Abstract
Background
Elevated left ventricular mass index (LVMI) and concentric left ventricular (LV) remodeling are related to adverse cardiovascular disease (CVD) events. The predictive utility of LV concentric remodeling and LV mass in the prediction of CVD events is not well characterized.
Methods and Results
Framingham Heart Study Offspring Cohort members without prevalent CVD (n=1715, 50% men, aged 65±9 years) underwent cardiovascular magnetic resonance for LVMI and geometry (2002–2006) and were prospectively followed for incident CVD (myocardial infarction, coronary insufficiency, heart failure, stroke) or CVD death. Over 13 808 person-years of follow-up (median 8.4, range 0.0 to 10.5 years), 85 CVD events occurred. In multivariable-adjusted proportional hazards regression models, each 10-g/m2 increment in LVMI and each 0.1 unit in relative wall thickness was associated with 33% and 59% increased risk for CVD, respectively (P=0.004 and P=0.009, respectively). The association between LV mass/LV end-diastolic volume and incident CVD was borderline significant (P=0.053). Multivariable-adjusted risk reclassification models showed a modest improvement in CVD risk prediction with the incorporation of cardiovascular magnetic resonance LVMI and measures of LV concentricity (C-statistic 0.71 [95% CI 0.65 to 0.78] for the model with traditional risk factors only, improved to 0.74 [95% CI 0.68 to 0.80] for the risk factor model additionally including LVMI and relative wall thickness).
Conclusions
Among adults free of prevalent CVD in the community, greater LVMI and LV concentric hypertrophy are associated with a marked increase in adverse incident CVD events. The potential benefit of aggressive primary prevention to modify LV mass and geometry in these adults requires further investigation.
Keywords: cardiovascular disease, cardiovascular magnetic resonance, epidemiology, left ventricular geometry, left ventricular mass
Greater left ventricular mass (LVM) and lower left ventricular (LV) systolic function, measured by echocardiography, are associated with excess adverse cardiovascular disease (CVD) events including coronary heart disease,1 heart failure (HF),2–4 stroke,5 and both CVD and all-cause mortality.6–10 Lower LV systolic function shown by LV ejection fraction is also associated with adverse outcomes.11,12 Volumetric cardiovascular magnetic resonance (CMR) is accurate, reproducible, and widely considered the gold standard for determination of LV volumes, LVM, and LV ejection fraction.
In addition, abnormal geometry, particularly concentric LV remodeling, is associated with systolic and diastolic dysfunction and portends a poor prognosis.13–17 Using CMR, a previous investigation reported positive associations of LV mass and concentric remodeling with CVD, including angina, myocardial infarction, HF, and stroke18; however, the analyses of LVM and concentricity were separate, and thus the prognostic impact of LV concentricity compared with mass alone is unclear. Some studies in hypertensive patients, including those with normal LVM, have demonstrated an association of concentric LV remodeling with CVD,13,19–21 whereas others have not.15,22 We sought to determine the incremental predictive value of LV concentricity on LVM index (LVMI) in the prediction of future CVD events in a community-dwelling adult population.
Methods
Study Population
The characteristics and examinations of the Framingham Heart Study (FHS) Offspring Cohort have been described.23 Briefly, the Offspring Cohort includes children of the original FHS cohort and the spouses of those children. Offspring have undergone serial examinations approximately every 4 years, beginning at examination 1 (1971–1975). Each examination has included routine medical history, physical examination, anthropometry, and electrocardiography. This study included Offspring Cohort members participating in examination 7 (1998–2001). A random sampling strategy was used to recruit from strata of sex, decade age, and Framingham Risk Score quintile.24 Potential study participants were excluded for contraindications to CMR (eg, pacemaker, implanted cardiodefibrillator) or residing in a state not contiguous with Massachusetts. In general, FHS participants who underwent CMR scanning had more favorable CVD risk factors than those who did not undergo CMR (Table 1). Of 3539 participant attendees of examination 7, 1776 participants completed CMR. An additional 18 participants completed CMR but did not attend examination 7. Of 1794 participants who completed CMR, 79 were excluded for prevalent CVD (coronary heart disease, HF, stroke, or transient ischemic attack), leaving a final sample size of 1715 participants.
Table 1.
Characteristics of FHS Offspring Participants at Examination 7 Who Did (n=1776) and Did Not (n=1763) Undergo CMR
| Clinical Characteristic (Examination 7) | No CMR (n=1763) | CMR (n=1776) |
|---|---|---|
| Age, y | 63±10 | 60±9 |
| Male sex, n (%) | 790 (45) | 835 (47) |
| Body mass index, kg/m2 | 28.5±5.7 | 27.9±4.9 |
| Body surface area, kg/m2 | 1.9±0.3 | 1.9±0.2 |
| Systolic blood pressure, mm Hg | 130±20 | 125±18 |
| Use of antihypertensive meds, n (%) | 700 (40) | 518 (29) |
| Diabetes, n (%) | 241 (15) | 154 (9) |
| Dyslipidemia, n (%) | 1005 (64) | 1087 (62) |
| Current smoking, n (%) | 299 (17) | 184 (10) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 84±20 | 86±18 |
Continuous data presented as mean±SD, and categorical data presented as n (%). Clinical characteristics from examination 7. Diabetes: fasting glucose ≥126 mg/dL or the use of hypoglycemic medications. Dyslipidemia: total cholesterol ≥200 mg/dL or the use of lipid-lowering medications. Estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. CMR indicates cardiovascular magnetic resonance; FHS, Framingham Heart Study.
