Abstract
Background
Adjunctive thrombus aspiration (TA) during primary percutaneous coronary intervention (PCI) was reported to promote better coronary and myocardial reperfusion. However, long-term mortality benefit of TA remains controversial. The objective of this study is to investigate the clinical impact of TA on long-term clinical outcomes in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary PCI.
Methods and Results
The CREDO-Kyoto AMI Registry is a large-scale cohort study of acute myocardial infarction patients undergoing coronary revascularization in 2005–2007 at 26 hospitals in Japan. Among 5429 patients enrolled in the registry, the current study population consisted of 3536 patients who arrived at the hospital within 12 hours after the symptom onset and underwent primary PCI. Clinical outcomes were compared between the 2 patient groups with or without TA. During primary PCI procedures, 2239 out of 3536 (63%) patients underwent TA (TA group). The cumulative 5-year incidence of all-cause death was significantly lower in the TA group than in the non-TA group (18.5% versus 23.9%, log-rank P<0.001). After adjusting for confounders, however, the risk for all-cause death in the TA group was not significantly lower than that in the non-TA group (hazard ratio: 0.90, 95% CI: 0.76 to 1.06, P=0.21). The adjusted risks for cardiac death, myocardial infarction, stroke, and target-lesion revascularization were also not significantly different between the 2 groups.
Conclusions
Adjunctive TA during primary PCI was not associated with better 5-year mortality in STEMI patients.
Keywords: acute coronary syndrome, coronary artery disease, no reflow, percutaneous coronary intervention, thrombus aspiration
Acute myocardial infarction (AMI) can be called the disease of thrombus: the plaque rupture and the subsequent thrombus formation results in the occlusion of a coronary artery. Therefore, primary percutaneous coronary intervention (PCI) is an established effective therapy for coronary reperfusion in AMI, and adjunctive thrombus aspiration (TA), which was presumed to improve microvascular perfusion, was introduced to reduce distal embolism in daily clinical practice. Several randomized control trials (RCTs) comparing PCI with or without adjunctive TA have reported conflicting results, and the mortality benefit of TA in ST segment elevation acute myocardial infarction (STEMI) patients treated with primary PCI still remains controversial.1–8 The Thrombus Aspiration during Percutaneous Coronary Intervention in Acute Myocardial Infarction Study was one of the largest trials suggesting 1-year mortality benefit of TA.9 On the other hand, the Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia trial has reported comparable 1-year mortality between primary PCI with TA versus PCI only.10 Recently, the Trial of Routine Aspiration Thrombectomy with PCI versus PCI Alone in Patients with STEMI (TOTAL) trial has shown no reduction of the risk of cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or New York Heart Association class IV heart failure within 180 days.11
In an attempt to evaluate whether adjunctive TA has clinical benefits in the real-world clinical practice, we examined the impact of adjunctive TA on long-term cardiovascular outcomes in a large-scale observational database of AMI patients undergoing primary PCI in Japan.
Methods
Study Population
The Coronary Revascularization Demonstrating Outcome study in Kyoto (CREDO-Kyoto) AMI registry is a physician-initiated, non-company-sponsored, multicenter registry enrolling consecutive AMI patients undergoing coronary revascularization within 7 days of symptom onset among 26 centers in Japan between January 2005 and December 2007 (Appendix 1). The relevant review boards or ethics committees in all participating centers approved the research protocol. Because of retrospective enrollment, written informed consents from the patients were waived; however, we excluded those patients who refused to participate in the study when contacted at follow-up. This strategy is concordant with the guidelines for epidemiological studies issued by the Ministry of Health, Labor and Welfare of Japan.
Among 5429 AMI patients enrolled in this registry, the current study population consisted of 3536 STEMI patients who had primary PCI within 12 hours after the onset after excluding 9 patients with refusal for study participation, 195 patients with coronary artery bypass grafting, 789 non-STEMI patients, 738 patients with PCI beyond 12 hours after the symptom onset, and 162 patients whose timing of PCI was unidentified (Figure 1).
Figure 1.
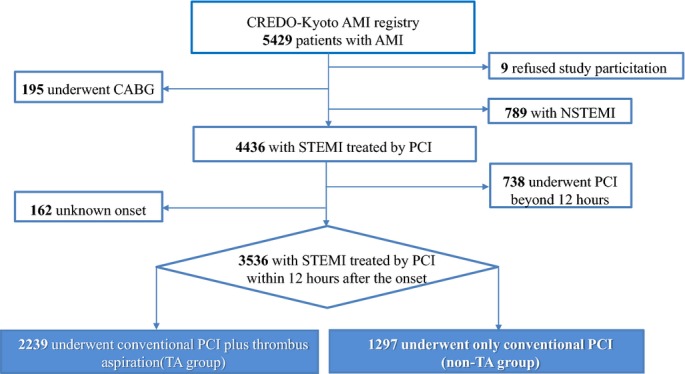
Study flow chart. AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; CREDO-Kyoto AMI Registry, Coronary Revascularization Demonstrating Outcome Study in Kyoto Acute Myocardial Infarction Registry; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TA, thrombus aspiration.
