Abstract
Background
The role of the CYP2C19 genotype on clopidogrel efficacy has been studied widely, with data suggesting reduced clopidogrel efficacy in loss-of-function variant carriers taking clopidogrel after percutaneous coronary intervention; however, data are limited regarding the association between CYP2C19 genetic variants and outcomes in stroke patients. We investigated whether CYP2C19 metabolizer status affects the risk of recurrent stroke or major bleeding in subcortical stroke patients taking dual antiplatelet therapy with aspirin and clopidogrel.
Methods and Results
CYP2C19*2 and CYP2C19*17 were genotyped in 522 patients treated with dual antiplatelet therapy from the Secondary Prevention of Small Subcortical Strokes (SPS3) study. CYP2C19 metabolizer status was inferred from genotype, and associations with the risk of recurrent stroke and major bleeding were assessed in the overall cohort and by race/ethnic group with logistic regression modeling. In the overall cohort, there were no differences in outcomes by CYP2C19 metabolizer status (recurrent stroke, odds ratio 1.81 [95% CI 0.76 to 4.30]; major bleeding, odds ratio 0.67 [95% CI 0.22 to 2.03]). In white participants, those with CYP2C19 intermediate or poor metabolizer status had higher odds of recurrent stroke (odds ratio 5.19 [95% CI 1.08 to 24.90]) than those with extensive or ultrarapid metabolizer status, but there was no evidence of difference in major bleeding.
Conclusions
There were significant differences in recurrent stroke by CYP2C19 genotype-inferred metabolizer status in white subcortical stroke patients receiving dual antiplatelet therapy with aspirin and clopidogrel, consistent with cardiovascular studies on CYP2C19 and clopidogrel; however, the bleeding risk that led to early termination of the antiplatelet arm of the SPS3 trial does not appear to be explained by CYP2C19 genotype. This study was relatively underpowered; therefore, these findings should be interpreted with caution and warrant replication.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00059306.
Keywords: clopidogrel, CYP2C19, pharmacogenomics, stroke prevention, subcortical stroke
Small subcortical strokes, also known as lacunar or small vessel strokes, comprise ≈25% of cerebral ischemic strokes.1–3 They often occur at younger ages than other stroke subtypes,4 are more frequent in persons of Hispanic descent,5–7 and are among the most common causes of cognitive impairment and vascular dementia.8–10 Studies have shown that the recurrence rate for patients experiencing a small subcortical stroke is 4% to 11% per year11,12 and that the incidence of vascular dementia is related to the rate of stroke recurrence.13,14 Consequently, reducing recurrence of small subcortical strokes may reduce progression of cognitive impairment, dementia, and functional decline.
The Secondary Prevention of Small Subcortical Strokes (SPS3) study was a clinical trial evaluating approaches to prevent stroke recurrence and included an antiplatelet arm testing aspirin plus placebo versus dual antiplatelet therapy (DAPT) with aspirin and clopidogrel, and another arm testing 2 different systolic blood pressure targets.15 No differences were noted between the 2 antiplatelet treatments for the study’s primary and secondary outcomes, which included all strokes, disabling or fatal stroke, transient ischemic attack without stroke, myocardial infarction, other thromboembolic events, and major vascular events.16 However, the risk of major bleeding was almost doubled in DAPT-treated patients compared with those who received aspirin plus placebo and led to early termination of the antiplatelet arm of the study at the recommendation of the data and safety monitoring committee.16
