Abstract
Background
Little is known about the incidence, predictors, or outcomes of intracranial hemorrhage (ICH) in patients with non–ST-segment elevation acute coronary syndromes (NSTE ACS). We aimed to determine the incidence and timing of ICH, characterize the location of ICH, and identify independent baseline predictors of ICH in NSTE ACS patients.
Methods and Results
We pooled patient-level data from 4 contemporary antithrombotic therapy trials. Multivariable modeling identified independent predictors of ICH. ICHs were adjudicated by a clinical events committee. Of 37 815 patients, 135 (0.4%) had an ICH. The median (25th, 75th percentiles) follow-up was 332 (184, 434) days but differed across trials. Locations of ICH were intracerebral (50%), subdural (31%), subarachnoid (18.5%), and intraventricular (11%). Independent predictors of ICH were older age (HR per 10 years, 1.61; 95% CI, 1.35 to 1.91); prior stroke/transient ischemic attack; HR, 1.95; 95% CI, 1.14 to 3.35), higher systolic blood pressure; HR per 10 mm Hg increase, 1.09; 95% CI, 1.01 to 1.18), and larger number of antithrombotic agents (HR per each additional agent, 2.06; 95% CI, 1.49 to 2.84). Of all ICHs, 45 (33%) were fatal.
Conclusions
In patients with NSTE ACS enrolled in recent clinical trials of antithrombotic therapies, ICH was uncommon. Patients with older age, prior transient ischemic attack/stroke, higher systolic blood pressure, or larger number of antithrombotic agents were at increased risk. One-third of patients with ICH died. These data may be useful to trialists and data and safety monitoring committees for trial conduct and monitoring.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifiers: TRACER: NCT00527943, PLATO: NCT00391872, APPRAISE-2: NCT00831441, TRILOGY ACS: NCT00699998.
Keywords: acute coronary syndromes, clinical outcomes, intracranial hemorrhage, systematic review
Little has been published about patients with non–ST-segment elevation acute coronary syndrome (NSTE ACS) who experience intracranial hemorrhage (ICH). Individual trials have reported ICH as part of bleeding or safety assessments. Proportions of patients with ICH have ranged from 0.2% to 0.5%.1–4 Heterogeneity of patient populations driven largely by inclusion and exclusion criteria, different management strategies, and different durations of follow-up have limited comparisons of ICH incidence across trials. Low event rates in individual trials also preclude statistical analyses.
We pooled patient-level data from 4 large global clinical trials coordinated at least in part at the Duke Clinical Research Institute in Durham, NC. The key objectives of these analyses are to determine the incidence and timing of ICH, to characterize the location of ICH, and to identify independent baseline predictors of ICH in NSTE ACS patients.
Methods
Study Population
The study population consisted of patients enrolled in 4 contemporary ACS trials: TRACER (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome), PLATO (Study of Platelet Inhibition and Patient Outcomes), APPRAISE-2 (Apixaban for Prevention of Acute Ischemic Events 2), and TRILOGY ACS (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes). The design and primary results of all 4 trials have been previously published.1–7 These trials were chosen because they were the 4 most recent, consecutive trials with individual patient-level data available at the DCRI that allowed comprehensive analyses to be performed. All studies were approved by the appropriate institutional review boards and ethics committees, and all participants provided informed consent.
Statistical Methods
Values are presented as N (percentage) and median with interquartile range (IQR) where appropriate. Time to ICH was explored using Kaplan–Meier methods. Rates of ICH are reported per 100 patient-years. Adjusted Cox multivariable regression modeling with both stepwise and backward elimination techniques were used to determine independent predictors of ICH. Candidate variables included age, sex, prior congestive heart failure, prior diabetes mellitus, prior hypercholesterolemia, prior hypertension, prior transient ischemic attack (TIA)/stroke, race, smoking status, baseline creatinine, baseline hemoglobin, systolic blood pressure (SBP), and weight. They were chosen after review of the baseline demographic and treatment factors to determine common variables across the 4 trials. They were also known risks factors for stroke or ICH for the ST-segment elevation myocardial infarction patient population.8 We created a candidate variable called “number of antithrombotic agents.” We included any oral antiplatelet agent or oral anticoagulant, including the randomized treatment assignment. This was done to assess the potential risk of ICH based on the number of agents but not to include comparisons across agents or trials. Missing values in candidate variables were not imputed, and multivariable models were based on complete case analysis.
