Abstract
Background
Patient sex and age may influence rates of death after receiving an implantable cardioverter-defibrillator for primary prevention. Differences in outcomes other than mortality and whether these differences vary by heart failure symptoms, etiology, and left ventricular ejection fraction are not well characterized.
Methods and Results
We studied 2954 patients with left ventricular ejection fraction ≤0.35 undergoing first-time implantable cardioverter-defibrillator for primary prevention within the Cardiovascular Research Network; 769 patients (26%) were women, and 2827 (62%) were aged >65 years. In a median follow-up of 2.4 years, outcome rates per 1000 patient-years were 109 for death, 438 for hospitalization, and 111 for heart failure hospitalizations. Procedure-related complications occurred in 8.36%. In multivariable models, women had significantly lower risks of death (hazard ratio 0.67, 95% CI 0.56 to 0.80) and heart failure hospitalization (hazard ratio 0.82, 95% CI 0.68 to 0.98) and higher risks for complications (hazard ratio 1.38, 95% CI 1.01 to 1.90) than men; patients aged >65 years had higher risks of death (hazard ratio 1.55, 95% CI 1.30 to 1.86) and heart failure hospitalization (hazard ratio 1.25, 95% CI 1.05 to 1.49) than younger patients. Age and sex differences were generally consistent in strata according to symptoms, etiology, and severity of left ventricular systolic dysfunction, except the higher risk of complications in women, which differed by New York Heart Association classification (P=0.03 for sex–New York Heart Association interaction), and the risk of heart failure hospitalization in older patients, which differed by etiology of heart failure (P=0.05 for age–etiology interaction).
Conclusions
The burden of adverse outcomes after receipt of an implantable cardioverter-defibrillator for primary prevention is substantial and varies according to patient age and sex. These differences in outcome generally do not vary according to baseline heart failure characteristics.
Keywords: elderly, implanted cardioverter-defibrillator, prognosis, women
Although implantable cardioverter-defibrillators (ICDs) are recommended to prevent cardiac death in selected patients with left ventricular systolic dysfunction (LVSD),1 controversies remain about the outcomes of this therapy in important patient subgroups, including women and elderly patients. The clinical trials that support existing guideline recommendations for primary-prevention ICD therapy2–4 enrolled relatively few women and patients who were younger than those typically seen in clinical practice.5 Studies of age and sex differences in outcomes have focused primarily on overall risks of death, with less attention to broader outcomes including hospitalizations; have assessed short-term outcomes; or have enrolled patients from single centers, limiting generalizability.6–12 Furthermore, the published literature has not explored the extent to which age and sex differences in outcomes vary according to measures of heart failure severity.
Accordingly, we studied outcomes in the Cardiovascular Research Network (CVRN) Longitudinal Study of Implantable Cardioverter-Defibrillators (LS-ICD), a contemporary community-based cohort receiving ICD therapy for primary prevention, with a focus on death, hospitalization, and complications in women and older patients. Our objectives were to characterize the risks of adverse outcomes in this cohort; to understand sex- and age-related differences in these outcomes; and to determine whether sex- and age-related differences vary as a function of characteristics of heart failure, including etiology, symptom burden, and severity of LVSD.
Methods
Data Sources
The CVRN LS-ICD has been described in detail previously.5 The data for this study were derived from 2 sources. Data on ICD eligibility, clinical characteristics, and device and provider details were collected from the National Cardiovascular Data Registry (NCDR) ICD Registry, a national registry that was formed as part of a coverage-with-evidence requirement of the Centers for Medicare and Medicaid Services.13 The NCDR implements a rigorous data-quality program that includes data and range checks, outlier analysis, and random audits.14 Data on outcomes including device-related complications, hospitalization, and death were obtained from the CVRN Virtual Data Warehouse (VDW), a standardized repository of clinically enriched administrative data generated in a distributed fashion using identical protocols at each participating health plan.15 Institutional review boards at participating sites approved the study. Waiver of informed consent was obtained because of the nature of the study. F.A.M., L.M.R., and K.A.G. had access to and affirm the accuracy of all data.
