Abstract
Background
Catheter–tissue contact is essential for effective lesion formation, thus there is growing usage of contact force (CF) technology in atrial fibrillation ablation. We conducted a meta-analysis to assess the impact of CF on clinical outcomes and procedural parameters in comparison to conventional catheter for atrial fibrillation ablation.
Methods and Results
An electronic search was performed using major databases. Outcomes of interest were recurrence rate, major complications, total procedure, and fluoroscopic times. Continuous variables were reported as standardized mean difference; odds ratios were reported for dichotomous variables. Eleven studies (2 randomized controlled studies and 9 cohorts) involving 1428 adult patients were identified. CF was deployed in 552 patients. The range of CF used was between 2 to 60 gram-force. The follow-up period ranged between 10 and 53 weeks. In comparing CF and conventional catheter groups, the recurrence rate was lower with CF (35.1% versus 45.5%, odds ratio 0.62 [95% CI 0.45–0.86], P=0.004). Shorter procedure and fluoroscopic times were achieved with CF (procedure time: 156 versus 173 minutes, standardized mean difference −0.85 [95% CI −1.48 to −0.21], P=0.009; fluoroscopic time: 28 versus 36 minutes, standardized mean difference −0.94 [95% CI −1.66; −0.21], P=0.01). Major complication rate was lower numerically in the CF group but not statistically significant (1.3% versus 1.9%, odds ratio 0.71 [95% CI 0.29–1.73], P=0.45).
Conclusions
The use of CF technology results in significant reduction of the atrial fibrillation recurrence rate after atrial fibrillation ablation in comparison to the conventional catheter group. CF technology is able to significantly reduce procedure and fluoroscopic times without compromising complication rate.
Keywords: ablation, atrial fibrillation, contact force, meta-analysis
Atrial fibrillation (AF) is the most common cardiac dysrhythmia, with a lifetime risk between 22% and 26% by 80 years of age.1 The current guidelines recommend catheter ablation in patients with symptomatic AF resistant to or intolerant of antiarrhythmic medications.2 AF ablation accounts for about one-third of the caseload in electrophysiology laboratories in the Western world.3 High recurrence rate remains a major concern for this complex ablation procedure, with up to 40% needing the procedure to be redone.4 In addition, the risks involved are substantial, with some devastating complications related to stroke, atrial esophageal fistula, and death.5,6 This risk would affect the decision of whether to repeat the procedure and mandates maximum efforts to ensure complete and durable isolation of the pulmonary veins during the initial procedure. Complete isolation of the pulmonary veins is related largely to the quality, size, and continuity of lesions delivered because recurrence is frequently related to recovery of conduction between the pulmonary veins and the surrounding left atrium after what is initially perceived to be complete isolation.4,7–9
Many advances have been introduced to enhance the quality of ablation applications in pulmonary vein isolation such as the use of irrigated and circular diagnostic catheters,10 3-dimensional tracking systems, robotics,11 testing with adenosine to reveal dormant conductions,12 and cardiac magnetic resonance imaging to assess the quality of lesions.13
Contact force (CF) is a fairly new technology that allows real-time contact feedback between the catheter tip and the targeted cardiac tissue. Theoretically, this approach improves the quality of the lesions and enhances safety outcomes.
We conducted a meta-analysis to assess the impact of CF on clinical outcomes and procedural parameters in comparison to conventional catheter (CC) for AF ablation.
Methods
Literature Search and Data Sources
An electronic literature search was performed by 3 investigators (M.S., D.B., A.K.) in accordance with the recommendations of the Cochrane Collaboration, using PubMed, Embase, Web of Science, Cochrane Central Register of Controlled Trials, and Scopus databases through March 25, 2015. The search terms were atrial fibrillation and ablation and contact force. Neither language nor demographic restrictions were applied. All references from papers obtained through the databases were reviewed manually. The electronic search was archived and is available on request. Our study was a systematic review and meta-analysis and thus did not require institutional review board approval.
Study Selection and Quality Assessment
The inclusion was limited to the studies (1) that compared CF with CC in radiofrequency catheter ablation of atrial fibrillation, (2) that included an adult population aged >18 years only, and (3) that provided data on outcomes of interest.
The selection of studies was assessed independently by 3 assessors (M.S., D.B., A.K.). We excluded noncomparative trials, case reports, editorials, letters, replies, and reviews. We also excluded any study that included other ablation technologies (eg, cryoablation or robotic navigation) that could affect our results and increase bias.
