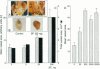Abstract
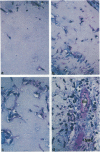
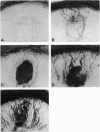
Scatter factor (also known as hepatocyte growth factor) is a glycoprotein secreted by stromal cells that stimulates cell motility and proliferation. In vitro, scatter factor stimulates vascular endothelial cell migration, proliferation, and organization into capillary-like tubes. Using two different in vivo assays, we showed that physiologic quantities of purified native mouse scatter factor and recombinant human hepatocyte growth factor induce angiogenesis (the formation of new blood vessels). The angiogenic activity was blocked by specific anti-scatter factor antibodies. Scatter factor induced cultured microvascular endothelial cells to accumulate and secrete significantly increased quantities of urokinase, an enzyme associated with development of an invasive endothelial phenotype during angiogenesis. We further showed that immunoreactive scatter factor is present surrounding sites of blood vessel formation in psoriatic skin. These findings suggest that scatter factor may act as a paracrine mediator in pathologic angiogenesis associated with human inflammatory disease.
Full text
PDF
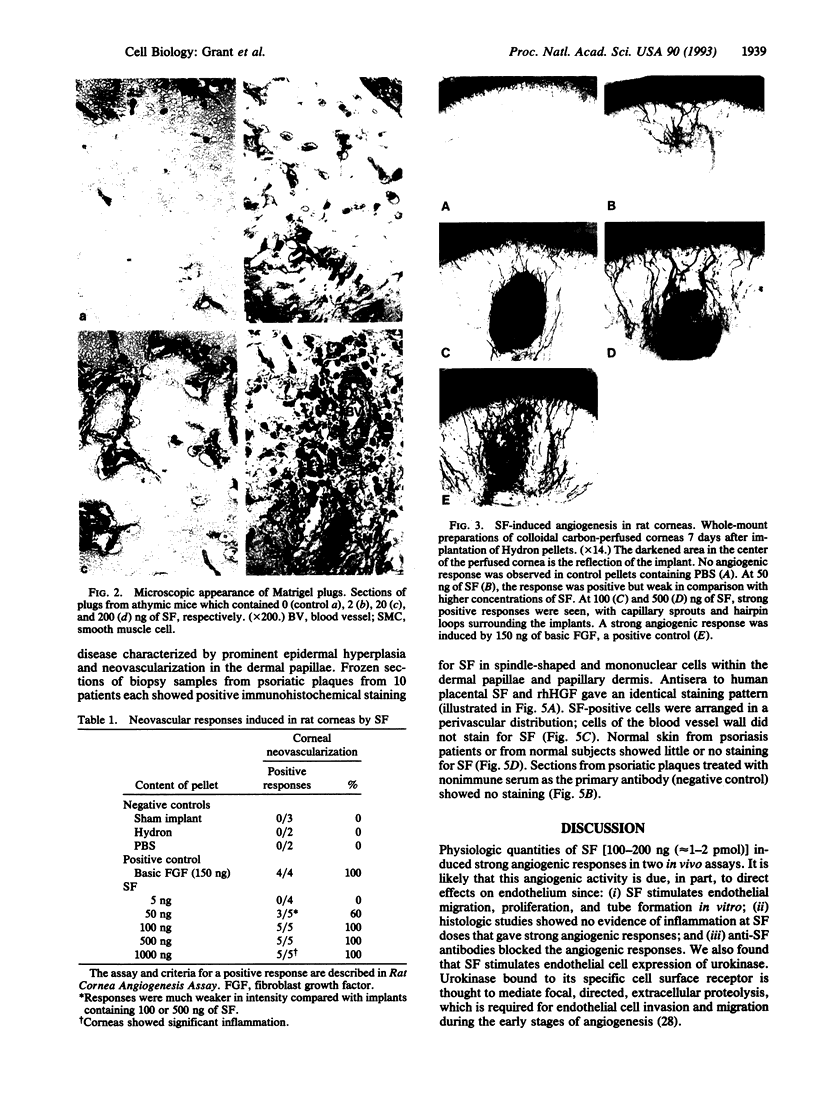
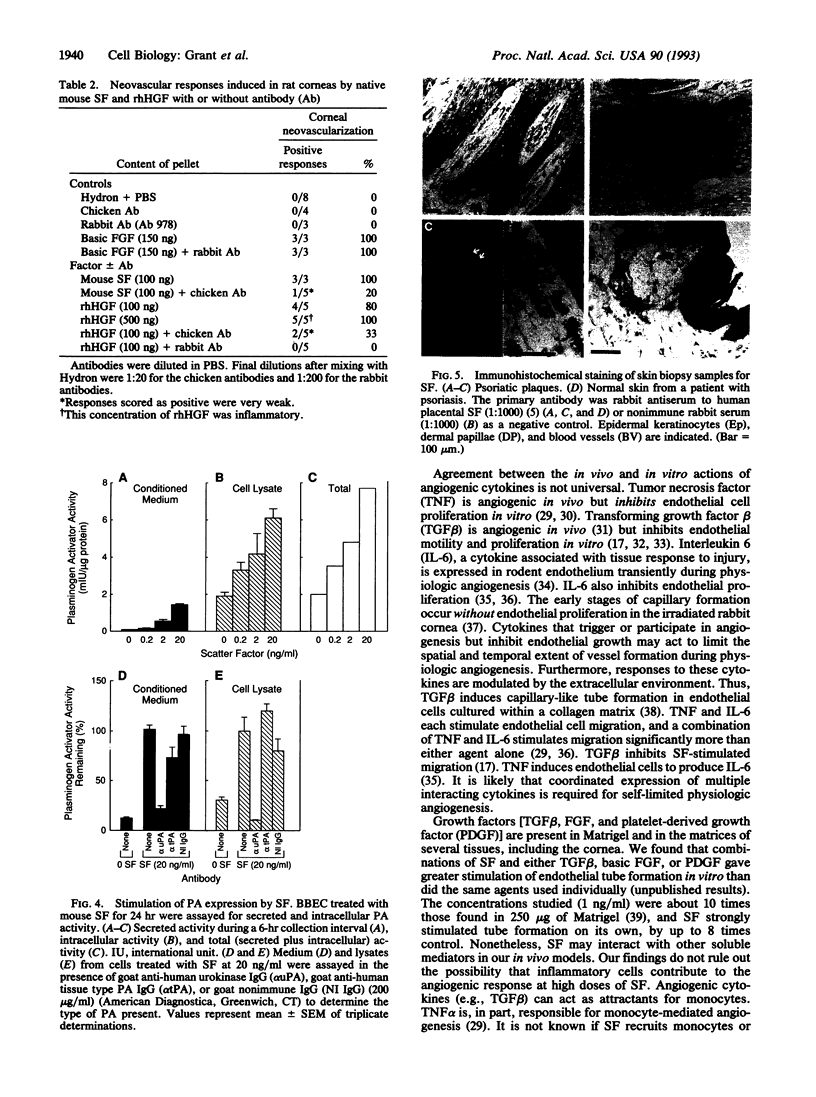

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Furlong R. A., Watt F. M. Production of scatter factor by ndk, a strain of epithelial cells, and inhibition of scatter factor activity by suramin. J Cell Sci. 1991 Mar;98(Pt 3):385–394. doi: 10.1242/jcs.98.3.385. [DOI] [PubMed] [Google Scholar]
- Bhargava M. M., Li Y., Joseph A., Pendergast M., Hofmann R., Rosen E. M., Goldberg I. D. Purification, characterization and mechanism of action of scatter factor from human placenta. EXS. 1991;59:63–75. doi: 10.1007/978-3-0348-7494-6_5. [DOI] [PubMed] [Google Scholar]
- Bhargava M., Joseph A., Knesel J., Halaban R., Li Y., Pang S., Goldberg I., Setter E., Donovan M. A., Zarnegar R. Scatter factor and hepatocyte growth factor: activities, properties, and mechanism. Cell Growth Differ. 1992 Jan;3(1):11–20. [PubMed] [Google Scholar]
- Bottaro D. P., Rubin J. S., Faletto D. L., Chan A. M., Kmiecik T. E., Vande Woude G. F., Aaronson S. A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991 Feb 15;251(4995):802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Coleman P. L., Green G. D. A sensitive, coupled assay for plasminogen activator using a thiol ester substrate for plasmin. Ann N Y Acad Sci. 1981;370:617–626. doi: 10.1111/j.1749-6632.1981.tb29768.x. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M., Thömmes P., Weiser T., Hübscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990 May;4(8):2528–2532. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]