The study was approved by the institutional review boards of both Boston University Medical Center and Beth Israel Deaconess Medical Center. All study participants provided written informed consent.
CMR Methods
Noncontrast supine CMR imaging was performed on a 1.5T CMR scanner (Gyroscan ACS NT, release 9, or Achieva, Release 1; Philips Medical Systems), with a 5-element commercial cardiac array receiver coil. Following localizing scans to determine the position and orientation of the heart within the thorax, end-expiratory breath hold, ECG-gated, steady-state free precession images were acquired in the LV short-axis orientation covering the entire left ventricle as well as 2-chamber and 4-chamber planes (temporal resolution 30 to 40 ms, Repetition time 3.2 ms=R-R interval, Echo time 1.6 ms, flip angle 60°, field-of-view 400 mm, matrix size 208×256, slice thickness 10 mm, gap=0).
Analysis of Cardiac Function and Structure
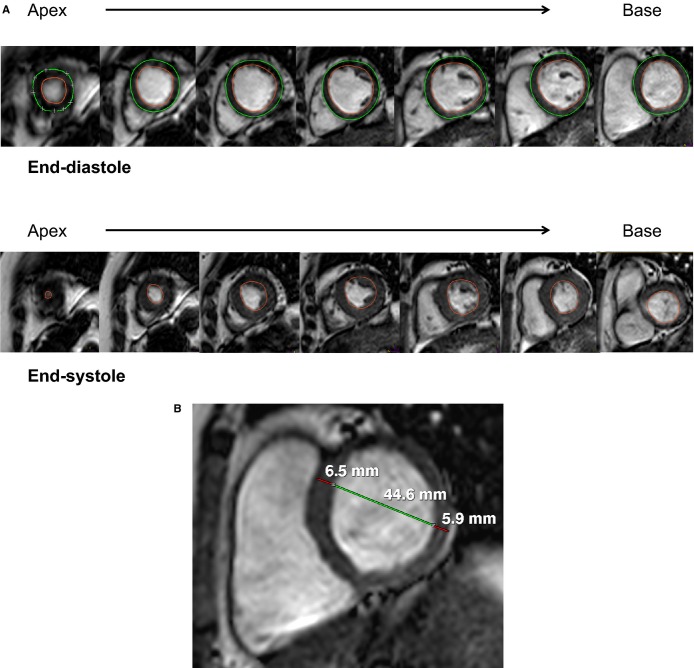
CMR data analysis was performed using dedicated software (EasyVision 5.1; Philips Medical Systems) by an observer blinded to all clinical data. Linear measurements of myocardial wall thickness were made in the short-axis view at the anteroseptal and inferolateral walls at end-diastole, using the myocardial slice just basal to the tips of the papillary muscles.24 Quantitative measures of LV systolic function and mass were obtained by manually tracing endocardial LV borders at end-diastole (initial images of the cine data set) and end-systole (the image phase with minimal cross-sectional area), as described previously24 and shown in Figure 1A. LV end-diastolic volume (LVEDV) and LV end-systolic volume were computed using the summation of discs method. LV ejection fraction was computed by (LVEDV−LV end-systolic volume)/LVEDV×100%. LVM was determined by tracing the epicardial and endocardial border of each slice at end-diastole, summing myocardial volume of all slices, and multiplying by myocardial density (1.05 g/mL). The papillary muscles were included in the LV cavity.18,24 LVM was indexed to body surface area (LVMI). The ratio of LVM to LVEDV (LVM/LVEDV) was calculated. Relative wall thickness (RWT) was computed as the ratio of LV anteroseptal plus inferolateral wall thickness to end-diastolic cavity dimension measured at the slice basal to the papillary muscles (Figure 1B). Greater LVM/LVEDV and RWT were considered indicative of more concentric geometry.
Figure 1.
Measurement of (A) left ventricular (LV) end-diastolic volume (top panel), end-systolic volume (bottom panel), and mass using manual tracing of endocardial (red) and epicardial (green) borders at end-diastole and tracing of endocardial border at end-systole and (B) LV wall thicknesses (red) and diameter (green) measurements taken at end-diastole from the short-axis slice immediately basal to the papillary muscles.