We used the Newcastle-Ottawa Scale to further assess the quality of the observational studies. Studies were judged on 3 broad perspectives: (1) selection of the study groups; (2) comparability of the groups; and (3) ascertainment of either the exposure or outcomes of interest for case–control or cohort studies, respectively.14 The quality of the randomized studies was evaluated based on the 5-point scale outlined by Jadad et al, with the following criteria: randomization with proper concealment of the allocation sequence, blinding of the patient and investigator to treatment allocation with description of the blinding method, and completeness of follow-up.15
Data Extraction
Three reviewers (M.S., D.B., A.K.) independently extracted the data from published sources; disagreements were resolved by discussion and, as necessary, in consultation with a third person (E.C., L.D., H.N., D.N.). The primary outcome measure was recurrence rate. Secondary outcomes included procedure and ablation times, total fluoroscopic time, and complication rates.
Whenever possible, direct communication with the authors of the papers was undertaken in an attempt to obtain the data of interest if presentation in the manuscript was incomplete.
Definition of Outcomes
The following outcomes were identified as relevant measures to compare for the studied groups: (1) rate of recurrence, defined as any symptomatic or asymptomatic atrial arrhythmia recurrence after ablation (density of monitoring and cutoff for duration is manuscript specific); (2) major complications, including embolic events, cardiac tamponade or pericardial effusion requiring intervention, phrenic nerve palsy, pulmonary vein stenosis, atrial esophageal fistula, and death; (3) minor complications, including pericardial effusion (not requiring intervention) or vascular access complications (including hematoma, arteriovenous fistula, or aneurysm); (4) procedural parameters, defined as total procedural time, ablation time, and fluoroscopy time according to the individual study protocols.
The study-specific definitions of outcomes were slightly variable. Although the assessment of outcomes across the trials was not standardized, the same criteria were applied equally to the groups within each trial.
Statistics
The software package RevMan (version 5), provided by the Cochrane Collaboration, was used for combining outcomes from the individual studies and for statistical analysis. Outcomes were pooled using a random-effects model described by DerSimonian and Laird.16 Summary estimates and 95% CI were reported for dichotomous variables as odds ratio (OR) and for continuous variables as standardized mean difference. The heterogeneity between studies was assessed using the Cochran Q test and I2. An I2 >50% was considered to represent significant heterogeneity.17 Statistical significance was set as P<0.05. We calculated the weighted mean for the variable baseline characteristics and complications outcomes.
Results
Summary of the Studies
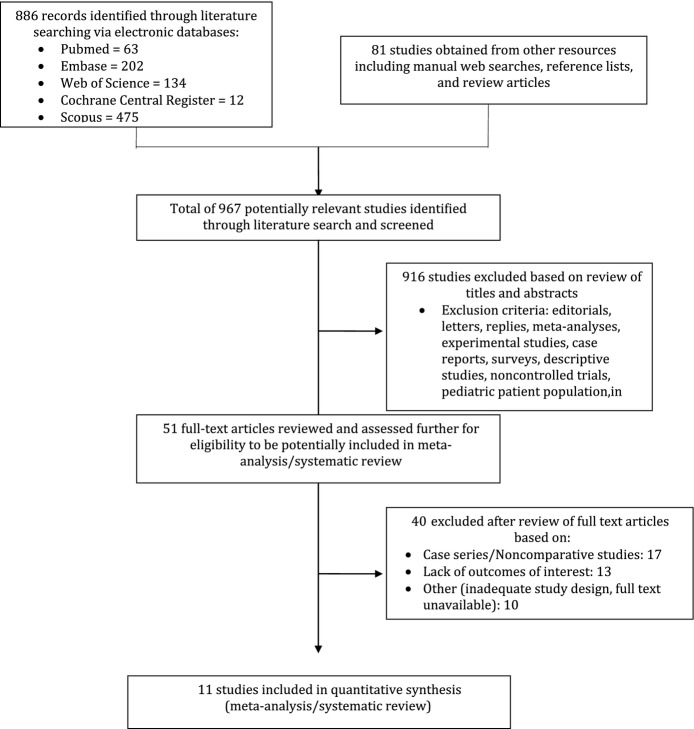
A thorough literature search resulted in 967 items (886 from electronic databases and 81 from other resources). Eleven studies (2 randomized controlled trials and 9 cohorts) were identified that compared the safety and efficacy of guided CF and CC in the setting of AF ablation.18–28 The studies met all applied inclusion criteria of this meta-analysis. The information relevant to the literature search is shown in Figure 1. Pulmonary vein isolation alone without additional ablation lesions was used as the targeted ablation procedural end point in most of the studies (7 studies); the ThermoCool SmartTouch Catheter (Biosense Webster Inc) was used in the majority of the studies for CF (8 studies). Different follow-up protocols were used among studies. The follow-up period ranged between 10 and 53 weeks (mean 42 weeks). Table 1 presents a summary of the included studies.
