Abstract
Background
Proprotein convertase subtilisin/kexin9 (PCSK9) monoclonal antibody significantly reduces low-density lipoprotein cholesterol level in patients with hypercholesterolemia. The goal of this study was to review recently reported randomized controlled trials to investigate the therapeutic effects and safety of PCSK9 inhibitors.
Methods and Results
The clinical randomized controlled trials published from inception to March 19, 2015 were identified from The Cochrane Library databases, PUBMED, and EBASE. Randomized controlled trials of at least 8 weeks duration using PCSK9 inhibitors in treating patients with hypercholesterolemia were included. Mean difference (MD) with a 95% CI was used to calculate the continuous data, the standardized mean difference with a 95% CI was used when the unit was not unified, and risk ratio with a 95% CI was used for dichotomous data. After screening, 20 trials fulfilled the inclusion criteria. PCSK9 inhibitors significantly decreased the levels of low-density lipoprotein cholesterol (MD=−65.29 mg/dL, 95% CI: −72.08 to −58.49), total cholesterol (MD=−60.04 mg/dL, 95% CI: −69.95 to −50.13), triglycerides (MD=−12.21 mg/dL, 95% CI: −16.21 to −8.22) and apolipoprotein-B (MD=−41.01 mg/dL, 95% CI: −46.07 to −35.94), lipoprotein(a) (standardized mean difference=−0.94, 95% CI: −1.12 to −0.77) and increased the levels of high-density lipoprotein cholesterol (MD=3.40 mg/dL, 95% CI: 3.12 to 3.68) and apolipoprotein-A1 (MD=6.75 mg/dL, 95% CI: 4.64 to 8.86). There was no significant difference in the incidence of treatment-emergent adverse events (risk ratio=1.01, 95% CI: 0.98 to 1.04), serious treatment-emergent adverse events (risk ratio=1.01, 95% CI: 0.88 to 1.17), and the discontinuation of treatment between the 2 groups (risk ratio=1.07, 95% CI: 0.86 to 1.34).
Conclusions
The meta-analysis indicated that PCSK9 inhibitors had a strong effect in lowering low-density lipoprotein cholesterol and other lipid levels with satisfactory safety and tolerability in patients with hypercholesterolemia.
Keywords: lipids, lipoproteins, meta-analysis, proprotein convertase subtilisin/kexin9 inhibitor
Despite advances in the detection and treatment of ischemic cardiovascular disease (CVD), such as myocardial infarction and stroke in recent years, it remains the leading cause of death worldwide.1 Low-density lipoprotein cholesterol (LDL-C) is the primary atherogenic lipoprotein, and LDL-C reduction is the target of primary or secondary prevention of CVD.2 Statins are considered the most effective agents for reducing LDL-C and decrease the risk for CVD events.3,4 It is recommended to prescribe high-intensity statin therapy for individuals with high risk of CVD.5 However, broad spectrums of high-risk patients fail to attain the guideline-recommended LDL-C goals due to statin intolerance and/or very high baseline levels (eg, familial hypercholesterolemia patients).6 Combination therapies that add nonstatin drugs are compromising methods in patients who are intolerant to high-intensity statin therapy.7 Recent studies revealed that adding ezetimibe to simvastatin modestly reduced LDL-C (15 mg/dL) and CVD risks.8 However, other effective therapies are needed as alternative methods to further decrease LDL-C and finally reduce the mortality and morbidity of CVD.
Proprotein convertase subtilisin/kexin9 (PCSK9) plays a pivotal role in regulating cholesterol homeostasis; it acts by binding to the LDL-receptor (LDL-R) at the surface of hepatocytes, hence promoting the clearance of LDL-R in lysosomes/endosomes, and results in decreased amount of LDL-R number and increased plasma HDL-C levels, so it has emerged as an attractive target for lowering LDL-C levels.9 The single-nucleotide polymorphism in PCSK9 gene are associated with LDL-C and risk of CVD, making PCSK9 inhibition a potential therapeutic modality.10–13 Statin therapy can increase plasma PCSK9 levels to some extent, while combination with PCSK9 inhibitors may compensate this secondary change.14 Various approaches have been tested to inhibit PCSK9 in active clinical and preclinical trials. Among those strategies, PCSK9 monoclonal antibody is of great interest because it blocks its binding to LDL-R via an allosteric mechanism.15 The human monoclonal antibodies against PCSK9 primarily include AMG145/Evolocumab, REGN727/SAR236553, and RN316/bococizumab.16 In the last 2 years, some early clinical trials have shown that PCSK9 inhibitors can reduce the plasma LDL-C level in patients with familial or nonfamilial hypercholesterolemia. The other lipids and lipoproteins such as total cholesterol (TC), triglycerides (TG), high-density lipoprotein-C (HDL-C), apolipoprotein-B (Apo-B), Apo-A1, and lipoprotein(a) could also benefit from this approach.