Clopidogrel is commonly used alone or in combination with aspirin for stroke prevention in at-risk individuals.17,18 It is a thienopyridine prodrug with a 2-stage activation mediated largely by the drug-metabolizing enzyme CYP2C19.19 The active metabolite of clopidogrel specifically and irreversibly binds to the platelet P2RY12 purinergic receptor, which inhibits ADP-mediated platelet activation and aggregation.20,21 The common loss-of-function *2 single nucleotide polymorphism (SNP) in CYP2C19 has been associated with decreased conversion of clopidogrel into its active metabolite,22,23 decreased antiplatelet activity based on ex vivo platelet reactivity testing in on-treatment patients,22,24,25 and a higher rate of recurrent cardiovascular events in the setting of percutaneous coronary intervention.24–26 The *17 gain-of-function SNP in CYP2C19 has been associated in some studies with a lower rate of recurrent cardiovascular events27–29 and an increased bleeding risk.28,29 This has led to CYP2C19 genetic testing to define clopidogrel treatment in patients following percutaneous coronary intervention in some health systems.30–32
Although evidence shows that the loss-of-function *2 allele in CYP2C19 significantly affects protection by clopidogrel against recurrent cardiovascular events, these data arise almost exclusively from patients with preexisting cardiovascular disease (eg, myocardial infarction, undergoing percutaneous coronary intervention, and/or history of coronary artery disease).24–26 Few data address the relevance of CYP2C19 SNPs in patients taking clopidogrel for stroke prevention. The primary SPS3 findings suggest an absence of efficacy and increased risk associated with DAPT versus aspirin alone in patients with small subcortical strokes, and genetic variants in CYP2C19 that contribute to the bioactivation of clopidogrel may have contributed to these findings. We sought to investigate whether SNPs in CYP2C19, particularly the well-described CYP2C19 *2 loss-of-function and *17 gain-of-function variants, affect the recurrence of stroke or bleeding in participants from SPS3 who were randomized to DAPT with aspirin and clopidogrel.
Methods
Study Population
The SPS3 study is an international multicenter randomized clinical trial evaluating antiplatelet and antihypertensive approaches to prevent stroke recurrence (ClinicalTrials.gov identifier NCT00059306). The design of the SPS3 has been described previously.15 Briefly, 3020 individuals with a recent (within the last 6 months) symptomatic small subcortical stroke/ or transient ischemic attack with a corresponding infarct on magnetic resonance imaging were recruited and randomized in to the study in a 2 × 2 factorial design to antiplatelet treatment of 325 mg aspirin daily plus placebo or 325 mg aspirin plus 75 mg clopidogrel daily, and to a systolic blood pressure target of higher (130 to 149 mm Hg), or lower (<130 mm Hg). The antiplatelet treatment arm was double-blinded. The primary outcome of the study was time to recurrent stroke (ischemic or hemorrhagic). Secondary outcomes included the rate of cognitive decline and major vascular events (including ischemic strokes, myocardial infarction, or vascular death). The primary safety outcome was major bleeding (major extracranial hemorrhage), which was defined as serious or life-threatening bleeding requiring transfusion of red cells or surgery, or resulting in permanent functional sequelae or death.16 All primary events, the primary safety outcome, and most secondary outcomes were adjudicated by a blinded events-adjudication committee.15 The average follow-up time in the antiplatelet arm was 3.4 years (range 0 to 8.2 years).16
The SPS3-Genetic Substudy (SPS3-GENES) collected DNA from saliva samples using OG-500 Oragene DNA self-collection kits (DNA Genotek Inc) from 1139 participants enrolled in SPS3, with 580 randomized to aspirin plus placebo and 559 randomized to DAPT with aspirin and clopidogrel. The genetic substudy was approved by the institutional review board at each study site, and all participants gave written informed consent for participation in the genetic substudy.