ICH was defined similarly across the trials and included primary hemorrhagic strokes, ischemic strokes with hemorrhagic conversion, and ICH events that were not strokes but had bleeding within the cranium. All suspected ICH events were adjudicated by a clinical events committee using similar procedures across all 4 trials.
Results
Enrollment, number of ICH events, median follow-up, and median time to ICH for each of the trials and overall are shown in Table1. In total, 48 286 patients were enrolled in the 4 trials and 10 471 (21.7%) were excluded because they were enrolled in the ST-segment elevation cohorts of the trials that included this patient population in addition to NSTE ACS (PLATO [N=7544] and APPRAISE-2 [N=2927]). Overall, 135 patients (0.4%) had ICH events. The median duration of follow-up varied across the trials because of protocol differences in planned duration, and 1 trial3 was stopped early by the data and safety monitoring board. Figure 1 shows the cumulative probability of ICH in each of the trials, with similar curves for all 4 studies. The ICH rate was 0.239 per 100 patient-years for patients receiving single antiplatelet therapy, 0.255 for those receiving dual antiplatelet therapy, and 0.602 for those receiving 3 antithrombotic agents. In patients with and without prior TIA/stroke, the ICH rates were 0.741 and 0.299 per 100 patient-years, respectively. The median (IQR) days from randomization to ICH was 205 days (85 to 367) in the entire dataset. Median times from randomization to ICH differed by trial (Table1).
Table 1.
Trials Included
| APPRAISE-23 | PLATO2 | TRACER1 | TRILOGY ACS4 | Total | |
|---|---|---|---|---|---|
| No. of patients included in analysis | N=4456 | N=11 080 | N=12 944 | N=9326 | N=37 815 |
| ICH, n/N (%) | 7/4465 (0.2) | 24/11 080 (0.2) | 68/12 897 (0.5) | 36/9326 (0.4) | 135/37 768 (0.4) |
| ICH rate per 100 patient-y follow-up | 0.264 | 0.263 | 0.393 | 0.272 | 0.319 |
| Median follow-up time (d), median (25th, 75th) | 180 (69, 299) | 282 (179, 371) | 481 (328, 651) | 513 (312, 731) | — |
| Median time to ICH (d), median (25th, 75th) | 56 (14, 214) | 118 (33, 180) | 224 (85, 369) | 278 (166, 445) | — |
| Investigative therapies | Apixaban vs placebo on SOC | Ticagrelor vs clopidogrel on ASA | Vorapaxar vs placebo on SOC | Prasugrel vs clopidogrel on ASA | — |
ASA indicates aspirin; ICH, intracranial hemorrhage; SOC, standard of care.
Figure 1.
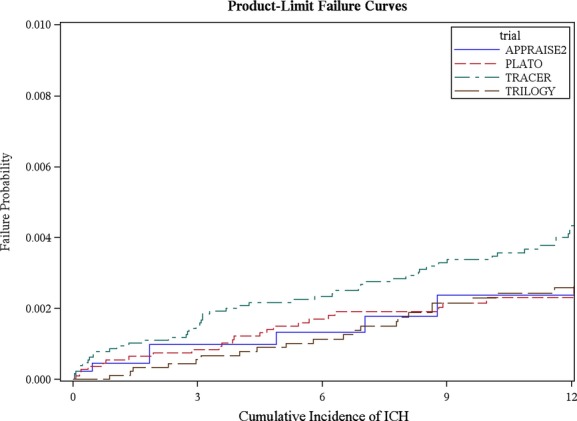
Product-limit failure curves.1–4 ICH indicates intracranial hemorrhage.
