Abstract
Background
Asymmetric dimethylarginine (ADMA) inhibits the production of nitric oxide, a key regulator of the vascular tone, and may be important in the development of cardiovascular disease (CVD). Our aim was to reliably quantify the association of ADMA and its isomer symmetric dimethylarginine (SDMA) with the risk of CVD outcomes in long-term cohort studies.
Methods and Results
Data were collated from 22 prospective studies involving a total of 19 842 participants, which have recorded 2339 CVD, 997 coronary heart disease, and 467 stroke outcomes during a mean follow-up of 7.1 years. In a comparison of individuals in the top with those in the bottom third of baseline ADMA values, the combined risk ratios were 1.42 (95% confidence interval: 1.29 to 1.56) for CVD, 1.39 for coronary heart disease (1.19 to 1.62), and 1.60 for stroke (1.33 to 1.91). Broadly similar results were observed according to participants’ baseline disease status (risk ratios for CVD: 1.35 [1.18 to 1.54] in general populations; 1.47 [1.16 to 1.87] in individuals with pre-existing CVD; and 1.52 [1.26 to 1.84] in individuals with pre-existing kidney disease) and by different study characteristics, including geographical location, sample type, assay method, number of incident outcomes, and level of statistical adjustment (all P values>0.05). In contrast, in 8 prospective studies involving 9070 participants and 848 outcomes, the corresponding estimate for SDMA concentration was 1.32 (0.92 to 1.90) for CVD.
Conclusions
Available prospective studies suggest associations between circulating ADMA concentration and CVD outcomes under a broad range of circumstances. Further research is needed to better clarify these associations, particularly in large general population studies.
Keywords: asymmetric dimethylarginine, cardiovascular diseases, meta-analysis, prospective studies
Asymmetric dimethylarginine (ADMA) is a naturally occurring modified amino acid in human blood. It inhibits the production of nitric oxide, a key regulator of the vascular tone, and may thereby contribute importantly to the process of atherosclerosis.1–3 ADMA has been shown to correlate with various measures of subclinical atherosclerosis, including carotid intima-media thickness4 and flow-mediated dilatation.5–8 Additionally, a growing number of studies suggest that high values of circulating ADMA concentration are associated with the incidence of cardiovascular disease (CVD) outcomes. However, interpretation of these studies has been complicated because they differ in relation to the population studied (eg, approximately general population versus patients with pre-existing CVD or kidney disease), the disease outcomes assessed (eg, “hard” CVD composed of coronary heart disease and stroke versus wider definitions), and/or the analytical approaches used (eg, different adjustment for potential confounders).9 Furthermore, as previously published reports typically comprised only a few hundred incident CVD outcomes, they were insufficiently powered to investigate associations by clinically relevant characteristics. Finally, the extent to which associations of ADMA are consistent with those of its related isomer symmetric dimethylarginine (SDMA) has never been quantitatively reviewed.
To help clarify the evidence, we have conducted a systematic review and meta-analysis of available data on ADMA and SDMA in relation to CVD outcomes. We had 3 principal aims. First, to quantify associations of circulating ADMA concentration with incident CVD, coronary heart disease (CHD), and stroke in a consistent manner. Second, to evaluate these associations under a wide range of circumstances. Third, to compare associations for ADMA with those for SDMA.
Methods
Literature Search and Study Selection
We sought prospective studies that had been published between January 1970 and January 2015 and reported on associations of dimethylarginines with incident CVD (defined as CHD or stroke). Systematic searches of PubMed, Web of Science, and EMBASE were supplemented by scanning reference lists of articles identified (including relevant reviews) and by correspondence with several study investigators. The search strategy is detailed in Table1. Studies were eligible for inclusion if they had recorded events over at least 1 year of follow-up and involved any of the following types of study populations: approximately general population (ie, participants not selected on the basis of preexisting disease at baseline), populations with pre-existing cardiovascular diseases (eg, people with CHD or stroke or peripheral artery disease), or populations with pre-existing kidney disease (eg, people with chronic kidney disease or a kidney transplant). We only included studies that conformed to our pre-specified CVD outcome definition, and excluded studies that used broader outcome definitions (involving incident heart failure, cardiac arrhythmia, peripheral arterial disease, venous thrombosis, pulmonary embolism, or all-cause mortality),10–22 had a follow-up of less than 1 year,23 or both.24–26 The meta-analysis was conducted following the PRISMA guidelines.
Table 1.
Search Terms Used for the Systematic Literature Search
| Database | Search Terms |
|---|---|
| PubMed | (“ADMA” [All Fields] OR “N, N-dimethylarginine” [Supplementary Concept] OR “N, N-dimethylarginine” [All Fields] OR “asymmetric dimethylarginine” [All Fields] OR “SDMA” [All Fields]) AND (“Cardiovascular Diseases” [Mesh] OR “Coronary Artery Disease” [MeSH] OR “Atherosclerosis” [MeSH] OR “Coronary Disease” [MeSH] OR “Myocardial Infarction” [MeSh] OR “Myocardial Ischemia” [MeSH] OR “Stroke” [MeSH] OR “Cerebrovascular” [All fields]) NOT (“Animals”[MeSH] NOT “Humans”[MeSH]) |
| Web of Science (SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH) | TS=(“ADMA” OR “N, N-dimethylarginine” OR “asymmetric dimethylarginine” OR “SDMA”) AND TS=(“Cardiovascular Diseases” OR “Coronary Artery Disease” OR “Atherosclerosis” OR “Coronary Disease” OR “Myocardial Infarction” OR “Myocardial Ischemia” OR “Stroke” OR “Cerebrovascular”) |
| EMBASE | (“ADMA” OR “N, N-dimethylarginine” OR “asymmetric dimethylarginine” OR “SDMA”).af AND (“Cardiovascular Diseases” OR “Coronary Artery Disease” OR “Atherosclerosis” OR “Coronary Disease” OR “Myocardial Infarction” OR “Myocardial Ischemia” OR “Stroke” OR “Cerebrovascular”).af |
The search was conducted on January 14, 2015. No language restrictions were applied.
Data Extraction
Descriptive and quantitative data were extracted by consensus among 2 independent reviewers using standardized data extraction protocols. If multiple publications on the same study were available, the most up-to-date or comprehensive information was used. Retrieved study characteristics included study design, geographical location, population source (ie, population registers, general practice registers, or hospital-based registers), baseline disease status, study size, average age at baseline, and proportion of male participants. Additionally, information on the measurement of dimethylarginines was obtained, including sample type (ie, plasma/serum), storage temperature, and assay details (ie, assay method and manufacturer/source). Finally, data in relation to follow-up were extracted, including duration of follow-up, the specific composition of reported endpoints, number of incident outcomes, effect sizes, and degree of statistical adjustment of reported associations. The degree of adjustment was classified as “o” when risk ratio (RRs) estimates were unadjusted; “+” when RRs were adjusted for age and sex; “++” when further adjusted for at least 2 conventional CVD risk factors (ie, smoking, diabetes, blood pressure, or circulating lipid levels); and “+++” when additionally adjusted for other factors.
Statistical Analysis
Analyses involved only within-study comparisons (ie, cases and controls were directly compared only within each cohort) to limit potential biases. To enable a consistent approach to analysis, RRs and 95% confidence intervals (CIs) in each study were standardized to a common scale, ie, to reflect a comparison of the top third with the bottom third of the population’s baseline distribution of circulating ADMA or SDMA concentrations, employing statistical methods described elsewhere.27 These comparisons correspond approximately to a difference of 0.67 μmol/L in ADMA and 0.53 μmol/L in SDMA concentrations, respectively. Summary RRs were calculated by pooling study-specific estimates by random-effects meta-analysis, with hazard ratios and odds ratios assumed to approximate the same measure of relative risk. One study provided supplementary unpublished tabular data on 10 years of follow-up (as opposed to 5 years in the original published report).28 When studies reported RRs of various levels of adjustment, the most adjusted estimate was used. Consistency of findings across studies was assessed with standard χ2 tests and the I2 statistic.29 Subgroup analyses were conducted using meta-regression across pre-specified study-level characteristics.30 Evidence of publication bias was assessed using funnel plots and Egger’s asymmetry test.31 Duval and Tweedie’s nonparametric “trim and fill” method was applied to take into account the effect of publication bias on pooled RRs.32 All analyses were performed using Stata release 12.1 (StataCorp, College Station, TX). All statistical tests were 2-sided and used a significance level of P<0.05.