Definitions and End Points
The primary outcome measure for the current analysis was all-cause death. Secondary outcome measures included cardiac death, noncardiac death, myocardial infarction (MI), stent thrombosis, stroke, and target-lesion revascularization. Death was regarded as cardiac in origin unless obvious noncardiac causes could be identified. MI was defined according to the Arterial Revascularization Therapy Study.12 Stent thrombosis was defined according to the Academic Research Consortium definition.13 Target-lesion revascularization was defined as either repeated percutaneous or surgical revascularization for a lesion anywhere within the stent or the 5-mm borders proximal or distal to the stent. The detailed definitions of baseline clinical characteristics were described previously.14
Data Collection for Baseline Characteristics and Follow-up Events
Demographic, angiographic, and procedural data were collected from hospital charts or hospital databases according to the prespecified definitions by experienced clinical research coordinators from the study management center (Research Institute for Production Development, Kyoto, Japan) (Appendix 1). In this retrospective cohort study, data collection for follow-up events was performed in 2010 and 2012. Collection of follow-up information was mainly conducted through review of inpatient and outpatient hospital charts by the clinical research coordinators, and additional follow-up information was collected through contact with patients, relatives, and/or referring physicians by sending mail with questions regarding vital status, subsequent hospitalizations, and status of antiplatelet therapy. Death, MI, stent thrombosis, and stroke were adjudicated by the clinical event committee (Appendix 1). Median follow-up duration was 1843 (interquartile range: 1496 to 2157) days. Complete 1-, 3-, and 5-year follow-up information was obtained in 98%, 95%, and 64% of patients.
Statistical Analysis
Categorical variables were presented as numbers and percentages and compared using the χ2 test or Fisher exact test. Continuous variables were presented as the mean and SD or the median and interquartile range. Continuous variables were compared using the Student t test or the Wilcoxon rank sum test based on their distributions. Cumulative incidences were estimated by the Kaplan–Meier method and differences were evaluated with the log-rank test. The effect of the TA group as compared with the non-TA group was expressed as hazard ratio (HR) and their 95% CI. Multivariable Cox proportional hazard models were employed to assess the HR of the TA group as compared with the non-TA group, adjusting for 41 clinically relevant factors listed in Table1. In addition, we computed the adjusted event curves of the 2 groups using the methods described by Ghali et al15 Consistent with our previous reports, continuous variables were dichotomized by clinically meaningful reference values or median values.14 We also evaluated the effect of TA on the primary outcome measure in several clinically relevant subgroups stratified by age (≥75 years or <75 years), gender (male or female), diabetes mellitus (with or without diabetes mellitus), total ischemic time (0 to 2, 2 to 6, 6 to 12 hours), culprit lesion (left anterior descending culprit or non–left anterior descending culprit), initial Thrombolysis In Myocardial Infarction (TIMI) flow grade (Thrombolysis In Myocardial Infarction flow grade 0 or Thrombolysis In Myocardial Infarction flow grade ≥1), and hemodynamic status (Killip 1 to 3 or Killip 4). Multivariable Cox proportional hazard models were similarly developed for the subgroup analysis. In addition to the adjunctive TA use, 24 variables with P<0.05 in the previously described full model were included in the multivariable models for the subgroup analysis, reflecting our preference for parsimonious models to avoid overfitting. All statistical analyses were conducted using JMP version 10.0.2 (SAS Institute Inc, Cary, NC) or SAS version 9.3 (SAS Institute Inc). All the statistical analyses were 2-tailed and P values <0.05 were considered statistically significant.
Table 1.
