at nonfederal healthcare institutions across California, Florida, and New York. Designated state agencies collect these data as part of their regulatory role and provide a de-identified version to the Agency for Healthcare Research and Quality.5 Each patient is assigned an anonymous identifier that allows tracking of patients across multiple visits and multiple years. This analysis was approved by the Weill Cornell Medical College institutional review board.
Patient Population
We identified patients at the time of a first-recorded hospital discharge with a primary diagnosis of AF, defined as International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code 427.31. We included patients with an initial visit between 2005 and 2010 in California, 2005 and 2011 in Florida, and 2006 and 2010 in New York; these dates were chosen to allow at least 1 year of follow-up for all patients. We excluded patients whose first visit involved an ablation procedure, because we were interested in assessing rates of ablation after hospitalization with symptomatic AF.
Measurements
Our outcome was catheter ablation of AF. Since no specific International Classification of Diseases, Ninth Revision, Clinical Modification procedure code exists for the ablation of AF, we assessed the sensitivity and specificity of procedure code 37.34 (ablation of heart tissue) in a sample of patients at New York-Presbyterian Hospital/Weill Cornell Medical Center. A single blinded investigator (G.G.) reviewed the medical records of 25 patients with a primary discharge diagnosis of AF and procedure code 37.34, as well as the records of 25 patients with a primary discharge diagnosis of AF and no procedure code 37.34, while blinded to the procedure code. Using the determination of whether an AF ablation procedure was performed as “gold standard,” we calculated the sensitivity and specificity of procedure code 37.34. Our initial review found this code to have a sensitivity of 100% (95% CI 84 to 100) and a specificity of 86% (95% CI 68 to 96). Post-hoc review of the false-positive procedure codes indicated that they were for ablation of ventricular tachycardia in patients who also had AF. We then tested a revised algorithm defining AF ablation as procedure code 37.34 during a hospitalization with a primary discharge diagnosis of AF and no discharge diagnosis of ventricular tachycardia. Using a further round of blinded medical record review validation, we found this revised algorithm to have a sensitivity of 96% (95% CI 80 to 100) and a specificity of 100% (95% CI 86 to 100). Given its superior combination of sensitivity and specificity, we then used this second algorithm to define catheter AF ablation in our analysis. To further ensure that we excluded procedures for ablation of common arrhythmias other than AF, we performed additional analyses that also excluded procedures in patients with diagnosis codes for paroxysmal supraventricular tachycardia (International Classification of Diseases, Ninth Revision, Clinical Modification code 427.0) or other unspecific cardiac arrhythmias (427.89).
In a secondary analysis, we examined rates of ablation procedures aimed at rhythm control (eg, pulmonary vein isolation) as opposed to rate control (eg, atrioventricular node ablation). Since atrioventricular node ablation requires the presence of a pacemaker, we repeated the chart review above in a different sample of patients to assess the sensitivity and specificity of our procedure code algorithm in the absence of any codes relating to a cardiac pacemaker. This revealed that procedure code 37.34 in the presence of a primary discharge diagnosis of AF and no codes for ventricular tachycardia or a cardiac pacemaker has a sensitivity of 92% (95% CI 74 to 99) and a specificity of 96% (95% CI 79 to 100) for ablation procedures designed to restore sinus rhythm, such as pulmonary vein isolation.
Our main goal was to assess the relationship between demographic characteristics and receipt of catheter ablation. Specifically, we were interested in age, sex, race, insurance type, and income level. Since the burden of medical comorbidity is likely to vary across demographic profiles and simultaneously be associated with selection of patients as appropriate candidates for an invasive procedure, we adjusted for all Elixhauser comorbidities9 as well as particularly relevant comorbidities not included in the Elixhauser list: stroke, transient ischemic attack, and coronary heart disease. To account for secular trends in use of catheter ablation,10 we adjusted for the year of index hospitalization.