Results
General Characteristics of the Included Studies
We screened 3180 records (Figure 1) and identified 22 eligible prospective studies28,33–53 reporting on a total of 19 842 participants (Tables2 and 3). During a mean follow-up duration of 7.1 years, a total of 2339 CVD, 997 CHD, and 467 stroke outcomes were recorded. Sixteen studies were based in Europe, 4 in North America, and 2 in Asia. Participants were typically sourced from population registers (7111 participants), general practitioner registers (2447 participants), or from hospitals (10 284 participants). Eight studies were based on participants from general populations (8298 participants); 9 studies involved people with pre-existing CVD (8043 participants); and 5 studies involved people with pre-existing kidney disease (3501 participants). All but 3 studies reported effect estimates adjusted for age, sex, and at least 2 other conventional CVD risk factors (ie, smoking, diabetes, blood pressure, or circulating lipid levels). Fourteen studies reported effect estimates further adjusted for additional characteristics, such as body mass index, C-reactive protein, social status, physical activity, or estimated glomerular filtration rate. On average, participants were 58 years old at baseline; 58% were male. The pooled mean and standard deviation was 0.71±0.31 μmol/L for circulating ADMA and 0.56±0.24 μmol/L for SDMA concentrations.
Figure 1.
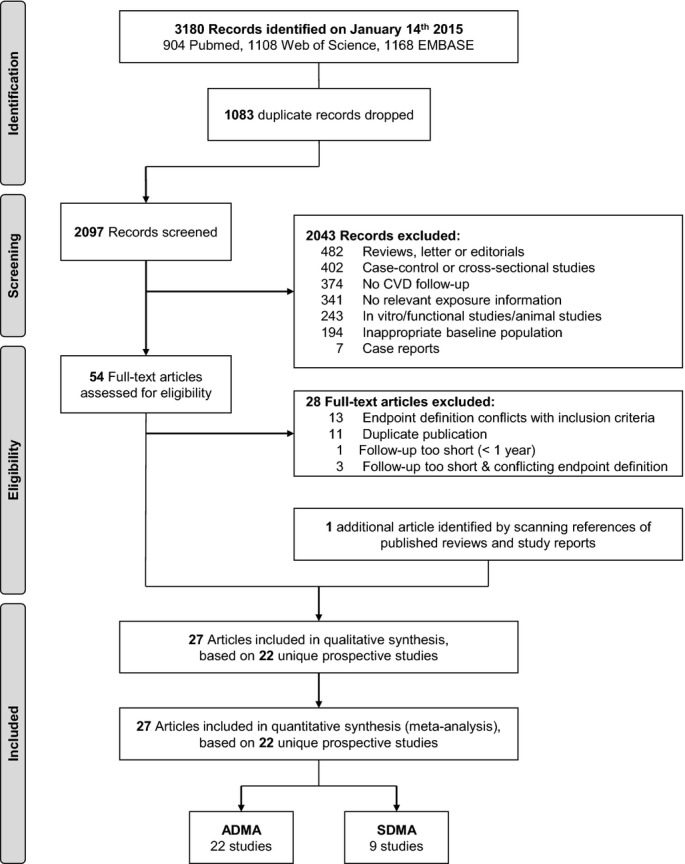
Study flow diagram. ADMA indicates asymmetric dimethylarginine; CVD, cardiovascular disease; SDMA, symmetric dimethylarginine.
Table 2.
Study Design and Assay Methods of the 22 Prospective Studies
| Study Acronym | Study Design | Measurement of Dimethylarginines | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Study Baseline | Population Source | Baseline Disease | No. of Participants | Mean Age, y | Male, % | Sample Type | Storage Temperature, °C | Assay Method | Manufacturers or Assay Source | Mean ADMA, μmol/L | Mean SDMA, μmol/L | |
| General population | |||||||||||||
| BRUNECK28 | Italy | 2000–2010 | Population register | — | 685 | 66 | 48 | Plasma | −70° | LC-MS/MS | ABSciex API 4000 | 0.97 | 0.65 |
| DHS33 | US | 2000–2002 | Population register | — | 3523 | 43 | 44 | Plasma | −70° | LC-MS/MS | Varian 1200L | N/R | N/R |
| GetABI (no PAD)34 | Germany | 2001–2006 | GP register | — | 1187 | 72 | 42 | Plasma | N/R | LC-MS/MS | Varian 1200L | 0.60 | 0.48 |
| INCHIANTI35 | Italy | 1998–2000 | Population register | — | 1025 | 75 | 44 | Serum | −80° | LC-MS/MS | Varian 1200L | 0.5 | — |
| KIHD36 | Finland | 1991–1993 | Population register | — | 150 | 58 | 100 | Serum | −80° | HPLC | N/R | 0.51 | — |
| KVINNOSTUDIEN37 | Sweden | 1968–1969 | Population register | — | 880 | 46 | 0 | Serum | −20° | HPLC | In house | 0.62 | — |
| MDC38 | Sweden | 1991–1996 | Population register | — | 506 | 58 | 40 | Plasma | N/R | LC-MS/MS | N/R | N/R | — |
| MONICA/KORA39 | Germany | 1989–1995 | Population register | — | 342 | 61 | 100 | Plasma | −70° | ELISA | DLD Diagnostica | 0.79 | — |
| Populations with pre-existing CVD | |||||||||||||
| AtheroGene40 | Germany | 1999–2004 | Hospital | Confirmed CAD | 1874 | 61 | 79 | Serum | −80° | ELISA | DLD Diagnostica | 0.68 | — |
| BECAC41 | Norway | 2000–2004 | Hospital | Suspected CAD | 1364 | 61 | 75 | Plasma | −80° | LC-MS/MS | Bevital AS | 0.59* | — |
| Cavalca et al42 | Italy | 2005–2007 | Hospital | NSTEMI | 104 | 67 | 74 | Plasma | −80° | HPLC | ESA Biosciences | 0.43 | 0.49 |
| Cavusoglu et al43 | US | 1999–2002 | Hospital | Suspected CAD | 182 | 65 | 100 | Plasma | −70° | ELISA | DLD Diagnostica | N/R | — |
| GeneBank44 | US | N/R | Hospital | Suspected CAD | 1011 | 64 | 47 | Plasma | −80° | HPLC | In-house | 1.03* | 0.65* |
| GetABI (PAD)34 | Germany | 2001–2006 | GP register | PAD | 1260 | 74 | 46 | Plasma | N/R | LC-MS/MS | Varian 1200L | 0.63 | 0.51 |
| KAROLA45 | Germany | 1999–2000 | Hospital | CHD | 1148 | 59 | 85 | Plasma | −80° | LC-MS/MS | Varian 1200L | 0.57 | 0.53 |
| Lu et al46 | Taiwan | 1999–2001 | Hospital | Suspected CAD | 103 | 71 | 87 | Plasma | −70° | HPLC | Waters 470 | 0.56 | 0.58 |
| Lu et al47 | Taiwan | 2006–2009 | Hospital | Suspected CAD | 997 | 67 | 79 | Plasma | −70° | HPLC | Waters 470 | 0.45 | — |
| Populations with pre-existing kidney disease | |||||||||||||
| ALERT48 | Multi-national | 1996–1997 | Hospital | KTx | 1847 | 50 | 66 | Serum | −80° | HPLC | N/R | 0.77* | — |
| CREED49,50 | Italy | 1997–1998 | Hospital | CKD stage 5 | 283 | 61 | 56 | Plasma | −80° | HPLC | Varian | 3.03* | — |
| Ignjatovic et al51 | Serbia | N/R | Hospital | Hemodialysis | 153 | 58 | 70 | Plasma | N/R | HPLC | Agilent 1200 | 0.44 | 0.94 |
| MDRD52 | US | 1989–1993 | Hospital | CKD stage 3/4 | 821 | 52 | 60 | Serum | −70° | ELISA | DLD Diagnostica | 0.73 | — |
| SDC53 | Denmark | 1993 | Hospital | Diab. nephropathy | 397 | 42 | 61 | Plasma | N/R | HPLC | N/R | 0.46 | — |
| Total | 1968–2010 | 19 842 | 58 | 58 | 0.71 | 0.56 | |||||||
Full study names: ALERT, Assessment of Lescol in Renal Transplantation Study; BECAC, Bergen coronary angiography cohort; BRUNECK, Bruneck Study; CREED, Cardiovascular Risk Extended Evaluation in Dialysis; DHS, Dallas Heart Study; GetABI, German Epidemiological Trial on Ankle Brachial Index; INCHIANTI, Invecchiare in Chianti Study; KAROLA, Langzeiterfolge der KARdiOLogischen Anschlussheilbehandlung; KIHD, Kuopio Ischaemic Heart Disease Study; KVINNOSTUDIEN, Kvinnostudien Population Study of Women in Gothenburg; MDC, Malmö Diet and Cancer Cardiovascular Cohort; MDRD, Modification of Diet in Renal Disease Study; MONICA/KORA, Monitoring of Trends and Determinants in Cardiovascular Disease Augsburg; SDC, Steno Diabetes Center. ADMA indicates asymmetric dimethylarginine; CAD, coronary artery disease; CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular disease; ELISA, enzyme-linked immunosorbent assay; GP, general practitioner; HPLC, high-performance liquid chromatography; KTx, kidney transplant; LC-MS/MS, liquid chromatography with tandem mass spectrometry; N/R, not reported; NSTEMI, non ST-elevation myocardial infarction; PAD, peripheral arterial disease; SDMA, symmetric dimethylarginine.