Baseline Patient Characteristics-TA Group Versus Non-TA Group
| Variables | TA Group N=2239 | Non-TA Group N=1297 | P Value |
|---|---|---|---|
| Clinical characteristics | |||
| Age | 66.6±12 | 68.9±12.1 | <0.001 |
| >75 years*† | 640 (28.6%) | 451 (34.8%) | <0.001 |
| Male gender* | 1700 (75.9%) | 933 (71.9%) | 0.009 |
| Body mass index | 23.8±3.5 | 23.3±3.4 | <0.001 |
| <25.0 kg/m2*† | 1557 (69.5%) | 977 (75.3%) | <0.001 |
| Hypertension*† | 1749 (78.1%) | 1011 (77.9%) | 0.91 |
| Diabetes mellitus | 659 (29.4%) | 459 (35.4%) | <0.001 |
| On insulin therapy*† | 83 (3.7%) | 72 (5.6%) | 0.01 |
| Current smoking* | 953 (42.6%) | 492 (37.9%) | 0.007 |
| Previous heart failure*† | 686 (30.6%) | 422 (32.5%) | 0.24 |
| Multivessel disease*† | 1054 (47.1%) | 738 (56.9%) | <0.001 |
| Mitral regurgitation 3-4/4*† | 55 (2.5%) | 43 (3.3%) | 0.14 |
| Previous myocardial infarction* | 196 (8.8%) | 129 (9.9%) | 0.24 |
| Previous stroke*† | 175 (7.8%) | 136 (10.5%) | 0.008 |
| Peripheral vascular disease* | 65 (2.9%) | 42 (3.2%) | 0.58 |
| Previous PCI or CABG | 215 (9.6%) | 121 (9.3%) | 0.79 |
| eGFR <30, without hemodialysis*† | 79 (3.5%) | 62 (4.8%) | 0.07 |
| Hemodialysis*† | 19 (0.9%) | 29 (2.2%) | <0.001 |
| Atrial fibrillation*† | 224 (10.0%) | 114 (8.8%) | 0.23 |
| Anemia (hemoglobin <11.0 g/dL)* | 185 (8.3%) | 136 (10.5%) | 0.03 |
| Thrombocytopenia (platelet <100×109/L)*† | 42 (1.9%) | 28 (2.2%) | 0.56 |
| COPD* | 69 (3.1%) | 41 (3.2%) | 0.90 |
| Liver cirrhosis*† | 52 (2.3%) | 32 (2.5%) | 0.79 |
| Malignancy*† | 173 (7.7%) | 118 (9.1%) | 0.16 |
| Presentation | |||
| Killip class ≤2 | 1873 (83.7%) | 1053 (81.2%) | 0.06 |
| Killip class 4*† | 324 (14.5%) | 206 (15.9%) | 0.26 |
| Initial TIMI flow grade=0* | 1620 (72.4%) | 664 (51.2%) | <0.001 |
| Total ischemic time (median hours) | 2.0 (1.0 to 3.9) | 2.3 (1.1 to 4.4) | 0.004 |
| IABP use | 369 (16.5%) | 218 (16.8%) | 0.80 |
| PCPS use | 62 (2.8%) | 39 (3.0%) | 0.68 |
| Lesion and procedural characteristics | |||
| Infarcted area | <0.001 | ||
| Anterior wall | 961 (42.9%) | 701 (54.0%) | |
| Inferior wall | 1066 (47.6%) | 397 (30.6%) | |
| Lateral wall | 36 (1.6%) | 73 (5.6%) | |
| Unprotected LMCA | 79 (3.5%) | 84 (6.5%) | <0.001 |
| Chronic total occlusion | 207 (9.3%) | 171 (13.2%) | <0.001 |
| Target lesion | |||
| Unprotected LMCA*† | 62 (2.8%) | 60 (4.6%) | 0.004 |
| Proximal LAD* | 1146 (51.2%) | 767 (59.1%) | <0.001 |
| LAD | 1184 (52.9%) | 825 (63.6%) | <0.001 |
| LCX | 394 (17.6%) | 278 (21.4%) | <0.001 |
| RCA | 1188 (53.1%) | 522 (40.2%) | <0.001 |
| Bifurcated lesion*† | 533 (23.8%) | 383 (29.5%) | <0.001 |
| Chronic total occlusion* | 61 (2.7%) | 50 (3.9%) | 0.07 |
| Side-branch stenting* | 58 (2.6%) | 52 (4.0%) | 0.02 |
| Implanted stents | |||
| Mean±SD | 1.6±1.0 | 1.8±1.2 | <0.001 |
| Median (IQR) | 1 (1 to 2) | 1 (1 to 2) | |
| Total stent length | |||
| Mean±SD | 34.0±23.1 | 36.8±27.7 | 0.48 |
| Median (IQR) | 24 (18 to 42) | 27 (18 to 44) | |
| >28 mm* | 839 (40.4%) | 517 (44.6%) | 0.02 |
| Minimal stent diameter | |||
| Mean±SD | 3.1±0.5 | 2.9±0.4 | <0.001 |
| Median (IQR) | 3.0 (3.0 to 3.5) | 3.0 (2.5 to 3.0) | |
| <3.0 mm* | 513 (24.7%) | 476 (41.1%) | <0.001 |
| Distal protection | 249 (11.1%) | 26 (2.0%) | <0.001 |
| Medication at discharge | |||
| Aspirin | 2210 (98.7%) | 1272 (98.1%) | 0.15 |
| Thienopyridine | 2157 (96.3%) | 1204 (92.8%) | <0.001 |
| Cilostazole* | 823 (36.8%) | 448 (34.5%) | 0.19 |
| Statin*† | 1220 (54.5%) | 671 (51.7%) | 0.11 |
| ACE-I/ARB*† | 1654 (73.9%) | 898 (69.2%) | 0.003 |
| β-Blocker*† | 946 (42.3%) | 517 (39.9%) | 0.16 |
| Calcium channel blocker* | 397 (17.7%) | 307 (23.7%) | <0.001 |
| Nitrate*† | 622 (27.8%) | 402 (31.0%) | 0.04 |
| Nicorandil*† | 595 (26.6%) | 406 (31.3%) | 0.003 |
| Warfarin* | 264 (11.8%) | 123 (9.5%) | 0.03 |
| PPI* | 786 (35.1%) | 406 (31.3%) | 0.02 |
| H2 blocker* | 760 (33.9%) | 429 (33.1%) | 0.60 |
Categorical variables are expressed as number (%) unless otherwise indicated. Continuous variables are shown as mean±SD or median (interquartile range). ACE-I/ARB indicates angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pumping; IQR, interquartile range; LAD, left anterior descending; LCX, left circumflex; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; PCPS, percutaneous cardiopulmonary support; PPI, proton-pump inhibitor; RCA, right coronary artery; TA, thrombus aspiration; TIMI, thrombolysis in myocardial infarction.
