Abstract
Background
Spironolactone, the only aldosterone antagonist available in China, improves outcomes in acute myocardial infarction (AMI) among patients with systolic dysfunction and either diabetes or heart failure (HF). However, national practice patterns in the use of spironolactone in China are unknown.
Methods and Results
From a nationally representative sample of AMI patients from in 2001, 2006, and 2011, we identified 6906 patients with either diabetes or HF and classified them into 1 of 4 groups according to their eligibility for spironolactone—“ideal”(left ventricular ejection fraction [LVEF] ≤40% and without contraindications), “contraindicated,” “not indicated” (neither ideal nor contraindicated), and “unknown indications” (LVEF unmeasured)—to determine how frequently patient eligibility for this drug is assessed in the hospital, how it is used in several groups, and to identify factors associated with the use in these groups. From 2001 to 2011, the proportion of patients whose eligibility for spironolactone was not assessed decreased (66.9% in 2001 to 32.8% in 2011). Spironolactone use significantly increased among ideal patients over this period (28.6% to 72.4%; P<0.001 for trend), but also in contraindicated patients (11.4% to 27.5%; P=0.002 for trend) and in other patients groups (not indicated: 27.5% to 38.3%; unknown indications: 21.3% to 35.1%; both P<0.01 for trend). In all 4 groups, patients presenting with HF on admission were more likely to receive spironolactone.
Conclusions
Although the appropriate use of spironolactone and assessment of eligibility increased in China over the past decade, there remains marked opportunities for improvement.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov Unique identifier: NCT01624883.
Keywords: acute myocardial infarction, quality of care, spironolactone
Aldosterone antagonists have the potential to improve outcomes in selected patients with acute myocardial infarction (AMI) and are recommended by many guidelines.1–3 Specifically, aldosterone antagonists have a proven benefit in patients with AMI and left ventricular ejection fraction (LVEF) ≤40%, with either heart failure (HF) or diabetes. However, their use requires careful patient selection, given that the benefit beyond this group is not established, and use in patients with contraindications, such as renal insufficiency or hyperkalemia, may even expose patients to significant risk.4,5
The application of cost-effective and evidence-based therapies is crucial for countries with limited resources, such as China. Furthermore, a core competency of any health system is the ability to respond to new evidence. We know little about the adoption of evidence-based therapies in the management of patients with AMI in China. The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) has proved the benefit of aldosterone antagonists in selected patients with AMI.6 Spironolactone, the only aldosterone antagonist available in China,7 is particularly worthy of assessment given its proven mortality benefit as well as low price (0.5 renminbi, or $0.08 per day). In addition, analysis of approaches used to determine patient eligibility for spironolactone may provide new insights into how patients with AMI in China are managed.
The China Patient-centered Evaluation Assessment of Cardiac Events Retrospective Study of Acute Myocardial Infarction (China PEACE-Retrospective AMI study) identified nationally representative samples of patients with AMI in 2001, 2006, and 2011. This study is an ideal tool to support detailed assessments of the quality of care provided in Chinese hospitals post-AMI, such as an analysis of spironolactone use. Accordingly, we conducted a comprehensive quality assessment of spironolactone therapy among patients with AMI, using data collected in this study. Specifically, our objectives were to characterize how eligibility for spironolactone is assessed, measure patterns and trends of spironolactone use—both in ideal candidates and in patients who are not ideal—and identify patient factors associated with the use of this drug in these groups.
Methods
Study Design of China PEACE-Retrospective AMI Study
The design of the China PEACE-Retrospective AMI Study has been published previously.8 In brief, we developed a nationally representative sample of hospitalizations for AMI in 2001, 2006, and 2011 using a 2-stage random sampling design. In the first stage, we identified hospitals using a simple random sampling procedure within 5 geographical-economic strata of China: Eastern-rural; Central-rural; Western-rural; Eastern-urban; and Central/Western-urban regions. We used these strata because hospital volumes and clinical capacities differ between urban and rural areas as well among the 3 official geographical regions (Eastern, Central, and Western) of Mainland China. We combined Central and Western urban regions given their similar per capita income and health services capacity. In the 3 rural strata, the sampling framework consisted of the central hospital in each of the predefined rural regions (2010 central hospitals in 2010 rural regions). In the 2 urban strata, the sampling framework consisted of the highest-level hospitals in each of the predefined urban regions (833 hospitals in 287 urban regions). We randomly selected representative hospitals from 2011 to assess current practices and traced this cohort backward to 2006 and 2001 to describe temporal trends. In the second stage, using systematic random sampling procedures, we drew cases from each sampled hospital using the local hospital database for patients with AMI in 2001, 2006, and 2011. AMI cases were identified using a principal discharge diagnosis of AMI based on International Classification of Diseases versions 9 or 10, given that hospitals in China are mandated by the Ministry of Health to list this information on the first page of the medical record, and in rare cases when such information was not available, we confirmed the diagnosis through medical record review.