CYP2C19 SNP Genotyping
DNA was isolated from all SPS3-GENES participants using the prepIT-L2P kit (PT-LP2-45; DNA Genotek Inc). There were 14 participants in SPS3-GENES with inadequate DNA due to poor sample collection (10 randomized to aspirin plus placebo and 4 randomized to DAPT). Participants who were randomized to the DAPT group with available DNA (n=555) were genotyped for CYP2C19*2 (rs4244285) and CYP2C19*17 (rs12248560) using TaqMan assays (Applied Biosystems). Genotyping was performed using the QuantStudio polymerase chain reaction system (Life Technologies). SNP call rates were 97.3% for CYP2C19*2 and 95.9% for CYP2C19*17. Overall, 106 samples were run in duplicate, and genotype concordance rates were >99% for both SNPs. Due to the racial and ethnic backgrounds of SPS3-GENES participants (the majority self-identified as white or black), we genotyped only CYP2C19*2 and CYP2C19*17 for this study because allele frequencies for other CYP2C19 polymorphisms were very low in these populations (eg, 1000 Genomes data show that CYP2C19*3 [rs4986893] is monomorphic in African, Native American, and European populations).33
Statistical Methods
Descriptive statistics were computed overall and by genetically defined race group as mean (SD) or frequency (percentage), as appropriate. Race groups were categorized as white, black, and Hispanic and were determined based on clustering results from a principal component analysis of genomewide SNP data. Hardy-Weinberg equilibrium was estimated for each SNP overall and in each race group using a chi-square test with 1 degree of freedom.
Participants were categorized into CYP2C19 metabolizer status based on CY2C19*2 and CYP2C19*17 genotypes as follows: extensive metabolizer (EM; *1/*1), ultrarapid metabolizer (UM; *1/*17, *17/*17), intermediate metabolizer (IM; *1/*2), and poor metabolizer (PM; *2/*2). There is controversy about the phenotype associated with 1 loss-of-function allele (*2) and 1 gain-of-function allele (*17) (*2/*17 genotype). The first Clinical Pharmacogenetics Implementation Consortium guidelines on CYP2C19 and clopidogrel did not classify this as a genotype with a recommended action.34 The revised guidelines (2013) included the *2/*17 genotype in the IM group but stated that ambiguity about the functional consequences remains when someone carries 1 of each of these alleles (*2 and *17).35 Because of this controversy and ambiguity, individuals with a *2/*17 genotype (n=29) were excluded from the analysis. Frequencies of events were calculated overall and by race group, and by CYP2C19 metabolizer status (ie, UM and EM versus IM and PM). Logistic regression models were fitted to examine the odds ratios (ORs; all recurrent strokes or major bleeding) in IM/PMs compared with EM/UMs (reference group). All models were adjusted for age and sex, and analyses conducted in the overall group were also adjusted for genetically defined race. The overall approach, with adjustment for race, was justified because no existing evidence suggests differences by race or ethnicity in the effect of these known variants on protein function.35 We did not adjust for blood pressure target group because there were no differences between targets with respect to reduction of all stroke, disabling or fatal stroke, or the composite outcome of myocardial infarction or vascular death.36 Because both CYP2C19*2 and CYP2C19*17 have been previously associated with other cardiovascular outcomes, a P value <0.05 was considered significant. All analyses were conducted using SAS version 9.3 (SAS Institute).
Based on the CYP2C19 metabolizer frequencies (22% IM/PM) and the expected event rate in SPS3-GENES (14%), we estimated that our combined sample of 522 subjects would provide 80% power to detect ORs of 1.8 in IM/PMs compared with EM/UMs at α=0.05.
Results
A total of 522 of the 555 subjects randomized to DAPT with available DNA were successfully genotyped for both CYP2C19*2 and CYP2C19*17 and available for analysis. Genotyping failed for 6 participants for CYP2C19*2, for 14 participants for CYP2C19*17, and for 9 participants for both CYP2C19*2 and CYP2C19*17; 4 participants were excluded due to a sex mismatch between their genetically defined sex and reported sex. The baseline demographics and characteristics for the 522 SPS3-GENES participants are shown in Table1. The largest genetically defined race group in SPS3-GENES was Hispanic (48%), followed by white (37%), and black (15%). Based on the design of the parent SPS3 trial, the vast majority of participants had a history of hypertension (88%) (Table1). In addition, almost all participants in the SPS3 trial had an ischemic stroke as the qualifying event (95%) (Table1). The SPS3-GENES participants included in this study were broadly similar to all SPS3 participants regarding clinical features.
Table 1.