Potential independent variables selected for multivariable analysis.
Potential independent variables selected for multivariable analysis in the specific subgroups.
Results
Among 3536 STEMI patients with primary PCI in the current study, 2239 patients (63%) received adjunctive TA during primary PCI (TA group). Baseline characteristics differed significantly in several aspects between the TA and the non-TA group (Table1).
The cumulative 5-year incidence of all-cause death was significantly lower in the TA group than in the non-TA group (18.5% versus 23.9%, log-rank P<0.001) (Table2 and Figure 2). However, after adjusting for confounders, the adjusted risk of the TA group relative to the non-TA group for all-cause death was not significantly different (HR: 0.90, 95% CI: 0.76 to 1.06, P=0.21) (Table2). Similarly, the adjusted risks for cardiac death, noncardiac death, and target-lesion revascularization were not significantly different between the 2 groups (HR: 0.99, 95% CI: 0.79 to 1.24, P=0.91; HR: 0.78, 95% CI: 0.62 to 1.03, P=0.08; and HR: 0.90, 95% CI: 0.76 to 1.07, P=0.23, respectively), although the cumulative 5-year incidences of cardiac death, noncardiac death, and target-lesion revascularization were significantly lower in the TA group (11.1% versus 14.5%, log-rank P=0.01, 8.3% versus 11.0%, log-rank P<0.001, and 21.6% versus 25.8%, log-rank P=0.007, respectively) (Table2). The cumulative 5-year incidences of and the adjusted risks for MI, stroke, and stent thrombosis were not significantly different between the TA and non-TA group (Table2).
Table 2.
Crude and Adjusted 5-Year Clinical Outcomes TA Group Versus Non-TA Group
| TA Group | Non-TA Group | Crude HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| No. of Events (Cumulative Incidence) N=2239 | No. of Events (Cumulative Incidence) N=1297 | |||||
| All-cause death | 393 (18.5%) | 297 (23.9%) | 0.74 (0.67 to 0.86) | <0.001 | 0.90 (0.76 to 1.06) | 0.21 |
| Cardiac death | 239 (11.1%) | 180 (14.5%) | 0.78 (0.65 to 0.95) | 0.01 | 0.99 (0.79 to 1.24) | 0.91 |
| Noncardiac death | 154 (8.3%) | 117 (11.0%) | 0.68 (0.54 to 0.85) | <0.001 | 0.78 (0.62 to 1.03) | 0.08 |
| Myocardial infarction | 115 (5.9%) | 78 (7.1%) | 0.83 (0.63 to 1.09) | 0.18 | 0.88 (0.65 to 1.20) | 0.42 |
| Stent thrombosis | 55 (2.6%) | 33 (2.9%) | 0.91 (0.60 to 1.40) | 0.68 | 0.92 (0.59 to 1.45) | 0.71 |
| Stroke | 108 (5.5%) | 77 (7.0%) | 0.74 (0.56 to 0.98) | 0.03 | 0.79 (0.58 to 1.10) | 0.16 |
| TLR | 436 (21.6%) | 294 (25.8%) | 0.82 (0.71 to 0.95) | 0.01 | 0.90 (0.76 to 1.07) | 0.23 |
Cumulative incidence was estimated by the Kaplan–Meier method. HR indicates hazard ratio; TA, thrombus aspiration; TLR, target-lesion revascularization.
Figure 2.