- Gherardi E., Gray J., Stoker M., Perryman M., Furlong R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5844–5848. doi: 10.1073/pnas.86.15.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Griffiths C. E., Voorhees J. J., Nickoloff B. J. Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989 Apr;20(4):617–629. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- Heimark R. L., Twardzik D. R., Schwartz S. M. Inhibition of endothelial regeneration by type-beta transforming growth factor from platelets. Science. 1986 Sep 5;233(4768):1078–1080. doi: 10.1126/science.3461562. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989 Sep 8;58(5):803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Kibbey M. C., Grant D. S., Kleinman H. K. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992 Nov 4;84(21):1633–1638. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Polverini P. J., Shepard H. M., Wiseman D. M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987 Oct 15;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Tucker A. M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988 Apr;106(4):1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L. T., Torcia G., Cozzolino F., Ray A., Tatter S. B., Santhanam U., Sehgal P. B., Stern D. Interleukin-6 gene expression in human endothelial cells: RNA start sites, multiple IL-6 proteins and inhibition of proliferation. Biochem Biophys Res Commun. 1989 Mar 31;159(3):991–998. doi: 10.1016/0006-291x(89)92206-7. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Tsubouchi H., Naka D., Takahashi K., Okigaki M., Arakaki N., Nakayama H., Hirono S., Sakiyama O., Takahashi K. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1989 Sep 15;163(2):967–973. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991 Nov 29;67(5):901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Motro B., Itin A., Sachs L., Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3092–3096. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Behrens J., Nussbaumer U., Böhlen P., Birchmeier W. Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5600–5604. doi: 10.1073/pnas.84.16.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989 Nov 23;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Naldini L., Vigna E., Narsimhan R. P., Gaudino G., Zarnegar R., Michalopoulos G. K., Comoglio P. M. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991 Apr;6(4):501–504. [PubMed] [Google Scholar]
- Polson A., von Wechmar M. B., Fazakerley G. Antibodies to proteins from yolk of immunized hens. Immunol Commun. 1980;9(5):495–514. doi: 10.3109/08820138009066011. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Leibovich S. J. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab Invest. 1984 Dec;51(6):635–642. [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E. M., Goldberg I. D., Kacinski B. M., Buckholz T., Vinter D. W. Smooth muscle releases an epithelial cell scatter factor which binds to heparin. In Vitro Cell Dev Biol. 1989 Feb;25(2):163–173. doi: 10.1007/BF02626174. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Goldberg I. D., Liu D., Setter E., Donovan M. A., Bhargava M., Reiss M., Kacinski B. M. Tumor necrosis factor stimulates epithelial tumor cell motility. Cancer Res. 1991 Oct 1;51(19):5315–5321. [PubMed] [Google Scholar]
