Abstract
Background
Optimal protocols for targeted temperature management are still unclear. This study investigated whether lower target temperatures and/or prolonged cooling could provide improved outcomes in comatose survivors of cardiac arrest.
Methods and Results
This observational study was conducted using the prospectively collected targeted temperature management database in Hiroshima, Japan. Between September 2003 and September 2014, 237 patients treated with TTM after cardiac arrest were enrolled in this study. The target temperatures and durations were assigned by the treating physicians regardless of the patients’ conditions. Favorable outcomes were defined as a cerebral performance category scale of 1 or 2 at the 90-day follow-up time point. The rate of favorable outcomes were similar between the patients whose protocols of target temperature were <34°C and ≥34°C (40% versus 35%, P=0.41), cooling durations were <28 and ≥28 hours (33% versus 44%, P=0.11), and rewarming durations were <28 and ≥28 hours (35% versus 41%, P=0.39). However, in patients treated with extracorporeal cardiopulmonary resuscitation, target temperatures <34°C were associated with more favorable outcomes (29% versus 8%, P=0.01). The cooling and rewarming durations >28 hours and target temperatures <34°C were associated with more frequent lethal arrhythmia, pneumonia, and/or bleedings.
Conclusions
Prolonged durations of cooling and rewarming ≥28 hours may not improve outcomes and may increase complications. Further studies are necessary to assess the hypothesis that target temperatures <34°C provide improved outcomes in patients treated with extracorporeal cardiopulmonary resuscitation.
Keywords: cardiopulmonary resuscitation, extracorporeal circulation, heart arrest, targeted temperature management, therapeutic hypothermia
Sudden cardiac arrest is a major cause of unexpected death in developed countries and is a major clinical issue. Despite cardiopulmonary resuscitation (CPR), only a few patients are able to return to their former lifestyle.1,2 Among out-of-hospital cardiac arrest victims, 80% were comatose after the return of spontaneous circulation (ROSC), and 6% to 8% survived to hospital discharge.3 Targeted temperature management (TTM), so-called therapeutic hypothermia, improves survival and neurological outcomes in comatose survivors of cardiac arrest.4,5 The current resuscitation guidelines recommended that comatose adult patients with ROSC after out-of-hospital ventricular fibrillation cardiac arrest should be cooled to 32°C to 34°C for 12 to 24 hours as class I, according to the protocols in the randomized clinical trials.4–6 However, there were many unsolved questions regarding TTM, such as the optimal target temperature (TT) and cooling and rewarming durations.7,8 Adverse postreperfusion cascades result in delayed neuronal death, which occurs minutes to days after reperfusion; on the other hand, TTM was usually discontinued within 12 to 24 hours of cooling, which was not long enough to finish some of the above cascade.6,9 In some animal models, longer cooling durations were associated with a more neuroprotective effect than shorter durations of cooling.10,11 Meanwhile, regarding the rewarming duration, rapid rewarming was associated with adverse outcomes in basic research.12 In the context of TTs, cerebral metabolic rates decrease by 7% for every 1°C reduction in an animal model, and it was reported that lower TTs may provide more neuroprotection in animal studies.13 We assessed the hypothesis that lower TTs and/or prolonged cooling was associated with improved neurological outcomes in comatose survivors of cardiac arrest.
Methods
Study Patients
This study is a retrospective analysis of the registry data. We collected data from TTM registries that consist of prospectively collected data from TTM patients at Hiroshima City Asa Hospital and Hiroshima City Hospital from September 2003 to September 2014 in Hiroshima, Japan. Additional data were obtained from previous reports on the same patients that did not include data focusing on the details of the TTs and durations of TTM.14–17 Written informed consent for clinical studies was obtained from family members. This study was approved by the local institutional review board.
Hypothermia Protocol
Both hospitals have similar clinical practices and similar equipment, including the catheter laboratories and intensive/coronary care units. In the study patients, the core temperatures were immediately monitored at the bladder and/or pulmonary artery and were used for the management of patients (Figure1). Because pulmonary artery catheters were not placed in all of the study patients, the bladder temperatures were analyzed in this study. The TTs and the durations of cooling and rewarming were determined by the treating physicians regardless of the patients’ illness and conditions. The hospital protocols of TTM encouraged the physicians to decrease the core temperature as early as possible to the TT.
Figure 1.
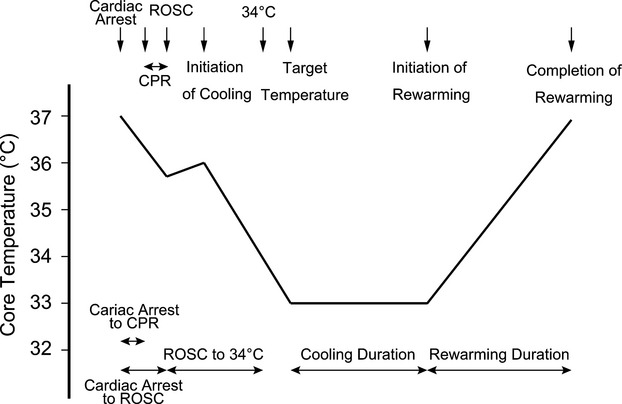
Shema of protocols for targeted temperature management. CPR indicates cardiopulmonary resuscitation; ROSC, return of spontaneous circulation.