Median.
Table 3.
Follow-Up and Incident Outcomes in the 22 Prospective Studies
| Study Acronym | Median Duration of Follow-Up, Years | Definition of Incident Cardiovascular Outcomes | No. of Incident Cardiovascular Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatal CVD | Non-Fatal MI | Fatal MI | Coronary Revascularization | Ischemic Stroke | Hemorrhagic Stroke | CVD | CHD | Strokes | ||
| General population | ||||||||||
| BRUNECK28 | 10.0* | No | Yes | Yes | Yes | Yes | No | 90 | 39 | 46 |
| DHS33 | 7.4 | Yes | No | No | No | No | No | 62 | — | — |
| GetABI (No PAD)34 | 5.0* | No | Yes | Yes | Yes | Yes | Yes | 131 | 99 | 33 |
| INCHIANTI35 | 9.2 | Yes | No | No | No | No | No | 141 | — | — |
| KIHD36 | 6.0* | Yes | Yes | No | No | No | No | — | 45 | — |
| KVINNOSTUDIEN37 | 24.0* | No | Yes | Yes | No | Yes | Yes | 101 | 58 | 43 |
| MDC38 | 12.0* | Yes | Yes | Yes | No | Yes | Yes | 253 | — | — |
| MONICA/KORA39 | 6.2 | Yes | Yes | No | No | No | No | — | 88 | — |
| Populations with pre-existing CVD | ||||||||||
| AtheroGene40 | 2.6† | Yes | Yes | No | No | Yes | Yes | 159 | — | 45 |
| BECAC41 | 5.3† | Yes | Yes | No | No | No | No | — | 129 | — |
| Cavalca et al42 | 1.8 | Yes | Yes | No | No | No | No | — | 24 | — |
| Cavusoglu et al43 | 2.0* | Yes | Yes | No | No | No | No | — | 37 | — |
| GeneBank44 | 3.0* | No | Yes | No | No | Yes | Yes | 64 | — | — |
| GetABI (PAD)34 | 5.0* | No | Yes | Yes | Yes | Yes | Yes | 263 | 197 | 65 |
| KAROLA45 | 8.1 | Yes | Yes | No | No | Yes | Yes | 150 | — | — |
| Lu et al46 | 1.3 | Yes | Yes | Yes | Yes | No | No | 51 | — | — |
| Lu et al47 | 2.4 | Yes | Yes | No | Yes | Yes | Yes | 144 | — | — |
| Populations with pre-existing kidney disease | ||||||||||
| ALERT48 | 6.7† | No | Yes | Yes | Yes | Yes | Yes | 455 | 281 | 174 |
| CREED49,50 | 10.9* | No | No | No | No | Yes | Yes | — | — | 61 |
| Ignjatovic et al51 | 3.0* | Yes | No | No | No | No | No | 37 | — | — |
| MDRD52 | 9.5 | Yes | No | No | No | No | No | 122 | — | — |
| SDC53 | 11.3 | No | Yes | Yes | Yes | Yes | Yes | 116 | — | — |
| Total | 7.1 | 2339 | 997 | 467 | ||||||
ALERT indicates Assessment of Lescol in Renal Transplantation Study; AtheroGene; BECAC, Bergen coronary angiography cohort; BRUNECK, Bruneck Study; CHD, coronary heart disease; CKD, chronic kidney disease; CREED, Cardiovascular Risk Extended Evaluation in Dialysis; CVD, cardiovascular disease; DHS, Dallas Heart Study; GetABI, German Epidemiological Trial on Ankle Brachial Index; INCHIANTI, Invecchiare in Chianti Study; KAROLA, Langzeiterfolge der KARdiOLogischen Anschlussheilbehandlung; KIHD, Kuopio Ischaemic Heart Disease Study; KVINNOSTUDIEN, Kvinnostudien Population Study of Women in Gothenburg; MDC, Malmö Diet and Cancer Cardiovascular Cohort; MDRD, Modification of Diet in Renal Disease Study; MI, myocardial infarction; MONICA/KORA, Monitoring of Trends and Determinants in Cardiovascular Disease Augsburg; PAD, peripheral arterial disease; SDC, Steno Diabetes Center.
Maximum.
Mean.
Circulating ADMA Concentration And Risk of Cardiovascular Outcomes
In a comparison of individuals in the top with those in the bottom third of baseline values of ADMA, the combined RRs were 1.42 (95% confidence interval: 1.29 to 1.56) for CVD, 1.39 (1.19 to 1.62) for CHD, and 1.60 (1.33 to 1.91) for stroke (Figures2 and 3). The level of between-study heterogeneity was low with I2 values ranging from 0% to 16%. The magnitude of association was comparable in studies conducted in the general population (RR for CVD, 1.35 [1.18 to 1.54]), studies of individuals with pre-existing CVD (1.47 [1.16 to 1.87]), and studies in individuals with pre-existing kidney disease (1.52 [1.26 to 1.84]) (Figure 4). Furthermore, there was no evidence for a difference in associations according to population source, geographical location, sample type, assay method, length of follow-up, number of CVD outcomes, and level of statistical adjustment employed (for meta-regression P>0.05 for all, Figure 4). The magnitude of association between ADMA and CVD risk did not differ according to mean age and sex distribution of the study population (Figure 4).
Figure 2.