Baseline Demographics and Characteristics in SPS3-GENES Participants Randomized to Dual Antiplatelet Therapy (Aspirin and Clopidogrel)
| Characteristic | White (n=191) | Black (n=80) | Hispanic (n=251) | Overall (n=522) |
|---|---|---|---|---|
| Age (y), mean±SD | 63.0±10.6 | 58.5±8.1 | 63.4±10.9 | 62.5±10.5 |
| Sex, male | 125 (65) | 45 (56) | 153 (61) | 323 (62) |
| History of | ||||
| Ischemic heart disease | 15 (8) | 10 (13) | 11 (4) | 36 (7) |
| Hypertension | 161 (84) | 78 (98) | 222 (88) | 461 (88) |
| Diabetes | 54 (28) | 28 (35) | 91 (36) | 173 (33) |
| Previous clinical stroke or transient ischemic attack | 9 (7) | 3 (4) | 7 (3) | 19 (4) |
| Qualifying event | ||||
| Ischemic stroke | 95 (91) | 33 (94) | 157 (94) | 285 (95) |
| Transient ischemic attack | 9 (9) | 2 (6) | 4 (3) | 15 (5) |
| Use of aspirin at time of qualifying event | 42 (23) | 21 (28) | 49 (20) | 112 (22) |
Values are presented as number (percentage) unless otherwise noted. SPS3-GENES indicates Secondary Prevention of Small Subcortical Strokes-Genetic Substudy.
Minor allele frequencies were consistent with the published literature, and both SNPs were in Hardy-Weinberg equilibrium in the overall population and in each race or ethnic group (white, black, and Hispanic). Table2 shows the frequency of CYP2C19 metabolizer status in SPS3-GENES participants. The metabolizer status frequencies are consistent with those reported previously.35
Table 2.
Frequency of Metabolizer Status in SPS3-GENES Participants Randomized to the Aspirin and Clopidogrel Dual Antiplatelet Therapy
| Metabolizer Status | White (n=176) | Black (n=73) | Hispanic (n=244) | Overall (n=493) |
|---|---|---|---|---|
| UM | 57 (32) | 25 (31) | 44 (18) | 126 (26) |
| EM | 78 (44) | 26 (33) | 156 (64) | 260 (52) |
| IM | 34 (19) | 18 (31) | 42 (17) | 94 (19) |
| PM | 7 (4) | 4 (5) | 2 (1) | 13 (3) |
| EM/UM | 135 (77) | 51 (70) | 200 (82) | 386 (78) |
| IM/PM | 41 (23) | 22 (30) | 44 (18) | 107 (22) |
Values are presented as number (percentage). Percentages may not add up to 100% because of rounding. *2/*17 individuals excluded from analysis (n=29). EM indicates extensive metabolizer (*1/*1); IM, intermediate metabolizer (*1/*2); PM, poor metabolizer (*2/*2); SPS3-GENES indicates Secondary Prevention of Small Subcortical Strokes-Genetic Substudy; UM, ultrarapid metabolizer (*1/*17, *17/*17).
Table3 shows the event rates and ORs for recurrent stroke and major bleeding in the SPS3-GENES participants. In the overall cohort, the OR suggested an increased risk for recurrent stroke in IM/PMs compared with EM/UMs but was not significantly different (OR 1.81 [95% CI 0.76 to 4.30]) (Table3). In white participants, there was a significantly increased risk of stroke (all of which were ischemic) in IM/PMs compared with EM/UMs (OR 5.19 [95% CI 1.08 to 24.90]) (Table3). Similar trends for an increased frequency of recurrent stroke in the IM/PM group were observed in black participants (Table3). There were no recurrent strokes among Hispanic IM/PMs. The ORs for recurrent stroke in each race group and overall are shown in the Figure 1. There were no differences in major bleeding by CYP2C19 metabolizer status overall or in any of the race groups individually (Table3).
Table 3.