Data were collected by centralized medical chart abstraction using standardized data definitions. We applied rigorous monitoring at each stage to ensure data quality. Data abstraction quality was monitored by randomly auditing 5% of the medical charts, with overall accuracy exceeding 98%.
The central ethics committee at the China National Center for Cardiovascular Diseases, or local internal ethics committees approved the China PEACE-Retrospective AMI Study. The study is registered at www.clinicaltrials.gov (NCT01624883). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Study Samples
We limited study samples to patients potentially eligible for spironolactone, namely, those with known HF or diabetes at discharge. Patients who had a length of hospital stay shorter than 24 hours were excluded to ensure that all patients had sufficient opportunity to receive spironolactone. Subsequently, we classified patients into 1 of 4 groups: the “ideal” group consisted of patients with a documented LVEF ≤40% and no contraindication to spironolactone; the “contraindicated” group consisted of patients with a contraindication (serum potassium >5 mmol/L, or serum creatinine >2.5 mg/dL [men] or >2.0 mg/dL [women], or documented allergy to spironolactone); the “not indicated” group consisted of patients with neither indication (ie, LVEF >40%) nor contraindication to spironolactone; and the “unknown indications” group consisted of patients whose LVEF was not measured during the hospitalization.
Variables
Data elements collected included demographic information, medical history, patient characteristics at presentation, hospital characteristics, laboratory parameters, concomitant therapy, and documented diagnosis. Cardiovascular risk (CVR) factors were coded as present if diagnosed before or during admission; clinical characteristics, including vital signs, represented those recorded on admission.
To capture the laboratory values likely to influence the decision about spironolactone therapy in patients who received the drug, we used the last potassium and creatinine values before administration of medication. For patients who ultimately did not receive spironolactone, we used the highest lab value recorded during hospitalization to ensure identification of any possible contraindication. LVEF values were based on echocardiography, radionuclide angiography, or computerized tomography coronary angiography.
Statistical Analysis
To examine temporal trends in spironolactone therapy, we used the Cochran-Armitage tests and applied weights proportional to the inverse sampling fraction of hospitals to account for differences in the sampling fraction for each time period. When exploring patient and hospital characteristics associated with the use of spironolactone, categorical variables were expressed as frequencies and percentages and analyzed using chi-square tests. Among all the variables, missing data were rare and occurred only for the age variable (0.1%), which was imputed to the overall median to avoid case-wise deletion.
We used logistic regression models to identify predictors independently associated with spironolactone use in different patient groups by indications. Variables in models include demographic characteristics, CVR factors, medical history, conditions and vital signs on admission, estimated glomerular filtration rate (eGFR), AMI type, economic-geographical region, rural/urban region, and years. In addition, we also included hospital characteristics, such as teaching status and percutaneous coronary intervention (PCI) capability (Table 1). A generalized estimating equation model was developed to account for clustering of patients within hospitals. All variables in the bivariate model were included in the multivariable model except those with frequencies under 1%. Odds ratios (ORs) and 95% confidential intervals (CIs) were reported.