Covariates
Clinical covariates were assessed at each of the serial cohort examinations, and covariates from the most contemporaneous antecedent examination (Examination 7) were used in the present study. Body mass index was calculated as the ratio of weight in kilograms and height in square meters. Manual blood pressure measurements were obtained twice during each clinic visit by a physician using a mercury sphygmomanometer with participants seated, and the average was recorded as the brachial blood pressure. We used the following definitions: hypertension, systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg at any visit or use of antihypertensive medications; diabetes mellitus, fasting glucose ≥126 mg/dL, nonfasting blood glucose ≥200 mg/dL, or the use of insulin or oral hypoglycemic medications; dyslipidemia, total cholesterol >200 mg/dL or the use of lipid-lowering medications. Glomerular filtration was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.25 Current smoking was defined as the use of cigarettes, pipes, or cigars at least once daily in the year prior to examination.
Ascertainment of Outcomes
Prevalent and incident CVD was adjudicated using standardized criteria by a 3–physician-investigator committee based on review of FHS physician assessment and all pertinent medical records. Incident CVD was defined by the occurrence of coronary heart disease, thrombotic stroke, hospitalized HF, or death from coronary heart disease–related myocardial infarction. We did not include stable angina, hemorrhagic stroke, transient ischemic attack, or claudication in the incident CVD definition. Coronary heart disease included myocardial infarction (diagnostic ECG, cardiac biomarkers, and clinical presentation) or coronary insufficiency (unstable angina). Established clinical criteria were used to define HF.26 The first episode of CVD symptoms was recorded as the date of onset in medical record review.
Statistical Analyses
Descriptive statistics for all covariates are presented as either percentage or mean±SD, with P<0.05 considered statistically significant. Time to incident CVD event was computed as the difference between the event date and CMR scan date. Participants who died or were lost to follow-up before they experienced an incident CVD event were censored at the date of death or the date of their last visit. CMR LV concentricity variables were first standardized within sex to a mean of 0 and SD 1 before fitting models. We used cubic splines to display continuous relationships between standardized LV concentricity measures and incident CVD.27 Splines were adjusted for age, sex, body mass index, systolic blood pressure, history of hypertensive medications, prevalent diabetes, dyslipidemia, estimated glomerular filtration rate, and current smoking.
We confirmed that proportional hazards assumptions were met with nonsignificant tests for time-dependent interactions of CMR measures and log-survival time. Cox proportional hazards regression models were used to examine the relationships between values and tertiles of LVMI, LVM/LVEDV, and RWT and with incident CVD. In addition, we evaluated the association of LVMI, LVM/LVEDV, and RWT as continuous variables (presented per 10-g/m2, 0.2-g/mL, and 0.1-unit increments, respectively) with CVD events. We chose to present effect sizes by quantitative increments rather than SD units because the former may represent more meaningful increments with better interpretability; however, the chosen increments also approximate a 1-SD unit of each respective measure. Cox models were first adjusted for age and sex and then included additional covariates (body mass index, systolic blood pressure, history of antihypertensive treatment, diabetes, dyslipidemia, estimated glomerular filtration rate, and current smoking).
The predictive utility of the various Cox models was compared using the C-statistic. The incremental effect of adding a CMR LV concentricity variable for predicting incident CVD was evaluated with the use of category-based net reclassification improvement index (NRI).26 The NRI is used to assess how well an exposure “reclassifies” patients from one risk category to another. We calculated NRI with an extension to survival analysis that uses Kaplan–Meier estimates of event probabilities with the following categories: low risk (0% to <3%), intermediate risk (3% to 6%), or high risk (>6%). A large NRI indicates that the additional exposure causes a large improvement in reclassification. Multivariable adjusted Kaplan–Meier plots stratified by sex-specific tertiles of each CMR LV concentricity variable were generated. We examined for effect modification in the relationships between CMR measures and incident CVD in secondary analyses. All analyses were conducted using SAS 9.3 (SAS Institute).
Results
Characteristics of the Sample
A total of 1715 FHS Offspring Cohort participants (mean age 65±9.0 years, 54% women) were followed for a median of 8.4 years (range 0.0 to 10.5 years). During this time, 85 participants (37 women) developed CVD. The majority of incident CVD events consisted of myocardial infarction (40%), hospitalized HF (26%), and ischemic stroke (24%) (Table 2). The characteristics of the study sample at baseline are shown in Table 3. More men than women experienced adverse CVD events. Those with incident CVD had higher systolic blood pressure; higher prevalence of antihypertensive medication use and diabetes; and greater LVM, LVM/LVEDV, and RWT.
Table 2.
Components of Incident CVD Events
| Number of CVD Events (%) | |
|---|---|
| Myocardial infarction | 34 (40.0) |
| Unstable angina | 3 (3.5) |
| Hospitalized heart failure | 22 (25.9) |
| Ischemic cerebrovascular accident | 20 (23.5) |
| CVD death | 6 (7.1) |
| Total | 85 |
CVD indicates cardiovascular disease.
Table 3.