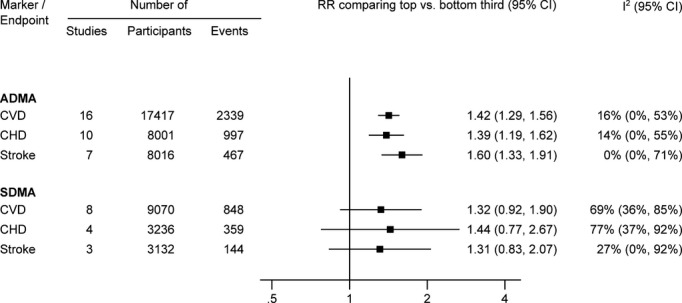
Combined RRs (95% CI) for cardiovascular outcomes in individuals in the top compared with those in the bottom third of ADMA and SDMA concentration. When analysis was restricted to studies that reported on both methylarginines (for direct comparison), the RR of ADMA was 1.40 (1.16, 1.68) for CVD, 1.24 (1.01, 1.52) for CHD, and 1.57 (1.12, 2.20) for stroke and the RR of SDMA was 1.32 (0.92, 1.90) for CVD, 1.44 (0.77, 2.67) for CHD, and 1.31 (0.83, 2.07) for stroke. ADMA indicates asymmetric dimethylarginine; CHD, coronary heart disease; CVD, cardiovascular disease; RR, risk ratio; SDMA, symmetric dimethylarginine.
Figure 3.
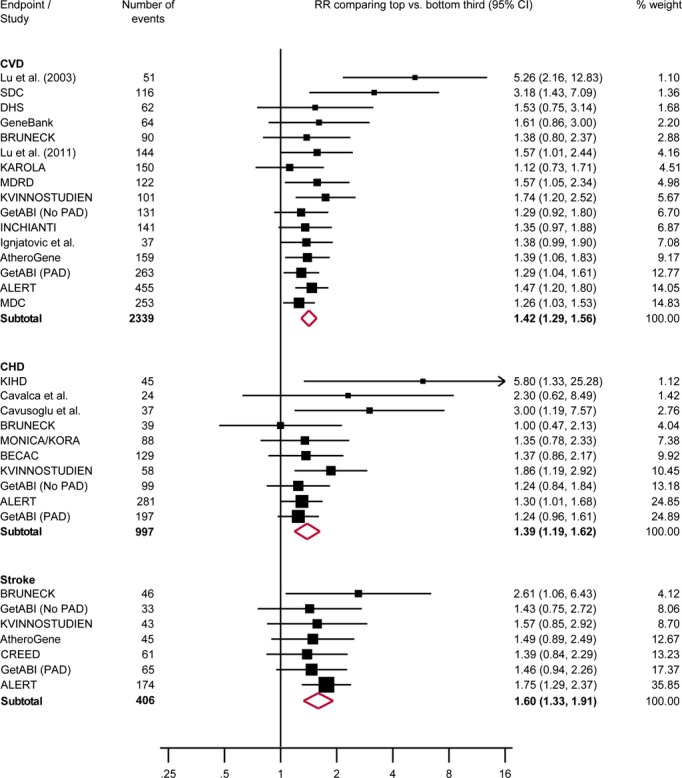
Reported RRs (95% CI) for cardiovascular outcomes in individuals in the top compared with those in the bottom third of ADMA concentration. I2 (95% CI) was 16% (0%, 53%) for CVD, 14% (0%, 55%) for CHD and 0% (0%, 71%) for stroke. ADMA indicates asymmetric dimethylarginine; ALERT, Assessment of Lescol in Renal Transplantation Study; BECAC, Bergen coronary angiography cohort; BRUNECK, Bruneck Study; CHD, coronary heart disease; CREED, Cardiovascular Risk Extended Evaluation in Dialysis; CVD, cardiovascular disease; DHS, Dallas Heart Study; GetABI, German Epidemiological Trial on Ankle Brachial Index; INCHIANTI, Invecchiare in Chianti Study; KAROLA, Langzeiterfolge der KARdiOLogischen Anschlussheilbehandlung; KIHD, Kuopio Ischaemic Heart Disease Study; KVINNOSTUDIEN, Kvinnostudien Population Study of Women in Gothenburg; MDC, Malmö Diet and Cancer Cardiovascular Cohort; MDRD, Modification of Diet in Renal Disease Study; MONICA/KORA, Monitoring of Trends and Determinants in Cardiovascular Disease Augsburg; PAD, peripheral arterial disease; RRs, risk ratios; SDC, Steno Diabetes Center.
Figure 4.
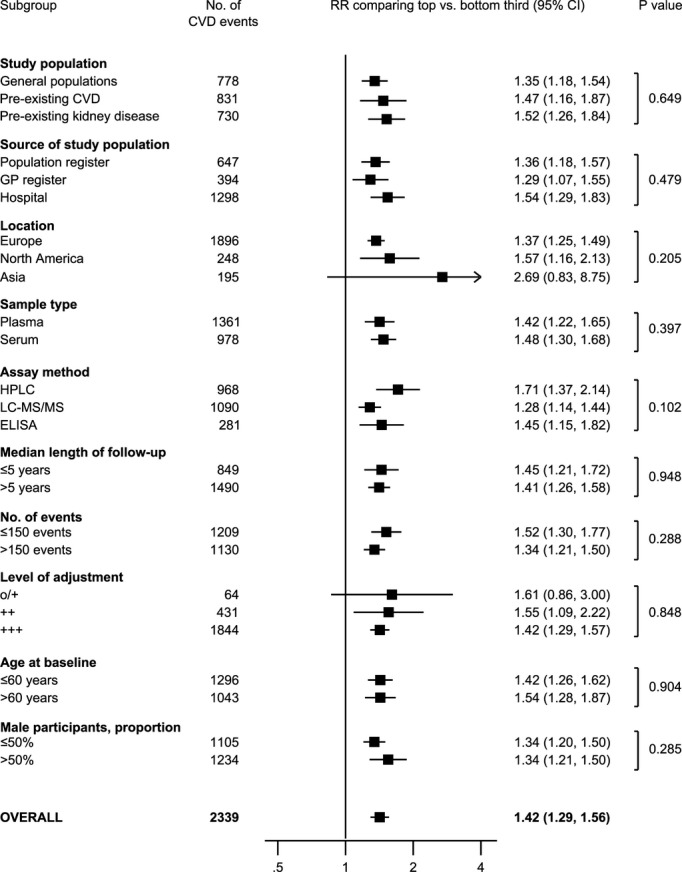
Association of ADMA concentration with CVD risk according to different clinically relevant characteristics. + indicates adjusted for age and sex; ++, further adjusted for at least 2 conventional CVD risk factors; +++, additionally adjusted for other factors; ADMA, asymmetric dimethylarginine; CVD, cardiovascular disease; ELISA, enzyme-linked immunosorbent assay; GP, general practitioner; HPLC, high-performance liquid chromatography; LC-MS/MS, liquid chromatography with tandem mass spectrometry; o, unadjusted; RR, risk ratio.
We observed some evidence for publication bias for the association of ADMA with risk of CVD and CHD outcomes (PEgger=0.009 and 0.033) (Figure 5). Application of the “trim and fill” method suggested that ≈5 studies for CVD and ≈2 studies for CHD were missing due to publication bias. Addition of these theoretical studies to the meta-analyses would attenuate RRs slightly, with RRs of 1.35 (1.20 to 1.51) and 1.35 (1.11 to 1.64) for CVD and CHD, respectively. There was no evidence for publication bias across studies reporting on stroke outcomes.
Figure 5.
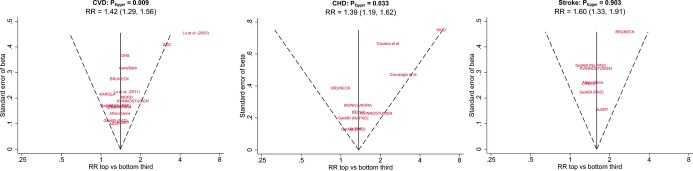
Funnel plots of reported associations between ADMA and cardiovascular outcomes. The dotted lines show pseudo 95% confidence intervals around the overall pooled estimate. P values are from Egger’s asymmetry test of associations. ADMA indicates asymmetric dimethylarginine; ALERT, Assessment of Lescol in Renal Transplantation Study; BECAC, Bergen coronary angiography cohort; BRUNECK, Bruneck Study; CHD, coronary heart disease; CREED, Cardiovascular Risk Extended Evaluation in Dialysis; CVD, cardiovascular disease; DHS, Dallas Heart Study; GetABI, German Epidemiological Trial on Ankle Brachial Index; KAROLA, Langzeiterfolge der KARdiOLogischen Anschlussheilbehandlung; KVINNOSTUDIEN, Kvinnostudien Population Study of Women in Gothenburg; MDC, Malmö Diet and Cancer Cardiovascular Cohort; MDRD, Modification of Diet in Renal Disease Study; MONICA/KORA, Monitoring of Trends and Determinants in Cardiovascular Disease Augsburg; PAD, peripheral arterial disease; RR, risk ratio; SDC, Steno Diabetes Center.