Table S1 shows the baseline demographics and patient characteristics by trial. Some differences are observed. The lower proportion of patients with prior stroke or TIA in TRILOGY ACS is due to planned exclusion of these patients.
Patients with ICH were older and more likely to have a history of hypertension, diabetes mellitus, smoking, coronary artery bypass graft surgery, prior stroke or TIA, and higher SBP (Table2). Patients with ICH were more often treated with antiplatelet and anticoagulant therapies.
Table 2.
Baseline Demographic and Clinical Characteristics by ICH
| No ICH (N=37 680) | ICH (N=135) | |
|---|---|---|
| Trial | ||
| APPRAISE-23 | 4458/37 680 (11.8%) | 7/135 (5.2%) |
| PLATO2 | 11 056/37 680 (29.3%) | 24/135 (17.8%) |
| TRACER1 | 12 876/37 680 (34.2%) | 68/135 (50.4%) |
| TRILOGY ACS4 | 9290/37 680 (24.7%) | 36/135 (26.7%) |
| Age, y | 65 (57, 72) | 69 (62, 77) |
| Race or ethnic group | ||
| White | 30 715/37 680 (81.5%) | 110/135 (81.5%) |
| Black | 770/37 680 (2.0%) | 3/135 (2.2%) |
| Other | 4861/37 680 (12.9%) | 15/135 (11.1%) |
| Male | 25 426/37 680 (67.5%) | 91/135 (67.4%) |
| Female | 12 254/37 680 (32.5%) | 44/135 (32.6%) |
| Body mass index, kg/m2 | 27 (25, 31) | 27 (24, 30) |
| Prior hypertension | 28 003/37 680 (74.3%) | 106/135 (78.5%) |
| Prior diabetes mellitus | 12 858/37 680 (34.1%) | 49/135 (36.3%) |
| Smoking status | ||
| Never smoked | 17 079/37 582 (45.4%) | 63/135 (46.7%) |
| Former smoker | 10 976/37 582 (29.2%) | 48/135 (35.6%) |
| Current smoker | 9527/37 582 (25.3%) | 24/135 (17.8%) |
| Prior dyslipidemia | 21 651/37 680 (57.5%) | 84/135 (62.2%) |
| Prior myocardial infarction | 12 481/37 680 (33.1%) | 49/135 (36.3%) |
| Prior congestive heart failure | 5007/37 680 (13.3%) | 22/135 (16.3%) |
| Prior transient ischemic attack/stroke | 2224/37 616 (5.9%) | 15/135 (11.1%) |
| Prior angina | 12 477/23 932 (52.1%) | 46/92 (50.0%) |
| Prior percutaneous coronary intervention | 8512/37 680 (22.6%) | 27/135 (20.0%) |
| Prior coronary artery bypass graft | 4399/37 680 (11.7%) | 23/135 (17.0%) |
| Prior chronic obstructive pulmonary disease | 1646/23 932 (6.9%) | 7/92 (7.6%) |
| Coronary artery disease | 10 385/33 222 (31.3%) | 44/128 (34.4%) |
| Baseline Killip score | ||
| I | 30 512/33 214 (91.9%) | 111/128 (86.7%) |
| II | 2258/33 214 (6.8%) | 13/128 (10.2%) |
| III | 411/33 214 (1.2%) | 4/128 (3.1%) |
| IV | 33/33 214 (0.1%) | 0/128 (0.0%) |
| Baseline heart rate, beats/min | 70 (62, 79) | 70 (62, 78) |
| Systolic blood pressure, mm Hg | 130 (120, 141) | 135 (120, 146) |
| Diastolic blood pressure, mm Hg | 77 (70, 82) | 79 (70, 85) |
| Glucose, mg/dL | 113 (96, 149) | 115 (93, 161) |
| Creatinine clearance, mL/min per 1.73 m² | 77 (60, 96) | 69 (52, 87) |
| Baseline hemoglobin, g/L | 138 (127, 148) | 136 (124, 147) |
| Baseline ST depression | 17 170/35 142 (48.9%) | 56/126 (44.4%) |