Baseline Characteristics of FHS Offspring Participants in the CMR Study (n=1715)
| Clinical Characteristic (Examination 7) | No CVD (n=1630) | Incident CVD (n=85) |
|---|---|---|
| Age at CMR, y | 64±9 | 70±9 |
| Male sex, n (%) | 731 (45) | 47 (56) |
| Body mass index, kg/m2 | 27.8±4.9 | 29.2±5.3 |
| Body surface area, kg/m2 | 1.90±0.23 | 1.98±0.25 |
| Systolic blood pressure, mm Hg | 124±18 | 131±16 |
| Use of antihypertensive meds, n (%) | 437 (27) | 32 (38) |
| Diabetes, n (%) | 120 (8) | 8 (10) |
| Dyslipidemia, n (%) | 980 (61) | 48 (59) |
| Current smoking, n (%) | 163 (10) | 7 (8) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 86±18 | 84±21 |
| Heart rate at CMR, beats/min | 65±11 | 66±14 |
| LVMI, g/m2 | 54±11 | 60±14 |
| LVEDV, mL | 125±30 | 132±36 |
| LVM/LVEDV, g/mL | 0.84±0.15 | 0.91±0.20 |
| Relative wall thickness | 0.29±0.05 | 0.32±0.06 |
| LVEF, % | 67±7 | 68±8 |
Continuous data presented as mean±SD, and categorical data presented as n (%). Clinical characteristics from examination 7. Diabetes: fasting glucose ≥126 mg/dL or the use of hypoglycemic medications. Dyslipidemia: total cholesterol ≥200 mg/dL or the use of lipid-lowering medications. Estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Relative wall thickness=(LV anteroseptal+inferolateral wall thickness)/LV end-diastolic dimension. CMR indicates cardiovascular magnetic resonance; CVD, cardiovascular disease; FHS, Framingham Heart Study; LV, left ventricular; LVEDV, left ventricular end-diastolic volume indexed to body surface area; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; LVMI, left ventricular mass index.
Associations of LVMI and Geometry With Incident CVD
Survival free of CVD by tertiles of LVMI, LVM/LVEDV, and RWT are shown in Figure 2. The third (highest) tertile of CMR LV concentricity variables demonstrated the lowest event-free survival over time and steepest slope curves. Hazard ratios (HRs) for LV remodeling with incident CVD are presented in Table 4. Survival free of CVD was lowest for the highest tertiles of each CMR measure. LVMI was significantly associated with greater hazard for incident CVD events in models adjusting for age and sex and after additional multivariable adjustment for CVD risk factors (HR 1.33 per 10-g/m2 increment, 95% CI 1.09 to 1.61, P=0.004 in multivariable-adjusted models). Analysis of multivariable-adjusted HRs for CVD events by tertiles revealed that excess risk was associated with the highest tertile of LVMI (HR 1.94, 95% CI 1.12 to 3.36, P=0.019) (Table 5).
Figure 2.
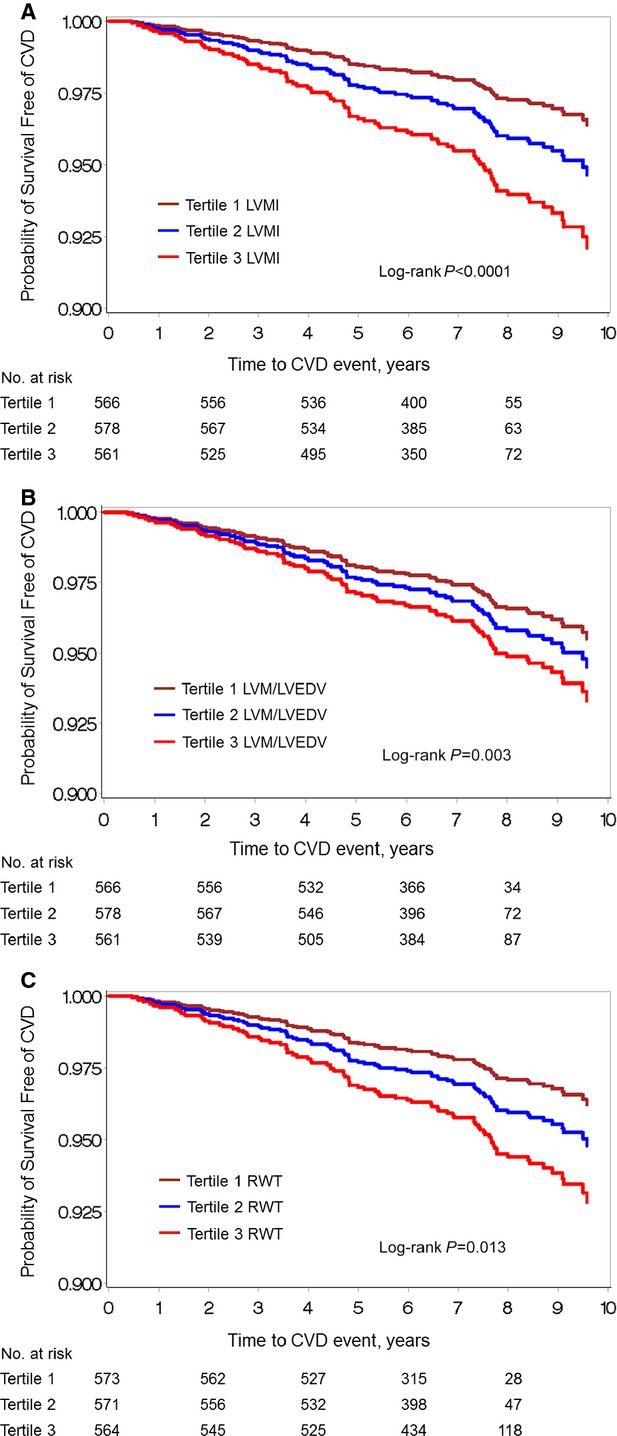
Survival free of CVD over time displayed by tertiles of (A) LVMI, (B) LVM/LVEDV, and (C) RWT. CVD indicates cardiovascular disease; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; LVMI, left ventricular mass index; RWT, relative wall thickness.