Circulating SDMA Concentration and Risk of Cardiovascular Outcomes
In a subset of 9 studies with available information on baseline SDMA concentration, the combined RRs comparing the top with the bottom third of SDMA concentration were 1.32 (0.92 to 1.90) for CVD, 1.44 (0.77 to 2.67) for CHD, and 1.31 (0.83 to 2.07) for stroke (Figures2 and 6). The level of between-study heterogeneity was moderate with I2 values ranging from 27% to 77%.
Figure 6.
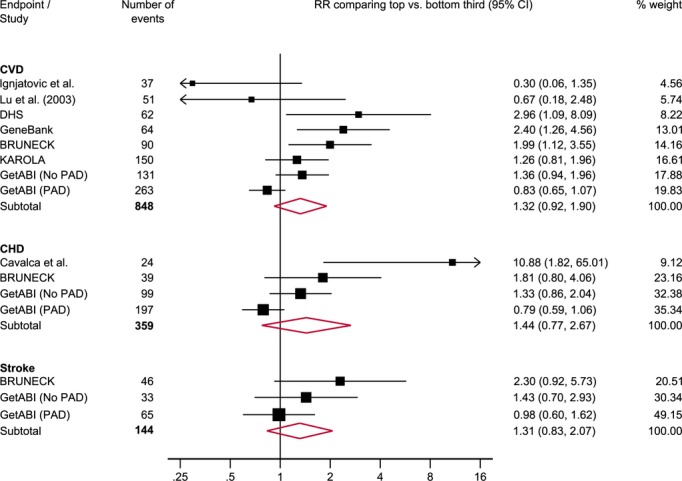
Reported RRs (95% CI) for cardiovascular outcomes in individuals in the top compared with those in the bottom third of SDMA concentration. I2 (95% CI) was 69% (36%, 85%) for CVD, 77% (37%, 92%) for CHD and 27% (0%, 92%) for stroke. BRUNECK indicates Bruneck Study; CHD, coronary heart disease; CVD, cardiovascular disease; DHS, Dallas Heart Study; GetABI, German Epidemiological Trial on Ankle Brachial Index; KAROLA, Langzeiterfolge der KARdiOLogischen Anschlussheilbehandlung; PAD, peripheral arterial disease; RRs, risk ratios; SDMA, symmetric dimethylarginine.
Discussion
The present review of about 20 000 non-overlapping participants from 22 prospective cohort studies across 9 countries has assessed strength and consistency of associations between circulating levels of ADMA and SDMA concentration and subsequent risk of cardiovascular outcomes. Overall, compared with individuals in the bottom third of baseline ADMA concentration, those in the top third of baseline ADMA concentration were at a ≈40% higher risk of CVD. This association was similar in participants with and without pre-existing CVD or kidney disease at baseline and across studies that used diverse methods to measure dimethylarginine levels. On the basis of somewhat limited existing data on SDMA, there was no significant association between SDMA concentration and the risk of cardiovascular outcomes.
Our epidemiological findings lend support to the suggested pathophysiological role of ADMA in atherogenesis. ADMA inhibits the production of nitric oxide (NO), a potent vasodilatator, from l-arginine.3,54 Accordingly, mice with genetically and chemically elevated levels of ADMA exhibit prompt increases in systemic vascular resistance and blood pressure55,56, whereas mice with low ADMA levels show decreases in these parameters.57 In addition to the effect on vascular tone, it has been proposed54,58 that the combination of high ADMA and low NO may promote vascular inflammation,59–62 low density lipoprotein oxidation,63 smooth muscle cell proliferation,64 endothelial cell apoptosis,65 generation of free radicals,66 and adhesion and aggregation of platelets.67,68 In the Framingham Study, elevated ADMA levels were associated with a higher risk of MRI-detected silent brain infarcts.69 Furthermore, compelling evidence from randomized controlled trials indicates that l-arginine supplementation reduces blood pressure70 and may recuperate vascular endothelial function.71 Altogether, ADMA and NO are regarded to play a pivotal role in endothelial dysfunction, the essential first step in atherogenesis.
On the other hand, despite its structural similarity to ADMA, the somewhat discrepant findings for SDMA may be explained by the notion that function and metabolism of SDMA are different. SDMA has very limited or no inhibitory effect on NO synthase.72 While >80% of circulating ADMA is eliminated through enzymatic degradation by the enzyme dimethylarginine dimethylaminohydrolase (DDAH),73 SDMA is primarily eliminated through renal filtration.74 A meta-analysis of 18 studies suggested that SDMA strongly correlates with both measured and estimated glomerular filtration rate and therefore can be regarded as an endogenous marker of renal function.75 Nonetheless, it is also possible that the apparent lack of significant associations for SDMA observed in the current review is due to low statistical power because concurrent data on this marker were available in only a handful of studies.
The strengths and limitations of our study merit consideration. We present the first meta-analysis on circulating ADMA and SDMA concentrations in relation to subsequent risk of cardiovascular outcomes. We applied pre-defined inclusion criteria to identify solely prospective, long-term studies (>1 year follow-up), thereby limiting potential misleading results owing to “reverse causation”. In the absence of individual-participant-level data, we used standardized estimates of ADMA and SDMA to allow consistent comparisons, and focused on clearly defined cardiovascular outcomes to meaningfully characterize the etiological associations. We have also presented data on a wide range of clinically relevant subgroups, which allowed us to explore in detail any potential sources of heterogeneity.
Nevertheless, our meta-analysis was still limited by the moderate amount of available data on cardiovascular outcomes. For example, there were only around 400 stroke events recorded among the ADMA studies. All studies measured dimethylarginines only once at baseline and were therefore unable to assess within-person variability of these biomarkers over time.76 Given these limitations in the existing literature, further investigation in large general population studies is needed, which would enable: (1) a further increase in the precision of estimates; (2) characterization of the shape of any dose-response relationships; (3) direct comparison of the magnitude of effect sizes for ADMA to those for traditional cardiovascular risk factors; (4) a more consistent approach to statistical adjustment; and (5) exploration of usefulness of these markers in CVD risk prediction by calculation of risk prediction metrics such as risk discrimination and risk reclassification. Finally, our meta-analysis was based on published data from prospective observational studies and therefore does not allow inferences to be made on the causal involvement of ADMA in cardiovascular disease development. Intervention studies that specifically target ADMA-related pathways (eg, via l-arginine and DDAH) will help judge causality.77,78 Furthermore, genetic loci discovered in recent genome-wide association studies of circulating levels of ADMA79,80 should provide further opportunities to evaluate specifically the causal association of ADMA with CVD through the “Mendelian randomization” approach81 (in analogy to the existing evidence for a causal role of NO signaling in the development of myocardial infarction82).
In conclusion, available evidence suggests significant positive associations of ADMA with cardiovascular disease outcomes under a broad range of circumstances. Further research is needed to better clarify these associations, particularly in large general population studies.
Acknowledgments
We thank Professors Stefan Kiechl and Johann Willeit for providing additional unpublished information for the Bruneck Study.
Sources of Funding
Dr Willeit is supported by an Erwin Schrödinger Fellowship in Epidemiology sponsored by the Austrian Science Fund (J 3679-B13). Professor Mayr is a Senior Fellow of the British Heart Foundation.