Statistical Analysis
Descriptive statistics were used to report and compare baseline patient characteristics between those who did or did not subsequently receive catheter ablation. We compared proportions using the χ2 test and continuous measures using the t test or rank-sum test as appropriate. Cumulative rates of catheter AF ablation after initial presentation were calculated using Kaplan–Meier survival statistics. Patients entered observation at the time of their first hospitalization with a primary AF diagnosis, and were censored at the time of catheter ablation, in-hospital death, or latest available follow-up. Cox proportional hazards analysis was used to assess the association between baseline demographic characteristics and receipt of ablation while adjusting for medical comorbidities. To assess the degree of healthcare utilization related to AF as well as the overall degree of utilization, we used the same models to assess the likelihood of repeated admissions for AF and for any cause. The threshold of statistical significance was set at α=0.05. All statistical analyses were performed using Stata/MP version 13 (College Station, TX).
Results
We identified a total of 397 612 patients who were hospitalized with AF, of whom 16 717 (4.2%, 95% CI 0.41 to 0.43) ultimately underwent ablation during a mean follow-up time of 3.16 years (SD, 1.9 years). Patients who underwent ablation were significantly younger, more often male, more often white, and more often privately insured (Table1). Patients undergoing ablation also had higher household income, and faced a lower burden of medical comorbidity. Cumulative rates of catheter ablation according to sex and race are shown in Figures1 and 2.
Table 1.
Characteristics of Patients Hospitalized for Atrial Fibrillation, Stratified by Subsequent Catheter Ablation
| Characteristic* | Ablation (N=16 717) | No Ablation (N=380 895) |
|---|---|---|
| Age, mean (SD), y | 64.2 (12.6) | 71.6 (14.2) |
| Female | 6695 (40.1) | 194 859 (51.2) |
| Race† | ||
| White | 13 855 (83.9) | 291 705 (77.8) |
| Black | 901 (5.5) | 26 020 (6.9) |
| Hispanic | 1139 (6.9) | 37 443 (10.0) |
| Asian | 265 (1.6) | 10 346 (2.8) |
| Other | 358 (2.2) | 9308 (2.5) |
| Payment source‡ | ||
| Medicare | 8522 (51.0) | 262 183 (68.8) |
| Medicaid | 666 (4.0) | 18 755 (4.9) |
| Private | 6649 (39.8) | 79 715 (20.9) |
| Self-pay | 372 (2.2) | 10 326 (2.7) |
| Other | 507 (3.0) | 9901 (2.6) |
| Quartile of median income | ||
| 1 | 3102 (19.0) | 86 413 (23.2) |
| 2 | 4052 (24.8) | 96 561 (25.9) |
| 3 | 4382 (26.8) | 96 993 (26.0) |
| 4 | 4830 (29.5) | 92 907 (24.9) |
| Year of index hospitalization | ||
| 2005 | 2617 (15.7) | 43 167 (11.3) |