Table 4.
Hazard Ratios for Incident CVD by CMR LV Metric, Per Unit LV Measure
| LV Characteristic | Model | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|
| LVMI, per 10 g/m2 | Age, sex-adjusted | 1.36 (1.13 to 1.62) | <0.001 |
| MV-adjusted | 1.33 (1.09 to 1.61) | 0.004 | |
| LVM/LVEDV, per 0.2 g/mL | Age, sex-adjusted | 1.40 (1.10 to 1.79) | 0.006 |
| MV-adjusted | 1.28 (1.0 to 1.65) | 0.053 | |
| RWT, per 0.1 unit | Age, sex-adjusted | 1.68 (1.21 to 2.33) | 0.002 |
| MV-adjusted | 1.59 (1.12 to 2.24) | 0.009 |
MV adjusted models were adjusted for age, sex, body mass index, systolic blood pressure, history of antihypertensive treatment, diabetes, dyslipidemia, estimated glomerular filtration rate, and current smoking. Clinical characteristics including height taken at examination 7. Evaluation for sex interactions were not significant for all CMR measures. CMR indicates cardiovascular magnetic resonance; CVD, cardiovascular disease; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; LVMI, left ventricular mass index; MV, multivariable; RWT, relative wall thickness.
Table 5.
Multivariable-Adjusted HR for Incident CVD by Tertile
| HR for Tertile 2 | HR for Tertile 3 | |
|---|---|---|
| LVMI | 1.05 (0.57 to 1.95) | 1.94 (1.12 to 3.36)* |
| LVM/LVEDV | 0.65 (0.35 to 1.20) | 1.24 (0.72 to 2.14) |
| RWT | 1.08 (0.58 to 2.02) | 1.69 (0.96 to 2.97)** |
Models were adjusted for age, sex, body mass index, systolic blood pressure, history of hypertensive medications, prevalent diabetes, dyslipidemia, estimated glomerular filtration rate, and current smoking. Clinical characteristics including height taken at examination 7. Tertile 1 was the referent. CVD indicates cardiovascular disease; HR, hazard ratio; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; LVMI, left ventricular mass index; RWT, relative wall thickness.
P=0.019.
P=0.07.
LVM/LVEDV was associated with an elevated HR for CVD in age- and sex-adjusted models (HR 1.40, 95% CI 1.10 to 1.79, P=0.006), but this association was attenuated with multivariable adjustment (P=0.053), and no significant association was seen in tertile analysis. Each 0.1-unit increment in RWT was associated with a nearly 60% increase in hazard for incident CVD (HR 1.59, 95% CI 1.12 to 2.24, P=0.009 in multivariable-adjusted models). Tertile analysis of the association between RWT and incident CVD demonstrated a suggestion of elevated risk with the highest tertile, but this did not attain statistical significance (P=0.07). Cubic splines revealed positive linear relations between CMR measures and incident CVD (Figure 3). The tests for nonlinearity of associations of CMR measures with incident CVD were nonsignificant (all P>0.05). We did not observe significant sex interactions between CMR measures and incident CVD (all P>0.05).
Figure 3.