Disclosures
None.
References
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- Bai Y, Sun L, Du L, Zhang T, Xin W, Lan X, Du G. Association of circulating levels of asymmetric dimethylarginine (ADMA) with carotid intima-media thickness: evidence from 6168 participants. Ageing Res Rev. 2013;12:699–707. doi: 10.1016/j.arr.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Böger GI, Rudolph TK, Maas R, Schwedhelm E, Dumbadze E, Bierend A, Benndorf RA, Böger RH. Asymmetric dimethylarginine determines the improvement of endothelium-dependent vasodilation by simvastatin: effect of combination with oral l-arginine. J Am Coll Cardiol. 2007;49:2274–2282. doi: 10.1016/j.jacc.2007.02.051. [DOI] [PubMed] [Google Scholar]
- Päivä H, Kähönen M, Lehtimäki T, Alfthan G, Viikari J, Laaksonen R, Hutri-Kähönen N, Laitinen T, Taittonen L, Raitakari OT, Juonala M. Levels of asymmetrical dimethylarginine are predictive of brachial artery flow-mediated dilation 6 years later. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2010;212:512–515. doi: 10.1016/j.atherosclerosis.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Ardigo D, Stüehlinger M, Franzini L, Valtueña S, Piatti PM, Pachinger O, Reaven GM, Zavaroni I. ADMA is independently related to flow-mediated vasodilation in subjects at low cardiovascular risk. Eur J Clin Invest. 2007;37:263–269. doi: 10.1111/j.1365-2362.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- Tsikas D. A critical review and discussion of analytical methods in the l-arginine/nitric oxide area of basic and clinical research. Anal Biochem. 2008;379:139–163. doi: 10.1016/j.ab.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Wallaschofski H, Atzler D, Dörr M, Nauck M, Völker U, Kroemer HK, Völzke H, Böger RH, Friedrich N. Incidence of all-cause and cardiovascular mortality predicted by symmetric dimethylarginine in the population-based study of health in pomerania. PLoS One. 2014;9:e96875. doi: 10.1371/journal.pone.0096875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–1600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Carlquist JF, Roberts WL, Horne BD, May HT, Schwarz EL, Pasquali M, Nielson R, Kushnir MM, Rockwood AL, Bair TL, Muhlestein JB Intermountain Heart Collaborative Study Group. Asymmetric dimethylarginine, cortisol/cortisone ratio, and C-peptide: markers for diabetes and cardiovascular risk? Am Heart J. 2007;153:67–73. doi: 10.1016/j.ahj.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Meinitzer A, Kielstein JT, Pilz S, Drechsler C, Ritz E, Boehm BO, Winkelmann BR, März W. Symmetrical and asymmetrical dimethylarginine as predictors for mortality in patients referred for coronary angiography: the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem. 2011;57:112–121. doi: 10.1373/clinchem.2010.150854. [DOI] [PubMed] [Google Scholar]
- Busch M, Fleck C, Wolf G, Stein G. Asymmetrical (ADMA) and symmetrical dimethylarginine (SDMA) as potential risk factors for cardiovascular and renal outcome in chronic kidney disease—possible candidates for paradoxical epidemiology? Amino Acids. 2006;30:225–232. doi: 10.1007/s00726-005-0268-8. [DOI] [PubMed] [Google Scholar]
- Cavusoglu E, Ruwende C, Chopra V, Poludasu S, Yanamadala S, Frishman WH, Eng C, Pinsky DJ, Marmur JD. Relation of baseline plasma ADMA levels to cardiovascular morbidity and mortality at two years in men with diabetes mellitus referred for coronary angiography. Atherosclerosis. 2010;210:226–231. doi: 10.1016/j.atherosclerosis.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Dückelmann C, Mittermayer F, Haider DG, Altenberger J, Eichinger J, Wolzt M. Asymmetric dimethylarginine enhances cardiovascular risk prediction in patients with chronic heart failure. Arterioscler Thromb Vasc Biol. 2007;27:2037–2042. doi: 10.1161/ATVBAHA.107.147595. [DOI] [PubMed] [Google Scholar]
- Krzyzanowska K, Mittermayer F, Wolzt M, Schernthaner G. Asymmetric dimethylarginine predicts cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2007;30:1834–1839. doi: 10.2337/dc07-0019. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Sakurai M, Takita T, Maruyama Y, Uno S, Ikegaya N, Kato A, Hishida A. Association of homocysteine and asymmetric dimethylarginine with atherosclerosis and cardiovascular events in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:797–805. doi: 10.1053/j.ajkd.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Mittermayer F, Krzyzanowska K, Exner M, Mlekusch W, Amighi J, Sabeti S, Minar E, Müller M, Wolzt M, Schillinger M. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol. 2006;26:2536–2540. doi: 10.1161/01.ATV.0000242801.38419.48. [DOI] [PubMed] [Google Scholar]
- Shi B, Ni Z, Zhou W, Yu Z, Gu L, Mou S, Fang W, Wang Q, Cao L, Yan Y, Qian J. Circulating levels of asymmetric dimethylarginine are an independent risk factor for left ventricular hypertrophy and predict cardiovascular events in pre-dialysis patients with chronic kidney disease. Eur J Intern Med. 2010;21:444–448. doi: 10.1016/j.ejim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Shin DS, Weatherby C, Harada RK, Ng MK, Nair N, Kielstein J, Cooke JP. Asymmetric dimethylarginine correlates with measures of disease severity, major adverse cardiovascular events and all-cause mortality in patients with peripheral arterial disease. Vasc Med. 2010;15:267–274. doi: 10.1177/1358863X10364552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucella F, Maas R, Vigilante M, Tripepi G, Schwedhelm E, Margaglione M, Gesualdo L, Boeger R, Zoccali C. Methylarginines and mortality in patients with end stage renal disease: a prospective cohort study. Atherosclerosis. 2009;207:541–545. doi: 10.1016/j.atherosclerosis.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Ari H, Ari S, Erdoğan E, Tiryakioğlu O, Ustündağ Y, Huysal K, Koca V, Bozat T. A novel predictor of restenosis and adverse cardiac events: asymmetric dimethylarginine. Heart Vessels. 2010;25:19–26. doi: 10.1007/s00380-009-1158-x. [DOI] [PubMed] [Google Scholar]
- Dückelmann C, Mittermayer F, Haider DG, Altenberger J, Wolzt M. Plasma asymmetric dimethylarginine and cardiovascular events in patients with acute decompensated heart failure. Transl Res. 2008;152:24–30. doi: 10.1016/j.trsl.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Lüneburg N, von Holten RA, Töpper RF, Schwedhelm E, Maas R, Böger RH. Symmetric dimethylarginine is a marker of detrimental outcome in the acute phase after ischaemic stroke: role of renal function. Clin Sci (Lond) 2012;122:105–111. doi: 10.1042/CS20110013. [DOI] [PubMed] [Google Scholar]
- Maas R, Dentz L, Schwedhelm E, Thoms W, Kuss O, Hiltmeyer N, Haddad M, Klöss T, Standl T, Böger RH. Elevated plasma concentrations of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine predict adverse events in patients undergoing noncardiac surgery. Crit Care Med. 2007;35:1876–1881. doi: 10.1097/01.CCM.0000277038.11630.71. [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Lee T, Santer P, Thompson G, Tsimikas S, Egger G, Holt DW, Willeit J, Xu Q, Mayr M. Asymmetric and symmetric dimethylarginines are of similar predictive value for cardiovascular risk in the general population. Atherosclerosis. 2009;205:261–265. doi: 10.1016/j.atherosclerosis.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–2708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, Tweedie R. A nonparametric, “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- Gore MO, Lüneburg N, Schwedhelm E, Ayers CR, Anderssohn M, Khera A, Atzler D, de Lemos JA, Grant PJ, McGuire DK, Böger RH. Symmetrical dimethylarginine predicts mortality in the general population: observations from the Dallas heart study. Arterioscler Thromb Vasc Biol. 2013;33:2682–2688. doi: 10.1161/ATVBAHA.113.301219. [DOI] [PubMed] [Google Scholar]