| 2006 | 3339 (20.0) | 61 701 (16.2) |
| 2007 | 3084 (18.5) | 60 747 (16.0) |
| 2008 | 2716 (16.3) | 62 770 (16.5) |
| 2009 | 2472 (14.8) | 64 751 (17.0) |
| 2010 | 1843 (11.0) | 64 898 (17.0) |
| 2011 | 646 (3.9) | 22 861 (6.0) |
| Coronary heart disease | 5054 (30.2) | 128 156 (33.7) |
| Transient ischemic attack | 79 (0.5) | 2897 (0.8) |
| CHA2DS2-VASc score, median (interquartile range) | 2 (1 to 3) | 3 (2 to 4) |
| Stroke | 31 (0.2) | 2171 (0.6) |
| Elixhauser comorbidities§ | ||
| Acquired immunodeficiency syndrome | 12 (0.1) | 239 (0.1) |
| Alcohol abuse | 586 (3.5) | 16 213 (4.3) |
| Deficiency anemias | 1059 (6.3) | 47 967 (12.6) |
| Rheumatoid arthritis/collagen vascular diseases | 359 (2.2) | 9311 (2.4) |
| Chronic blood loss anemia | 46 (0.3) | 2003 (0.5) |
| Congestive heart failure | 20 (0.1) | 1536 (0.4) |
| Chronic pulmonary disease | 2998 (17.9) | 78 025 (20.5) |
| Coagulopathy | 273 (1.6) | 10 906 (2.9) |
| Depression | 1037 (6.2) | 25 781 (6.8) |
| Diabetes, uncomplicated | 3134 (18.8) | 76 278 (20.0) |
| Diabetes with chronic complications | 292 (1.8) | 12 669 (3.3) |
| Drug abuse | 175 (1.1) | 4540 (1.2) |
| Hypertension | 9895 (59.2) | 246 144 (64.6) |
| Hypothyroidism | 2055 (12.3) | 54 733 (14.4) |
| Liver disease | 178 (1.1) | 5813 (1.5) |
| Lymphoma | 91 (0.5) | 3216 (0.8) |
| Fluid and electrolyte disorders | 1723 (10.3) | 69 534 (18.3) |
| Metastatic cancer | 40 (0.2) | 6058 (1.6) |
| Other neurological disorders | 426 (2.6) | 21 781 (5.7) |
| Obesity | 2115 (12.7) | 39 872 (10.5) |
| Paralysis | 88 (0.5) | 5695 (1.5) |
| Peripheral vascular disorders | 689 (4.1) | 24 418 (6.4) |
| Psychoses | 212 (1.3) | 8012 (2.1) |
| Pulmonary circulation disorders | <10 (0.0) | 417 (0.1) |
| Renal failure | 954 (5.7) | 40 283 (10.6) |
| Solid tumor without metastasis | 171 (1.0) | 8680 (2.3) |
| Peptic ulcer disease excluding bleeding | <10 (0.0) | 195 (0.1) |
| Valvular disease | 22 (0.1) | 797 (0.2) |
| Weight loss | 53 (0.3) | 6590 (1.7) |
Data are presented as number (%) unless otherwise specified.
Self-reported by patients or their surrogates. Numbers do not sum to group totals because of missing race/ethnicity data in 1.6% of patients.
Numbers do not sum to group totals because of missing payment-source data in <0.01% of patients.
Numbers represent the number of Elixhauser comorbid conditions, which comprise a comprehensive set of 28 comorbidity measures for use with large administrative datasets.
Figure 1.
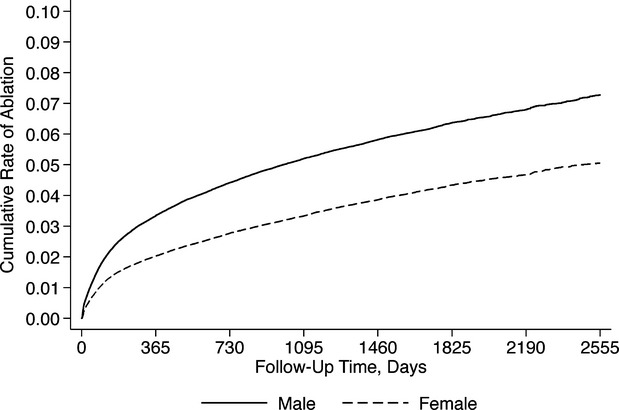
Cumulative rate of catheter ablation, stratified by sex.
Figure 2.
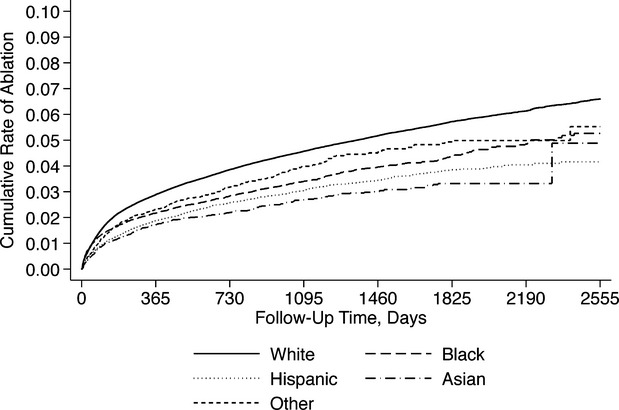
Cumulative rate of catheter ablation, stratified by race.