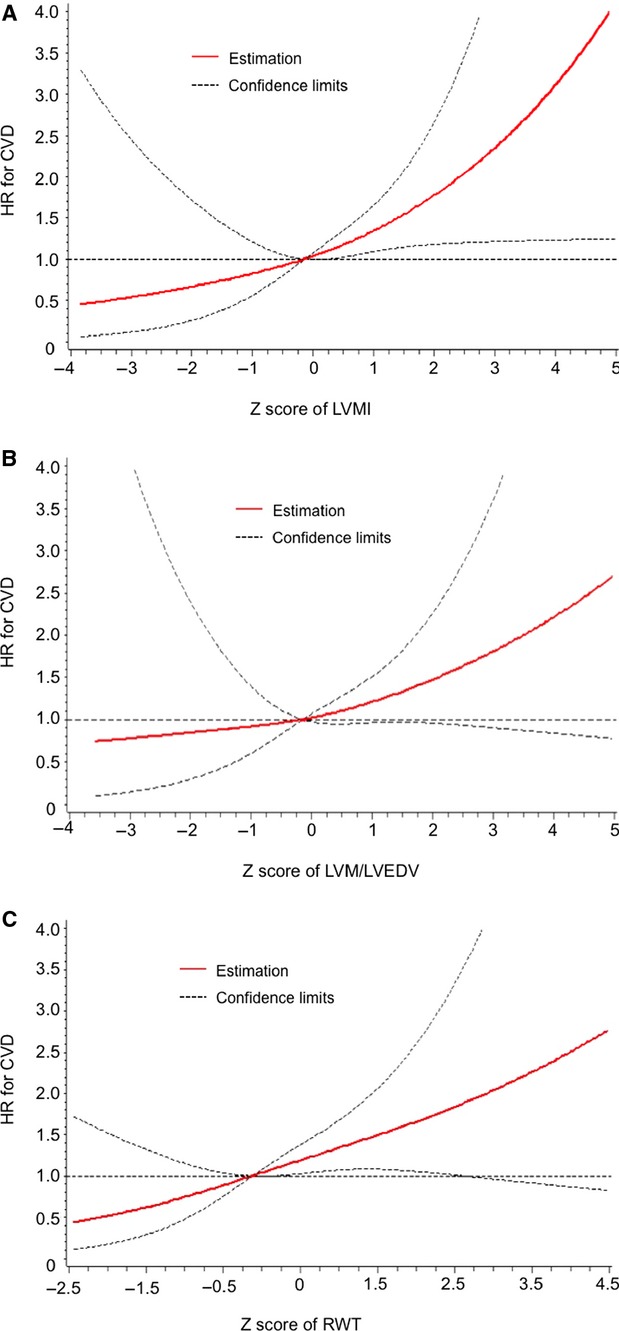
Multivariable-adjusted cubic splines modeling the relations between incident CVD and (A) LVMI, (B) LVM/LVEDV, and (C) RWT. CVD indicates cardiovascular disease; HR, hazard ratio; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; LVMI, left ventricular mass index; RWT, relative wall thickness.
LVMI and LV Geometry in Prediction of Incident CVD
The predictive utility of CMR measures of LVMI and geometry in CVD risk prediction are presented in Table 6. The C-statistic for a prediction model incorporating CVD risk factors was 0.71 (95% CI 0.65 to 0.77) and modestly improved with the addition of LVMI, LVM/LVEDV, or RWT to the risk factor model. When RWT was combined with LVMI in the traditional risk factor model, we observed a greater C-statistic than either variable used individually (0.74 [95% CI 0.68 to 0.80]). RWT (NRI 0.13, P=0.0002) and the combination of LVMI and RWT (NRI 0.13, P=0.004) had the greatest risk reclassification for CVD. The improvement in risk reclassification in the model incorporating traditional risk factors, LVMI, and RWT originated from both down- and upclassification of risk groups in those with nonevents as well as upclassification of risk groups in those with CVD events (Table 7).
Table 6.
Reclassification Metrics With Addition of LVMI to Traditional Risk Factor Model to Predict Incident CVD
| Reclassification Metric | Model for CVD | |||||
|---|---|---|---|---|---|---|
| CVD Risk Factors | +LVMI | +LVM/LVEDV | +RWT | +LVMI and LVM/LVEDV | +LVMI and RWT | |
| C-statistic value (95% CI) | 0.71 (0.65 to 0.77) | 0.73 (0.67 to 0.78) | 0.72 (0.67 to 0.78) | 0.73 (0.67 to 0.78) | 0.73 (0.67 to 0.79) | 0.74 (0.68 to 0.80) |
| NRI, categorical (<3%, 3% to 6%, >6%) | 0.09 (P=0.03) | 0.10 (P=0.01) | 0.13 (0.0002) | 0.09 (P=0.049) | 0.13 (P=0.004) | |
Columns represent reclassification measures with addition of standardized LVMI, LVM/LVEDV, and RWT each added separately to CVD prediction model containing traditional CVD risk factors only, then added in combination to traditional CVD risk factor model (right-most 2 columns). CVD indicates cardiovascular disease; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; LVMI, left ventricular mass index; NRI, net reclassification improvement index; RWT, relative wall thickness.
Table 7.
Net Reclassification Indices Comparing Models With Traditional Risk Factors Versus Risk Factors Plus CMR Measures
| Risk Group, Model 1 | Risk Group, Model 2, n (%) | Total n | ||
|---|---|---|---|---|
| <3% | 3% to 6% | >6% | ||
| No CVD event | ||||
| <3% | 640 (92.6) | 49 (7.1) | 2 (0.3) | 691 |
| 3% to 6% | 128 (27.2) | 283 (60.1) | 60 (12.7) | 471 |
| >6% | 2 (0.5) | 102 (24.3) | 315 (75.2) | 419 |
| Total n | 770 | 434 | 377 | 1581 |
| CVD event | ||||
| <3% | 34 (91.9) | 3 (8.1) | 0 (0.0) | 37 |
| 3% to 6% | 3 (13.0) | 15 (65.2) | 5 (21.7) | 23 |
| >6% | 0 (0.0) | 1 (4.6) | 21 (95.5) | 22 |
| Total n | 37 | 19 | 26 | 82 |
Model 1: age, sex, body mass index, systolic blood pressure, use of antihypertensive medications, diabetes, dyslipidemia, estimated glomerular filtration rate, and current smoking. Model 2: Model 1 plus left ventricular mass index and relative wall thickness. CMR indicates cardiovascular magnetic resonance; CVD, cardiovascular disease.