| Baseline transient ST elevation | 3192/35 176 (9.1%) | 9/126 (7.1%) |
| Baseline T-wave inversion | 10 338/35 142 (29.4%) | 31/126 (24.6%) |
| Selected medication administered between hospital admission and randomization by intracranial hemorrhage | ||
| Aspirin | 35 664/37 680 (94.6%) | 131/135 (97.0%) |
| Thienopyridines | 26 750/37 680 (71.0%) | 103/135 (76.3%) |
| Heparin (all forms) | 23 539/37 680 (62.5%) | 86/135 (63.7%) |
| Glycoprotein IIb/IIIa | 4671/37 680 (12.4%) | 19/135 (14.1%) |
| Direct thrombin inhibitors | 1298/37 680 (3.4%) | 7/135 (5.2%) |
| Statin | 31 202/37 680 (82.8%) | 107/135 (79.3%) |
| β-blockers | 28 720/37 680 (76.2%) | 112/135 (83.0%) |
| No. of antithrombotics* | ||
| 0 | 89/37 302 (0.2%) | 0/135 (0.0%) |
| 1 | 2511/37 302 (6.7%) | 7/135 (5.2%) |
| 2 | 27 467/37 302 (73.6%) | 78/135 (57.8%) |
| 3 | 7230/37 302 (19.4%) | 50/135 (37.0%) |
| 4 | 5/37 302 (0.0%) | 0/135 (0.0%) |
Data presented as n/N (%) or median (25th, 75th); 1341 patients (3.8%) had missing race. ICH indicates intracranial hemorrhage.
Definition of number of antithrombotics: PLATO—1 for ticagrelor or clopidogrel (randomized treatment)+1 if the patient is taking aspirin at randomization+1 if the patient is taking vitamin K antagonists at randomization; APPRAISE2—1 if the patient is taking aspirin at randomization+1 if the patient is taking clopidogrel/ticlopidine/prasugrel at randomization+1 if the patient was randomized to apixaban; TRACER—1 if the patient is taking aspirin at randomization+1 if the patient is taking thienopyridines at randomization+1 if the patient is taking oral anticoagulants at randomization+1 if patient randomized to vorapaxar; TRILOGY ACS—1 for clopidogrel or prasugrel (randomized treatment)+1 if the patient is taking aspirin at randomization.
The anatomic locations of the ICH events are shown in Table3. One-half of all ICHs were intracerebral (50.4%), and nearly one-third (31.1%) were subdural hematomas. In total, 45 of the 135 (33.3%) ICH events were fatal.
Table 3.
ICH, ICH Type, and Significant Mortality Following ICH by Clinical Trial
| APPRAISE-23 (N=7) | PLATO2 (N=24) | TRACER1 (N=68) | TRILOGY ACS4 (N=36) | Total (N=135) | |
|---|---|---|---|---|---|
| Intracerebral hemorrhage | 3/7 (42.9%) | 14/24 (58.3%) | 40/68 (58.8%) | 11/36 (30.6%) | 68/135 (50.4%) |
| Subdural hematoma | 1/7 (14.3%) | 1/24 (4.2%) | 23/68 (33.8%) | 17/36 (47.2%) | 42/135 (31.1%) |
| Subarachnoid hemorrhage | 0/7 (0.0%) | 3/24 (12.5%) | 15/68 (22.1%) | 7/36 (19.4%) | 25/135 (18.5%) |
| Intraventricular hemorrhage | 1/7 (14.3%) | 0/24 (0.0%) | 14/68 (20.6%) | 0/36 (0.0%) | 15/135 (11.1%) |
| Fatal intracranial hemorrhage | 4/7 (57.1%) | 9/24 (37.5%) | 23/68 (33.8%) | 9/36 (25.0%) | 45/135 (33.3%) |
Data are presented as n/N (%). ICH indicates intracranial hemorrhage.