Data Collection
Data pertaining to CPR and TTM were collected from the medical records. The primary goal was to examine the effect of TTs and cooling and rewarming durations on the outcomes. Favorable outcomes were defined as a cerebral performance category (CPC) of 1 or 2 on a 5-point scale 90 days after the index cardiac arrest.18 The CPC scales include the following: CPC 1, good cerebral performance: conscious, alert, able to work, and might have mild neurologic or psychologic deficit; CPC 2, moderate cerebral disability: conscious, sufficient cerebral function for independent activities of daily life, and able to work in sheltered environment; CPC 3, severe cerebral disability: conscious, dependent on others for daily support because of impaired brain function, and ranges from ambulatory state to severe dementia or paralysis; CPC 4, coma or vegetative state: not conscious, unaware of surroundings, no cognition, and no verbal or psychological interactions with environment; and CPC 5, death: certified brain dead or dead by traditional criteria.
Safety end points were the rate of complications during the first 7 days after the index cardiac arrest. Data on complications, including lethal arrhythmia, pneumonia, and bleeding of any severity, were collected from the medical records. Lethal arrhythmia was defined as ventricular fibrillation, ventricular tachycardia, torsade de pointes, hemodynamic unstable supraventricular tachycardia, or atrioventricular block. Pneumonia was assessed by ≥2 physicians according to physical examinations, laboratory data, and/or imaging modalities. The sites of bleeding were the catheter or cannulation sites, mucous membranes, the nose, the urinary tract, the gastrointestinal tract, subcutaneous tissue, and skin, as well as intracerebral and intraabdominal sites.4
Statistical Analysis
All of the study patients were analyzed in an intention-to-treat fashion by using temperatures and durations that were decided at the time of TTM initiation. Continuous variables are presented as medians (with interquartile range), and categorical variables are presented as numbers (with percentages). To assess the TT and durations of cooling and rewarming, we divided the study patients into 2 groups according to the thresholds as follows: the TTs were divided by 34°C because most patients were treated at 33°C or 34°C. The durations of cooling and rewarming were divided by 28 hours because the majority of patients were treated for 24 or 48 hours to assess the prolonged durations of cooling and rewarming that were listed in the current guidelines. To assess the changes in the protocols in this study period, the cohort was divided into 2 eras according to the date of cardiac arrest (2003 to 2008 [early period] or 2009 to 2014 [late period]), and the clinical characteristics, protocols, and outcomes were assessed. The Mann–Whitney U test for continuous variables and the χ2 test or Fisher exact test for categorical variables were used as appropriate to identify the factors associated with favorable outcomes. As a subgroup analysis, the odds ratio (OR) for favorable outcomes of TT <34°C relative to ≥34°C was evaluated in subgroups that included patients with out-of-hospital cardiac arrest, in-hospital cardiac arrest, shockable initial recorded rhythms, unshockable initial recorded rhythms, acute coronary syndromes (ACS), non-ACS, conventional CPR, and ECPR (i.e., CPR with initiation of venoarterial extracorporeal membrane oxygenation [ECMO] during CPR) by using a univariable logistic-regression model. ACS was diagnosed by ≥2 on-site cardiologists according to previous symptoms of the index cardiac arrest, laboratory data, ECG, and/or coronary angiography. Tests for interaction between TTs <34°C and the subgroups for each outcome were conducted by using logistic regression. Multivariable logistic regression was used to assess the durations and TTs as predictors of a favorable outcome. To select variables, a univariable analysis was performed to identify potentially relevant covariates (P<0.2) to predict favorable outcomes, including age ≥55 years, shockable initial recorded rhythms, out-of-hospital cardiac arrest, ACS, cardiac arrest to CPR ≥5 minutes, cardiac arrest to ROSC ≥32 minutes, ROSC to 34°C ≥167 minutes, TT <34°C, duration of cooling ≥28 hours, duration of rewarming ≥28 hours, and the late period (after 2009). Safety end points were compared between the patients of different protocols by using the χ2 test. Statistical analysis was performed with the JMP 8.0.2.2 statistical package (SAS Institute). A value of P<0.05 was considered statistically significant.
Results
Patients
A total of 237 patients were enrolled in the study. The clinical characteristics are shown in Table1. The median age of the study patients was 62 years (interquartile range, 52 to 72 years), 180 patients (76%) were male, 193 patients (81%) experienced out-of-hospital cardiac arrest, and 126 patients (53%) showed an initial shockable rhythm. In this study, 144 patients (61%) underwent immediate coronary angiography, and 76 patients (32%) underwent subsequent percutaneous coronary revascularization. Among the study patients, cold saline was administered rapidly in 156 patients (66%), surface cooling with ethanol evaporation was performed in 4 (2%) patients, gastric lavage with cold saline was performed in 19 (8%) patients, water circulating mattress was used in 142 (60%) patients, Arctic Sun (Medivance Inc) was used in 38 (16%) patients, coil cooling device attached to a hemodiafiltration system was used in 22 (9%) patients, and heat exchanger with ECMO was used in 65 (28%) patients.
Table 1.