Event Rates and Odds Ratios for Recurrent Stroke and Major Bleeding in SPS3-GENES Participants Randomized to Dual Antiplatelet Therapy (Aspirin and Clopidogrel) by CYP2C19 Metabolizer Phenotype
| Event | White (n=176) | Black (n=73) | Hispanic (n=244) | Overall (n=493) | ||||
|---|---|---|---|---|---|---|---|---|
| EM/UM (n=135) | IM/PM (n=41) | EM/UM (n=51) | IM/PM (n=22) | EM/UM (n=200) | IM/PM (n=44) | EM/UM (n=386) | IM/PM (n=107) | |
| Recurrent stroke | ||||||||
| n (%) | 3 (2.2) | 4 (9.8) | 6 (11.8) | 5 (22.7) | 8 (4.0) | 0 (0.0) | 17 (4.4) | 9 (8.4) |
| OR (95% CI) | Ref | 5.19 (1.08 to 24.90) | Ref | 3.45 (0.80 to 14.90) | Ref | † | Ref | 1.81 (0.76 to 4.30) |
| Major bleeding | ||||||||
| n (%) | 6 (4.4) | 3 (7.3) | 6 (11.8) | 0 (0.0) | 7 (3.5) | 1 (2.3) | 19 (4.9) | 4 (3.7) |
| OR (95% CI) | Ref | 1.54 (0.36 to 6.61) | Ref | † | Ref | 0.67 (0.08 to 5.76) | Ref | 0.67 (0.22 to 2.03) |
*2/*17 individuals excluded from analysis (n=29). EM indicates extensive metabolizer (*1/*1); IM, intermediate metabolizer (*1/*2); OR, odds ratio adjusted for age and sex and genetically defined race in the overall analysis; PM, poor metabolizer (*2/*2); SPS3-GENES indicates Secondary Prevention of Small Subcortical Strokes-Genetic Substudy; UM, ultrarapid metabolizer (*1/*17, *17/*17).
No events in the IM/PM group, so the OR and 95% CI cannot be estimated.
Figure 1.
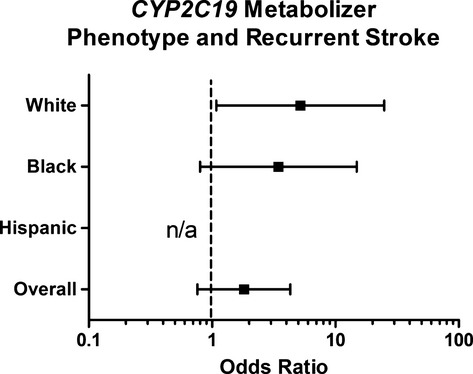
Odds ratios for recurrent stroke in the CYP2C19IM/PM phenotype compared with the EM/UM phenotype in genetically defined white, black, and Hispanic participants. All odds ratios were adjusted for age and sex. The odds ratio for Hispanic participants was not estimable because of 0 events in IM/PM participants. EM indicates extensive metabolizer; IM, intermediate metabolizer; n/a, not estimable; PM, poor metabolizer; UM, ultrarapid metabolizer.
Sensitivity analyses that included *2/*17 participants in the IM category revealed data consistent with those described in Table3, although data were no longer statistically significant in white participants (OR for recurrent stroke was 3.56 [95% CI 0.76 to 16.67]). Additional sensitivity analyses investigating *17 carriers versus noncarriers were conducted and did not reveal any statistically significant results (data not shown).
Discussion
The SPS3 trial showed that addition of clopidogrel to standard aspirin treatment did not reduce the risk of stroke recurrence but increased the risk of major hemorrhage and death.16 These results of the SPS3 trial led to a recommendation against routine use of DAPT with clopidogrel and aspirin for secondary prevention after a subcortical infarct.18 Nonetheless, despite the findings of the antiplatelet therapy arm of SPS3, we believed it was critical to complete our planned study of the role of CYP2C19 metabolizer status on outcomes during the SPS3 trial. Most important, given the increased risk of bleeding observed with DAPT in SPS3, in the context of existing literature suggesting higher bleeding risk in those lacking a *2 allele,28,29 it was critical to examine the role of CYP2C19 on major bleeding events. Furthermore, although the main antiplatelet trial results demonstrated a lack of efficacy of DAPT in the small subcortical stroke patient population, we felt it would be valuable to clarify any additional contribution of CYP2C19*2 to the risk of vascular events in SPS3.