The results of the modeling to determine the independent predictors of ICH are shown in Tables S2 and4. Table S2 shows the full model, and Table4 shows the model after backward selection. The same model was obtained by using stepwise model selection. Four independent predictors of ICH were identified: older age, prior stroke or TIA, higher SBP, and treatment with a larger number of antithrombotic agents. Because patients with prior stroke or TIA were supposed to be excluded from TRILOGY ACS but 23 patients with prior stroke or TIA were enrolled and randomized, treatment was stopped as soon as this information became known, the modeling was performed without patients from the TRILOGY ACS trial, and the results were similar (data not shown). None of these 23 patients had an ICH.
Table 4.
Multivariable Analyses of ICH Model After Backward Selection
| Parameter | χ2 | HR | 95% CI | ||
|---|---|---|---|---|---|
| Value | P Value | Value | Lower | Upper | |
| Older age (HR in units of 10 years) | 28.63 | <0.001 | 1.61 | 1.35 | 1.91 |
| Number of antithrombotics (HR per 1-unit increase) | 13.48 | 0.002 | 2.10 | 1.41 | 3.13 |
| Prior stroke/transient ischemic attack (yes vs no) | 6.94 | 0.008 | 2.10 | 1.21 | 3.66 |
| Systolic blood pressure (HR in units of 10 mm/Hg) | 4.12 | 0.042 | 1.09 | 1.01 | 1.18 |
Analysis based on 37 241 complete cases. HR indicates hazard ratio; ICH, intracranial hemorrhage.
Discussion
We aggregated patient-level data from 4 large, contemporary trials of patients with ACS. In nearly 38 000 patients with NSTE ACS, ICH was uncommon and occurred in 0.4% of patients. However, 33% of ICH events were fatal. Four independent predictors of ICH were identified: older age, history of stroke or TIA, higher SBP, and larger number of antithrombotics. Investigators designing clinical trials of antithrombotic therapies in NSTE ACS populations or experts involved in data and safety monitoring committees may find these data useful, even though the overall event rate is low.
Prior large trials with similar NSTE ACS populations have individually reported ICH occurrences of 0.1% to 0.3% with different follow-up periods and patient populations.9–12 The proportions of patients with ICH from these other trials are consistent with the current analysis, particularly the TRITON–TIMI 38 trial, which was conducted in a contemporary era and (unlike SYNERGY and PURSUIT) had a longer follow-up period, similar to the 4 trials included in these analyses. ICH has been an important safety signal in several recent trials, including TRITON–TIMI 38,9 which supported approval of prasugrel for NSTE ACS but not in patients with prior stroke or TIA, as well as the TRA-2P TIMI 50 trial,13 in which the data and safety monitoring board stopped enrollment of patients with prior stroke due to excess ICH with vorapaxar compared with placebo. The follow-up phase of TRACER was also stopped, due to excess bleeding including ICH.1
In the design of trials, careful consideration of inclusion and exclusion criteria may be important to minimize risk to some patients while still being broad enough in patient recruitment to optimize generalizability of the trial results. Point estimates of ICH occurrence provided from these data of nearly 38 000 patients may also aid data and safety monitoring committees that are charged with monitoring human subject safety, as benchmarks of expected rates of ICH may be informative. From a clinical perspective, more work is needed before these data can be used to definitively guide treatment decisions for specific ACS patients. While we believe these data are robust, making patient treatment decisions based on 135 events is potentially hazardous.
Patient demographics and clinical characteristics, such as those used in our modeling, have been shown to be predictive of ICH events in other patient populations, including patients with ST-segment elevation myocardial infarction9 and for general populations.14 However, other important predictors of ICH are known that we could not include in these analyses, such as amyloid angiopathy.15 More work is needed to potentially integrate biomarker, proteomic, and genomic information that is available for some of the 4 trials included in these analyses.