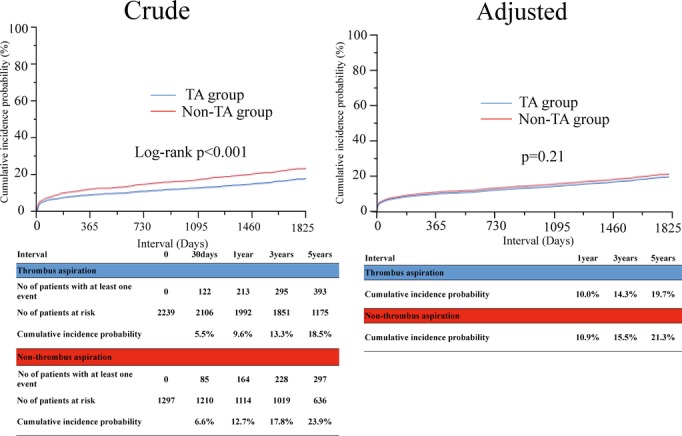
Crude and adjusted Kaplan–Meier curves for cumulative incidence of all-cause death. TA indicates thrombus aspiration.
The comparable adjusted risk for all-cause death between the TA and non-TA groups was consistently observed across subgroups stratified by gender, diabetes mellitus, and location of culprit lesion (Figure 3). In the subgroups of patients <75 years of age, total ischemic time 0 to 2 hours, initial thrombolysis in myocardial infarction flow grade 1 to 3, and Killip class 4, the adjusted risk for all-cause death in the TA group was significantly lower than that in the non-TA group. However, there was not a significant interaction between those 4 subgroup factors and the effect of TA on the risk for all-cause death (Figure 3).
Figure 3.
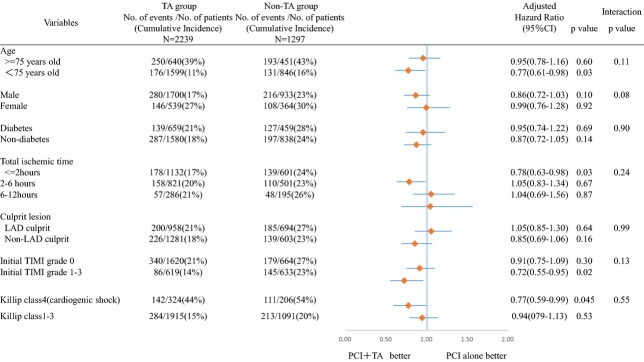
Subgroup analyses and forest plots of hazard ratio for all-cause death. LAD indicates left anterior descending; PCI, percutaneous coronary intervention; TA, thrombus aspiration; TIMI, thrombolysis in myocardial infarction.
Discussion
The main finding of the current analysis is that mortality benefit of adjunctive TA during primary PCI was not observed in STEMI patients with primary PCI in real-world clinical practice. Several RCTs reported the benefits of adjunctive use of TA during primary PCI.4,7,9 The Thrombus Aspiration during Percutaneous Coronary Intervention in Acute Myocardial Infarction Study trial demonstrated significantly lower 1-year mortality by TA use. Reflecting these results, the current STEMI guidelines recommend the use of adjunctive TA during primary PCI as class IIa indication with a level of evidence B.16 Most of these trials, however, did not have adequate power to detect mortality benefit of TA and evaluated surrogate end points such as myocardial blush grade or resolution of ST-segment elevation instead of mortality.4–8 Indeed, the recent 3 RCTs reported the absence of clinical benefit of TA in STEMI patients with primary PCI.2,3,11 First, the INFUSE-AMI trial, comparing primary PCI plus adjunctive TA with primary PCI alone in 452 STEMI patients, reported no benefit of TA use in terms of infarct size at 30 days assessed by cardiac magnetic resonance imaging.3 Second, the Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia trial is a multicenter, randomized-controlled clinical trial assessing the mortality benefit of TA with adequate power (enrolling 7244 patients) and characterized by using the national comprehensive registry. The Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia trial failed to show that routine TA could reduce 1-year mortality of STEMI patients treated with primary PCI.10 Finally, in the TOTAL trial, which has been the most recently presented, routine TA plus primary PCI, as compared with conventional PCI alone, did not reduce the risk of cardiovascular death, recurrent MI, cardiogenic shock, or class IV heart failure within 180 days. The finding of the TOTAL trial concerning the mortality benefit of thrombectomy is consistent with that of the Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia trial.11 Moreover, another important finding in the TOTAL trial is that routine TA was associated with a significantly higher rate of stroke. In this respect, previous studies including trials of rheolytic thrombectomy reported a similar finding.17,18 Certainly the mechanism of stroke might be embolization of thrombus or air during the procedure, but the explanation sounded unreasonable because the period of a continued increase in the rate of stroke was between 30 and 180 days, but not within 24 hours after the procedure. As the possibility of a chance finding as the explanation cannot be eliminated because of the relatively small number of events, further studies should be warranted to clarify the safety of TA for stroke risk. In spite of the TOTAL trial, there is no denying that the procedure of TA has the potential to make intervention easier in selected cases without any complex manipulation.1,4–6,19 However, judging from the results of major trials including the safety concern about potential stroke risk, prudent attitudes should be taken toward the procedure in daily clinical practice.3,10,11,17–19
Several previous observational studies in real-world clinical practice reported the mortality benefit of TA.20–23 Consistent with the findings of the 3 RCTs, however, long-term mortality benefit of TA during primary PCI could not be observed in the current study, reflecting real-world clinical practice. The possibility cannot be ruled out that TA might be beneficial in high-risk patients excluded from the trials, but in our analysis, the benefit of TA could not be observed in any subsets of patients, including high-risk patients such as elderly people or cardiogenic-shock cases. As in the INFUSE-AMI trial, the efficacy of TA was evaluated according to the total ischemic time as subgroup analysis in our study. In the patients with total ischemic time 0 to 2 hours, the adjusted risk for all-cause death in the TA group was significantly lower than that in the non-TA group, but there was not a significant interaction between the total ischemic time and the effect of TA. Therefore, a mortality benefit of adjunctive TA cannot be expected in most STEMI patients undergoing primary PCI in the current clinical practice, where the management of STEMI patients has achieved great improvement with respect to both reperfusion therapy and adjunctive medical therapy.