Because of differences in study design and clinical outcomes, including dyslipidemia types, medicine dosage and therapeutic duration, and the efficiency and safety of PCSK9 inhibitors that each author reported, greatly vary. To date, there is no report of any comprehensive and quantitative evaluation of the efficiency and safety of PCSK9 inhibitors therapy. The purpose of this meta-analysis is to compare the efficiency and safety of all published randomized controlled trials (RCTs) using PCSK9 inhibitors with various background lipid therapies versus placebo for treating patients with familial or nonfamilial hypercholesterolemia. In total, 18 articles were assessed for efficacy and 20 articles were assessed for safety analyses.
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (MOOSE group).17
Data Source, Search Strategy, and Inclusion Criteria
The Cochrane Library databases, PUBMED, and EBASE were searched for original articles from inception to March 19, 2015 to identify all RCTs using PCSK9 inhibitor therapy. The following search items were used: ((((AMG 145*) OR evolocumab*) OR REGN727*) OR SAR236553*) OR RN316*) OR PF04950615*) OR bococizumab*) OR antibody to proprotein convertase subtilisin/kexin type 9*) OR antibody to PCSK9*) AND (((randomized controlled trial [pt]) OR (controlled clinical trial [pt]) OR (randomized [tiab]) OR (placebo [tiab]) OR (drug therapy [sh]) OR (randomly [tiab]) OR (trial [tiab]) OR (groups [tiab])) NOT (animals[mh] NOT humans [mh])). All the relevant articles were published in English, conducted on human subjects, and classified as RCTs. The references of the studies and trials registries on ClinicalTrial.gov were also searched for additional articles. Original trials were eligible for the present meta-analysis if they met the following criteria: (1) study design: RCT; (2) study population: patients with familial or nonfamilial hypercholesterolemia; (3) study intervention: Patients in the treatment group received PCSK9 inhibitors versus patients in the control group received placebo with or without other lipid-lowering therapy; (4) lipid parameters: Trials in which LDL-C, HDL-C, TC, TG, Apo-B, Apo-A1 and lipoprotein(a) were measured at baseline and during PCSK9 inhibitors therapy in the entire study; and (5) treatment duration: longer than 8 weeks. We excluded the case reports, nonhuman studies, and studies without adequate information on outcomes and lacking control group.
Data Extraction and Study Quality Assessment
Two investigators (C.L., L.L.) individually performed screening the titles and abstracts, duplicate checking, and reviewed full articles that met the inclusion criteria and extracted the data and managed according to the intention-to-treat principle. We extracted data from the published RCTs mainly because it is still controversial to include data from unpublished trials. The following items were extracted from included studies: first author’s name, year of publication, study design, characteristic of patients, sample size, duration of intervention and type of control, drug dose, clinical outcomes, and adverse events. If a trial is reported at several time points, we included the final reported follow-up point. We assessed the risk of bias of included studies based on the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The judgments were expressed simply as “low risk,” “high risk,” or “unclear risk” of bias. Discrepancies were resolved by extensive discussions between the 2 authors or a third person. The last-observation-carried-forward method was used to deal with the missing values. The quality of eligible RCTs was qualified independently using the 5-point Jadad score, which evaluates 3 criteria: basis of randomization (0 to 2 points), double blinding (0 to 2 points), and withdrawals and dropouts (0 to 1 points). Studies with a score ≥3 points are considered to be high quality.