In multivariate Cox regression models adjusting for demographic characteristics and medical comorbidities, the likelihood of ablation was lower in women (hazard ratio [HR] 0.83; 95% CI 0.80 to 0.86), Hispanics (HR 0.60; 95% CI 0.56 to 0.64), Asians (HR 0.58; 95% CI 0.52 to 0.66), and blacks (HR 0.68; 95% CI 0.64 to 0.73) even after adjustment for insurance status and income level (Table2). Compared to those with Medicare insurance, ablation was more likely in those with private insurance (HR 1.17; 95% CI 1.11 to 1.22) and less likely in those with Medicaid (HR 0.74, 95% CI 0.68 to 0.81) or no insurance (HR 0.52; 95% CI 0.46 to 0.58). The likelihood of ablation rose linearly with household income (HR for top versus bottom income quartile, 1.21; 95% CI 1.16 to 1.27).
Table 2.
Multivariate Cox Regression Analysis, Adjusted for Medical Comorbidities
| Characteristic (Comparison Group) | Hazard Ratio | P Value | 95% CI |
|---|---|---|---|
| Age | 0.97 | <0.001 | 0.97 to 0.972 |
| Sex (male) | |||
| Female | 0.83 | <0.001 | 0.80 to 0.86 |
| Race (white) | |||
| Black | 0.68 | <0.001 | 0.63 to 0.73 |
| Hispanic | 0.60 | <0.001 | 0.56 to 0.64 |
| Asian | 0.58 | <0.001 | 0.51 to 0.66 |
| Other | 0.77 | <0.001 | 0.69 to 0.86 |
| Insurance type (Medicare) | |||
| Medicaid | 0.74 | <0.001 | 0.67 to 0.81 |
| Private | 1.17 | <0.001 | 1.11 to 1.22 |
| Self-pay | 0.52 | <0.001 | 0.46 to 0.58 |
| Other | 0.83 | <0.001 | 0.75 to 0.91 |
| Median income quartile (1st quartile) | |||
| 2nd | 1.08 | 0.001 | 1.03 to 1.14 |
| 3rd | 1.12 | <0.001 | 1.07 to 1.17 |
| 4th | 1.22 | <0.001 | 1.16 to 1.27 |
Although women were less likely to undergo ablation, they were more likely than men to have repeat hospitalizations for AF (HR 1.23; 95% CI 1.21 to 1.24) or for any cause (HR 1.10; 95% CI 1.09 to 1.10). Compared to whites, blacks had a slightly lower likelihood of AF-related repeat hospitalization (HR 0.97; 95% CI 0.94 to 0.99) and a higher likelihood of repeat hospitalization for any cause (HR 1.38; 95% CI 1.37 to 1.39). The same pattern existed for Hispanics. We found nearly identical patterns when excluding procedures in patients with diagnoses for paroxysmal supraventricular tachycardia or other unspecified arrhythmias, and when specifically assessing ablation procedures designed for rhythm control.
Discussion
In a large, population-based sample of patients hospitalized for AF, we found that the likelihood of subsequent catheter ablation was greater in whites and in men than in nonwhites and women. Additionally, we found that likelihood of catheter ablation was lower in individuals who had lower incomes and were insured through government-sponsored insurance programs. Moreover, lower rates of ablation in women and nonwhites occurred despite similar or higher rates of readmissions related to AF. These differences were seen after adjustment for income, insurance status, and medical comorbidities.
Our results fit the well-established demographic trend in American cardiovascular care. In comparison to whites, black patients are less likely to undergo invasive cardiac procedures,11,12 cardiac catheterizations following acute myocardial infarction,13 and cardiac resynchronization therapy in systolic heart failure.14 Female patients with coronary artery disease have been shown to receive less aggressive care than men,15,16 and female diabetic veterans are less likely to receive lipid-lowering therapies than their male counterparts.17
The results of our study are also consistent with recently documented patterns favoring white male patients in the treatment of AF in particular. Naderi et al showed that black patients hospitalized with AF received inpatient AF-targeted therapies less frequently than whites.18 As described elsewhere, Hispanics and women with newly diagnosed AF were less likely to receive catheter ablation in comparison to whites and men despite adjusting for medical comorbidities, age, and income; in this same study, black patients were also significantly less likely than white patients to receive rate control, rhythm control, or anticoagulant therapies.8 Although this study examined an older patient cohort and a wider range of AF treatments than our present study, these results parallel our current findings with respect to catheter ablation. It is important to note that racial disparities are likely the results of complex interactions between socioeconomic characteristics, clinical conditions, and available healthcare provider characteristics—not all of which were known to us because of the nature of our data.