Clinical Implications
The 3 latest RCTs demonstrated no clinical benefit of routine TA, the results of which are consistent with those of the current analysis. From these clinical trials, the recommendation of routine TA in the current guideline should be reconsidered. However, the clinical efficacy of TA cannot be totally denied, and further investigation evaluating the clinical benefit of selective TA should be performed. In some selective cases, TA could facilitate the primary PCI procedure by more clearly delineating the true lesion length for appropriate stenting. In addition, one of the most important findings in the TOTAL trial is an increased rate of stroke. The safety concern about stroke associated with TA should also be investigated in future studies.
Limitations
Our study has several limitations. First, this is not a RCT but an observational study. The indication of TA was at the discretion of the operator or of the hospital, so that outcomes might be affected by the effect of the operator’s skill or of the hospital’s practice level. In addition, baseline patient characteristics differed significantly between the TA and non-TA groups. Despite the appropriate statistical adjustment for potential confounders, unmeasured confounding factors might have influenced the results of the current study. Second, the current study did not evaluate detailed angiographic findings such as thrombus burden or myocardial blush grade. Third, as glycoprotein IIa/IIIb inhibitors are not currently available in Japan, much caution is required in generalizing these results to patients outside Japan.
Conclusions
Adjunctive TA during primary PCI was not associated with better 5-year mortality in STEMI patients with primary PCI.
Acknowledgments
We appreciate the support and collaboration of the co-investigators participating in the CREDO-Kyoto AMI registry. We are indebted to the outstanding effort of the clinical research coordinators for data collection.
Appendix
List of Participating Centers and Investigators for the CREDO-Kyoto AMI Registry
Kyoto University Hospital: Takeshi Kimura, Ryuzo Sakata, Akira Marui; Kishiwada City Hospital: Mitsuo Matsuda, Hirokazu Mitsuoka, Masahiko Onoe; Tenri Hospital: Yoshihisa Nakagawa, Kazuo Yamanaka; Hyogo Prefectural Amagasaki Hospital: Hisayoshi Fujiwara, Yoshiki Takatsu, Nobuhisa Ohno; Kitano Hospital: Ryuji Nohara; Koto Memorial Hospital: Tomoyuki Murakami, Teruki Takeda; Kokura Memorial Hospital: Masakiyo Nobuyoshi, Masashi Iwabuchi, Michiya Hanyu; Maizuru Kyosai Hospital: Ryozo Tatami, Tsutomu Matsushita; Nara Hospital, Kinki University Faculty of Medicine: Manabu Shirotani, Noboru Nishiwaki; Kobe City Medical Center General Hospital: Toru Kita, Yutaka Furukawa, Yukikatsu Okada; Nishi-Kobe Medical Center: Hiroshi Kato, Hiroshi Eizawa; Kansai Denryoku Hospital: Katsuhisa Ishii; Osaka Red Cross Hospital: Masaru Tanaka, Shogo Nakayama; University of Fukui Hospital: Jong-Dae Lee, Akira Nakano, Takaaki Koshiji, Koichi Morioka; Shizuoka City Shizuoka Hospital: Akinori Takizawa, Mitsuomi Shimamoto, Fumio Yamazaki; Hamamatsu Rosai Hospital: Masaaki Takahashi, Junichiro Nishizawa; Shiga University of Medical Science Hospital: Minoru Horie, Hiroyuki Takashima; Japanese Red Cross Wakayama Medical Center: Takashi Tamura, Masaki Aota; Shimabara Hospital: Mamoru Takahashi, Takafumi Tabata; Kagoshima University Medica and Dental Hospital: Chuwa Tei, Shuichi Hamasaki, Yutaka Imoto, Hiroyuki Yamamoto; Shizuoka General Hospital: Hirofumi Kambara, Osamu Doi, Katsuhiko Matsuda, Masafumi Nara; Kurashiki Central Hospital: Kazuaki Mitsudo, Kazushige Kadota, Tatsuhiko Komiya; Mitsubishi Kyoto Hospital: Shinji Miki, Tetsu Mizoguchi, Hiroyuki Nakajima; Kumamoto University Hospital: Hisao Ogawa, Seigo Sugiyama, Michio Kawasuji, Syuji Moriyama; Shimada Municipal Hospital: Ryuichi Hattori, Takeshi Aoyama, Makoto Araki; Juntendo University Shizuoka Hospital: Satoru Suwa, Keiichi Tanbara.