Figure 1.
Flow diagram of literature search and study selection.
Table 1.
Summary of the Included Studies
| No. of Patients | |||||||
|---|---|---|---|---|---|---|---|
| Study (n=11) | Year | Type of Study | CF (n=552) | Control (n=876) | Follow-up (months) (mean 10.6±3.34) | Ablation | CF Catheter |
| Martinek23 | 2012 | Prospective nonrandomized study | 25 | 25 | n/a | Circumferential PVI | ThermoCool SmartTouch |
| Casella19 | 2013 | Randomized controlled trial | 20 | 35 | 12 | Circumferential PVI | Tacticath or Contact Therapy Cool Path |
| Andrade18 | 2014 | Prospective nonrandomized study | 25 | 50 | 13.3 | Circumferential PVI | ThermoCool SmartTouch |
| Kimura21 | 2014 | Randomized controlled trial | 19 | 19 | 6.7 | Circumferential PVI | ThermoCool SmartTouch |
| Marijon22 | 2014 | Prospective nonrandomized study | 30 | 30 | 12 | Circumferential PVI | ThermoCool SmartTouch |
| Sciarra24 | 2014 | Prospective nonrandomized study | 21 | 21 | 2.5 | Circumferential PVI and additional RF applications | ThermoCool SmartTouch |
| Wakili27 | 2014 | Prospective nonrandomized study | 32 | 35 | 12 | Circumferential PVI | TactiCath |
| Wutzler28 | 2014 | Prospective nonrandomized study | 31 | 112 | 12 | Circumferential PVI | TactiCath |
| Jarman20 | 2014 | Retrospective case–control study | 200 | 400 | 11.4 | PVI (for paroxysmal AF: additional linear ablation was performed only exceptionally; nonparoxysmal AF: use of additional lesions varied by operator, including linear lesions at the roof, mitral isthmus, posterior wall and CTI, targeting of complex fractionated electrograms, and ablation at the endocardial and epicardial aspects of the coronary sinus) | ThermoCool SmartTouch |
| Ullah26 | 2014 | Prospective nonrandomized study | 50 | 50 | 12 | PVI or WACA plus CTI plus mitral isthmus plus roof line (CTI line added in patients with AFL hx; if remained in AF linear lesions added at mitral isthmus and roof, both point-to-point and drag) | ThermoCool SmartTouch |
| Sigmund25 | 2015 | Prospective case-matched control trial | 99 | 99 | 12 | Circumferential PVI plus linear ablation plus CFAE (PVI only, PVI with lines, PVI with lines and CFAE, PVI with CFAE) | ThermoCool SmartTouch |
AF indicates atrial fibrillation; AFL, atrial flutter; CF, contact force; CFAE, complex fractionated atrial electrogram; CTI, cavotricuspid isthmus; hx, history; PVI, pulmonary vein isolation; RF, radiofrequency ablation; WACA, wide area circumferential ablation.
Baseline Characteristics of Patients
A total of 1428 patients were enrolled in both study and control groups; CF was deployed in 552 patients. Patients in the CF group were slightly older in comparison to the CC group (61±2 versus 60±2 years; P=0.046), and this might be related to selection bias in nonrandomized studies. The patients were predominantly male in both CF and CC groups (73% and 72%; P=0.343). The baseline clinical characteristics were similar between both groups. There were no significant differences in left ventricular ejection fraction (60%±5.4% versus 59%±4.5% P=0.609) or left atrial diameter (41±3.8 mm versus 43±2.7 mm P=0.594) between the 2 groups. Similar numbers of patients in the CF and CC groups had hypertension (43.5% versus 37.9% P=0.695) and diabetes mellitus (8.4% versus 7.7% P=0.894). Paroxysmal AF accounted for 59% of patients in the CF group and 60% in the CC group (P=0.948). Summary of the baseline characteristics are presented in Table 2.
Table 2.
Summary of the Baseline Characteristics
| Variable | CF | Control | P Value |
|---|---|---|---|
| Total patients, n | 552 | 876 | n/a |
| Paroxysmal AF no. (% mean) | 59% | 60% | 0.948 |
| Age, y (mean±SD) | 61±2 | 60±2 | 0.046 |
| Male sex (% mean) | 73% | 72% | 0.343 |
| Left ventricular ejection fraction, % (mean±SD) | 60±5.4 | 59±4.5 | 0.609 |
| Left atrial diameter, mm (mean±SD) | 41±3.8 | 43±2.7 | 0.594 |
| Hypertension (% mean) | 43.5% | 37.9% | 0.695 |
| Diabetes mellitus (% mean) | 8.4% | 7.7% | 0.894 |
All means calculated as weighted means. AF indicates atrial fibrillation; CF, contact force; n/a, not applicable.