From an economic perspective, it is clear that physician reimbursements impact physician behavior.19 Likewise, electrophysiologists may have been more reluctant to pursue catheter ablation in Medicaid or Medicare patients due to the comparatively lower physician fee reimbursements from government-sponsored plans when compared to private insurance plans. Medicare physician fee schedules for percutaneous cardiac ablation procedures are generally higher than those of Medicaid,20–22 but while Medicaid and Medicare physician fee reimbursements for catheter ablation are publicly available, similar data from private payers are not.23 The effect of these reimbursement differences on the demographic incongruities we found is therefore clouded by the paucity of data on private insurance plan reimbursements nationwide, but could theoretically provide an explanation for the comparatively lower likelihood of catheter ablation in Medicaid-insured patients.
Similarly, hospital reimbursements for catheter ablation procedures could have also driven some of the observed demographic differences in treatment. In 2012, US hospitals were reimbursed an average of $10 338 by Medicare for an admission in which the principal procedure was a percutaneous cardiovascular procedure without a coronary stent.24 As discussed elsewhere, however, private payer reimbursement data to hospitals are largely unavailable. While the ratio between reimbursement and per-procedure cost would hypothetically be of high importance, multiple issues make the concept of catheter ablation “cost” difficult to pinpoint. Procedure cost likely fluctuates between healthcare institutions24 and cannot be reliably generalized from the existing cost-effectiveness studies on catheter ablation.25,26 Greater transparency for insurance payer reimbursements to hospital systems and further research on generalizable cost-effectiveness data would be useful in definitively making conclusions on the relationship between institutional costs, reimbursements, and demographic disparities in the provision of catheter ablation.
Although insurance types may play an important role in explaining our results, they do not entirely explain the differences in catheter ablation according to income, race, and sex that we found after adjusting for insurance type. The relatively lower likelihood of ablation seen in uninsured patients and women in our study may have been confounded by low income levels and subsequent inability to afford the procedure. Data from the US Census show that individuals with low household incomes are more frequently uninsured, and that a larger percentage of adult women than men lived under the poverty threshold in the year 2011.27 Out-of-pocket costs to undergo catheter ablation are not well defined and probably depend on variable factors such as location, hospital fee schedules, and individual electrophysiologist preferences. Despite this, a relative unaffordability of catheter ablation in lower-income groups, including women, may partially explain the lower likelihood of ablation in these groups.
While associations between race, income, and sex do exist in nationwide data, they do not fully account for the lower likelihood of receiving catheter ablation in nonwhites and women. Additional unmeasured factors, such as language barriers, may have affected physician decision making in Hispanic patients,28 and women may also have been more reluctant to communicate concerns to their physicians.29 In light of the extensive literature on racial and sex-based disparities in cardiovascular care, conscious or unconscious biases on the part of treating physicians should also be considered as plausible explanations for the differences we found in our study.
The strengths of this study are its large, generalizable study population, relatively long period of follow-up, and its use of a multivariate model to adjust for multiple confounders. However, our study is limited by several factors. First, we lacked detailed clinical data and thus could not differentiate between patients with paroxysmal, persistent, and permanent AF. Catheter ablation is approved by the Food and Drug Administration for paroxysmal AF but not persistent AF,2 and it is unknown whether AF type may have driven patient selection in our sample. Similarly, not all episodes of AF provoke the same severity of patient symptoms, and we were unable to account for sex and racial differences in symptomatology,30–32 which is likely a key driver in the decision for patients to present to the hospital, as well as for providers to perform ablation. The lack of clinical data also means that we could not account for antithrombotic therapy status, which may have affected decisions about ablation. Second, we lacked the detailed clinical information needed to assess whether patients who did not ultimately undergo ablation either were not offered it, or declined to proceed with treatment. Third, our study design, which focused on predictors of ablation, did not allow us to assess the impact of the disparities we describe on healthcare costs or on patient outcomes, such as mortality, or freedom from symptoms. Fourth, we cannot be sure that patients had not previously undergone ablation in the years prior to the time period for which we have data. Fifth, we cannot rule out sex and racial differences in rates of loss to follow-up due to emigration out of state. Future studies will be needed to assess demographic differences in utilization of ablation for AF while adjusting for important clinical covariates that are not available in administrative claims data.