Characteristics of the Study Patients According to the Different Protocols
| TT <34°C (n=90) | TT ≥34°C (n=147) | P Value | Cooling <28 h (n=162) | Cooling ≥28 h (n=75) | P Value | Rewarming <28 h (n=158) | Rewarming ≥28 h (n=79) | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 58 (52 to 70) | 63 (52 to 72) | 0.116 | 64 (53 to 72) | 58 (49 to 69) | 0.042 | 64 (53 to 72) | 56 (52 to 68) | 0.013 |
| Male | 70 (78) | 110 (75) | 0.606 | 116 (72) | 64 (85) | 0.022 | 113 (72) | 67 (85) | 0.024 |
| Out-of-hospital cardiac arrest | 124 (84) | 69 (77) | 0.140 | 123 (76) | 70 (93) | 0.001 | 119 (75) | 74 (94) | <0.001 |
| Witnessed arrest | 81 (90) | 112 (76) | 0.008 | 138 (85) | 55 (73) | 0.029 | 134 (85) | 59 (75) | 0.059 |
| Bystander cardiopulmonary resuscitation | 57 (63) | 70 (48) | 0.019 | 92 (57) | 35 (47) | 0.146 | 88 (56) | 39 (49) | 0.357 |
| Initial shockable rhythm | 52 (58) | 74 (50) | 0.265 | 82 (51) | 44 (59) | 0.248 | 81 (51) | 45 (57) | 0.407 |
| Acute coronary syndrome | 44 (49) | 53 (36) | 0.051 | 61 (38) | 36 (48) | 0.132 | 58 (37) | 39 (49) | 0.062 |
| Immediate coronary angiography | 76 (84) | 68 (46) | <0.001 | 102 (63) | 42 (56) | 0.307 | 99 (63) | 45 (57) | 0.397 |
| Subsequent percutaneous coronary intervention | 40 (44) | 36 (24) | 0.001 | 52 (32) | 24 (32) | 0.988 | 49 (31) | 27 (34) | 0.623 |
| Extracorporeal membrane oxygenation | 48 (53) | 39 (27) | <0.001 | 72 (44) | 15 (20) | <0.001 | 68 (43) | 19 (24) | 0.004 |
| Patients survived up to completion of rewarming | 66 (73) | 113 (77) | 0.539 | 42 (26) | 16 (21) | 0.444 | 117 (74) | 62 (78) | 0.455 |
Data presented as number (%) of patients or median (interquartile range). TT indicates target temperature.
The distributions of the TTM protocols by the cooling and rewarming durations and TTs are shown in Figure2A. In the TT protocols, 90% of the study patients were subjected to 33°C (32%) or 34°C (62%). In terms of the cooling duration TTM protocols, 94% of the patients were subjected to durations of cooling for 24 hours (62%) or 48 hours (31%). Regarding the rewarming duration TTM protocols, 97% of the patients were subjected to rewarming durations of 12 (28%), 24 (36%), 48 (23%), or 72 hours (10%). Among 237 patients, 58 patients died before the completion of rewarming. The distributions of the durations and TTs in the patients who survived through the completion of rewarming (n=179) are also shown in Figure2B.
Figure 2.
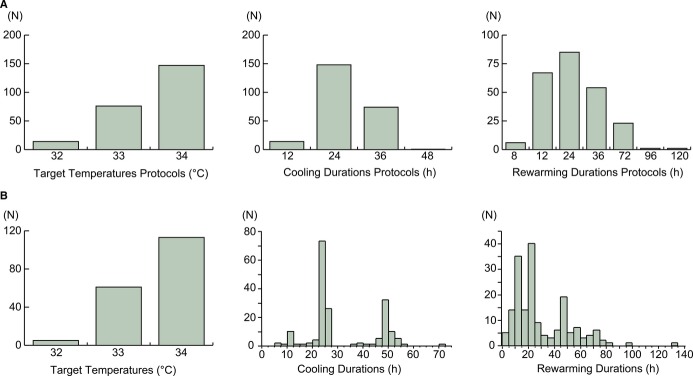
The distributions of cooling and rewarming durations (in hours, h) and target temperatures. A, The distributions of all of the patients according to the protocols. B, The distributions of the patients who survived up to the completion of rewarming according to the practical durations and temperatures.
The clinical characteristics, protocols of TTM, and outcomes according to the study period are shown in Table2. The patients in the late period (2009 to 2014) were older, had more initial nonshockable rhythms, had a higher usage of ECMO, had a longer duration from cardiac arrest to ROSC, and had a shorter duration from ROSC to 34°C. Regarding the TTM protocols, the prevalence of a target temperature <34°C was similar; however, prolonged durations of cooling (62% versus 2%, P<0.001) and rewarming (64% versus 3%, P<0.001) were used less often in the late period.
Table 2.
The Clinical Characteristics, Protocols, and Outcomes According to the Study Period
| Early Period (2003 to 2008, n=118) | Late Period (2009 to 2014, n=119) | P Value | |
|---|---|---|---|
| Age, y | 58 (49 to 69) | 64 (54 to 73) | 0.016 |
| Male | 96 (81) | 84 (71) | 0.053 |
| Out-of-hospital cardiac arrest | 103 (87) | 90 (76) | 0.021 |
| Witnessed arrest | 92 (78) | 101 (85) | 0.171 |
| Bystander cardiopulmonary resuscitation | 57 (48) | 70 (59) | 0.105 |
| Initial shockable rhythm | 72 (61) | 54 (45) | 0.016 |
| Acute coronary syndrome | 53 (45) | 44 (37) | 0.214 |
| Immediate coronary angiography | 65 (55) | 79 (66) | 0.075 |
| Subsequent percutaneous coronary intervention | 35 (30) | 41 (34) | 0.429 |
| Extracorporeal membrane oxygenation | 29 (25) | 58 (49) | <0.001 |
| Patients survived up to completion of rewarming | 99 (84) | 80 (67) | 0.003 |
| Cardiac arrest to CPR, min | 6 (1 to 11) | 3 (1 to 11) | 0.541 |
| Cardiac arrest to ROSC, min | 32 (19 to 45) | 43 (24 to 57) | 0.004 |
| ROSC to 34°C, min | 190 (116 to 343) | 70 (15 to 135) | <0.001 |
| Protocol | |||
| Target temperature <34°C | 44 (38) | 46 (39) | 0.823 |
| Cooling ≥28 h | 73 (62) | 2 (2) | <0.001 |
| Rewarming ≥28 h | 75 (64) | 4 (3) | <0.001 |
| 90-Day survival | 64 (54) | 41 (34) | 0.002 |
| Favorable outcomes | 55 (47) | 32 (27) | 0.002 |
Data presented as number (%) of patients or median (interquartile range). CPR indicates cardiopulmonary resuscitation; ROSC, return of spontaneous circulation.