Table 1.
Bivariate Analysis of Characteristics Associated With Spironolactone Therapy Among Ideal Patients
| Characteristics | No. of Patients With Characteristic | % of Receiving Spironolactone | P Value |
|---|---|---|---|
| All ideal patients | 637 | 66.4 | |
| Demographics | |||
| Age, y | |||
| <65 | 216 | 55.1 | <0.0001 |
| ≥65 | 421 | 72.2 | |
| Gender | |||
| Male | 431 | 64.5 | 0.141 |
| Female | 206 | 70.4 | |
| Cardiovascular risk factors | |||
| Hypertension | |||
| No | 265 | 61.1 | 0.017 |
| Yes | 372 | 70.2 | |
| Diabetes | |||
| No | 388 | 67.5 | 0.455 |
| Yes | 249 | 64.7 | |
| Current smoking | |||
| No | 438 | 69.2 | 0.028 |
| Yes | 199 | 60.3 | |
| Medical history | |||
| Myocardial infarction | |||
| No | 510 | 66.1 | 0.727 |
| Yes | 127 | 67.7 | |
| Clinical characteristics at admission | |||
| Cardiogenic shock | |||
| No | 600 | 66.5 | 0.838 |
| Yes | 37 | 64.9 | |
| Heart failure | |||
| No | 201 | 52.2 | <0.0001 |
| Yes | 436 | 72.9 | |
| AMI type | |||
| STEMI | 530 | 64.5 | 0.026 |
| NSTEMI/uncertain | 107 | 75.7 | |
| SBP, mm Hg | |||
| <90 | 20 | 75 | 0.464 |
| 90 to 139 | 410 | 64.9 | |
| ≥140 | 207 | 68.6 | |
| Heart rate, beats/min | |||
| <60 | 38 | 60.5 | 0.003 |
| 60 to 90 | 327 | 60.9 | |
| >90 | 272 | 73.9 | |
| eGFR, mL/min per 1.73 m2 | |||
| <60 | 169 | 63.3 | <0.0001 |
| 60 to 89 | 207 | 63.8 | |
| ≥90 | 131 | 56.5 | |
| Unmeasured | 130 | 84.6 | |
| Treatment | |||
| ACE inhibitor/ARB use | |||
| No | 161 | 59.0 | 0.021 |
| Yes | 476 | 68.9 | |
| Beta-blocker use | |||
| No | 179 | 71.0 | 0.129 |
| Yes | 458 | 64.6 | |
| ACE inhibitor/ARB+beta-blocker | |||
| No | 270 | 67.0 | 0.772 |
| Yes | 367 | 65.9 | |
| Hospital level | |||
| Teaching hospital | |||
| No | 86 | 58.1 | 0.081 |
| Yes | 551 | 67.7 | |
| PCI-capable hospital | |||
| No | 153 | 51.6 | <0.0001 |
| Yes | 484 | 71.1 | |
| Economic-geographical region | |||
| Eastern | 391 | 67.3 | 0.532 |
| Central | 136 | 67.6 | |
| Western | 110 | 61.8 | |
| Urban/rural | |||
| Urban | 180 | 61.7 | 0.112 |
| Rural | 457 | 68.3 | |
| Year | |||
| 2001 | 38 | 31.6 | <0.0001 |
| 2006 | 174 | 66.7 | |
| 2011 | 425 | 69.4 | |
ACE inhibitor indicates angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction.
All comparisons were 2-tailed, with P<0.05 considered statistically significant. All statistical analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC) and R software (version 3.0.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Cohort
The nationally representative samples described in the China PEACE-Retrospective AMI study consisted of 16 100 patients hospitalized for AMI in 162 hospitals across China (Figure 1A), with the 2011 sample representing 245 720 patients across China. After excluding patients with a length of stay shorter than 24 hours, and those without HF or diabetes when discharged, we identified 6906 patients (12.2% in 2001, 27.7% in 2006, and 60.1% in 2011) who were potentially eligible for spironolactone (Figure 1B). Across all years, median age was 69 years (interquartile range, 59 to 76) and 35.8% were female. Among these patients, 44.2% had diabetes, and almost three quarters of patients (73.9%) had HF - among them the rate of loop diuretic use rose slightly during the study period. CVR factors were common: 57.9% had hypertension (HTN), 29.8% were current smokers, and 28.0% had coronary artery disease.
Figure 1.
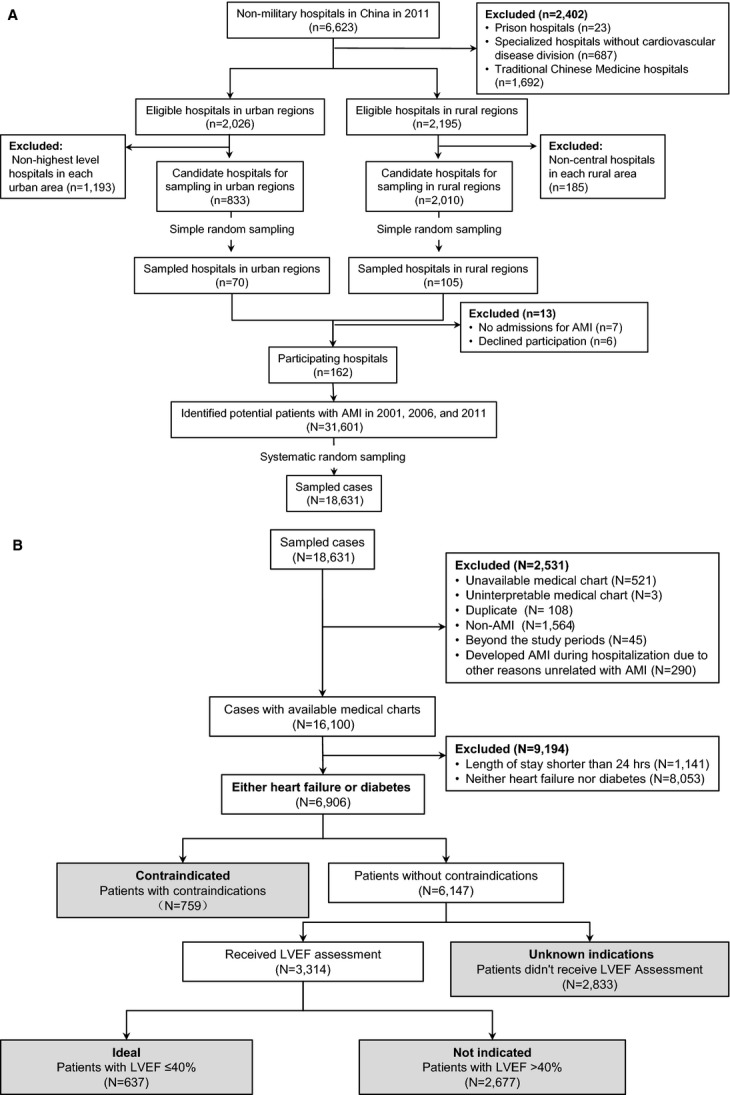
A, Flow diagram showing the process used to produce a nationally representative sampling of hospitals in China. “N” represents number of patients; “n” represents number of hospitals. B, Flow diagram showing the approach to classify patients into 4 groups according to their indications for spironolactone. “N” represents number of patients. AMI indicates acute myocardial infarction; LVEF, left ventricular ejection fraction.