- Böger RH, Endres HG, Schwedhelm E, Darius H, Atzler D, Lüneburg N, von Stritzky B, Maas R, Thiem U, Benndorf RA, Diehm C. Asymmetric dimethylarginine as an independent risk marker for mortality in ambulatory patients with peripheral arterial disease. J Intern Med. 2011;269:349–361. doi: 10.1111/j.1365-2796.2010.02322.x. [DOI] [PubMed] [Google Scholar]
- Pizzarelli F, Maas R, Dattolo P, Tripepi G, Michelassi S, D’Arrigo G, Mieth M, Bandinelli S, Ferrucci L, Zoccali C. Asymmetric dimethylarginine predicts survival in the elderly. Age (Dordr) 2013;35:2465–2475. doi: 10.1007/s11357-013-9523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkonen VP, Päivä H, Salonen JT, Lakka TA, Lehtimäki T, Laakso J, Laaksonen R. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–2128. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- Leong T, Zylberstein D, Graham I, Lissner L, Ward D, Fogarty J, Bengtsson C, Björkelund C, Thelle D Swedish-Irish-Norwegian Collaboration. Asymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women: 24-year follow-up of the population study of women in Gothenburg. Arterioscler Thromb Vasc Biol. 2008;28:961–967. doi: 10.1161/ATVBAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- Magnusson M, Wang T, Hedblad B, Engstrom G, Ostling G, Gerszten R, Melander O. High levels of arginine, citrulline and ADMA are independent predictors of cardiovascular disease. Eur Heart J. 2013;34:1058–1059. doi: 10.1093/eurheartj/ehs424. . Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R, Schulze F, Baumert J, Löwel H, Hamraz K, Schwedhelm E, Koenig W, Böger RH. Asymmetric dimethylarginine, smoking, and risk of coronary heart disease in apparently healthy men: prospective analysis from the population-based Monitoring of Trends and Determinants in Cardiovascular Disease/Kooperative Gesundheitsforschung in der Region Augsburg study and experimental data. Clin Chem. 2007;53:693–701. doi: 10.1373/clinchem.2006.081893. [DOI] [PubMed] [Google Scholar]
- Schnabel R, Rupprecht HJ, Lackner KJ, Lubos E, Bickel C, Meyer J, Münzel T, Cambien F, Tiret L, Blankenberg S AtheroGene Investigators. Analysis of N-terminal-pro-brain natriuretic peptide and C-reactive protein for risk stratification in stable and unstable coronary artery disease: results from the AtheroGene study. Eur Heart J. 2005;26:241–249. doi: 10.1093/eurheartj/ehi036. [DOI] [PubMed] [Google Scholar]
- Borgeraas H, Strand E, Ringdal Pedersen E, Dierkes J, Ueland PM, Seifert R, Wilberg ER, Bohov P, Berge RK, Nilsen DWT, Nygård O. Omega-3 status and the relationship between plasma asymmetric dimethylarginine and risk of myocardial infarction in patients with suspected coronary artery disease. Cardiol Res Pract. 2012;2012:201742. doi: 10.1155/2012/201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalca V, Veglia F, Squellerio I, De Metrio M, Rubino M, Porro B, Moltrasio M, Tremoli E, Marenzi G. Circulating levels of dimethylarginines, chronic kidney disease and long-term clinical outcome in non-ST-elevation myocardial infarction. PLoS One. 2012;7:e48499. doi: 10.1371/journal.pone.0048499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Pinsky DJ, Marmur JD. Relationship of baseline plasma ADMA levels to cardiovascular outcomes at 2 years in men with acute coronary syndrome referred for coronary angiography. Coron Artery Dis. 2009;20:112–117. doi: 10.1097/MCA.0b013e328323982f. [DOI] [PubMed] [Google Scholar]
- Wang Z, Tang WHW, Cho L, Brennan DM, Hazen SL. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: potential mechanisms beyond nitric oxide synthase inhibition. Arterioscler Thromb Vasc Biol. 2009;29:1383–1391. doi: 10.1161/ATVBAHA.109.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegerink B, Maas R, Vossen CY, Schwedhelm E, Koenig W, Böger R, Rothenbacher D, Brenner H, Breitling LP. Asymmetric and symmetric dimethylarginine and risk of secondary cardiovascular disease events and mortality in patients with stable coronary heart disease: the KAROLA follow-up study. Clin Res Cardiol. 2013;102:193–202. doi: 10.1007/s00392-012-0515-4. [DOI] [PubMed] [Google Scholar]
- Lu TM, Ding YA, Lin SJ, Lee WS, Tai HC. Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J. 2003;24:1912–1919. doi: 10.1016/j.ehj.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Lu TM, Chung MY, Lin MW, Hsu CP, Lin SJ. Plasma asymmetric dimethylarginine predicts death and major adverse cardiovascular events in individuals referred for coronary angiography. Int J Cardiol. 2011;153:135–140. doi: 10.1016/j.ijcard.2011.06.120. [DOI] [PubMed] [Google Scholar]
- Abedini S, Meinitzer A, Holme I, März W, Weihrauch G, Fellstrøm B, Jardine A, Holdaas H. Asymmetrical dimethylarginine is associated with renal and cardiovascular outcomes and all-cause mortality in renal transplant recipients. Kidney Int. 2010;77:44–50. doi: 10.1038/ki.2009.382. [DOI] [PubMed] [Google Scholar]
- Tripepi G, Mattace-Raso F, Rapisarda F, Stancanelli B, Malatino L, Witteman J, Zoccali C, Mallamaci F. Traditional and nontraditional risk factors as predictors of cerebrovascular events in patients with end stage renal disease. J Hypertens. 2010;28:2468–2474. doi: 10.1097/HJH.0b013e32833eaf49. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J, Böger R. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- Ignjatovic AM, Cvetkovic TP, Pavlovic RM, Dordevic VM, Milosevic ZG, Dordevic VB, Pavlovic DD, Stojanovic IR, Bogdanovic D. Endothelial dysfunction, inflammation and malnutrition markers as predictors of mortality in dialysis patients: multimarker approach. Int Urol Nephrol. 2013;45:1715–1724. doi: 10.1007/s11255-013-0439-6. [DOI] [PubMed] [Google Scholar]
- Young JM, Terrin N, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Sarnak MJ, Menon V. Asymmetric dimethylarginine and mortality in stages 3 to 4 chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1115–1120. doi: 10.2215/CJN.06671208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajer M, Tarnow L, Jorsal A, Teerlink T, Parving HH, Rossing P. Plasma concentration of asymmetric dimethylarginine (ADMA) predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2008;31:747–752. doi: 10.2337/dc07-1762. [DOI] [PubMed] [Google Scholar]
- Böger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824–833. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Wakino S, Tatematsu S, Yoshioka K, Homma K, Sugano N, Kimoto M, Hayashi K, Itoh H. Role of asymmetric dimethylarginine in vascular injury in transgenic mice overexpressing dimethylarginie dimethylaminohydrolase 2. Circ Res. 2007;101:e2–e10. doi: 10.1161/CIRCRESAHA.107.156901. [DOI] [PubMed] [Google Scholar]
- Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang BY, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation. 2003;108:3042–3047. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- Caplin B, Leiper J. Endogenous nitric oxide synthase inhibitors in the biology of disease: markers, mediators, and regulators? Arterioscler Thromb Vasc Biol. 2012;32:1343–1353. doi: 10.1161/ATVBAHA.112.247726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Böger RH, Bode-Böger SM, Tangphao O, Tsao PS, Blaschke TF, Cooke JP. Asymmetric dimethylarginine increases mononuclear cell adhesiveness in hypercholesterolemic humans. Arterioscler Thromb Vasc Biol. 2000;20:1040–1046. doi: 10.1161/01.atv.20.4.1040. [DOI] [PubMed] [Google Scholar]
- Böger RH, Bode-Böger SM, Tsao PS, Lin PS, Chan JR, Cooke JP. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J Am Coll Cardiol. 2000;36:2287–2295. doi: 10.1016/s0735-1097(00)01013-5. [DOI] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PS, Buitrago R, Chan JR, Cooke JP. Fluid flow inhibits endothelial adhesiveness. Nitric oxide and transcriptional regulation of VCAM-1. Circulation. 1996;94:1682–1689. doi: 10.1161/01.cir.94.7.1682. [DOI] [PubMed] [Google Scholar]
- Hogg N, Kalyanaraman B, Joseph J, Struck A, Parthasarathy S. Inhibition of low-density lipoprotein oxidation by nitric oxide. Potential role in atherogenesis. FEBS Lett. 1993;334:170–174. doi: 10.1016/0014-5793(93)81706-6. [DOI] [PubMed] [Google Scholar]
- Böger RH, Bode-Böger SM, Kienke S, Stan AC, Nafe R, Frölich JC. Dietary l-arginine decreases myointimal cell proliferation and vascular monocyte accumulation in cholesterol-fed rabbits. Atherosclerosis. 1998;136:67–77. doi: 10.1016/s0021-9150(97)00183-4. [DOI] [PubMed] [Google Scholar]
- Jiang DJ, Jia SJ, Dai Z, Li YJ. Asymmetric dimethylarginine induces apoptosis via p38 MAPK/caspase-3-dependent signaling pathway in endothelial cells. J Mol Cell Cardiol. 2006;40:529–539. doi: 10.1016/j.yjmcc.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Böger RH, Bode-Böger SM, Mügge A, Kienke S, Brandes R, Dwenger A, Frölich JC. Supplementation of hypercholesterolaemic rabbits with l-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis. 1995;117:273–284. doi: 10.1016/0021-9150(95)05582-h. [DOI] [PubMed] [Google Scholar]
- Willoughby SR, Rajendran S, Chan WP, Procter N, Leslie S, Liberts EA, Heresztyn T, Chirkov YY, Horowitz JD. Ramipril sensitizes platelets to nitric oxide: implications for therapy in high-risk patients. J Am Coll Cardiol. 2012;60:887–894. doi: 10.1016/j.jacc.2012.01.066. [DOI] [PubMed] [Google Scholar]
- Wolf A, Zalpour C, Theilmeier G, Wang BY, Ma A, Anderson B, Tsao PS, Cooke JP. Dietary l-arginine supplementation normalizes platelet aggregation in hypercholesterolemic humans. J Am Coll Cardiol. 1997;29:479–485. doi: 10.1016/s0735-1097(97)00523-8. [DOI] [PubMed] [Google Scholar]
- Pikula A, Böger RH, Beiser AS, Maas R, DeCarli C, Schwedhelm E, Himali JJ, Schulze F, Au R, Kelly-Hayes M, Kase CS, Vasan RS, Wolf PA, Seshadri S. Association of plasma ADMA levels with MRI markers of vascular brain injury: Framingham offspring study. Stroke. 2009;40:2959–2964. doi: 10.1161/STROKEAHA.109.557116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W. Effect of oral l-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959–965. doi: 10.1016/j.ahj.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Bai Y, Sun L, Yang T, Sun K, Chen J, Hui R. Increase in fasting vascular endothelial function after short-term oral l-arginine is effective when baseline flow-mediated dilation is low: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2009;89:77–84. doi: 10.3945/ajcn.2008.26544. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Böger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol. 2011;7:275–285. doi: 10.1038/nrneph.2011.31. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Böger RH, Bode-Böger SM, Martens-Lobenhoffer J, Lonnemann G, Frölich JC, Haller H, Fliser D. Low dialysance of asymmetric dimethylarginine (ADMA)—in vivo and in vitro evidence of significant protein binding. Clin Nephrol. 2004;62:295–300. doi: 10.5414/cnp62295. [DOI] [PubMed] [Google Scholar]
- Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—a meta-analysis. Nephrol Dial Transplant. 2006;21:2446–2451. doi: 10.1093/ndt/gfl292. [DOI] [PubMed] [Google Scholar]
- Fibrinogen Studies Collaboration. Wood AM, White I, Thompson SG, Lewington S, Danesh J. Regression dilution methods for meta-analysis: assessing long-term variability in plasma fibrinogen among 27,247 adults in 15 prospective studies. Int J Epidemiol. 2006;35:1570–1578. doi: 10.1093/ije/dyl233. [DOI] [PubMed] [Google Scholar]
- Leiper J, Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov. 2011;10:277–291. doi: 10.1038/nrd3358. [DOI] [PubMed] [Google Scholar]
- Sun T, Zhou WB, Luo XP, Tang YL, Shi HM. Oral l-arginine supplementation in acute myocardial infarction therapy: a meta-analysis of randomized controlled trials. Clin Cardiol. 2009;32:649–652. doi: 10.1002/clc.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüneburg N, Lieb W, Zeller T, Chen MH, Maas R, Carter AM, Xanthakis V, Glazer NL, Schwedhelm E, Seshadri S, Ikram MA, Longstreth WT, Jr, Fornage M, König IR, Loley C, Ojeda FM, Schillert A, Wang TJ, Sticht H, Kittel A, König J, Benjamin EJ, Sullivan LM, Bernges I, Anderssohn M, Ziegler A, Gieger C, Illig T, Meisinger C, Wichmann HE, Wild PS, Schunkert H, Psaty BM, Wiggins KL, Heckbert SR, Smith N, Lackner K, Lunetta KL, Blankenberg S, Erdmann J, Munzel T, Grant PJ, Vasan RS, Böger RH. Genome-wide association study of l-arginine and dimethylarginines reveals novel metabolic pathway for symmetric dimethylarginine. Circ Cardiovasc Genet. 2014;7:864–872. doi: 10.1161/CIRCGENETICS.113.000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä I, Kleber ME, Lyytikäinen LP, Hernesniemi JA, Mäkelä KM, Oksala N, Laaksonen R, Pilz S, Tomaschitz A, Silbernagel G, Boehm BO, Grammer TB, Koskinen T, Juonala M, Hutri-Kähönen N, Alfthan G, Viikari JSA, Kähonen M, Raitakari OT, März W, Meinitzer A, Lehtimäki T AtheroRemo Consortium. Genome-wide association study on dimethylarginines reveals novel AGXT2 variants associated with heart rate variability but not with overall mortality. Eur Heart J. 2014;35:524–531. doi: 10.1093/eurheartj/eht447. [DOI] [PubMed] [Google Scholar]
- Ding H, Wu B, Wang H, Lu Z, Yan J, Wang X, Shaffer JR, Hui R, Wang DW. A novel loss-of-function DDAH1 promoter polymorphism is associated with increased susceptibility to thrombosis stroke and coronary heart disease. Circ Res. 2010;106:1145–1152. doi: 10.1161/CIRCRESAHA.109.215616. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Stark K, Esslinger UB, Rumpf PM, Koesling D, de Wit C, Kaiser FJ, Braunholz D, Medack A, Fischer M, Zimmermann ME, Tennstedt S, Graf E, Eck S, Aherrahrou Z, Nahrstaedt J, Willenborg C, Bruse P, Brænne I, Nöthen MM, Hofmann P, Braund PS, Mergia E, Reinhard W, Burgdorf C, Schreiber S, Balmforth AJ, Hall AS, Bertram L, Steinhagen-Thiessen E, Li SC, März W, Reilly M, Kathiresan S, McPherson R, Walter U CARDIoGRAM. Ott J, Samani NJ, Strom TM, Meitinger T, Hengstenberg C, Schunkert H. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature. 2013;504:432–436. doi: 10.1038/nature12722. [DOI] [PubMed] [Google Scholar]