Favorable Outcomes
Among the study patients, 110 patients (46%) survived up to 30 days, 106 patients (45%) were discharged alive, and 108 patients (46%) survived up to 90 days. At 90 days, 84 patients (35%) had a CPC of 1, 3 (1%) had a CPC of 2, 9 (4%) had a CPC of 3, 12 (5%) had a CPC of 4, 129 (54%) had a CPC of 5, and 87 patients (37%) had favorable outcomes. The prevalence of TT <34°C (36% versus 40%, P=0.589), durations of cooling ≥28 hours (35% versus 29%, P=0.284), and durations of rewarming ≥28 hours (35% versus 32%, P=0.580) were similar between the patients who survived up to 90 days and those who did not. The durations and TTs of TTM by favorable or unfavorable outcomes are shown in Table3. The durations from cardiac arrest to ROSC in the patients with favorable outcomes were significantly shorter than those in the patients with unfavorable outcomes (22 minutes versus 45 minutes, P<0.001). The durations from ROSC to 34°C were significantly longer in patients with favorable outcomes than in those with unfavorable outcomes (209 minutes versus 98 minutes, P<0.001). The TT and the cooling and rewarming durations protocols were similar between the patients with favorable and unfavorable outcomes. We conducted a post-hoc power calculation, and we had a 99.6% statistical power to detect such an effect size of Cohen’s w 0.3 at an α level of 0.05. The results were consistent across all subgroups, except for the significantly higher rate of TT <34°C in ECPR patients with favorable outcomes than in those with unfavorable outcomes (82% versus 49%, P=0.01, Table4). There were no significant interactions between TT <34°C and the subgroups. In the ECPR subsets, the baseline characteristics were similar between the patients with TTs of <34°C and those with TTs >34°C (Table5); however, the rates of favorable outcomes in patients with TTs <34°C were significantly higher than those with TTs of ≥34°C (29% versus 8%, P=0.01). The rate of favorable outcomes was higher in the patients in the early period compared with those of the late period (47% versus 27%, P=0.002, Table2). In the subsets of ACS patients, favorable outcomes were similar between the patients who received immediate coronary angiography and subsequent coronary revascularization and those who did not (31% versus 36%, P=0.615). The ORs for favorable outcomes in the subgroups with TTs <34°C are shown in Figure3. In the subsets of patients who were treated with ECPR, TTs <34°C were significantly more favorable (OR 4.94, 95% CI 1.46 to 22.81, P=0.009). The univariable and multivariable analyses for predicting favorable outcomes are shown in Table6. The TTM protocols of TTs <34°C, durations of cooling ≥28 hours, and durations of rewarming ≥28 hours were not independently associated with favorable outcomes. The covariates that reached statistically significance included a shockable initial recorded rhythm, time intervals from collapse to ROSC ≥32 minutes, and time intervals from ROSC to 34°C ≥167 minutes.
Table 3.
Durations and Target Temperatures With or Without Favorable Outcomes
| Favorable (n=87) | Unfavorable (n=150) | P Value | |
|---|---|---|---|
| Cardiac arrest to CPR, min | 5 (2 to 9) | 4 (1 to 13) | 0.556 |
| Cardiac arrest to ROSC, min | 22 (15 to 32) | 45 (32 to 58) | <0.001 |
| ROSC to 34°C, min | 209 (114 to 340) | 98 (35 to 164) | <0.001 |
| Protocol | |||
| Target temperature <34°C | 36 (41) | 54 (36) | 0.411 |
| Cooling ≥28 h | 33 (38) | 42 (28) | 0.113 |
| Rewarming ≥28 h | 32 (37) | 47 (31) | 0.391 |
| Practical temperatures and durations | |||
| Target temperature <34°C | 36 (41) | 54 (36) | 0.411 |
| Cooling, h | 24.5 (23.7 to 48.0) | 24.0 (11.1 to 26.6) | 0.002 |
| Rewarming, h | 24.0 (12.0 to 48.0) | 13.0 (0.0 to 25.0) | <0.001 |
Data presented as number (%) of patients or median (interquartile range). CPR indicates cardiopulmonary resuscitation; ROSC, return of spontaneous circulation.
Table 4.