There were notable changes in the relative proportion of the 4 patient groups over time (Figure 2). For example, the proportion of “ideal” patients doubled from 2001 to 2006 (4.5% to 9.1%) and remained stable thereafter (10.2% in 2011). In contrast, the proportion of “contraindicated” patients varied little across the 3 years (13.0%, 11.9%, and 10.2% in 2001, 2006, and 2011, respectively). The proportion of “not indicated” patients increased markedly over the years (from 15.6% in 2001 to 46.8% in 2011; P<0.001 for trend), whereas that of “unknown indications” patients showed a reciprocal decrease.
Figure 2.
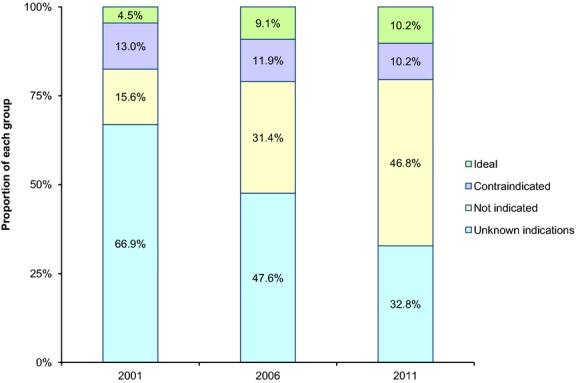
Acute myocardial infarction patients with heart failure or diabetes grouped by their eligibility for spironolactone in 2001, 2006, and 2011. Ideal: patients with a left ventricular ejection fraction (LVEF) ≤40% and without contraindications to spironolactone; contraindicated: patients with a contraindication (serum potassium >5 mmol/L, or serum creatinine >2.5 mg/dL [men] or >2.0 mg/dL [women], or documented allergy to spironolactone); not indicated: patients with neither indication (ie, LVEF >40%) nor contraindication to spironolactone; unknown indications: patients whose LVEF was not measured during the hospitalization.
Use of Spironolactone Therapy Among Different Groups
Overall, the weighted rate of spironolactone use in 2011 differed among patients in each group: 72.4% in “ideal”; 27.5% in “contraindicated”; 38.3% in “not indicated”; and 35.1% in “unknown indications.” Spironolactone use increased in all groups over the past decade: among “ideal” patients, the weighted rate of use increased from 28.6% in 2001 to 68.5% in 2006 and to 72.4% in 2011 (P<0.001 for trend), whereas for “contraindicated” patients it increased from 11.4% in 2001 to 22.4% in 2006 and to 27.5% in 2011 (P=0.002 for trend). Similar increases were observed among “not indicated” patients (P=0.007 for trend) and “unknown indications” patients (P<0.001 for trend; Figure 3). Given that spironolactone can also be used to treat HTN or as a concomitant therapy in HF with reduced LVEF, we performed a post-hoc analysis describing spironolactone use in a specific subgroup of “not indicated” patients, namely, those with neither HTN nor HF. In this subgroup of patients in 2011, 17.2% received spironolactone.
Figure 3.
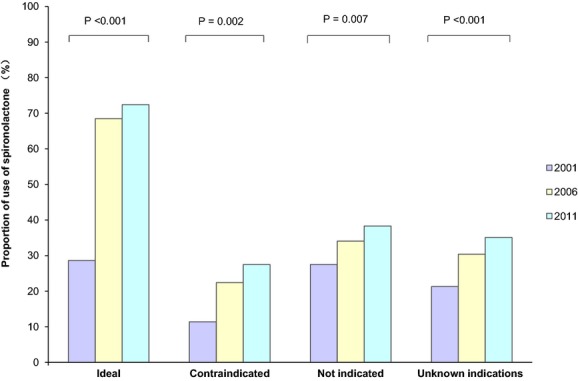
Spironolactone use (weighted) among different groups of acute myocardial infarction patients with heart failure or diabetes according to their eligibility for spironolactone in 2001, 2006, and 2011. Ideal: patients with a left ventricular ejection fraction (LVEF) ≤40% and without contraindications to spironolactone; contraindicated: patients with a contraindication (serum potassium >5 mmol/L, or serum creatinine >2.5 mg/dL [men] or >2.0 mg/dL [women], or documented allergy to spironolactone); not indicated: patients with neither indication (ie, LVEF >40%) nor contraindication to spironolactone; unknown indications: patients whose LVEF was not measured during the hospitalization.