Procedural Outcomes
Recurrence rate was reported in the majority of the studies (10 studies). In comparing CF and CC groups, a significantly lower recurrence rate was noted with CF (35.1% versus 45.5%, OR 0.62 [95% CI 0.45–0.86], P=0.004). No significant heterogeneity was noted for the comparison (I2=23%, P=0.23) (Figure 2. The CF used ranged between 2 and 60 gram-force (mean 17±5 g). There were not enough studies on persistent AF to support a separate analysis of the recurrence rate. We had 4 studies that reported recurrence rate in patients with only paroxysmal AF, which showed a lower recurrence rate in the CF group, in line with our overall analysis (15% versus 31%, OR 0.38 [95% CI 0.19–0.76], P=0.007). The small number of the studies and patients for either paroxysmal or persistent AF did not support this subgroup analysis.
Figure 2.

Forest plot of the individual and combined rates of recurrence. CF indicates contact force; M-H, Mantel-Haenszel test.
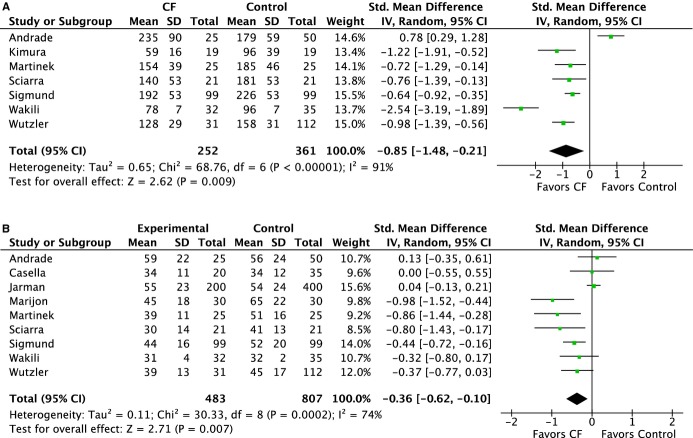
Shorter total procedure and ablation times were achieved with CF (total procedure time: 156 versus 173 minutes, SDM −0.85 [95% CI −1.48 to −0.21], P=0.009; ablation time: 47 versus 51 minutes, SDM −0.36 [95% CI −0.62 to −0.10], P=0.007) (Figure 3A and 3B). The use of CF technology was associated with reduced fluoroscopy time (28 versus 36 minutes, SDM −0.94 [95% CI −1.66 to −0.21] P=0.01) (Figure 4.
Figure 3.
Forest plots of the individual and combined rates of (A) total procedure time and (B) ablation time. CF indicates contact force; IV, inverse variance.
Figure 4.
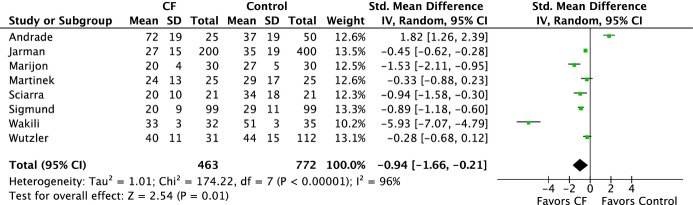
Forest plots of the individual and combined rates of total fluoroscopic time. CF indicates contact force; IV, inverse variance.
Complication Rates
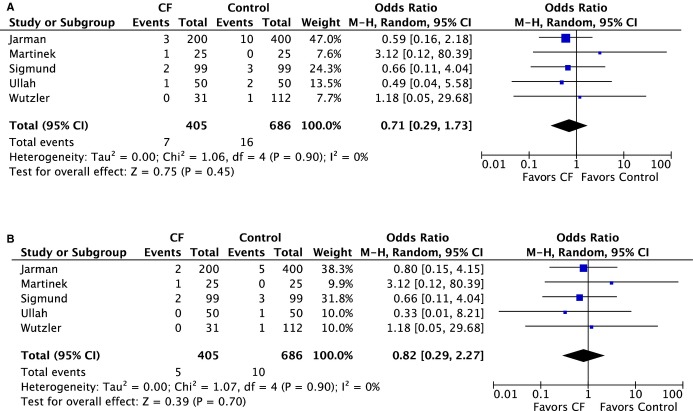
The major complication rate was numerically lower in the CF group versus CC group; however, this did not reach statistical significance (1.3% versus 1.9%, OR 0.71 [95% CI 0.29 to 1.73], P=0.45) (Figure 5A). There were 5 cases of cardiac tamponade or effusion requiring intervention in the CF group versus 10 cases in the CC group (1.2% versus 1.4%, OR 0.82 [95% CI 0.29–2.27], P=0.70) (Figure 5B). Minor complications, mainly related to vascular access, were similar between both groups (2.9% versus 2.6%, OR 0.99 [95% CI 0.48–2.05], P=0.98) (Figure 6.