Outcomes
We calculated the net change in lipid and apolipoprotein levels before and after PCSK9 inhibitors treatment as the primary outcomes. We then compared the clinical and laboratory treatment-emergent adverse events (TEAE)s, serious TEAEs, and the discontinuation of drug treatment between the treatment group and control group. The laboratory adverse events included hepatotoxicity (alanine aminotransferase and aspartate aminotransferase levels ≥3-fold upper limit of normal), musculoskeletal injury (creatine kinase ≥5-folds upper limit of normal).The most common clinical TEAEs included injection-site reaction (eg, generalized pruritis, hypersensitive reaction, erythema, rash, swelling, discoloration, or pain), nasopharyngitis, upper respiratory tract infections, influenza, cough, nausea, myalgia, myositis, headache, diarrhea, fatigue, abnormal pain, rectal bleeding, dehydration, arthralgia, and back pain.18–37 The serious TEAEs were defined as an adverse event that was fatal, life threatening, required admission to the hospital or prolonged stay in the hospital, or that caused persistent or significant disability.35
Data Synthesis and Statistical Analysis
We used mean difference with corresponding 95% CI for continuous outcomes (lipids and apolipoproteins of participants in the trials), standardized mean difference with a 95% CI for continued outcomes when the unit was not unified (lipoprotein[a]) and relative risk (RR) with 95% CI for dichotomous outcome (TEAEs, serious TEAEs, and discontinuation of participants in the trials). All quantitative variables are listed in the form of mean±SD. We contacted the original author of the study to obtain the missing data if necessary. The following data were needed to measure the weighted mean difference: the mean absolute change of lipoproteins and apolipoprotein levels (LDL-C, TC, HDL-C, TG, ApoB, Apo-A1) from baseline to the longest follow-up time point in milligrams per deciliter (mg/dL). All the units of these variables were standardized when TC, HDL-C, and LDL-C are expressed by mmol/L, multiplied by 38.6 to convert to mg/dL, and TG is converted to mg/dL by multiplying by 88.5. If the results were expressed by median and range, the mean and SD were calculated according to the methods listed in our previous study.38–40 Treatment or control groups with multiple doses were combined, respectively, to create a single pairwise comparison, with the primary comparisons being treatment versus placebo. We calculated the average mean and SD of multiple dose–response groups by the methods described in previous studies. If the authors did not list the mean differences but provided the mean and/or SD instead, we calculated the mean difference from the other studies in this review by the methods described previously.38
Heterogeneity and Publication Bias
The results of the included studies were performed with fixed-effect model or random effect in the computer program Review Manager (REVMAN) from the Cochrane Collaboration. We used the Cochrane Q test to measure the heterogeneity across included trials, χ2 tests, and I2 statistics to assess the magnitude of heterogeneity. We selected a fixed-effect model if there was no unexplained statistical heterogeneity. If heterogeneity existed, then the random-effect model was used.41 We considered I2≥50% to indicate statistically significant heterogeneity between trials.42 When I2≥50% was observed, we would take some measures to reduce the heterogeneity, such as performed subgroups by age, dyslipidemia types, PCSK9 inhibitor types, treatment duration (≤12 weeks and >12 weeks), monotherapy or coadministration with other therapies, and study quality to identify the reasons for the diverseness. Publication bias, which includes selection bias, performance bias, attrition bias, reporting bias, and other risk of bias, was assessed by using the funnel plots, and Egger’s regression test was also used to assay the possibility of publication bias.43 This meta-analysis was performed by REVMAN software, version 5.3 (The Cochrane Collaboration, Nordic Cochrane center, Copenhagen, Denmark). A 2-sided P-value <0.05 was considered to be statistically significant.
Results
Search Results and Study Characteristics
A total of 290 articles were obtained via searches on the databases, of which 66 records were excluded after determination of duplication and 196 articles were excluded after screening the titles and abstracts. A group of 28 relevant articles were reviewed in-depth by 2 independent authors, of which the following studies were excluded: 5 were not RCTs, 1 lacked a control group, and the other 1 were open-label trials (Figure 1). Finally, 20 RCTs met the eligible criteria including 6464 hypercholesterolemia cases, and 3416 controls were selected into the meta-analysis study.18–37 The characteristics of these trials included are shown in Table 1. Overall, these studies had a relatively high quality judged as by the Jadad score (5 scores=6, 4 scores=5, 3 scores=6, 2 scores=3). The summaries of risk of bias of included studies are shown in Figure 2. The funnel plot results did not show any publication bias in all the analyses performed (data not shown). The enrolled studies included 3 kinds of PCSK9 inhibitors (AMG145/Evolocumab=12, REGN727/SAR236553/Alirocumab=7, RN316/bococizumab=1). Ten studies used PCSK9 inhibitors as monotherapy and another 10 studies were coadministered with statins, ezetimibe, or standard of care. Participants of 16 studies were hypercholesterolemia patients, 2 of these studies contained some heterozygous familial hypercholesterolemia (HeFH) patients. Three studies enrolled HeFH patients and 1 study consisted of homozygous familial hypercholesterolemia patients. Most of the treatment durations ranged from 8 to 24 weeks except for the study of Blom,32 which had treatment durations of as long as 52 weeks.
Figure 1.

Preferred reporting items for systematic review and meta-analysis (PRISMA) flowchart of the process of study selection.
Table 1.