Discussion
In this prospective study of 1715 community-dwelling adults free of prevalent CVD, we observed that greater LVM and concentricity measured by CMR were associated with incident CVD events. The risks appeared continuous but were most significant at the highest tertiles. The largest incremental improvement in risk prediction beyond traditional risk factors was attributed to LVMI, but the addition of RWT to LVMI further improved risk stratification. Collectively, our results suggest that both CMR measures of LVM and concentricity are important for risk stratification.
LVM in CVD Risk
With elevated afterload, resultant LV hypertrophy (LVH) is thought to reduce wall stress via Laplace’s law, wherein wall stress is proportional to LV pressure and cavity radius and inversely proportional to wall thickness; however, the persistence or progression of LVH may prove maladaptive. The associations between elevated LVM and CVD morbidity and mortality have long been recognized. Earlier epidemiological studies using electrocardiographic and M-mode echocardiography definitions of LVH, with their inherent diagnostic limitations, nevertheless showed significant associations with coronary artery disease, HF, arrhythmias, and CVD mortality.6,13,28,29 Contemporary echocardiographic and CMR measures, with superior image quality, have confirmed similar results.18,30 Consistent with these results, we observed a significantly greater risk of CVD with greater LVMI. We also examined continuous relationships between CMR measures and incident CVD using spline models. Although tests for nonlinearity were not significant, the relationship between LVMI and CVD risk was greatest for the highest LVMI group, consistent with a prior report.18
LV Geometry in CVD Risk
Alterations in LV geometry may occur in the presence or absence of LVH and are the basis of the categorical classification scheme of normal geometry, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy adopted by the American Society of Echocardiography.31 The plasticity of LV geometry involves changes on both macrocytic and microcytic levels in response to adverse stimuli and prolonged exposure to CVD risk factors in addition to afterload. With greater age, LVM/LVEDV rises due to lower LVEDV disproportionate to the decline in LVM, and greater LVM/LVEDV confers risk for CVD events in community samples.32 Given differing influences on LVH and remodeling, it is biologically plausible that LV remodeling may add prognostic information for CVD risk not captured by LVMI. The degree of contribution of LV concentricity to CVD risk has been debated, with evidence both for and against the incremental prognostic impact of LV concentricity on the presence of LVH.13,19–21 The larger hazards for CVD observed with linear (RWT), rather than volumetric (LVM/LVEDV), measures may reflect that indices of remodeling captured at the LV base are more strongly associated with CVD. Alternatively, the larger confidence intervals of RWT compared with LVM/LVEDV may indicate more variability in the measurement, possibly related to single-linear rather than integrated volumetric assessment of LV size. Consequently, our findings suggest the need for further investigation. In a separate investigation using CMR, the Multi-Ethnic Study of Atherosclerosis investigators observed differential magnitudes of associations of LVMI and LVM/LVEDV with CVD, but the effect of accounting for both LVMI and LV remodeling together was not reported.18 Subsequently, the Cardiovascular Health Study investigators, using a modified algorithm with echocardiography to classify LV remodeling by cavity size and mass, suggested that measurement of LV geometry carries risk separate from that conferred by LVMI.30 We extended these observations using CMR by studying the additive effect of these measures using risk reclassification indices. With models including both LVMI and concentricity, we observed an increase in the NRI and C-statistic, measures of the model’s capability for discrimination and calibration in risk prediction. Our results from the well-characterized, community-dwelling FHS Offspring cohort using CMR are consistent with and expand on the prior studies.
We studied adults initially free of prevalent CVD. Measures of LVM and remodeling reflect gross biological adaptations to prolonged levels of adverse CVD risk factors and hemodynamic derangements. Our findings in this sample thus underscore the significance of the degree of subclinical cardiac remodeling in CVD prognostication. Our results suggest the need for further investigation regarding refined risk stratification algorithms to identify persons at risk for CVD and evaluation of potential therapies to prevent adverse LV remodeling.
Strengths and Limitations
The FHS is a prospective, longitudinal study with meticulous follow-up. We studied incident CVD events validated by a physician-investigator end point committee review, excluding outcomes with a potential lack of definitive clinical evidence that could lead to possible misdiagnosis (eg, angina, HF symptoms without hospitalization, transient ischemic attack). Although this decision limited our number of events and statistical power and may be relatively conservative compared with other studies, focusing on diagnoses for which there was objective clinical evidence increased the robustness of our findings. Advantages of the FHS end point review include lack of reliance on diagnosis codes, which may have inherent inaccuracies, and the consistent application of standardized criteria over time in all FHS participants. In addition, we evaluated LVM and concentricity using a modern CMR sequence, the current gold standard imaging technique with high spatial resolution that circumvents many limitations of echocardiography. Application of additional CMR methods for characterization of myocardial tissue in future studies may help elucidate the mechanisms by which architectural and structural changes in the LV occur and better define the etiology of CVD risk associated with LV remodeling.