Durations and Target Temperatures With or Without Favorable Outcomes
| Initial Recorded Rhythm Was Shockable (n=126) | Out-of-Hospital Cardiac Arrest (n=193) | Acute Coronary Syndrome (n=97) | Conventional Cardiopulmonary Resuscitation (n=150) | Extracorporeal Cardiopulmonary Resuscitation (n=87) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Favorable (n=64) | Unfavorable (n=62) | P Value | Favorable (n=72) | Unfavorable (n=121) | P Value | Favorable (n=31) | Unfavorable (n=66) | P Value | Favorable (n=70) | Unfavorable (n=80) | P Value | Favorable (n=17) | Unfavorable (n=70) | P Value | |
| Cardiac arrest to CPR, min | 6 (2 to 9) | 6 (2 to 12) | 0.274 | 7 (2 to 9) | 6 (1 to 14) | 0.522 | 7 (1 to 11) | 4 (1 to 10) | 0.658 | 7 (2 to 10) | 10 (2 to 15) | 0.057 | 0 (0 to 5) | 2 (1 to 9) | 0.058 |
| Cardiac arrest to ROSC, min | 21 (15 to 29) | 49 (33 to 60) | <0.001 | 23 (15 to 35) | 48 (37 to 61) | <0.001 | 25 (19 to 36) | 44 (24 to 58) | 0.001 | 21 (15 to 30) | 42 (31 to 53) | <0.001 | 25 (18 to 44) | 51 (34 to 62) | 0.002 |
| ROSC to 34°C, min | 231 (127 to 401) | 108 (55 to 189) | <0.001 | 231 (136 to 373) | 109 (55 to 168) | <0.001 | 178 (105 to 320) | 94 (22 to 148) | 0.002 | 237 (151 to 394) | 153 (98 to 242) | <0.001 | 28 (6 to 90) | 34 (10 to 90) | 0.805 |
| Protocol | |||||||||||||||
| Target temperature <34°C | 26 (41) | 26 (42) | 0.881 | 26 (36) | 43 (36) | 0.936 | 17 (55) | 27 (41) | 0.199 | 22 (31) | 20 (25) | 0.381 | 14 (82) | 34 (49) | 0.012 |
| Cooling ≥28 h | 25 (39) | 19 (31) | 0.322 | 32 (44) | 38 (31) | 0.068 | 16 (52) | 20 (30) | 0.043 | 29 (41) | 31 (39) | 0.738 | 4 (24) | 11 (16) | 0.48 |
| Rewarming ≥28 h | 24 (38) | 21 (34) | 0.671 | 31 (43) | 43 (36) | 0.299 | 15 (48) | 24 (36) | 0.26 | 28 (40) | 32 (40) | 1.000 | 4 (24) | 15 (21) | 1.000 |
| Practical | |||||||||||||||
| Target temperature <34°C | 26 (41) | 26 (42) | 0.881 | 26 (36) | 43 (36) | 0.936 | 17 (55) | 27 (41) | 0.199 | 22 (31) | 20 (25) | 0.381 | 14 (82) | 34 (49) | 0.012 |
| Cooling, h | 24.6 (23.8 to 48.0) | 24.0 (6.4 to 26.4) | 0.009 | 25.8 (24.0 to 48.0) | 24.5 (12.9 to 36.5) | 0.002 | 26.0 (23.8 to 48.0) | 23.7 (6.2 to 31.9) | 0.005 | 24.9 (24.0 to 48.0) | 24.5 (23.1 to 48.0) | 0.269 | 23.7 (12.0 to 25.8) | 19.6 (5.6 to 25.1) | 0.13 |
| Rewarming, h | 23.0 (12.0 to 48.0) | 12.0 (0 to 24.3) | <0.001 | 25.3 (17.3 to 48.8) | 15.5 (0 to 29.5) | <0.001 | 26.0 (15.5 to 66.3) | 12.0 (0 to 45.8) | <0.001 | 24.0 (15.4 to 48.0) | 21.0 (11.0 to 46.1) | 0.052 | 12.0 (11.5 to 42.3) | 5 (0 to 19.0) | 0.002 |
CPR indicates cardiopulmonary resuscitation; ROSC, return of spontaneous circulation.
Table 5.
Baseline Characteristics in Patients Treated With Extracorporeal Cardiopulmonary Resuscitation According to the Target Temperature Protocols
| Target Temperatures <34°C (n=48) | Target Temperatures ≥34°C (n=39) | P Value | |
|---|---|---|---|
| Age, y | 60 (54 to 69) | 64 (60 to 72) | 0.138 |
| Male | 36 (75) | 28 (72) | 0.736 |
| Out-of-hospital cardiac arrest | 28 (58) | 26 (67) | 0.426 |
| Witnessed arrest | 45 (94) | 37 (95) | 1.000 |
| Bystander cardiopulmonary resuscitation | 38 (79) | 28 (72) | 0.424 |
| Initial shockable rhythm | 22 (46) | 18 (46) | 0.976 |
| Acute coronary syndrome | 26 (54) | 22 (56) | 0.834 |
| Immediate coronary angiography | 37 (77) | 31 (79) | 0.787 |
| Subsequent percutaneous coronary intervention | 22 (46) | 21 (54) | 0.457 |
| Patients survived up to completion of rewarming | 20 (42) | 20 (51) | 0.371 |
Figure 3.
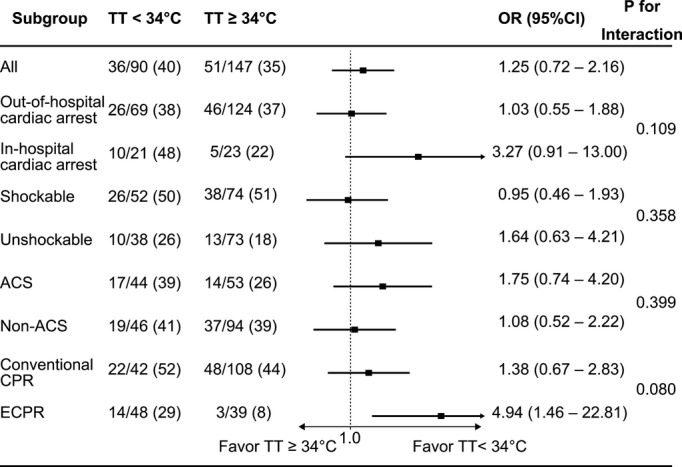
Odds ratio of favorable outcomes of TT <34°C or ≥34°C in the subgroups. The odds ratios were evaluated using a univariable logistic regression. Patients with out-of-hospital cardiac arrest, in-hospital cardiac arrest, shockable initial recorded rhythms, unshockable initial recorded rhythms, acute coronary syndrome, nonacute coronary syndrome, conventional cardiopulmonary resuscitation, and extracorporeal cardiopulmonary resuscitation were included in the subgroups. ACS indicates acute coronary syndrome; ECPR, extracorporeal cardiopulmonary resuscitation; OR, odds ratio; TT, target temperature.