Figure 5.
Forest plots of the individual and combined rates of (A) major complications and (B) cardiac tamponade. CF indicates contact force; M-H, Mantel-Haenszel test.
Figure 6.
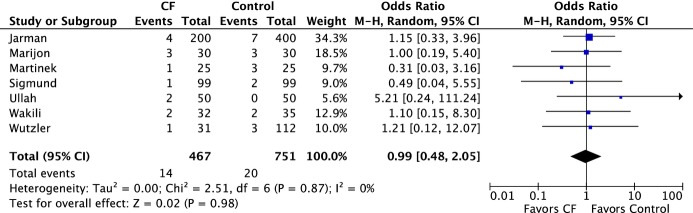
Forest plots of the individual and combined rates of minor complications. CF indicates contact force; M-H, Mantel-Haenszel test.
Discussion
Our meta-analysis demonstrated the following key findings: (1) A lower recurrence rate was noted with CF in comparison to CC; (2) shorter procedure, ablation, and fluoroscopic times were achieved with CF; and (3) major and minor complication rates were similar between both groups.
Many studies have demonstrated the importance of the CF technology as a determinant of adequate ablation lesion quality and size,29–32 with a significant reduction in the prevalence of dormant conduction.18 Measures like intracardiac electrograms, tactile feedback, and impedance are usually used to assess the catheter tip–tissue contact, but on many occasions, these measures are less accurate and do not provide real-time feedback.33,34 CF technology provides operators with instant feedback allowing for adequate maneuvering of the catheter and avoiding inadequate lesion formation, suboptimal contact, or excessive contact with possible mechanical injury. The challenge remains to identify the optimal CF that should be applied during AF ablation to ensure adequate lesion formation and superior outcomes. Reddy et al in the TOCCATA study showed that 80% of patients treated with an average CF >20 g were free from AF recurrence at 12 months, whereas all patients treated with an average CF of <10 g experienced recurrences.35 In contrast, it has been suggested that CF >5 g is associated with adequate lesion formation with impedance fall during 60 seconds of ablation.36 In addition, CF varies widely in certain anatomic locations.37 Our study provides important information regarding the optimal average CF of 17±5 g with acceptable recurrence and complication rates, especially the risk of pericardial tamponade or effusion requiring intervention.
There was significant reduction in procedure and ablation times not only in comparison to point-by-point manual radiofrequency ablation but also similar to what is seen in “single-shot” devices such as the Cryoballoon.38 Using CF feedback as a main determinant of adequate lesion formation would minimize ineffective lesion formation and thus achieve pulmonary vein isolation faster without the need for additional ablation lesions to confirm isolation. Furthermore, operators would spend less time assessing signal or impedance drop during ablation. All of these factors would explain the shorter procedure and ablation times.
Radiation safety remains a major concern in invasive electrophysiology. In this analysis, fluoroscopic time was reduced by 8 minutes in CF compared with CC groups, with an average reported fluoroscopy time of 28 minutes in CF versus 36 minutes in CC groups. This reduction is largely related to the continuous monitoring of the catheter while using CF, enabling operators to manipulate and advance the catheter without the need of excessive fluoroscopic guidance. In contrast, more frequent visualization with fluoroscopy is typically used with CCs in an effort to prevent perforation or to assess contact by the tip of catheter appearance and movements. Attention to radiation exposure and a statistically significant decrease in exposure have clinical relevance for both operators and patients undergoing ablation procedures.39,40
This meta-analysis has proven the enhanced safety of using the CF technology with acceptable rates of minor and major complications and reduced risk of cardiac perforation (although it did not reach statistical significance). This is related mainly to the ability to continuously monitor the catheter while manipulating it in the cardiac chambers, with real-time instant feedback of the catheter tip–tissue contact. Moreover, avoiding ablation at suboptimal CF would reduce the need for excessive ablations and subsequent related complications.
Limitations
Some studies were of limited quality, given their retrospective and single-center designs. Assessing outcomes like procedural time and complication rate is complex and multifactorial. Factors like different levels of experience among operators, catheters used, instrumentation, ablation energy and duration, magnitude of ablation performed, antiarrhythmic drugs, and incomplete data may have altered our conclusions. Some operators performed, in addition to pulmonary vein isolation ablation, more complex lesion sets (eg, complex atrial fractionated electrogram, left atrial roof line, mitral isthmus line), and that may have affected the outcomes of these procedures compared with only pulmonary vein isolation procedures. We could not address this issue, given the heterogeneity of ablation protocols among different operators. There could have been a lack of statistical power for some outcomes studied. Some of the outcomes had high I2 representing significant heterogeneity such as procedure, ablation, and fluoroscopic times. That said, outcomes like recurrence rate, major complications, cardiac tamponade, and minor complications had insignificant heterogeneity that could reflect some similarities among studies.