Limitations
These analyses have limitations. Baseline information common to trials was included, but we did not include important postrandomization factors such as medication therapy and dosing or procedures. In addition, only blood pressure at time of randomization was systematically collected in the trials. Comparisons across the trials were not performed given the differences in patient populations and follow-up durations that were driven in part by protocol specifications. These trials included experiment arms with antithrombotic therapies with different mechanisms of action and different background therapies defined by the standard of care. The approach used to combine these different agents in a composite number of antithrombotic therapies, therefore, has limitations. Despite the large patient population, the number of ICH events remained modest and limits the confidence of the inferences that can be made from the data. We did not systematically assess stroke morbidity in all the trials and, thus, have limited information on patient outcomes after nonfatal ICH.
Conclusions
ICH was an uncommon event in patients with NSTE ACS and occurred in 0.4% of patients. Many events occurred early after enrollment, but there was continued risk for ICH over time. In total, 33% of patients with ICH died within 30 days of the event. Patients with older age, prior stroke or TIA, higher SBP, or treatment with larger number of antithrombotic agents were at an increased risk of ICH. Investigators designing clinical trials of antithrombotic therapies in NSTE ACS populations or experts involved in data and safety monitoring committees may find these data useful. Strategies to better identify patients at risk of ICH after NSTE ACS are needed and are part of ongoing clinical investigations, including incorporation of biomarker and genetic information.
Acknowledgments
We thank Morgan deBlecourt for her editorial assistance, provided as part of her regular duties as an employee of the Duke Clinical Research Institute. We also thank all the investigators who worked on the trials and the participants who participated.
Sources of Funding
This work, as well as the TRACER trial, was supported by Merck & Co, Inc. Ms Hager was supported by National Institutes of Health training grant T32HL079896.
Disclosures
Dr Mahaffey’s full disclosures for before August 1, 2013, are available at www.dcri.org; disclosures for after August 1, 2013, are available at http://med.stanford.edu/profiles/kenneth_mahaffey. Professor White has received research grants from Sanofi Aventis, Eli Lilly, NIH, Merck Sharpe & Dohme, AstraZeneca, GSK, Daiichi Sankyo Pharma Development; and consulting fees from AstraZeneca. Dr Armstrong has received a research grant from Merck & Co and consulting or other fees from Eli Lilly and AstraZeneca. Dr Alexander’s disclosures are available at https://dcri.org/about-us/conflict-of-interest. Dr Tricoci has a consultant agreement and received a research grant from Merck & Co. Dr Lopes has received research grants from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, and Pfizer. Dr Ohman has received grant funding and travel expenses from Daiichi Sankyo and Lilly; consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Liposcience, Merck, Pozen, Roche, Sanofi-Aventis, The Medicines Company, and WebMD; grant funding from Gilead Sciences; and lecture fees from Gilead Sciences, Boehringer Ingelheim, and The Medicines Company. Dr Roe has received consulting fees from Bristol-Myers Squibb, GlaxoSmithKline, Merck, Janssen Pharmaceuticals, KAI Pharmaceuticals, Sanofi-Aventis, Helsinn Pharmaceuticals, Regeneron, Novartis, AstraZeneca, Orexigen, Eli Lilly, and Daiichi Sankyo; research grants from Bristol-Myers Squibb, Hoffmann-La Roche, Novartis, Schering-Plough, KAI Pharmaceuticals, Eli Lilly, AstraZeneca, and Janssen Pharmaceuticals; and speakers’ bureau payments from AstraZeneca and Janssen. Dr Harrington has received research grants and consultant/advisory fees from Merck & Co. Full disclosures are available at http://med.stanford.edu/profiles/Robert_Harrington/. Dr Wallentin has received research grants from AstraZeneca, Merck & Co, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline; was a consultant for Abbott, Merck & Co, Regado Biosciences, Athera Biotechnologies, Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, and Bristol-Myers Squibb/Pfizer; received lecture fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, and GlaxoSmithKline; received honoraria from Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb/Pfizer, and GlaxoSmithKline; and received travel support from AstraZeneca, Bristol-Myers Squibb/Pfizer, and GlaxoSmithKline. The other authors have no disclosures.
Supporting Information
Table S1. Baseline demographic and clinical characteristics by clinical trial.
Table S2. Multivariable analyses of ICH full model.