Patient and Hospital Characteristics Associated With Spironolactone Therapy
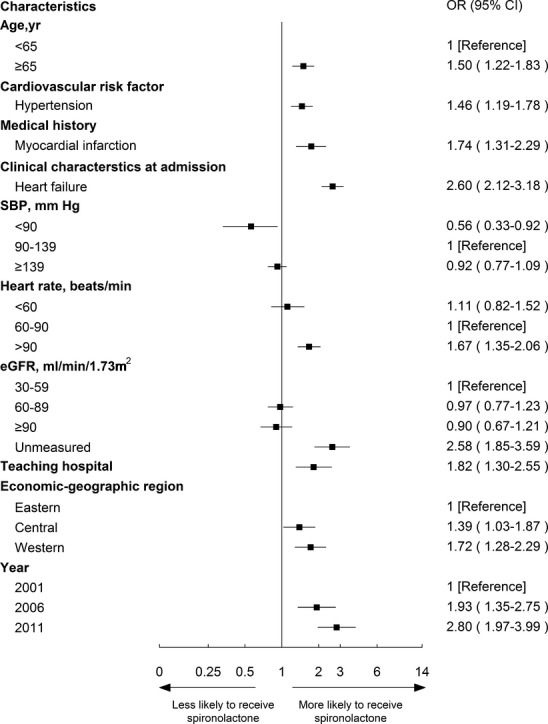
Bivariate analysis of the factors associated with the use of spironolactone among ideal patients is shown in Table 1. All characteristics were entered into the multivariable model to determine independent predictors of use (Figure 4). Specifically, older patients (≥65 year) were more likely to be treated than younger patients (72.2% vs. 55.1%; OR, 2.07; 95% CI, 1.30 to 3.30). Patients with HTN or with symptoms of HF at admission were both more likely to receive spironolactone (70.2% vs. 61.1%; OR, 1.57; 95% CI, 1.11 to 2.23 and 72.9% vs. 52.1%; OR, 2.00; 95% CI, 1.18 to 3.36, respectively) than those without these comorbidities. Patients in PCI-capable hospitals were more likely to be treated (71.1% vs. 51.6%; OR, 2.12; 95% CI, 1.12 to 4.02).
Figure 4.
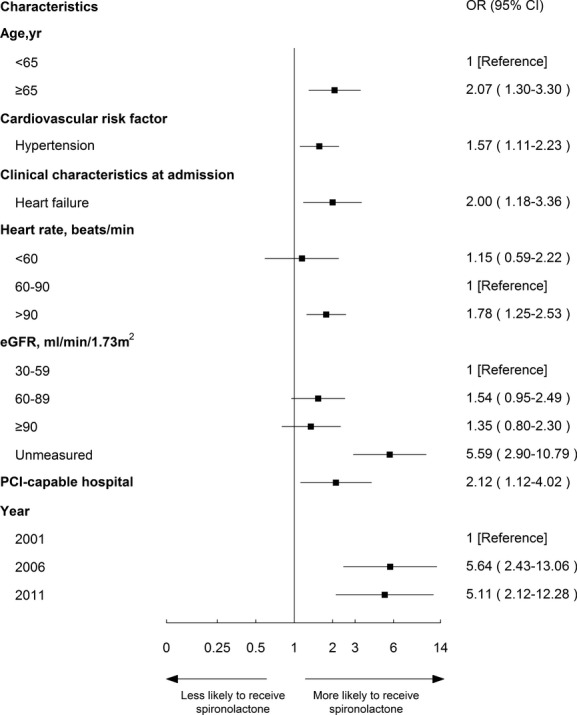
Factors associated with spironolactone therapy among “ideal” patients in the multivariable model. Variables associated with spironolactone therapy among ideal patients are shown along the vertical axis. The adjusted odds ratio of 1 shows no difference to receive spironolactone therapy among ideal patients. Each dot represents the point estimate of the effect of that variable in the model; the line shows the 95% confidence interval (CI). eGFR indicates estimated glomerular filtration rate; OR, odds ratio; PCI, percutaneous coronary intervention.