Baseline Characteristics of Trials Included in Meta-Analysis
| Study, Author, Year | Design | Diagnosis | Control | Drug Regimen | Duration | n | Mean Age (y) |
|---|---|---|---|---|---|---|---|
| Roth EM, 2012 18 | M, R, DB, PC, PG | Hypercholesterolemia | Atorvastatin; placebo | A: NR | 8 weeks | 92 | 56.9 (9.8) |
| Stein EA, 2012(1) 19 | M, R, PC, AD, MD | Healthy; HeFH; hypercholesterolemia | Placebo | A: 0.3, 1.0, 3.0, 6.0, 12.0 mg/kg IV and 50, 100, 150, 250 mg SC at days 1, 2, 4, 8, 11, 15, 22, 29, 43, 64, 85, and 106; 50, 100, or 150 mg SC on days 1, 29, and 43 | 106/148 days | 133 | 41.9 |
| Sullivan D, 2012 20 | M, R, DB, PC, EC, DR | Hypercholesterolemia | Placebo; ezetimibe | E: 280, 350, or 420 mg SC q4w | 12 weeks | 157 | 61.8 (8.4) |
| Stein EA, 2012(2) 21 | M, R, DB, PC | HeFH | Placebo | E: 150 mg SC q2w;150, 200, or 300 mg SC q4w | 12 weeks | 77 | 53.4 (9.7) |
| Dias CS, 2012 22 | S, DB, PC, AD | Healthy; hypercholesterolemia, HeFH | Placebo | E: Healthy adults: 7, 21, 70, 210, or 420 mg SC; 21 or 420 mg IV; hypercholesterolemia adults: 14 or 35 mg SC qw; 140 or 280 mg q2w; 420 mg q4w | 80 days | 113 | 44 (8.5) |
| Koren MJ, 2012 23 | M, R, DB, PC | Hypercholesterolemia | Placebo; ezetimibe | E: 70, 105, or 140 mg SC q2w; 280, 350 or 420 mg SC q4w | 12 weeks | 406 | 50.6 (11.8) |
| Cannon CP, 2015 24 | M, R, DB, PG, AC, DD, EC | High cardiovascular risk and elevated LDL-C | Ezetimibe | A: 75 mg SC q2w | 24 weeks | 720 | 61.5 (9.3) |
| Koren MJ, 2014 25 | R, DB, PC | Hypercholesterolemia | Placebo; ezetimibe | E: 140 mg SC q2w or 420 mg q4w | 12 weeks | 614 | 53.3 (11.8) |
| Giugliano RP, 2012 26 | M, R, DB, PC, DR | Hypercholesterolemia | Placebo | E: 70, 105, or 140 mg SC q2w; 280, 350 or 420 mg SC q4w | 12 weeks | 631 | 62.0 |
| Raal F, 2012 27 | M, R, DB, PC, DR | HeFH | Placebo | E: 350 or 420 mg SC q4w | 12 weeks | 167 | 49.6 (12.6) |
| McKenney JM, 2012 28 | S, R, DB, PC, P | Hypercholesterolemia | Placebo | A: 50, 100, or 150 mg SC q2w; 200, 300 mg SC q4w | 12 weeks | 183 | 56.7 (10.0) |
| Robinson JG, 2014 29 | M, R, DB, PC, EC | Hypercholesterolemia and mixed dyslipidemia | Placebo; ezetimibe | E: 140 mg SC q2w or 420 mg SC q4w | 12 weeks | 1896 | 60.1 (9.8) |
| Stroes E, 2014 30 | M, R, DB, PC, EC | Hypercholesterolemia | Placebo; ezetimibe | E: 140 mg SC q2w or 420 mg SC q4w | 12 weeks | 307 | 61.5 (9.8) |
| Hirayama A, 2014 31 | M, R, DB, PC, DR | High risk for cardiovascular events | Placebo | E: 70 or 140 mg SC q2w; 280 or 420 mg q4w | 12 weeks | 307 | 61.5 (9.7) |
| Blom DJ, 2014 32 | M, R, DB, PC | Hyperlipidemia | Placebo | E: 420 mg SC q4w | 52 weeks | 901 | 56.3 (10.5) |
| Roth EM, 2014 33 | M, R, DB, AC, DD | 10-year risk of fatal cardiovascular events ≥1% to b5%, LDL-C 100 to 190 mg/dL | Ezetimibe | A: 75 mg SC q2w with dose up-titrated to 150 mg q2w | 24 weeks | 103 | 60.2 (4.9) |
| Robinson JG, 2015 34 | M, R, DB, PC,PG | LDL-C >70 mg/dL | Placebo | A: 150 mg SC q2w | 24 weeks | 2341 | 60.5 (10.4) |
| Raal F, 2015 35 | M, R, DB, PC | HeFH | Placebo | E: 140 mg SC q2w, 420 mg SC q4w | 12 weeks | 331 | 50.6 (12.7) |
| Raal F, 2015 36 | M, R, DB, PC | HoFH | Placebo | E: 420 mg SC q4w | 12 weeks | 50 | 31 (13) |
| Ballantyne CM, 2015 37 | M, R, DB, PC, DR | LDL-C >80 mg/dL | Placebo | B: 50, 100 or 150 mg SC q2w; 200 or 300 mg SC q4w | 24 weeks | 354 | 60.1 (10.1) |
A indicates Alirocumab/REGN727; AC, active control; AD, ascending dose; B, bococizumab/RN316; DB, double blind; DD, double dummy; DR, dose ranging; E, Evolocumab/AMG145; EC, ezetimibe control; HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous familial hypercholesterolemia; IV, intravenous; LDL-C, low-density lipoprotein cholesterol; M, multicenter; MD, multiple dose; NR, not reported; PC, placebo control; PG, parallel group; q2w, every 2 weeks; q4w, every 4 weeks; qw, once weekly; R, randomized; S, single-center; SC, subcutaneous.