Table 6.
Univariable Analysis for Favorable Outcomes
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| All patients (n=237) | ||||||
| Age ≥55 y | 0.50 | 0.28 to 0.87 | 0.015 | 0.53 | 0.26 to 1.07 | 0.078 |
| Initial recorded rhythm was shockable | 3.95 | 2.24 to 7.14 | <0.001 | 2.59 | 1.31 to 5.19 | 0.006 |
| Out-of-hospital cardiac arrest | 1.15 | 0.59 to 2.34 | 0.689 | |||
| Acute coronary syndrome | 0.70 | 0.41 to 1.21 | 0.205 | |||
| Cardiac arrest to CPR ≥5 min | 0.93 | 0.55 to 1.57 | 0.777 | |||
| Cardiac arrest to ROSC ≥32 min | 0.11 | 0.06 to 0.21 | <0.001 | 0.16 | 0.08 to 0.30 | <0.001 |
| ROSC to 34°C ≥167 min | 5.49 | 3.11 to 9.89 | <0.001 | 4.07 | 1.86 to 9.22 | <0.001 |
| Protocol of targeted temperature management | ||||||
| Target temperature <34°C | 1.25 | 0.73 to 2.16 | 0.412 | 1.80 | 0.86 to 3.85 | 0.120 |
| Duration of cooling ≥28 h | 1.57 | 0.89 to 2.75 | 0.115 | 3.20 | 0.37 to 70.83 | 0.312 |
| Duration of rewarming ≥28 h | 1.28 | 0.73 to 2.22 | 0.393 | 5.13 | 0.61 to 113.23 | 0.142 |
| The late period (after 2009) | 0.42 | 0.24 to 0.72 | 0.002 | 0.72 | 0.30 to 1.75 | 0.467 |
CPR indicates cardiopulmonary resuscitation; ROSC, return of spontaneous circulation.
Complications
The complications during the first 7 days after the index cardiac arrest are shown in Table7. Episodes of lethal arrhythmia and pneumonia were more frequent in patients with protocols cooling and rewarming durations ≥28 hours than in those with durations <28 hours. The rates of lethal arrhythmia and pneumonia were similar among patients with protocols TTs <34°C and those with TTs ≥34°C. The rate of bleeding was significantly higher in patients treated with protocols TTs <34°C than in those with TTs ≥34°C (53% versus 31%, P<0.001).
Table 7.
Complications During the First 7 Days After Cardiac Arrest
| Target Temperature Protocol | Cooling Duration Protocol | Rewarming Duration Protocol | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <34°C (n=90) | ≥34°C (n=147) | P Value | <28 hours (n=162) | ≥28 hours (n=75) | P Value | <28 hours (n=158) | ≥28 hours (n=79) | P Value | |
| Lethal arrhythmia | 14 (16) | 16 (11) | 0.294 | 15 (9) | 15 (20) | 0.021 | 12 (8) | 18 (23) | <0.001 |
| Pneumonia | 25 (28) | 37 (25) | 0.658 | 31 (19) | 31 (41) | <0.001 | 29 (18) | 33 (42) | <0.001 |
| Bleeding | 48 (53) | 45 (31) | <0.001 | 62 (38) | 31 (41) | 0.654 | 60 (38) | 33 (42) | 0.573 |
Data are presented as number (%) of patients.
Discussion
Our study showed that there were no significant differences in the neurological outcomes of patients treated with or without prolonged cooling and rewarming durations. In all of the study patients, similar neurological outcomes were observed in patients who were treated with TTs <34°C and ≥34°C; however, TTs <34°C were associated with improved outcomes in patients treated with ECPR. Prolonged cooling and rewarming durations and lower TTs were associated with more frequent complications including lethal arrhythmia, pneumonia, and/or bleedings.
This study has several strengths for hypothesis generation to provide some light on doubts concerning the optimum TT and durations, although it was observational. First, the majority of study patients were evaluated with the use of immediate coronary angiography and subsequent coronary intervention. Many observational studies reported that immediate coronary angiography and revascularization were associated with improved outcomes, and the current guidelines recommended immediate coronary angiography and revascularization for resuscitated patients when indicated.19–21 Our study patients received the current optimal care for ischemic heart disease and revascularization.
Second, this study includes patients treated with novel CPR using ECMO and analyzed the difference of associations of TTs and durations between the patients treated with or without ECPR.22,23 Recently, Nielsen et al reported that there were no differences between the mortality rates of patients treated with TTs of 33°C and 36°C, and our data in non-ECPR patients support these results.24 However, in patients treated with ECPR in our study, TTs <34°C was associated with significantly improved neurological outcomes compared with patients treated with TTs ≥34°C.
The possible cause of the different lower TT results between patients treated with and without ECPR may be as follows: first, optimal target temperatures may vary by the severity of ischemic injury. However, if only the severity of illness plays a role, different outcomes should have been observed in the TTM trial.24 Therefore, other factors/mechanisms must be present in the differences of outcomes in our study. Second, the early application of hypothermia increased intact survival, and the timing of hypothermia determined the organ protective effect of hypothermia in animal studies.25,26 Rapid cooling by ECMO may be able to provide more neuroprotective and cardioprotective effects at lower temperatures that depend on the depth of cooling compared with the non-ECMO technique.27 Third, ECMO could provide hemodynamic stability just after the initiation of ECMO, and the neuroprotective effect may be different between TTs in such conditions.28 Fourth, reperfusion with ECMO may be closer to “controlled reperfusion,” and in such conditions, the organ-protective effects may vary by the TTs.28 In patients treated with ECMO, reperfused blood was oxygenated and carbon dioxide was removed by using a membrane oxygenator, and the blood was relatively cold due to a loss of heat by heat radiation and conduction at the ECMO circuit, which was cooled by a using heat exchanger at the time of initiation of ECMO. On the other hand, in patients treated with conventional CPR, the blood was relatively hypoxic, hypercapnic, and acidic, and the blood temperature was equal to the core temperature at the time of ROSC.