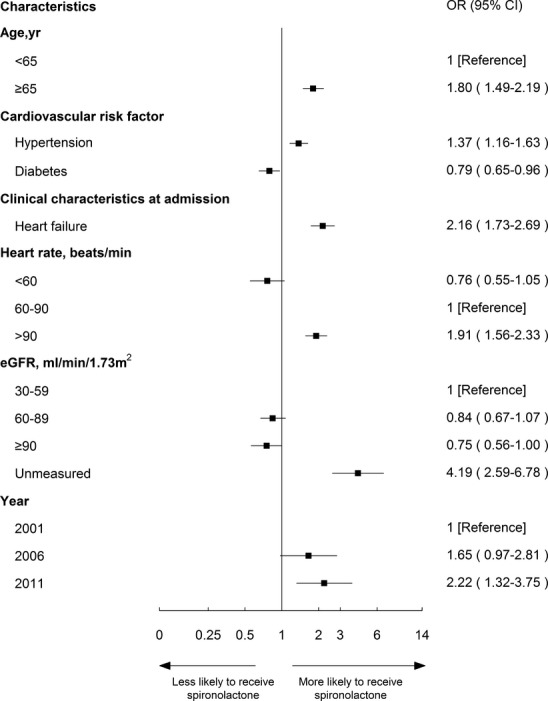
Characteristics in multivariable analysis that remained independently associated with the use of spironolactone in the other 3 groups are shown in Figures5 through 7. Spironolactone use was more common among patients with history of myocardial infarction in both “contraindicated” and “unknown indications” groups (OR, 1.84; 95% CI, 1.15 to 2.94 and OR, 1.74; 95% CI, 1.31 to 2.29, respectively). Patients >65 years old and patients with HTN had a high likelihood of receiving spironolactone among both “not indicated” and “unknown indication” groups. Across all groups, patients with symptoms of HF at admission to the hospital were more likely to be treated with spironolactone (contraindicated: OR, 3.26; 95% CI, 2.05 to 5.16; unknown indications: OR, 2.60; 95% CI, 2.12 to 3.18; not indicated: OR, 2.16; 95% CI, 1.73 to 2.69).
Figure 5.
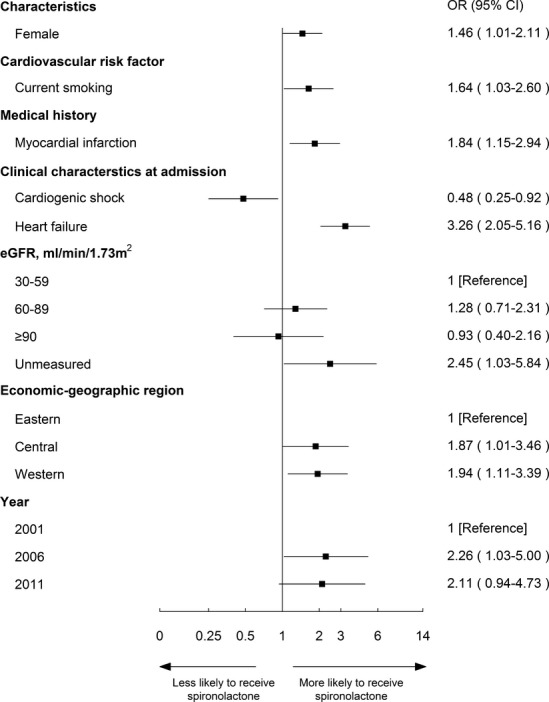
Factors associated with spironolactone therapy among “contraindicated” patients in multivariable model. Variables associated with spironolactone therapy among ideal patients are shown along the vertical axis. The adjusted odds ratio of 1 shows no difference to receive spironolactone therapy among ideal patients. Each dot represents the point estimate of the effect of that variable in the model; the line shows the 95% confidence interval (CI). eGFR indicates estimated glomerular filtration rate; OR, odds ratio.
Figure 7.
Factors associated with spironolactone therapy among “unknown indications” patients in the multivariable model. Variables associated with spironolactone therapy among ideal patients are shown along the vertical axis. The adjusted odds ratio of 1 shows no difference to receive spironolactone therapy among ideal patients. Each dot represents the point estimate of the effect of that variable in the model; the line shows the 95% confidence interval (CI). eGFR indicates estimated glomerular filtration rate; OR, odds ratio; SBP, systolic blood pressure.
Figure 6.
Factors associated with spironolactone therapy among “not indicated” patients in the multivariable model. Variables associated with spironolactone therapy among ideal patients are shown along the vertical axis. The adjusted odds ratio of 1 shows no difference to receive spironolactone therapy among ideal patients. Each dot represents the point estimate of the effect of that variable in the model; the line shows the 95% confidence interval (CI). eGFR indicates estimated glomerular filtration rate; OR, odds ratio.
Discussion
In this national quality assessment analyzing spironolactone use among patients with AMI in China, we found that spironolactone use increased over time. However, suboptimal patient identification and selection were detected throughout the study period and persisted in 2011. Although more patients underwent LVEF assessment, which is necessary to determine their eligibility for spironolactone, one third of patients did not have an LVEF assessment during their hospitalization for AMI in 2011. Spironolactone use among patients who may not benefit and those with contraindications was common and such use increased significantly over time. Our findings indicate that the Chinese health care system rapidly responded to new information that highlighted the utility of spironolactone, but also appears to have driven increased use among patients who lack a strong indication, albeit at a lower rate than in ideal patients. These findings highlight an opportunity for hospitals in China to improve the translation of evidence into clinical practice.