Figure 2.

Risk-of-bias graph: review authors’ judgments about each risk-of-bias item presented as percentages across all included studies.
Lipid-Modifying Effects
Our analysis showed that PCSK9 inhibitors therapy significantly reduced LDL-C levels whether or not in combination with other lipid-lowing therapy. As shown in Figure 3, significant decrease in LDL-C was found in the intervention group; the weighted mean net change was −65.29 mg/dL (95% CI −72.08 to −58.49). Corresponding changes in TC, HDL-C, TG, Apo-B, and Apo-A1 were −60.04 mg/dL (95% CI −69.95 to −50.13) (Figure 4), 3.40 mg/dL (95% CI 3.12 to 3.68) (Figure 5), −12.21 mg/dL (95% CI −16.21 to −8.22) (Figure 6), −41.01 mg/dL (95% CI −46.07 to −35.94) (Figure 7), and 6.75 mg/dL (95% CI 4.64 to 8.86) (Figure 8), respectively. Also, the standardized mean difference change in lipoprotein(a) was −0.94 (95% CI −1.12 to −0.77) (Figure 9).
Figure 3.

Forest plots depicting the effect of PCSK9 monoclonal antibodies on LDL-C. LDL-C indicates low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin9.
Figure 4.
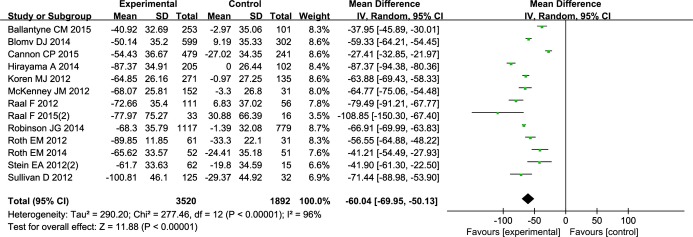
Forest plots depicting the effect of PCSK9 monoclonal antibodies on TC. PCSK9 indicates proprotein convertase subtilisin/kexin9; TC, total cholesterol.
Figure 5.

Forest plots depicting the effect of PCSK9 monoclonal antibodies on HDL-C. HDL-C indicates high-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin9.
Figure 6.

Forest plots depicting the effect of PCSK9 monoclonal antibodies on TG. PCSK9 indicates proprotein convertase subtilisin/kexin9; TG, triglycerides.
Figure 7.

Forest plots depicting the effect of PCSK9 monoclonal antibodies on APO-B. APO-B indicates apolipoprotein-B; PCSK9, proprotein convertase subtilisin/kexin9.
Figure 8.

Forest plots depicting the effect of PCSK9 monoclonal antibodies on APO-A1. APO-A1 indicates apolipoprotein-A1; PCSK9, proprotein convertase subtilisin/kexin9.
Figure 9.

Forest plots depicting the effect of proprotein convertase subtilisin/kexin9 monoclonal antibodies on lipoprotein(a).