The TTM protocols in our study resulted in longer durations of cooling and rewarming, which were less frequent after 2009. This finding may be caused by the guideline’s recommendation. The patient characteristics treated with TTM were also altered so that the patients with more severe conditions, such as nonshockable rhythms, longer duration from cardiac arrest to ROSC, or refractory cardiac arrest treated with ECMO, were treated with TTM in the late period. The more severe conditions of the patients in the late period may cause a lower rate of favorable outcomes compared with those in the early period (Tables2 and 6).
In our study, a shockable initial recorded rhythm, a time interval from collapse to ROSC <32 minutes, and a time interval from ROSC to 34°C ≥167 minutes were independently associated with more favorable outcomes. Predictors, such as a shockable rhythm and the time interval from collapse to ROSC, were reported previously.5,16,29 Nagao et al reported that early attainment of 34°C was associated with improved outcomes.30 On the other hand, Haugk et al reported that a faster decline in body temperature to 34°C was associated with unfavorable outcomes.31 Though it seems contradictory, this discrepancy may be caused by the patient populations and the cooling methods used in the studies. Preserved homeostasis may act to better maintain body temperature in patients whose neurological injuries are minor and who had favorable outcomes, resulting in a delay to the TT. The patients in the study by Haugk et al were cooled by using nonextracorporeal cooling methods, and the median duration from ROSC to 34°C was 144 minutes. On the other hand, ECMO and its attached heat exchanger could provide faster cooling and easy and precise temperature control.32 One-half of the patients in Nagao et al’s study were cooled by cold infusion and ECMO, and their duration from the initiation of ECMO to 34°C was ≈30 minutes less than that of Haugk et al’s study. In Nagao et al’s study, the difference of the time to TT between patients with and without favorable outcomes might be relatively small due to powerful cooling by ECMO, and the major differences between the times to TT were based on the timing of initiation of cooling between post-ROSC cooling and intra-arrest cooling. Our study supports both results. Recent studies reported that the early attainment of hypothermia did not result in favorable outcomes in patients treated with or without prehospital cooling under conventional CPR.33 We were able to generate a hypothesis that the early attainment of TT could result in more favorable outcomes with ECPR but not with conventional CPR.
Possible explanations, implications, and hypotheses for clinicians and future trials from this study are that optimal durations of cooling in TTM may be <28 hours. In patients treated with conventional CPR, TTs <34°C and ≥34°C may provide similar outcomes, and if extrapolated from the report by Nielsen et al, the optimal TT may be 36°C24; however, in selected patients such those treated with ECPR, this study could generate a hypothesis that TTs <34°C provide improved outcomes, and the optimal TT may be different according to the patient conditions after cardiac arrest. Can what was old be new again? Randomized controlled trials are necessary to clarify these issues. We calculated that forthcoming randomized trials would need to enroll 100 patients in each group to have 80% power to show favorable outcomes 15% higher in the TT <34°C group than those in the TT ≥34°C group with a 2-sided α level of 0.05, assuming that the frequency of favorable outcomes at TTs <34°C is 25%.
Limitations
This study was a nonrandomized controlled study and had the inherent limitations of any observational investigation. The study was prone to biases related to unmeasured factors. The TTs and durations were decided at the time of initiation of TTM by the treating physicians, and there could be selection biases, such as longer durations of cooling or lower TTs for patients treated with prolonged resuscitation. However, the baseline characteristics, such as time interval from collapse to ROSC, of the study patients were similar between the TT and duration subsets, and the severity of illness might not affect the discretion of the treating physicians. Patient selection for ECPR might have the potential to include selection biases, but the choice of protocols for TTM and selection of candidates for ECPR were different, while the baseline characteristics in patients treated with ECMO were similar (Table5).
Conclusions
Prolonged cooling and rewarming durations ≥28 hours were not associated with improved outcomes and were associated with higher rates of lethal arrhythmia and pneumonia. TTs <34°C were not associated with improved outcomes and were associated with a higher prevalence of bleeding in patients treated with conventional CPR. However, TTs <34°C may be beneficial for patients who were CPR nonresponders and were treated with ECPR. On the basis of these findings, randomized studies are needed to answer the question of whether we should turn down the thermostat in patients treated with ECPR.
Acknowledgments
We appreciate the efforts of the coronary care units, the cardiology wards, the emergency depsrtments, the catheter laboratories, and the heart team staff in the hospitals, which made this study possible.
Disclosures
None.