Conclusions
This meta-analysis suggests that the use of CF technology results in a significant reduction of AF recurrence rate after AF ablation in comparison to the CC group. CF technology is able to significantly reduce procedure and fluoroscopic times without compromising the complication rate.
Disclosures
Dr Di Biase is a consultant for Biosense Webster, Boston Scientific, Stereotaxis and St Jude Medical, and has received speaker honoraria/travel from Medtronic, Atricure, EPiEP and Biotronik. Rest of the authors: None.
References
- Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW Members AATF. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- Shurrab M, Fishman E, Kaoutskaia A, Birnie D, Ayala-Paredes F, Sultan O, Chauhan V, Skanes A, Parkash R, Morillo C, Janmohamed A, Toal S, Essebag V, Sterns L, Veenhuyzen G, Mangat I, Redfearn D, Philippon F, Connors S, Healey J, Verma A, Crystal E. Snapshot of adult invasive cardiac electrophysiology in Canada: results of the web-based registry. J Interv Card Electrophysiol. 2014;40:93–98. doi: 10.1007/s10840-014-9899-6. [DOI] [PubMed] [Google Scholar]
- Cappato R, Negroni S, Pecora D, Bentivegna S, Lupo PP, Carolei A, Esposito C, Furlanello F, De Ambroggi L. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108:1599–1604. doi: 10.1161/01.CIR.0000091081.19465.F1. [DOI] [PubMed] [Google Scholar]
- Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53:1798–1803. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Danon A, Shurrab M, Nair KM, Latcu DG, Arruda MS, Chen X, Szili-Torok T, Rossvol O, Wissner EE, Lashevsky I, Crystal E. Atrial fibrillation ablation using remote magnetic navigation and the risk of atrial-esophageal fistula: international multicenter experience. J Interv Card Electrophysiol. 2015;43:169–174. doi: 10.1007/s10840-015-0003-7. [DOI] [PubMed] [Google Scholar]
- Mesas CE, Augello G, Lang CC, Gugliotta F, Vicedomini G, Sora N, De Paola AA, Pappone C. Electroanatomic remodeling of the left atrium in patients undergoing repeat pulmonary vein ablation: mechanistic insights and implications for ablation. J Cardiovasc Electrophysiol. 2006;17:1279–1285. doi: 10.1111/j.1540-8167.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- Nanthakumar K, Plumb VJ, Epstein AE, Veenhuyzen GD, Link D, Kay GN. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation. 2004;109:1226–1229. doi: 10.1161/01.CIR.0000121423.78120.49. [DOI] [PubMed] [Google Scholar]
- Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, Liu X, Bansch D, Kuck KH. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- Marrouche NF, Dresing T, Cole C, Bash D, Saad E, Balaban K, Pavia SV, Schweikert R, Saliba W, Abdul-Karim A, Pisano E, Fanelli R, Tchou P, Natale A. Circular mapping and ablation of the pulmonary vein for treatment of atrial fibrillation: impact of different catheter technologies. J Am Coll Cardiol. 2002;40:464–474. doi: 10.1016/s0735-1097(02)01972-1. [DOI] [PubMed] [Google Scholar]
- Shurrab M, Danon A, Lashevsky I, Kiss A, Newman D, Szili-Torok T, Crystal E. Robotically assisted ablation of atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2013;169:157–165. doi: 10.1016/j.ijcard.2013.08.086. [DOI] [PubMed] [Google Scholar]
- Andrade JG, Pollak SJ, Monir G, Khairy P, Dubuc M, Roy D, Talajic M, Deyell M, Rivard L, Thibault B, Guerra PG, Nattel S, Macle L. Pulmonary vein isolation using a pace-capture-guided versus an adenosine-guided approach: effect on dormant conduction and long-term freedom from recurrent atrial fibrillation–a prospective study. Circ Arrhythm Electrophysiol. 2013;6:1103–1108. doi: 10.1161/CIRCEP.113.000454. [DOI] [PubMed] [Google Scholar]
- Celik H, Ramanan V, Barry J, Ghate S, Leber V, Oduneye S, Gu Y, Jamali M, Ghugre N, Stainsby JA, Shurrab M, Crystal E, Wright GA. Intrinsic contrast for characterization of acute radiofrequency ablation lesions. Circ Arrhythm Electrophysiol. 2014;7:718–727. doi: 10.1161/CIRCEP.113.001163. [DOI] [PubMed] [Google Scholar]