In the overall cohort, we did not observe any significant differences; however, among white participants, we found that CYP2C19 IM/PMs had higher risk for stroke recurrence compared with EM/UMs during the follow-up period (average 3.4 years). These findings are consistent with the published cardiovascular literature.24–29,37 We did not observe significant differences in the odds of major bleeding between the 2 groups, overall or by race or ethnic group. To our knowledge, this study is the first to investigate the influence of SNPs in CYP2C19 on stroke recurrence and major bleeding in clopidogrel-treated patients with small subcortical stroke. Although DAPT with clopidogrel is unlikely to be used commonly in small subcortical stroke patients, our data support the suggestion that the CYP2C19 genotype may have played a role in the outcomes observed in the SPS3 trial.
The effect size we observed in whites for recurrent stroke in small subcortical stroke patients (OR 5.19) was larger than those reported previously for recurrent cardiovascular disease in patients with preexisting cardiovascular disease (ORs for major adverse cardiovascular events: 1.26 to 1.96; relative risk for stent thrombosis: 2.58 to 3.82).27,37–39 The study is relatively small, thus it should not be presumed that a larger trial in a similar population would lead to an OR of this magnitude. In addition, many statistical tests were performed, and although the finding in white participants is consistent with the published literature on cardiovascular outcomes in white populations, it is possible that our finding represents a type 1 statistical error. Evidence that it might not be a type 1 error is supported by other studies that have shown the CYP2C19 genotype may influence clopidogrel efficacy in stroke patients with other stroke subtypes.40–42 A study in 625 Chinese ischemic stroke patients found that CYP2C19 loss-of-function allele carriers had a higher risk of subsequent vascular events versus noncarriers (hazard ratio 2.16 [95% CI 1.31 to 3.56]).40 Similar results were observed in a smaller study of 53 patients with stroke or transient ischemic attack,41 and a study of 259 Chinese ischemic stroke patients showed that CYP2C19 loss-of-function allele carriers had poorer functional outcomes, using the National Institutes of Health Stroke Scale and the modified Rankin Scale, compared with noncarriers.42 These studies, however, were not specific to subcortical events and likely included many individuals with atherosclerotic large artery or cardioembolic mechanisms.
A major limitation of our study is that it was underpowered to detect genotype effect sizes in black and Hispanic participants because of the small sample size for black participants and lower-than-expected event rates for Hispanic participants. The ORs were not estimable in Hispanic participants because there were no events among IM/PMs. For black participants, even with small sample sizes, we still observed effect sizes similar to those observed in white participants.
In conclusion, we observed significant differences in recurrent stroke but not major bleeding in white participants by CYP2C19 metabolizer status in small subcortical stroke patients taking aspirin plus clopidogrel DAPT. These findings are consistent with prior studies of CYP2C19 and clopidogrel in patients with preexisting cardiovascular disease; however, when patients from all race groups were combined, we did not observe a significant difference in outcomes by metabolizer status. The influence of CYP2C19 metabolizer status on recurrent stroke is likely to have minimal clinical implications because DAPT is not recommended in small subcortical stroke patients based on the outcome of the primary SPS3 trial.
Acknowledgments
We thank the SPS3 site investigators and the SPS3-GENES participants.
Sources of Funding
This project was supported by NIH grants R01 NS073346, U01 GM074492-05S109, and U01 NS038529. Lewis was supported also supported by NIH grant K23 GM102678.
Disclosures
The authors have no disclosures other than funding from the National Institutes of Health.