We observed significant heterogeneity in the analysis for LDL-C, TC, TG, Apo-B, Apo-A1, and lipoprotein(a) (P<0.00001, I2=96%; P<0.00001, I2=96%; P<0.00001, I2=80%; P<0.00001, I2=96%; P=0.001, I2=63%; P<0.00001, I2=91%, respectively). I2 from the I2 test was ≥50%, so a random-effect model was used. To investigate the potential discrepancy, we divided subgroups by age, methods of treatment, treatment duration, specific drugs, baseline lipid levels, and study quality, but we failed to find any association with these factors. Fix-effect models were chosen to analyze the HDL-C, because low heterogeneity was obtained (P=0.01, I2=49%). We also performed sensitivity analysis; however, the exclusion of any single study did not change the P-value of pooled estimates for either outcome. Heterogeneity was still significant in LDL-C forest plots after grouping trials by methods of treatment (PSCK9 inhibitor monotherapy or combined therapy) (Figure 10), enrolled patients (HeFH or non-HeFH) (Figure 1), and treatment duration (≤12 weeks or >12 weeks) (Figure 2). The LDL-C reduction was more obvious in patients who received PCSK9 inhibitor monotherapy (−69.84 mg/dL, 95% CI −79.9 to −59.78) than coadministered with other therapy (−60.16 mg/dL, 95% CI −70.25 to −50.06). Patients with HeFH (−79.02 mg/dL, 95% CI −99.45 to −58.59) and treatment duration ≤12 weeks (−70.89 mg/dL, 95% CI −77.28 to −64.51) also experienced significant reduction in LDL-C levels. We showed no evidence of publication bias for either outcome as indicated by Egger’s linear regression test and funnel plot results. Most of the cholesterol diversities might be ascribed to clinical dissimilarities, as different dyslipidemia types have varying lipid-modifying effects, and different doses of PCSK9 inhibitors may show varying lipid-lowering effects.
Figure 10.
Forest plots depicting the effect on low-density lipoprotein cholesterol for subgroup analysis after grouping by methods of treatment.
Figure 11.

Forest plots depicting the effect on low-density lipoprotein cholesterol for subgroup analysis after grouping by enrolled patients.
Figure 12.
Forest plots depicting the effect on low-density lipoprotein cholesterol for subgroup analysis after grouping by treatment duration.
Safety and Tolerability Outcomes
The most commonly reported TEAEs in the included studies were nasopharyngitis, upper respiratory tract infections, influenza, cough, headache, and back pain; most of them were mild or moderate in intensity. There were 4048 patients receiving PCSK9 inhibitor and 3416 patients receiving placebo or statins monotherapy who experienced TEAEs (relative risk: 1.01, 95% CI [0.98 to 1.04]) (Figure 3), which implies that there was no significant difference between the 2 groups, and the serious TEAEs between the 2 groups also did not show a difference (relative risk: 1.01, 95% CI 0.88 to 1.17) (Figure 4). No significant heterogeneity was found in the analysis of TEAEs (P=0.03, I2=41%) and serious TEAEs (P=0.99, I2=0), so the fixed model was chosen. There was no significant difference in the rates of discontinuation of the treatment between the 2 groups (relative risk: 1.07, 95% CI 0.86 to 1.34) (Figure 5).
Figure 13.

Forest plot depicting the treatment-emergent adverse events of proprotein convertase subtilisin/kexin9 monoclonal antibodies.
Figure 14.

Forest plots depicting the serious treatment-emergent adverse events of proprotein convertase subtilisin/kexin9 monoclonal antibodies.
Figure 15.

Forest plots depicting the treatment-associated withdrawal.
Discussion
Recently, RCTs indicated that the PCSK9 inhibitors significantly reduced LDL-C levels with or without other lipid-lowing therapies. In the present study, we performed this meta-analysis with a total of 20 studies enrolling 9880 participants with hypercholesterolemia. Our study showed that PCSK9 inhibitor therapy significantly reduced the levels of LDL-C, TC, TG, Apo-B, and lipoprotein(a). We also found that PCSK9 inhibitors not only reduced the absolute LDL-C levels, but also increased the HDL-C and Apo-A1 levels.
The test of heterogeneity of LDL-C, TG, Apo-B, Apo-A1, and lipoprotein(a) failed to reach statistical significance. It may be argued that these studies should not be combined in a meta-analysis because they contained varying interventions and controls. However, the absence of statistical significance does not necessarily rule out clinical diversity in aspects such as study design and dose. Other possible reasons could ascribe the heterogeneity to the selection of enrolled patients, as some enrolled patients had combined statin therapy while others did not. For example, all patients in Stein’s21 study had experienced a 6-week washout or statin stabilization run-in period, while Raal’s27 study did not have the statin run-in period. All patients, whether with or without statin therapy, went into the experimental studies immediately. Thus, Raal’s study obtained a significant reduction in the levels of LDL-C, TC, Apo-B, and lipoprotein(a). Therefore, heterogeneity would be expected. Genetic polymorphisms and dietary habits may also influence the response to PSCK9 inhibitors, but they were reported in the research studies. In terms of safety, PCSK9 inhibitors may lead to many TEAEs, such as injection-site reaction, nasopharyngitis, upper respiratory tract infections, influenza, cough, headache, and so on. While none of these TEAEs is life threatening and the serious TEAEs were not increased more than in the control group, more large RCTs are needed to further confirm the safety. Furthermore, there was also a low rate of TEAEs leading to discontinuation.