In conclusion, we found suggestions of significant demographic differences—mainly in race, sex, and insurance type—in the provision of catheter ablation after hospital presentation for AF in the United States. While these differences were consistent with well-described national delivery patterns for cardiovascular procedures, the causes remain unclear and may be multifactorial. It is important for treating physicians to be aware that such differences in treatment may exist, not only for the goal of providing their patients with optimal medical care, but also to help reduce the large problem of US healthcare inequality.
Sources of Funding
This study was supported by grant K23NS082367 (Kamel) from the National Institute of Neurological Disorders and Stroke.
Disclosures
None.
References
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:2305–2307. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, Klein G, Weerasooriya R, Clementy J, Haissaguerre M. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hiendricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–696. [Google Scholar]
- Agency for Healthcare Research and Quality. Healthcare cost and utilization project. Available at: http://hcupnet.ahrq.gov. Accessed March 10, 2015. [DOI] [PubMed]
- Chan AK, McGovern RA, Brown LT, Sheehy JP, Zacharia BE, Mikell CB, Bruce SS, Ford B, McKhann GM. Disparities in access to deep brain stimulation surgery for Parkinson disease: interaction between African American race and Medicaid use. JAMA Neurol. (2nd) 2014;71:291–299. doi: 10.1001/jamaneurol.2013.5798. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Huynh T, Des Lauriers J. Gender and other disparities in referral to specialized heart failure clinics following emergency department visits. J Womens Health (Larchmt) 2013;22:526–531. doi: 10.1089/jwh.2012.4107. [DOI] [PubMed] [Google Scholar]
- Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin M. Race and gender related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12:1406–1412. doi: 10.1016/j.hrthm.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Quality AfHRa. Healthcare cost and utilization project. Available at: http://hcupnet.ahrq.gov. Accessed March 10, 2015.
- Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med. 2001;135:352–366. doi: 10.7326/0003-4819-135-5-200109040-00012. [DOI] [PubMed] [Google Scholar]
- Lillie-Blanton M, Maddox TM, Rushing O, Mensah GA. Disparities in cardiac care: rising to the challenge of Healthy People 2010. J Am Coll Cardiol. 2004;44:503–508. doi: 10.1016/j.jacc.2004.04.043. [DOI] [PubMed] [Google Scholar]
- Chen J, Rathore S, Radford MJ, Wang Y, Krumholz HM. Racial differences in the use of cardiac catheterization after acute myocardial infarction. N Engl J Med. 2001;344:1443–1449. doi: 10.1056/NEJM200105103441906. [DOI] [PubMed] [Google Scholar]
- Casale JC, Wolf F, Pei Y, Devereux RB. Socioeconomic and ethnic disparities in the use of biventricular pacemakers in heart failure patients with left ventricular systolic dysfunction. Ethn Dis. 2013;23:275–280. [PubMed] [Google Scholar]
- Steingart RM, Packer M, Hamm P, Coglianese ME, Gersh B, Geltman EM, Sollano J, Katz S, Moyé L, Basta LL, Lewis SJ, Gottlieb SS, Bernstein V, McEwan P, Jacobson K, Brown EJ, Kukin ML, Kantrowitz NE, Pfeffer MA. Sex differences in the management of coronary artery disease. Survival and Ventricular Enlargement Investigators. N Engl J Med. 1991;325:226–230. doi: 10.1056/NEJM199107253250402. [DOI] [PubMed] [Google Scholar]
- Ayanian JZ, Epstein AM. Differences in the use of procedures between women and men hospitalized for coronary heart disease. N Engl J Med. 1991;325:221–225. doi: 10.1056/NEJM199107253250401. [DOI] [PubMed] [Google Scholar]
- Vimalananda VG, Miller DR, Palnati M, Christiansen CL, Fincke BG. Gender disparities in lipid-lowering therapy among veterans with diabetes. Womens Health Issues. 2011;21:S176–S181. doi: 10.1016/j.whi.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Naderi S, Rodriguez F, Wang Y, Foody JM. Racial disparities in hospitalizations, procedural treatments and mortality of patients hospitalized with atrial fibrillation. Ethn Dis. 2014;24:144–149. [PubMed] [Google Scholar]
- Long SK. Physicians may need more than high reimbursements to expand Medicaid participation: findings from Washington State. Health Aff. 2013;32:1560–1567. doi: 10.1377/hlthaff.2012.1010. [DOI] [PubMed] [Google Scholar]
- CMS Fee Schedule Lookup. Available at: http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 10, 2015.