References
- Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LH, Ambrosio G, Bode C, Cequier A, Cornel JH, Diaz R, Erkan A, Huber K, Hudson MP, Jiang L, Jukema JW, Lewis BS, Lincoff AM, Montalescot G, Nicolau JC, Ogawa H, Pfisterer M, Prieto JC, Ruzyllo W, Sinnaeve PR, Storey RF, Valgimigli M, Whellan DJ, Widimsky P, Strony J, Harrington RA, Mahaffey KW TRACER Investigators. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33. doi: 10.1056/NEJMoa1109719. [DOI] [PubMed] [Google Scholar]
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA PLATO Investigators. Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez-Sendon J, De Caterina R, Jansky P, Darius H, Vinereanu D, Cornel JH, Cools F, Atar D, Leiva-Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin L APPRAISE-2 Investigators. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, Ardissino D, Nicolau JC, Boden WE, Gurbel PA, Ruzyllo W, Dalby AJ, McGuire DK, Leiva-Pons JL, Parkhomenko A, Gottlieb S, Topacio GO, Hamm C, Pavlides G, Goudev AR, Oto A, Tseng CD, Merkely B, Gasparovic V, Corbalan R, Cinteză M, McLendon RC, Winters KJ, Brown EB, Lokhnygina Y, Aylward PE, Huber K, Hochman JS, Ohman EM TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–1309. doi: 10.1056/NEJMoa1205512. [DOI] [PubMed] [Google Scholar]
- TRACER Executive and Steering Committees. The Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRA*CER) trial: study design and rationale. Am Heart J. 2009;158:327–334.e4. doi: 10.1016/j.ahj.2009.07.001. [DOI] [PubMed] [Google Scholar]
- James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R, Becker R, Wallentin L. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157:599–605. doi: 10.1016/j.ahj.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Chin CT, Roe MT, Fox KA, Prabhakaran D, Marshall DA, Petitjean H, Lokhnygina Y, Brown E, Armstrong PW, White HD, Ohman EM TRILOGY ACS Steering Committee. Study design and rationale of a comparison of prasugrel and clopidogrel in medically managed patients with unstable angina/non-ST-segment elevation myocardial infarction: the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Am Heart J. 2010;160:16–22.e1. doi: 10.1016/j.ahj.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Sloan MA, Sila CA, Mahaffey KW, Granger CB, Longstreth WT, Jr, Koudstaal P, White HD, Gore JM, Simoons ML, Weaver WD, Green CL, Topol EJ, Califf RM. Prediction of 30-day mortality among patients with thrombolysis-related intracranial hemorrhage. Circulation. 1998;98:1376–1382. doi: 10.1161/01.cir.98.14.1376. [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, Kereiakes DJ, Langer A, Mahaffey KW, Nessel CC, Armstrong PW, Avezum A, Aylward P, Becker RC, Biasucci L, Borzak S, Col J, Frey MJ, Fry E, Gulba DC, Guneri S, Gurfinkel E, Harrington R, Hochman JS, Kleiman NS, Leon MB, Lopez-Sendon JL, Pepine CJ, Ruzyllo W, Steinhubl SR, Teirstein PS, Toro-Figueroa L, White H SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292:45–54. doi: 10.1001/jama.292.1.45. [DOI] [PubMed] [Google Scholar]
- Vavalle JP, Clare R, Chiswell K, Rao SV, Petersen JL, Kleiman NS, Mahaffey KW, Wang TY. Prognostic significance of bleeding location and severity among patients with acute coronary syndromes. JACC Cardiovasc Interv. 2013;6:709–717. doi: 10.1016/j.jcin.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy. N Engl J Med. 1998;339:436–443. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA TRA 2P–TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- Samarasekera N, Smith C, Al-Shahi Salman R. The association between cerebral amyloid angiopathy and intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2012;83:275–281. doi: 10.1136/jnnp-2011-300371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline demographic and clinical characteristics by clinical trial.
Table S2. Multivariable analyses of ICH full model.