The causes of CVD are multifactorial, and one of its main risk factors is dyslipidemia. Current guidelines consequently identify TC and LDL-C level as the primary target for lipid-modifying therapy.3,44 Statins are by far the most effective lipid-lowering drugs to significantly decrease CVD risk by lowering LDL-C levels.45–47 The LDL-C goal is <70 mg/dL and/or a ≥50% LDL-C reduction in very high CVD risk patients when the target levels cannot be reached.48 In order to reach the goal, high-intensity statin therapy is recommended. High-intensity statin was confirmed to be safe and tolerable for most patients, but at least 10% of patients experienced side effects such as myalgia, muscle aches, weakness, or other symptoms. Therefore, some people are unwilling to continue their statin therapy.49–51 For these patients, alternative lipid-lowering therapy must be used to obtain their target LDL-C level.
Our meta-analysis revealed that PCSK9 inhibitor treatment received satisfactory lipid-modifying effects with acceptable safety in patients with familial or nonfamilial hypercholesterolemia. Although the current results are encouraging, some issues should be taken into consideration. First, the monoclonal antibody is injected subcutaneously, making it unattractive for long-term treatment. Second, some animal and cellular studies revealed that LDL-R can act as the entry point for some viruses, including hepatitis C virus. It is unknown whether PCSK9 inhibitors would increase the risk of hepatitis C virus infection.52 Finally, the main concern of hypercholesterolemia is the increased risk for atherosclerotic disease; it still remains unknown whether the reduced LDL-C with PCSK9 inhibitors can finally improve the clinical outcomes. In our meta-analysis, only Koren’s25 phase III clinical trial mentioned that there were no deaths or cardiovascular events in their trial. Hopefully, some large multicenter RCTs are under investigation, which could provide more evidence to prove its long-term effect, safety, and clinical outcomes.53 In conclusion, PCSK9 inhibitors exert excellent effects on the lipid parameters in patients with familial or nonfamilial hypercholesterolemia even in combination with statin therapy. Given the fact that LDL-C reduction is correlated with reduced CVD mortality, PCSK9 inhibition could potentially be another attractive therapeutic option for CVD treatment. The study has some limitations. We observed significant heterogeneities across most of the reported outcomes, but we failed to reveal the heterogeneities by dividing subgroups or sensitivities methods. Therefore, caution should be used in interpreting the results of the meta-analysis when combining the heterogeneous data sets. Hopefully, more RCTs in progress could add new evidence to PSCK9 inhibitors investigations.
Sources of Funding
This work was supported by the grants from the National Natural Science Foundation of China (31430043, 31130029) and the National Basic Research Program of China (973 Program, 2008CB517308, 2012CB517801).
Disclosures
None.
References
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel members. An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia—full report. J Clin Lipidol. 2014;8:29–60. doi: 10.1016/j.jacl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- Waters DD, Brotons C, Chiang CW, Ferrières J, Foody J, Jukema JW, Santos RD, Verdejo J, Messig M, McPherson R, Seung KB, Tarasenko L Lipid Treatment Assessment Project 2 Investigators. A multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120:28–34. doi: 10.1161/CIRCULATIONAHA.108.838466. [DOI] [PubMed] [Google Scholar]
- Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf C, Holme I, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- Blazing MA, Giugliano RP, Cannon CP, Musliner TA, Tershakovec AM, White JA, Reist C, McCagg A, Braunwald E, Califf RM. Evaluating cardiovascular event reduction with ezetimibe as an adjunct to simvastatin in 18,144 patients after acute coronary syndromes: final baseline characteristics of the IMPROVE-IT study population. Am Heart J. 2014;168:205–212. doi: 10.1016/j.ahj.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res. 2012;53:2515–2524. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50 suppl:S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derré A, Villéger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, Davey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta E, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, Prat A. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2004;24:1454–1459. doi: 10.1161/01.ATV.0000134621.14315.43. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, Hutabarat RM, Clausen VA, Karsten V, Cehelsky J, Nochur SV, Kotelianski V, Horton J, Mant T, Chiesa J, Ritter J, Munisamy M, Vaishnaw AK, Gollob JA, Simon A. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–68. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artenstein AW, Opal SM. Proprotein convertases in health and disease. N Engl J Med. 2011;365:2507–2518. doi: 10.1056/NEJMra1106700. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–1900. doi: 10.1056/NEJMoa1201832. [DOI] [PubMed] [Google Scholar]
- Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, Du Y, Kranz T, Gasparino E, Swergold GD. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, Wasserman SM, Stein EA. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497–2506. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- Dias CS, Shaywitz AJ, Wasserman SM, Smith BP, Gao B, Stolman DS, Crispino CP, Smirnakis KV, Emery MG, Colbert A, Gibbs JP, Retter MW, Cooke BP, Uy ST, Matson M, Stein EA. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888–1898. doi: 10.1016/j.jacc.2012.08.986. [DOI] [PubMed] [Google Scholar]
- Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, Bolognese M, Wasserman SM. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995–2006. doi: 10.1016/S0140-6736(12)61771-1. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM for the ODYSSEY COMBO II Investigators. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186–1194. doi: 10.1093/eurheartj/ehv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM, Bays H MENDEL-2 Investigators. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, Liu T, Mohanavelu S, Hoffman EB, McDonald ST, Abrahamsen TE, Wasserman SM, Scott R, Sabatine MS LAPLACE-TIMI 57 Investigators. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–2017. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the reduction of LDL-C with PCSK9 inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R LAPLACE-2 Investigators. Effect of evolocumab or ezetimibe added to moderate or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia the LAPLACE-2 randomized clinical trial. JAMA. 2014;311:1870–1882. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, Xue A, Scott R, Wasserman SM, Rocco M GAUSS-2 Investigators. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–2548. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Hirayama A, Honarpour N, Yoshida M, Yamashita S, Huang F, Wasserman SM, Teramoto T. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk—primary results from the phase 2 YUKAWA study. Circ J. 2014;78:1073–1082. doi: 10.1253/circj.cj-14-0130. [DOI] [PubMed] [Google Scholar]
- Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA DESCARTES Investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, Merlet L, Pordy R, Baccara-Dinet MT. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized phase 3 trial. Int J Cardiol. 2014;176:55–61. doi: 10.1016/j.ijcard.2014.06.049. [DOI] [PubMed] [Google Scholar]
- Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, Shahawy ME, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–340. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA TESLA Investigators. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–350. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Neutel J, Cropp A, Duggan W, Wang EQ, Plowchalk D, Sweeney K, Kaila N, Vincent J, Bays H. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115:1212–1221. doi: 10.1016/j.amjcard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol. 2007;49:1642–1648. doi: 10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- Li C, Zhang W, Zhou F, Chen C, Zhou L, Li Y, Liu L, Pei F, Luo H, Hu Z, Cai J, Zeng C. Cholesteryl ester transfer protein inhibitors in the treatment of dyslipidemia: a systematic review and meta-analysis. PLoS One. 2013;8:e77049. doi: 10.1371/journal.pone.0077049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D. Liberati A, Petticrew M, Shekelle P, Stewart LA the PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon R, Leeflang MM, McDonald S, Eisinga A, Mitchell RL, Whiting P, Glanville JM. Search strategies to identify diagnostic accuracy studies in MEDLINE and EMBASE. Cochrane Database Syst Rev. 2013;9:MR000022. doi: 10.1002/14651858.MR000022.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for Cardiovascular Prevention & Rehabilitation; Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholesterol Treatment Trialists’ (CTT) Collaborators. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- Reiner Z. New ESC/EAS guidelines for the management of dyslipidaemias: any controversies behind the consensus? Eur J Cardiovasc Prev Rehabil. 2011;18:724–727. doi: 10.1177/1741826711418946. [DOI] [PubMed] [Google Scholar]
- LaRosa JC, Pedersen TR, Somaratne R, Wasserman SM. Safety and effect of very low levels of low-density lipoprotein cholesterol on cardiovascular events. Am J Cardiol. 2013;111:1221–1229. doi: 10.1016/j.amjcard.2012.12.052. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D, Dallongeville J, Sabouret P, Bruckert E. Discontinuation of statin therapy due to muscular side effects: a survey in real life. Nutr Metab Cardiovasc Dis. 2013;23:871–875. doi: 10.1016/j.numecd.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K, Aronow WS, Athyros V, Djuric DM, Ezhov MV, Greenfield RS, Hovingh GK, Kostner K, Serban C, Lighezan D, Fras Z, Moriarty PM, Muntner P, Goudev A, Ceska R, Nicholls SJ, Broncel M, Nikolic D, Pella D, Puri R, Rysz J, Wong ND, Bajnok L, Jones SR, Ray KK, Mikhailidis DP. Statin intolerance—an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11:1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed GH, Tang H, Khan M, Hassanein T, Liu J, Siddiqui A. Hepatitis C virus stimulates low-density lipoprotein receptor expression to facilitate viral propagation. J Virol. 2014;88:2519–2529. doi: 10.1128/JVI.02727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragan S, Serban MC, Banach M. Proprotein convertase subtilisin/kexin 9 inhibitors: an emerging lipid-lowering therapy? J Cardiovasc Pharmacol Ther. 2015;20:157–168. doi: 10.1177/1074248414539562. [DOI] [PubMed] [Google Scholar]