Conclusions
In this prospective study of middle-aged and older community-dwelling adults initially free of prevalent CVD, the addition of LVM and concentricity to models based on traditional risk factors improved CVD risk prediction and suggests the use of CMR imaging for risk stratification. Further investigation will be necessary to determine the clinical impact of these methods of risk stratification on CVD outcomes.
Sources of Funding
The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute (NO1-HC-25195 and HHSN268201500001I). This work was also supported by NHLBI R01-HL70279 (to Manning), 1K23 HL118259 (to Tsao), and the Harvard Medical School Fellowship (to Tsao).
Disclosures
None.
References
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110:101–107. doi: 10.7326/0003-4819-110-2-101. [DOI] [PubMed] [Google Scholar]
- Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- Mazza A, Tikhonoff V, Casiglia E, Pessina AC. Predictors of congestive heart failure mortality in elderly people from the general population. Int Heart J. 2005;46:419–431. doi: 10.1536/ihj.46.419. [DOI] [PubMed] [Google Scholar]
- Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199–1205. doi: 10.1016/s0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Ahn C, Kronzon I, Koenigsberg M. Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am J Cardiol. 1991;67:295–299. doi: 10.1016/0002-9149(91)90562-y. [DOI] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- Ghali JK, Liao Y, Simmons B, Castaner A, Cao G, Cooper RS. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med. 1992;117:831–836. doi: 10.7326/0003-4819-117-10-831. [DOI] [PubMed] [Google Scholar]
- Gillum RF. Epidemiology of heart failure in the United States. Am Heart J. 1993;126:1042–1047. doi: 10.1016/0002-8703(93)90738-u. [DOI] [PubMed] [Google Scholar]
- Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- Wong M, Staszewsky L, Latini R, Barlera S, Glazer R, Aknay N, Hester A, Anand I, Cohn JN. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure: valsartan heart failure trial (Val-HeFT) echocardiographic data. J Am Coll Cardiol. 2004;43:2022–2027. doi: 10.1016/j.jacc.2003.12.053. [DOI] [PubMed] [Google Scholar]
- Quinones MA, Greenberg BH, Kopelen HA, Koilpillai C, Limacher MC, Shindler DM, Shelton BJ, Weiner DH. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 2000;35:1237–1244. doi: 10.1016/s0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- Milani RV, Lavie CJ, Mehra MR, Ventura HO, Kurtz JD, Messerli FH. Left ventricular geometry and survival in patients with normal left ventricular ejection fraction. Am J Cardiol. 2006;97:959–963. doi: 10.1016/j.amjcard.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Ventura HO, Cardenas GA, Mehra MR, Messerli FH. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. Am J Cardiol. 2007;100:1460–1464. doi: 10.1016/j.amjcard.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Ventura HO, Messerli FH. Left ventricular geometry and mortality in patients >70 years of age with normal ejection fraction. Am J Cardiol. 2006;98:1396–1399. doi: 10.1016/j.amjcard.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, Poisa P, Rizzoni D, Castellano M, Agabiti-Rosei E. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004;43:731–738. doi: 10.1161/01.HYP.0000121223.44837.de. [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Bartoccini C, Santucci A, Santucci C, Reboldi G, Porcellati C. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol. 1995;25:871–878. doi: 10.1016/0735-1097(94)00424-O. [DOI] [PubMed] [Google Scholar]
- Pierdomenico SD, Lapenna D, Bucci A, Manente BM, Cuccurullo F, Mezzetti A. Prognostic value of left ventricular concentric remodeling in uncomplicated mild hypertension. Am J Hypertens. 2004;17:1035–1039. doi: 10.1016/j.amjhyper.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Santucci A, Santucci C, Reboldi G, Porcellati C. Prognostic value of left ventricular mass and geometry in systemic hypertension with left ventricular hypertrophy. Am J Cardiol. 1996;78:197–202. doi: 10.1016/s0002-9149(96)90395-1. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- Salton CJ, Chuang ML, O’Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, Edelman RR, Levy D, Manning WJ. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39:1055–1060. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813–822. doi: 10.7326/0003-4819-72-6-813. [DOI] [PubMed] [Google Scholar]
- Levy D, Anderson KM, Savage DD, Balkus SA, Kannel WB, Castelli WP. Risk of ventricular arrhythmias in left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987;60:560–565. doi: 10.1016/0002-9149(87)90305-5. [DOI] [PubMed] [Google Scholar]
- Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse left ventricular remodeling in community-dwelling older adults predicts incident heart failure and mortality. JACC Heart Fail. 2014;2:512–522. doi: 10.1016/j.jchf.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing G, American Society of Echocardiography’s G, Standards C and European Association of E. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]