- Wells GASB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed September 16, 2015. [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011) Available at: http://community.cochrane.org/handbook. Accessed September 16, 2015. [Google Scholar]
- Andrade JG, Monir G, Pollak SJ, Khairy P, Dubuc M, Roy D, Talajic M, Deyell M, Rivard L, Thibault B, Guerra PG, Nattel S, Macle L. Pulmonary vein isolation using “contact force” ablation: the effect on dormant conduction and long-term freedom from recurrent atrial fibrillation–a prospective study. Heart Rhythm. 2014;11:1919–1924. doi: 10.1016/j.hrthm.2014.07.033. [DOI] [PubMed] [Google Scholar]
- Casella M, Dello RA, Russo E, Al-Mohani G, Santangeli P, Riva S, Fassini G, Moltrasio M, Innocenti E, Colombo D, Bologna F, Izzo G, Gallinghouse JG, Di Biase L, Natale A, Tondo C. Biomarkers of myocardial injury with different energy sources for atrial fibrillation catheter ablation. Cardiol J. 2014;21:516–523. doi: 10.5603/CJ.a2013.0153. [DOI] [PubMed] [Google Scholar]
- Jarman JW, Panikker S, Das M, Wynn GJ, Ullah W, Kontogeorgis A, Haldar SK, Patel PJ, Hussain W, Markides V, Gupta D, Schilling RJ, Wong T. Relationship between contact force sensing technology and medium-term outcome of atrial fibrillation ablation: a multicenter study of 600 patients. J Cardiovasc Electrophysiol. 2015;26:378–384. doi: 10.1111/jce.12606. [DOI] [PubMed] [Google Scholar]
- Kimura M, Sasaki S, Owada S, Horiuchi D, Sasaki K, Itoh T, Ishida Y, Kinjo T, Tomita H, Okumura K. Comparison of lesion formation between contact force-guided and non-guided circumferential pulmonary vein isolation: a prospective, randomized study. Heart Rhythm. 2014;11:984–991. doi: 10.1016/j.hrthm.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Marijon E, Fazaa S, Narayanan K, Guy-Moyat B, Bouzeman A, Providencia R, Treguer F, Combes N, Bortone A, Boveda S, Combes S, Albenque JP. Real-time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: procedural and 1-year results. J Cardiovasc Electrophysiol. 2014;25:130–137. doi: 10.1111/jce.12303. [DOI] [PubMed] [Google Scholar]
- Martinek M, Lemes C, Sigmund E, Derndorfer M, Aichinger J, Winter S, Nesser HJ, Purerfellner H. Clinical impact of an open-irrigated radiofrequency catheter with direct force measurement on atrial fibrillation ablation. Pacing Clin Electrophysiol. 2012;35:1312–1318. doi: 10.1111/j.1540-8159.2012.03503.x. [DOI] [PubMed] [Google Scholar]
- Sciarra L, Golia P, Natalizia A, De Ruvo E, Dottori S, Scara A, Borrelli A, De Luca L, Rebecchi M, Fagagnini A, Bandini A, Guarracini F, Galvani M, Calo L. Which is the best catheter to perform atrial fibrillation ablation? A comparison between standard ThermoCool, SmartTouch, and Surround Flow catheters. J Interv Card Electrophysiol. 2014;39:193–200. doi: 10.1007/s10840-014-9874-2. [DOI] [PubMed] [Google Scholar]
- Sigmund E, Puererfellner H, Derndorfer M, Kollias G, Winter S, Aichinger J, Nesser HJ, Martinek M. Optimizing radiofrequency ablation of paroxysmal and persistent atrial fibrillation by direct catheter force measurement-a case-matched comparison in 198 patients. Pacing Clin Electrophysiol. 2015;38:201–208. doi: 10.1111/pace.12549. [DOI] [PubMed] [Google Scholar]
- Ullah W, Hunter RJ, Haldar S, McLean A, Dhinoja M, Sporton S, Earley MJ, Lorgat F, Wong T, Schilling RJ. Comparison of robotic and manual persistent AF ablation using catheter contact force sensing: an international multicenter registry study. Pacing Clin Electrophysiol. 2014;37:1427–1435. doi: 10.1111/pace.12501. [DOI] [PubMed] [Google Scholar]
- Wakili R, Clauss S, Schmidt V, Ulbrich M, Hahnefeld A, Schussler F, Siebermair J, Kaab S, Estner HL. Impact of real-time contact force and impedance measurement in pulmonary vein isolation procedures for treatment of atrial fibrillation. Clin Res Cardiol. 2014;103:97–106. doi: 10.1007/s00392-013-0625-7. [DOI] [PubMed] [Google Scholar]