To our knowledge, this is the first comprehensive, nationally representative quality assessment of spironolactone use in AMI in China. Previous studies evaluating the use of aldosterone antagonists among AMI patients in other countries have focused only on patients with definite indications for treatment.9–11 In contrast, our study describes to what extent patients with AMI are evaluated for spironolactone and shows that spironolactone is used not only among patients with indications, but also those with contraindications, without indications, and with indications unknown. Additionally, the use of a nationally representative sample ensures that the findings of this analysis are broadly applicable across China and can serve as the basis for future quality improvement initiatives.
LVEF assessment among patients with AMI improved over the past decade, which created more opportunities to consider spironolactone therapy; however, further improvement is possible. In 2001, indications for two thirds of patients were unknown and only 4.5% of the cohort was classified as “ideal,” whereas in 2011, with wider LVEF assessment, “ideal” patients increased to 10%; however, one third of patients still lacked an LVEF assessment. In comparison, among patients in the United States with AMI from 2007 to 2009, the rate of LVEF assessment was reported to be 91.0%.12 It should be noted that LVEF assessment is a critical component of the care of patients with AMI because it enables risk stratification and guides the prescription of other therapies as well, for instance, inhibitors of angiotensin converting enzyme (ACE).
The increasing use of spironolactone among ideal patients in China over time is encouraging. In 2001, spironolactone use among “ideal” patients was no better than other groups, but it increased sharply thereafter, possibly in response to the EPHESUS study, which was published after 2001 and clearly supported the use of aldosterone antagonists in this patient population.6 There are no previous studies in China with which to compare our results. However, a registry-based study in Spain reported that 54.8% patients with AMI and HF received aldosterone antagonist in hospital between 2006 and 20089; and in the United States, the prescription rate of aldosterone antagonists at discharge among patients with AMI and reduced LVEF was only approximately 15% from 2009 to 2010.10,11 Although the utilization seems to be better in China than in other countries, there remains room for further improvement, given the potential benefit of this agent.
The substantial use of spironolactone among patients with contraindications—nearly 1 in 4 patients—was concerning and indicates a gap in the patient selection process that can expose patients to potential harm, such as worsening hyperkalemia or significant renal dysfunction.4,13 It must be noted that there may be circumstances in which spironolactone use is contraindicated according to the guidelines, yet clinicians perceive that the benefit of the drug will outweigh its risks. For example, some recently published studies demonstrate that the benefit of aldosterone antagonists may offset its risk in the setting of moderate hyperkalemia.14,15 Nonetheless, the rising proportion of “contraindicated” patients being treated with spironolactone is consistent with a study in the United States and suggests that the growth in use may, at times, be indiscriminate.16
Spironolactone was frequently used among patients without clear indications, namely, those with LVEF >40%. It should be noted that the recent Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist study found no benefit of spironolactone use in patients with HF and a preserved LVEF.17 In the subgroup of patients without any identifiable indication, including HTN or systolic HF, 17.2% were treated, suggesting that a substantial number of patients are receiving a drug that has limited benefit for their condition, and with a potential for adverse effects.
In all 4 groups, patients presenting with HF on admission were more likely to receive spironolactone. Such a pattern indicates that the presence of HF was a common and important trigger for spironolactone prescription by clinicians and implies a wide acceptance of the drug in HF. Additionally, the steady rate of loop diuretic use among patients with HF indicates that spironolactone was not being systematically substituted for loop diuretics, and that other factors are driving spironolactone uptake. The selection issues identified in this analysis may impede the transfer of benefit to appropriate patients. Quality improvement initiatives emphasizing the appropriate use of spironolactone are warranted to improve patient selection and avoid adverse events.
Limitations
Our study has some limitations. First, our analysis used data abstracted from medical records. However, to ensure accurate abstraction, we had strict definitions for all variables and employed quality control procedures to insure that the abstraction accuracy reached 98%. Second, we may not have captured all patients with contraindications owing to inadequate physician documentation or unmeasured serum chemistry, which may lead to errors in the estimation of ideal patients and contraindicated patients. Third, the benefit of aldosterone antagonists for AMI patients was demonstrated in the EPHESUS study, which studied eplerenone, rather than spironolactone, which is the only aldosterone antagonist available in China. However, the structural similarity of spironolactone and eplerenone suggests that they may have similar efficacy, and guidelines endorse the use of both drugs for patients with AMI. Last, because data were abstracted from deidentified medical charts, we were unable to determine whether some patients were included in multiple study years, or had multiple admissions during the same year; however, given the 5-year difference between the 3 time points, and the random sampling method, the number of such patients is likely to be minimal.
Conclusion
We identified opportunities to optimize the use of spironolactone post-AMI in Chinese clinical practice, including wider LVEF assessment, more-careful selection of patients, and increasing the utilization among ideal patients. Our findings shed light on existing practice patterns in the treatment of AMI in China, serve as the basis for future quality assessment efforts, and illuminate the barriers to more-appropriate use of evidence-based therapies for all countries seeking opportunity to optimize care.