References
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- Wolfe CD, Rudd AG, Howard R, Coshall C, Stewart J, Lawrence E, Hajat C, Hillen T. Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2002;72:211–216. doi: 10.1136/jnnp.72.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiten J, Lodder J. Prognosis for survival, handicap and recurrence of stroke in lacunar and superficial stroke. Cerebrovasc Dis. 1993;3:221–226. [Google Scholar]
- Del Brutto OH, Mosquera A, Sanchez X, Santos J, Noboa CA. Stroke subtypes among Hispanics living in Guayaquil, Ecuador. Results from the Luis Vernaza Hospital Stroke Registry. Stroke. 1993;24:1833–1836. doi: 10.1161/01.str.24.12.1833. [DOI] [PubMed] [Google Scholar]
- Lavados PM, Sacks C, Prina L, Escobar A, Tossi C, Araya F, Feuerhake W, Galvez M, Salinas R, Alvarez G. Incidence, 30-day case-fatality rate, and prognosis of stroke in Iquique, Chile: a 2-year community-based prospective study (PISCIS project) Lancet. 2005;365:2206–2215. doi: 10.1016/S0140-6736(05)66779-7. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, White LR, Masaki KH, Li CY, Curb JD, Yano K, Rodriguez BL, Foley DJ, Blanchette PL, Havlik R. Characterization of risk factors for vascular dementia: the Honolulu-Asia Aging Study. Neurology. 1999;53:337–343. doi: 10.1212/wnl.53.2.337. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Paik M, Figueroa M, Gropen TI, Stern Y, Sano M, Remien R, Williams JB, Mohr JP, Mayeux R. Clinical determinants of dementia related to stroke. Ann Neurol. 1993;33:568–575. doi: 10.1002/ana.410330603. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- Bamford J, Sandercock P, Jones L, Warlow C. The natural history of lacunar infarction: the Oxfordshire Community Stroke Project. Stroke. 1987;18:545–551. doi: 10.1161/01.str.18.3.545. [DOI] [PubMed] [Google Scholar]
- Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- Samuelsson M, Soderfeldt B, Olsson GB. Functional outcome in patients with lacunar infarction. Stroke. 1996;27:842–846. doi: 10.1161/01.str.27.5.842. [DOI] [PubMed] [Google Scholar]
- Loeb C, Gandolfo C, Bino G. Intellectual impairment and cerebral lesions in multiple cerebral infarcts. A clinical-computed tomography study. Stroke. 1988;19:560–565. doi: 10.1161/01.str.19.5.560. [DOI] [PubMed] [Google Scholar]
- Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, Coffey C, McClure LA, Szychowski JM, Conwit R, Heberling PA, Howard G, Bazan C, Vidal-Pergola G, Talbert R, Hart RG. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke. 2011;6:164–175. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators SPS. Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA American Heart Association Stroke Council CoC, Stroke Nursing CoCC and Council on Peripheral Vascular D. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genomics. 2010;20:463–465. doi: 10.1097/FPC.0b013e3283385420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med. 2003;3:113–122. doi: 10.1055/s-2003-40669. [DOI] [PubMed] [Google Scholar]
- Kim KA, Park PW, Hong SJ, Park JY. The effect of CYP2C19 polymorphism on the pharmacokinetics and pharmacodynamics of clopidogrel: a possible mechanism for clopidogrel resistance. Clin Pharmacol Ther. 2008;84:236–242. doi: 10.1038/clpt.2008.20. [DOI] [PubMed] [Google Scholar]
- Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, Steg PG, Ferrieres J, Danchin N, Becquemont L French Registry of Acute STE and Non STEMII. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, Stratz C, Schmiebusch P, Bestehorn HP, Buttner HJ, Neumann FJ. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51:1925–1934. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- Liu YP, Hao PP, Zhang MX, Zhang C, Gao F, Zhang Y, Chen YG. Association of genetic variants in CYP2C19 and adverse clinical outcomes after treatment with clopidogrel: an updated meta-analysis. Thromb Res. 2011;128:593–594. doi: 10.1016/j.thromres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Tang HL, Hu YF, Xie HG. The gain-of-function variant allele CYP2C19*17: a double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J Thromb Haemost. 2012;10:199–206. doi: 10.1111/j.1538-7836.2011.04570.x. [DOI] [PubMed] [Google Scholar]