- Wutzler A, Huemer M, Parwani AS, Blaschke F, Haverkamp W, Boldt LH. Contact force mapping during catheter ablation for atrial fibrillation: procedural data and one-year follow-up. Arch Med Sci. 2014;10:266–272. doi: 10.5114/aoms.2014.42578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar S, Jarman JW, Panikker S, Jones DG, Salukhe T, Gupta D, Wynn G, Hussain W, Markides V, Wong T. Contact force sensing technology identifies sites of inadequate contact and reduces acute pulmonary vein reconnection: a prospective case control study. Int J Cardiol. 2013;168:1160–1166. doi: 10.1016/j.ijcard.2012.11.072. [DOI] [PubMed] [Google Scholar]
- Kuck KH, Reddy VY, Schmidt B, Natale A, Neuzil P, Saoudi N, Kautzner J, Herrera C, Hindricks G, Jais P, Nakagawa H, Lambert H, Shah DC. A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm. 2012;9:18–23. doi: 10.1016/j.hrthm.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, Lambert H, Yulzari A, Wissner E, Kuck KH. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol. 2013;6:327–333. doi: 10.1161/CIRCEP.113.000374. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, Ikeda A, Pitha JV, Sharma T, Lazzara R, Jackman WM. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1:354–362. doi: 10.1161/CIRCEP.108.803650. [DOI] [PubMed] [Google Scholar]
- Di Biase L, Paoletti Perini A, Mohanty P, Goldenberg AS, Grifoni G, Santangeli P, Santoro F, Sanchez JE, Horton R, Joseph Gallinghouse G, Conti S, Mohanty S, Bailey S, Trivedi C, Garg A, Grogan AP, Wallace DT, Padeletti L, Reddy V, Jais P, Haissaguerre M, Natale A. Visual, tactile, and contact force feedback: which one is more important for catheter ablation? Results from an in vitro experimental study. Heart Rhythm. 2014;11:506–513. doi: 10.1016/j.hrthm.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Kumar S, Haqqani HM, Chan M, Lee J, Yudi M, Wong MC, Morton JB, Ling LH, Robinson T, Heck PM, Kelland NF, Halloran K, Spence SJ, Kistler PM, Kalman JM. Predictive value of impedance changes for real-time contact force measurements during catheter ablation of atrial arrhythmias in humans. Heart Rhythm. 2013;10:962–969. doi: 10.1016/j.hrthm.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, Jais P, Hindricks G, Peichl P, Yulzari A, Lambert H, Neuzil P, Natale A, Kuck KH. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012;9:1789–1795. doi: 10.1016/j.hrthm.2012.07.016. [DOI] [PubMed] [Google Scholar]
- De Bortoli A, Sun LZ, Solheim E, Hoff PI, Schuster P, Ohm OJ, Chen J. Ablation effect indicated by impedance fall is correlated with contact force level during ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:1210–1215. doi: 10.1111/jce.12215. [DOI] [PubMed] [Google Scholar]
- Kumar S, Morton JB, Lee J, Halloran K, Spence SJ, Gorelik A, Hepworth G, Kistler PM, Kalman JM. Prospective characterization of catheter-tissue contact force at different anatomic sites during antral pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2012;5:1124–1129. doi: 10.1161/CIRCEP.112.972208. [DOI] [PubMed] [Google Scholar]
- Jourda F, Providencia R, Marijon E, Bouzeman A, Hireche H, Khoueiry Z, Cardin C, Combes N, Combes S, Boveda S, Albenque JP. Contact-force guided radiofrequency vs. second-generation balloon cryotherapy for pulmonary vein isolation in patients with paroxysmal atrial fibrillation-a prospective evaluation. Europace. 2015;17:225–231. doi: 10.1093/europace/euu215. [DOI] [PubMed] [Google Scholar]
- Kovoor P, Ricciardello M, Collins L, Uther JB, Ross DL. Risk to patients from radiation associated with radiofrequency ablation for supraventricular tachycardia. Circulation. 1998;98:1534–1540. doi: 10.1161/01.cir.98.15.1534. [DOI] [PubMed] [Google Scholar]
- Perisinakis K, Damilakis J, Theocharopoulos N, Manios E, Vardas P, Gourtsoyiannis N. Accurate assessment of patient effective radiation dose and associated detriment risk from radiofrequency catheter ablation procedures. Circulation. 2001;104:58–62. doi: 10.1161/hc2601.091710. [DOI] [PubMed] [Google Scholar]