References
- Safar P. Cerebral resuscitation after cardiac arrest: research initiatives and future directions. Ann Emerg Med. 1993;22:324–349. doi: 10.1016/s0196-0644(05)80463-9. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Iwami T, Kawamura T, Nagao K, Tanaka H, Hiraide A. Nationwide public-access defibrillation in Japan. N Engl J Med. 2010;362:994–1004. doi: 10.1056/NEJMoa0906644. [DOI] [PubMed] [Google Scholar]
- Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879. doi: 10.1001/jama.291.7.870. [DOI] [PubMed] [Google Scholar]
- The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL. Part 9: post–cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Sunde K. Rewarming after therapeutic hypothermia. Resuscitation. 2012;83:930–931. doi: 10.1016/j.resuscitation.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CM, Ahern Kv, Cheng ML, Lee JE, Yenari MA, Steinberg GK, Kirsch JR. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654:265–272. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Enomoto S, Hindman BJ, Dexter F, Smith T, Cutkomp J. Rapid rewarming causes an increase in the cerebral metabolic rate for oxygen that is temporarily unmatched by cerebral blood flow. A study during cardiopulmonary bypass in rabbits. Anesthesiology. 1996;84:1392–1400. doi: 10.1097/00000542-199606000-00016. [DOI] [PubMed] [Google Scholar]
- Steen PA, Newberg L, Milde JH, Michenfelder JD. Hypothermia and barbiturates: individual and combined effects on canine cerebral oxygen consumption. Anesthesiology. 1983;58:527–532. [PubMed] [Google Scholar]
- Kagawa E, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S, Nakama Y, Maruhashi T, Dai K, Matsushita J, Ikenaga H. History of diabetes mellitus as a neurologic predictor in comatose survivors of cardiac arrest of cardiac origin treated with mild hypothermia. Resuscitation. 2009;80:881–887. doi: 10.1016/j.resuscitation.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Kagawa E, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S, Nakama Y, Dai K, Takayuki O, Ikenaga H, Morimoto Y, Ejiri K, Oda N. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation. 2010;81:968–973. doi: 10.1016/j.resuscitation.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Kagawa E, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S, Nakama Y, Dai K, Otani T, Ikenaga H, Morimoto Y, Ejiri K, Oda N. Who benefits most from mild therapeutic hypothermia in coronary intervention era? A retrospective and propensity-matched study. Crit Care. 2010;14:R155. doi: 10.1186/cc9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa E, Dote K, Kato M, Sasaki S, Nakano Y, Kajikawa M, Higashi A, Itakura K, Sera A, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S. Should we emergently revascularize occluded coronaries for cardiac arrest? Rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation. 2012;126:1605–1613. doi: 10.1161/CIRCULATIONAHA.111.067538. [DOI] [PubMed] [Google Scholar]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Spaulding CM, Joly LM, Rosenberg A, Monchi M, Weber SN, Dhainaut JF, Carli P. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- Dumas F, Cariou A, Manzo-Silberman S, Grimaldi D, Vivien B, Rosencher J, Empana J-P, Carli P, Mira J-P, Jouven X, Spaulding C. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (parisian region out of hospital cardiac arrest) registry. Circ Cardiovasc Interv. 2010;3:200–207. doi: 10.1161/CIRCINTERVENTIONS.109.913665. [DOI] [PubMed] [Google Scholar]
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- Nagao K, Hayashi N, Kanmatsuse K, Arima K, Ohtsuki J, Kikushima K, Watanabe I. Cardiopulmonary cerebral resuscitation using emergency cardiopulmonary bypass, coronary reperfusion therapy and mild hypothermia in patients with cardiac arrest outside the hospital. J Am Coll Cardiol. 2000;36:776–783. doi: 10.1016/s0735-1097(00)00779-8. [DOI] [PubMed] [Google Scholar]
- Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, Chen LC, Tsai PR, Wang SS, Hwang JJ, Lin FY. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Kober L, Langorgen J, Lilja G, Moller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- Nozari A, Safar P, Stezoski SW, Wu X, Kostelnik S, Radovsky A, Tisherman S, Kochanek PM. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113:2690–2696. doi: 10.1161/CIRCULATIONAHA.106.613349. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Gotberg M, Lang I, Holzer M, Noc M, Clemmensen P, Jensen U, Metzler B, James S, Botker HE, Omerovic E, Engblom H, Carlsson M, Arheden H, Ostlund O, Wallentin L, Harnek J, Olivecrona GK. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: a randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J Am Coll Cardiol. 2014;63:1857–1865. doi: 10.1016/j.jacc.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Trummer G, Foerster K, Buckberg GD, Benk C, Mader I, Heilmann C, Liakopoulos O, Beyersdorf F. Superior neurologic recovery after 15 minutes of normothermic cardiac arrest using an extracorporeal life support system for optimized blood pressure and flow. Perfusion. 2014;29:130–138. doi: 10.1177/0267659113497776. [DOI] [PubMed] [Google Scholar]
- Reynolds JC, Frisch A, Rittenberger JC, Callaway CW. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: when should we change to novel therapies? Circulation. 2013;128:2488–2494. doi: 10.1161/CIRCULATIONAHA.113.002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao K, Kikushima K, Watanabe K, Tachibana E, Tominaga Y, Tada K, Ishii M, Chiba N, Kasai A, Soga T, Matsuzaki M, Nishikawa K, Tateda Y, Ikeda H, Yagi T. Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circ J. 2010;74:77–85. doi: 10.1253/circj.cj-09-0502. [DOI] [PubMed] [Google Scholar]
- Haugk M, Testori C, Sterz F, Uranitsch M, Holzer M, Behringer W, Herkner H. Relationship between time to target temperature and outcome in patients treated with therapeutic hypothermia after cardiac arrest. Crit Care. 2011;15:R101. doi: 10.1186/cc10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata A, Magnet IA, Drabek T, Stezoski JP, Janesko-Feldman K, Popp E, Garman RH, Tisherman SA, Kochanek PM. Extracorporeal versus conventional cardiopulmonary resuscitation after ventricular fibrillation cardiac arrest in rats: a feasibility trial. Crit Care Med. 2013;41:e211–e222. doi: 10.1097/CCM.0b013e318287f51e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, Copass MK, Carlbom D, Deem S, Longstreth WT, Jr, Olsufka M, Cobb LA. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311:45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]