- Zabalza M, Subirana I, Sala J, Lluis-Ganella C, Lucas G, Tomas M, Masia R, Marrugat J, Brugada R, Elosua R. Meta-analyses of the association between cytochrome CYP2C19 loss- and gain-of-function polymorphisms and cardiovascular outcomes in patients with coronary artery disease treated with clopidogrel. Heart. 2012;98:100–108. doi: 10.1136/hrt.2011.227652. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Elsey AR, Clare-Salzler MJ, Nessl D, Conlon M, Nelson DR. Institutional profile: University of Florida and Shands Hospital Personalized Medicine Program: clinical implementation of pharmacogenetics. Pharmacogenomics. 2013;14:723–726. doi: 10.2217/pgs.13.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulley JM, Denny JC, Peterson JF, Bernard GR, Vnencak-Jones CL, Ramirez AH, Delaney JT, Bowton E, Brothers K, Johnson K, Crawford DC, Schildcrout J, Masys DR, Dilks HH, Wilke RA, Clayton EW, Shultz E, Laposata M, McPherson J, Jirjis JN, Roden DM. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin Pharmacol Ther. 2012;92:87–95. doi: 10.1038/clpt.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuldiner AR, Palmer K, Pakyz RE, Alestock TD, Maloney KA, O’Neill C, Bhatty S, Schub J, Overby CL, Horenstein RB, Pollin TI, Kelemen MD, Beitelshees AL, Robinson SW, Blitzer MG, McArdle PF, Brown L, Jeng LJ, Zhao RY, Ambulos N, Vesely MR. Implementation of pharmacogenetics: the University of Maryland Personalized Anti-platelet Pharmacogenetics Program. Am J Med Genet C Semin Med Genet. 2014;166C:76–84. doi: 10.1002/ajmg.c.31396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomes Project C. Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, Roden DM, Klein TE, Shuldiner AR Clinical Pharmacogenetics Implementation C. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90:328–332. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, Klein TE, Sabatine MS, Johnson JA, Shuldiner AR Clinical Pharmacogenetics Implementation C. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM Group SS. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulot JS, Collet JP, Silvain J, Pena A, Bellemain-Appaix A, Barthelemy O, Cayla G, Beygui F, Montalescot G. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J Am Coll Cardiol. 2010;56:134–143. doi: 10.1016/j.jacc.2009.12.071. [DOI] [PubMed] [Google Scholar]
- Sofi F, Giusti B, Marcucci R, Gori AM, Abbate R, Gensini GF. Cytochrome P450 2C19*2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J. 2011;11:199–206. doi: 10.1038/tpj.2010.21. [DOI] [PubMed] [Google Scholar]
- Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Li Y, Li J, Zhang Z, Zhu W, Liu W, Cai Q, Wang X, Cao L, Bai W, Fan X, Ma M, Guo R, Liu X, Xu G. Variant recurrent risk among stroke patients with different CYP2C19 phenotypes and treated with clopidogrel. Platelets. 2014 doi: 10.3109/09537104.2014.953044. doi: 10.3109/09537104.2014.953044 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Spokoyny I, Barazangi N, Jaramillo V, Rose J, Chen C, Wong C, Tong D. Reduced clopidogrel metabolism in a multiethnic population: prevalence and rates of recurrent cerebrovascular events. J Stroke Cerebrovasc Dis. 2014;23:694–698. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Jia DM, Chen ZB, Zhang MJ, Yang WJ, Jin JL, Xia YQ, Zhang CL, Shao Y, Chen C, Xu Y. CYP2C19 polymorphisms and antiplatelet effects of clopidogrel in acute ischemic stroke in China. Stroke. 2013;44:1717–1719. doi: 10.1161/STROKEAHA.113.000823. [DOI] [PubMed] [Google Scholar]