- Rosen E. M., Grant D., Kleinman H., Jaken S., Donovan M. A., Setter E., Luckett P. M., Carley W., Bhargava M., Goldberg I. D. Scatter factor stimulates migration of vascular endothelium and capillary-like tube formation. EXS. 1991;59:76–88. doi: 10.1007/978-3-0348-7494-6_6. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Jaken S., Carley W., Luckett P. M., Setter E., Bhargava M., Goldberg I. D. Regulation of motility in bovine brain endothelial cells. J Cell Physiol. 1991 Feb;146(2):325–335. doi: 10.1002/jcp.1041460218. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Liu D., Setter E., Bhargava M., Goldberg I. D. Interleukin-6 stimulates motility of vascular endothelium. EXS. 1991;59:194–205. doi: 10.1007/978-3-0348-7494-6_13. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Meromsky L., Setter E., Vinter D. W., Goldberg I. D. Purified scatter factor stimulates epithelial and vascular endothelial cell migration. Proc Soc Exp Biol Med. 1990 Oct;195(1):34–43. doi: 10.3181/00379727-195-43115. [DOI] [PubMed] [Google Scholar]
- Rubin J. S., Chan A. M., Bottaro D. P., Burgess W. H., Taylor W. G., Cech A. C., Hirschfield D. W., Wong J., Miki T., Finch P. W. A broad-spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):415–419. doi: 10.1073/pnas.88.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Sholley M. M., Ferguson G. P., Seibel H. R., Montour J. L., Wilson J. D. Mechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cells. Lab Invest. 1984 Dec;51(6):624–634. [PubMed] [Google Scholar]
- Stern C. D., Ireland G. W., Herrick S. E., Gherardi E., Gray J., Perryman M., Stoker M. Epithelial scatter factor and development of the chick embryonic axis. Development. 1990 Dec;110(4):1271–1284. doi: 10.1242/dev.110.4.1271. [DOI] [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987 May 21;327(6119):239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985 Aug;77:209–223. doi: 10.1242/jcs.77.1.209. [DOI] [PubMed] [Google Scholar]
- Vukicevic S., Kleinman H. K., Luyten F. P., Roberts A. B., Roche N. S., Reddi A. H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992 Sep;202(1):1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- Weidner K. M., Arakaki N., Hartmann G., Vandekerckhove J., Weingart S., Rieder H., Fonatsch C., Tsubouchi H., Hishida T., Daikuhara Y. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7001–7005. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner K. M., Behrens J., Vandekerckhove J., Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990 Nov;111(5 Pt 1):2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]