List of Clinical Research Coordinators
Research Institute for Production Development: Kumiko Kitagawa, Misato Yamauchi, Naoko Okamoto, Yumika Fujino, Saori Tezuka, Asuka Saeki, Miya Hanazawa, Yuki Sato, Chikako Hibi, Hitomi Sasae, Emi Takinami, Yuriko Uchida, Yuko Yamamoto, Satoko Nishida, Mai Yoshimoto, Sachiko Maeda, Izumi Miki, Saeko Minematsu.
List of Clinical Event Committee Members
Mitsuru Abe (Kyoto Medical Center), Hiroki Shiomi (Kyoto University Hospital), Tomohisa Tada (Kyoto University Hospital), Junichi Tazaki (Kyoto University Hospital), Yoshihiro Kato (Kyoto University Hospital), Mamoru Hayano (Kyoto University Hospital), Akihiro Tokushige (Kyoto University Hospital), Masahiro Natsuaki (Kyoto University Hospital), Tetsu Nakajima (Kyoto University Hospital).
Sources of Funding
The CREDO-Kyoto AMI Registry was supported by the Ministry of Health, Labor and Welfare, Japan and Pharmaceuticals and Medical Devices Agency, Japan.
Disclosures
None.
References
- Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–567. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK, Trial T. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- Stone GW, Maehara A, Witzenbichler B, Godlewski J, Parise H, Dambrink JH, Ochala A, Carlton TW, Cristea E, Wolff SD, Brener SJ, Chowdhary S, El-Omar M, Neunteufl T, Metzger DC, Karwoski T, Dizon JM, Mehran R, Gibson CM Investigators I-A. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. 2012;307:1817–1826. doi: 10.1001/jama.2012.421. [DOI] [PubMed] [Google Scholar]
- Ikari Y, Sakurada M, Kozuma K, Kawano S, Katsuki T, Kimura K, Suzuki T, Yamashita T, Takizawa A, Misumi K, Hashimoto H, Isshiki T Investigators V. Upfront thrombus aspiration in primary coronary intervention for patients with st-segment elevation acute myocardial infarction: report of the VAMPIRE (VAcuuM asPIration thrombus REmoval) trial. JACC Cardiovasc Interv. 2008;1:424–431. doi: 10.1016/j.jcin.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Silva-Orrego P, Colombo P, Bigi R, Gregori D, Delgado A, Salvade P, Oreglia J, Orrico P, de Biase A, Piccalo G, Bossi I, Klugmann S. Thrombus aspiration before primary angioplasty improves myocardial reperfusion in acute myocardial infarction: the DEAR-MI (Dethrombosis to Enhance Acute Reperfusion in Myocardial Infarction) study. J Am Coll Cardiol. 2006;48:1552–1559. doi: 10.1016/j.jacc.2006.03.068. [DOI] [PubMed] [Google Scholar]
- Burzotta F, Trani C, Romagnoli E, Mazzari MA, Rebuzzi AG, De Vita M, Garramone B, Giannico F, Niccoli G, Biondi-Zoccai GG, Schiavoni G, Mongiardo R, Crea F. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol. 2005;46:371–376. doi: 10.1016/j.jacc.2005.04.057. [DOI] [PubMed] [Google Scholar]
- Sardella G, Mancone M, Bucciarelli-Ducci C, Agati L, Scardala R, Carbone I, Francone M, Di Roma A, Benedetti G, Conti G, Fedele F. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol. 2009;53:309–315. doi: 10.1016/j.jacc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Migliorini A, Stabile A, Rodriguez AE, Gandolfo C, Rodriguez Granillo AM, Valenti R, Parodi G, Neumann FJ, Colombo A, Antoniucci D Investigators JT. Comparison of AngioJet rheolytic thrombectomy before direct infarct artery stenting with direct stenting alone in patients with acute myocardial infarction. The JETSTENT trial. J Am Coll Cardiol. 2010;56:1298–1306. doi: 10.1016/j.jacc.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Vlaar PJ, Svilaas T, van der Horst IC, Diercks GFH, Fokkema ML, de Smet BJGL, van den Heuvel AFM, Anthonio RL, Jessurun GA, Tan E-S, Suurmeijer AJH, Zijlstra F. Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- Lagerqvist B, Frobert O, Olivecrona GK, Gudnason T, Maeng M, Alstrom P, Andersson J, Calais F, Carlsson J, Collste O, Gotberg M, Hardhammar P, Ioanes D, Kallryd A, Linder R, Lundin A, Odenstedt J, Omerovic E, Puskar V, Todt T, Zelleroth E, Ostlund O, James SK. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111–1120. doi: 10.1056/NEJMoa1405707. [DOI] [PubMed] [Google Scholar]
- Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, Kedev S, Thabane L, Stankovic G, Moreno R, Gershlick A, Chowdhary S, Lavi S, Niemela K, Steg PG, Bernat I, Xu Y, Cantor WJ, Overgaard CB, Naber CK, Cheema AN, Welsh RC, Bertrand OF, Avezum A, Bhindi R, Pancholy S, Rao SV, Natarajan MK, Ten Berg JM, Shestakovska O, Gao P, Widimsky P, Dzavik V Investigators T. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389–1398. doi: 10.1056/NEJMoa1415098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- Shiomi H, Nakagawa Y, Morimoto T, Furukawa Y, Nakano A, Shirai S, Taniguchi R, Yamaji K, Nagao K, Suyama T, Mitsuoka H, Araki M, Takashima H, Mizoguchi T, Eisawa H, Sugiyama S, Kimura T Investigators CR-KA. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: observational study. BMJ. 2012;344:e3257. doi: 10.1136/bmj.e3257. [DOI] [PubMed] [Google Scholar]
- Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, Galbraith PD, Knudtson ML. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- American College of Emergency P, Society for Cardiovascular A, Interventions. O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Ali A, Cox D, Dib N, Brodie B, Berman D, Gupta N, Browne K, Iwaoka R, Azrin M, Stapleton D, Setum C, Popma J Investigators A. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J Am Coll Cardiol. 2006;48:244–252. doi: 10.1016/j.jacc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Tamhane UU, Chetcuti S, Hameed I, Grossman PM, Moscucci M, Gurm HS. Safety and efficacy of thrombectomy in patients undergoing primary percutaneous coronary intervention for acute ST elevation MI: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2010;10:1017. doi: 10.1186/1471-2261-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbhani DJ, Bavry AA, Desai MY, Bangalore S, Byrne RA, Jneid H, Bhatt DL. Aspiration thrombectomy in patients undergoing primary angioplasty: totality of data to 2013. Catheter Cardiovasc Interv. 2014;84:973–977. doi: 10.1002/ccd.25532. [DOI] [PubMed] [Google Scholar]
- Noman A, Egred M, Bagnall A, Spyridopoulos I, Jamieson S, Ahmed J. Impact of thrombus aspiration during primary percutaneous coronary intervention on mortality in ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:3054–3061. doi: 10.1093/eurheartj/ehs309. [DOI] [PubMed] [Google Scholar]
- Nakatani D, Sato H, Sakata Y, Mizuno H, Shimizu M, Suna S, Nanto S, Hirayama A, Ito H, Fujii K, Hori M. Effect of intracoronary thrombectomy on 30-day mortality in patients with acute myocardial infarction. Am J Cardiol. 2007;100:1212–1217. doi: 10.1016/j.amjcard.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Kikkert WJ, Claessen BE, van Geloven N, Baan J, Jr, Vis MM, Koch KT, Piek JJ, Tijssen JG, Henriques JP. Adjunctive thrombus aspiration versus conventional percutaneous coronary intervention in ST-elevation myocardial infarction. Catheter Cardiovasc Interv. 2013;81:922–929. doi: 10.1002/ccd.24592. [DOI] [PubMed] [Google Scholar]
- Hachinohe D, Jeong MH, Saito S, Kim MC, Cho KH, Ahmed K, Hwang SH, Lee MG, Sim DS, Park KH, Kim JH, Hong YJ, Ahn Y, Kang JC, Kim JH, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi D, Cho MC, Kim CJ, Seung KB, Chung WS, Jang YS, Rha SW, Bae JH, Park SJ Korea Acute Myocardial Infarction Registry I. Clinical impact of thrombus aspiration during primary percutaneous coronary intervention: results from Korea Acute Myocardial Infarction Registry. J Cardiol. 2012;59:249–257. doi: 10.1016/j.jjcc.2011.12.005. [DOI] [PubMed] [Google Scholar]