Sources of Funding
This project was partly supported by the Research Special Fund for Public Welfare Industry of Health (201202025) from the National Health and Family Planning Commission of China. Dr Krumholz is supported by grant U01HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Dr Ross is supported by the National Institute on Aging (K08AG032886) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors appreciate the multiple contributions made by study teams at the China Oxford Center for International Health Research and the Yale-New Haven Hospital Center for Outcomes Research and Evaluation in the realms of study design and operations, particularly the data collection by Yi Pi, Jiamin Liu, Wuhanbilige Hundei, Haibo Zhang, Lihua Zhang, Xue Du, Xin Zheng, and Yuanlin Guo. The authors appreciate the analytical advice of Zhenqiu Lin, Yongfei Wang, and the analytical review check by Meng Su. The authors appreciate the editing by Sisi Wang, Sudhakar Nuti, and Erica Spatz. The authors are grateful for the support provided by the Chinese government.
Disclosures
Dr Krumholz reports being the recipient of research grants from Medtronic and Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing, and the chair of a cardiac scientific advisory board for UnitedHealth. Dr Ross reports that he is a member of a scientific advisory board for FAIR Health, Inc. Dr Masoudi receives salary support from the American College of Cardiology for his role as the Senior Medical Officer of the National Cardiovascular Data Registries. The authors declare no other relevant conflicts of interest.
Supporting Information
Appendix S1. Members of the China PEACE Collaborative Group-Retrospective AMI Study Site Investigators and China PEACE Study Consultants.
References
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1999 Guidelines for the management of patients with Acute Myocardial Infarction) Circulation. 2004;110:e82–e292. [PubMed] [Google Scholar]
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology. The Chinese guideline for the diagnose and treatment of patients with ST-segment elevation myocardial infarction. Chin J Cardiol. 2010;38:675–690. [Google Scholar]
- Lachaine J, Beauchemin C, Ramos E. Use, tolerability and compliance of spironolactone in the treatment of heart failure. BMC Clin Pharmacol. 2011;11:4. doi: 10.1186/1472-6904-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:211–214. doi: 10.1016/s0735-1097(02)02694-3. [DOI] [PubMed] [Google Scholar]
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- CFDA. 2013. National essential drugs list of China: edition 2012 (in Chinese)
- Dharmarajan K, Li J, Li X, Lin Z, Krumholz HM, Jiang L. The China Patient-Centered Evaluative Assessment of Cardiac Events (China PEACE) retrospective study of acute myocardial infarction: study design. Circ Cardiovasc Qual Outcomes. 2013;6:732–740. doi: 10.1161/CIRCOUTCOMES.113.000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-de-Sa E, Martinez A, Anguita M, Dobarro D, Jimenez-Navarro M. Aldosterone receptor antagonist use after myocardial infarction. Data from the REICIAM registry. Rev Esp Cardiol. 2011;64:981–987. doi: 10.1016/j.recesp.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Rao KK, Enriquez JR, de Lemos JA, Alexander KP, Chen AY, McGuire DK, Fonarow GC, Das SR. Use of aldosterone antagonists at discharge after myocardial infarction: results from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get with the Guidelines (GWTG) Am Heart J. 2013;166:709–715. doi: 10.1016/j.ahj.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Rassi AN, Cavender MA, Fonarow GC, Cannon CP, Hernandez AF, Peterson ED, Peacock WF, Laskey WK, Rosas SE, Zhao X, Schwamm LH, Bhatt DL. Temporal trends and predictors in the use of aldosterone antagonists post-acute myocardial infarction. J Am Coll Cardiol. 2013;61:35–40. doi: 10.1016/j.jacc.2012.08.1019. [DOI] [PubMed] [Google Scholar]
- Miller AL, Dib C, Li L, Chen AY, Amsterdam E, Funk M, Saucedo JF, Wang TY. Left ventricular ejection fraction assessment among patients with acute myocardial infarction and its association with hospital quality of care and evidence-based therapy use. Circ Cardiovasc Qual Outcomes. 2012;5:662–671. doi: 10.1161/CIRCOUTCOMES.112.965012. [DOI] [PubMed] [Google Scholar]
- Shah KB, Rao K, Sawyer R, Gottlieb SS. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol. 2005;46:845–849. doi: 10.1016/j.jacc.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) Circ Heart Fail. 2014;7:51–58. doi: 10.1161/CIRCHEARTFAILURE.113.000792. [DOI] [PubMed] [Google Scholar]
- Clarke KW, Gray D, Hampton JR. Evidence of inadequate investigation and treatment of patients with heart failure. Br Heart J. 1994;71:584–587. doi: 10.1136/hrt.71.6.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi FA, Spertus JA, Krumholz HM, Jiang L. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet. 2015;385:441–451. doi: 10.1016/S0140-6736(14)60921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Members of the China PEACE Collaborative Group-Retrospective AMI Study Site Investigators and China PEACE Study Consultants.