- Medi-Cal Rates as of 2/15/2015. Available at: http://files.medi-cal.ca.gov/pubsdoco/Rates/rates_download.asp. Accessed March 10, 2015.
- Florida Medicaid Portal. Provider fee schedules. Available at: http://portal.flmmis.com/FLPublic/Provider_ProviderServices/Provider_ProviderSupport/Provider_ProviderSupport_FeeSchedules/tabId/51/Default.aspxhttp://portal.flmmis.com/FLPublic/Provider_ProviderServices/Provider_ProviderSupport/Provider_ProviderSupport_FeeSchedules/tabId/51/Default.aspx. Accessed March 19, 2015.
- Institute for Clinical and Economic Review (ICER) Ablation strategies for atrial fibrillation: supplemental data and analyses to the comparative effectiveness review of the Agency for Healthcare Research and Quality—Final Meeting Report. New England Comparative Effectiveness Public Advisory Council (CEPAC) Public Meeting. 2011; 1-52. Available at: http://cepac.icer -review.org/wp-content/uploads/2011/04/RAPiD-Afib-FINAL-Meeting-Report.pdf. Accessed April 15, 2015.
- Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. Physician-controlled costs: the choice of equipment used for atrial fibrillation ablation. J Interv Card Electrophysiol. 2013;36:157–165. doi: 10.1007/s10840-013-9782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaykin Y, Shamiss Y. Cost of atrial fibrillation: invasive vs non-invasive management in 2012. Curr Cardiol Rev. 2012;8:368–373. doi: 10.2174/157340312803760730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyt M, Van Brabandt H, Devos C. The cost-utility of catheter ablation of atrial fibrillation: a systematic review and critical appraisal of economic evaluations. BMC Cardiovasc Disord. 2013;13:1–9. doi: 10.1186/1471-2261-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. Census QuickFacts. Available at: http://quickfacts.census.gov. Accessed March 10, 2015.
- Samuels-Kalow ME, Stack AM, Porter SC. Parental language and dosing errors after discharge from the pediatric emergency department. Pediatr Emerg Care. 2013;29:982–987. doi: 10.1097/PEC.0b013e3182a269ec. [DOI] [PubMed] [Google Scholar]
- Arber S, McKinlay J, Adams A, Marceau L, Link C, O’Donnell A. Influence of patient characteristics on doctors’ questioning and lifestyle advice for coronary heart disease: a UK/US video experiment. Br J Gen Pract. 2004;54:673–678. [PMC free article] [PubMed] [Google Scholar]
- Humphries KH, Kerr CR, Connolly SJ, Klein G, Boone JA, Green M, Sheldon R, Talajic M, Dorian P, Newman D. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–2370. doi: 10.1161/01.cir.103.19.2365. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Lavelle T, Essebag V, Cohen DJ, Zimetbaum P. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152:1097–1103. doi: 10.1016/j.ahj.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Ellis E, Zimetbaum P. Quality of life in atrial fibrillation: measurement tools and impact of interventions. J Cardiovasc Electrophysiol. 2008;19:762–768. doi: 10.1111/j.1540-8167.2007.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