Study Cohort
We identified 3649 consecutive adult patients (aged >18 years) undergoing first-time ICD implantation for the primary prevention of sudden cardiac death in 15 hospitals within 7 integrated health systems in the CVRN between January 1, 2006, and December 31, 2010. We excluded patients not enrolled in 1 of the 7 CVRN health plans at the time of implantation (n=610) and those with a left ventricular ejection fraction (LVEF) >0.35 (n=85). The study cohort, when compared with patients not enrolled, were older on average (median age 69 versus 67 years, P<0.001), included a lower proportion of women (25.8% versus 30.2%, P=0.02), were more often white (63.0% versus 43.7%, P<0.001), and had New York Heart Association (NYHA) I to II symptoms more often (61.5% versus 49.3%, P<0.001).
Outcomes
The end points included time to death from any cause, time to hospitalization from any cause and from heart failure, and device-related complications within the first 90 days after implantation. Death was ascertained through the VDW, which uses health system databases, Social Security Administration vital status files, and state death certificate registries, depending on the participating CVRN site. Hospitalizations were assessed using the VDW, which includes admission and discharge dates and diagnoses for all hospitalizations. Because health plans are responsible for coverage of all health services for members, hospitalizations outside of health plan facilities are included in the VDW. Hospitalizations for heart failure were identified using the principal discharge diagnosis for heart failure (Table1), an approach that has been applied widely in studies of heart failure.16 Follow-up for death and hospitalization was obtained through December 31, 2011, providing up to 6 years of follow-up.
Table 1.
ICD-9-CM, CPT, and HCPSC Codes Used to Ascertain Clinical Events in the Cardiovascular Research Network Virtual Data Warehouse
| Clinical Event (Ascertainment Time Frame) | Codes |
|---|---|
| Heart failure hospitalization* (3 years) | 428-428.99, 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93 |
| Pneumothorax/hemothorax requiring chest tube (30 days) | ICD-9-CM 512.x or 511.8 AND |
| ICD-9 Procedure 34.04, 34.05, 34.06, or 34.09 | |
| Hematoma requiring transfusion or evacuation (30 days) | ICD-9-CM 998.1x, 287.4x, 518.7, V58.2, or V59.01 AND |
| ICD-9 Procedure 99.00, 99.03. 99.04, 34.04, or 34.09 | |
| Tamponade or pericardiocentesis (30 days) | ICD-9-CM 420, 423.0, 423.3, or 423.9 AND |
| ICD-9-CM Procedure 37.0 or 37.12 | |
| Mechanical complication of ICD with system revision (90 days) | ICD-9-CM 996.0x AND |
| ICD-9-CM Procedure 37.75, 37.79, 37.97, 37.99, 00.52, or 37.95 OR CPT 33215, 33225, 33233, 33235, 71090, 33224, 33242 | |
| OR HCPCS C1777, C1895, C1898, C1900, C1899, G0299, G0300 | |
| ICD Re-implantation (90 days) | ICD-9-CM Procedure 00.52, 00.50, 00.51, 00.53, 00.54, 37.94, 37.89, 37.96, 37.98 OR |
| CPT 33216, 33217, 33218, 33220, 33223, 33240, 33241, 33249, 33207, 33208, 33214, 33226, 33243, 33244 OR | |
| HCPCS C1721, C1722, C1785, C1786, C1882, C1882 | |
| Device related infection (90 days) | ICD-9-CM 996.61 |
CPT indicates Current Procedural Terminology Codes; HCPCS, Healthcare Common Procedure Coding System; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
To ascertain hospitalizations attributed to heart failure, only the principal discharge diagnosis was considered.
Device-related complications were ascertained using a modification of the approach developed for purposes of reporting complications after ICD implantation used for hospital public reporting.17 This approach applies a variable ascertainment time frame (either 30 or 90 days) depending on the complication. We identified events using codes from the International Classification of Diseases, Ninth Revision, Clinical Modification; the Current Procedural Terminology; and the Healthcare Common Procedure Coding System, in any position on an inpatient claim (Table1). Complications ascertained over the first 30 days after ICD implantation included pneumothorax or hemothorax requiring a chest tube, hematoma requiring transfusion or evacuation, cardiac tamponade or pericardiocentesis, or death. Complications ascertained over the first 90 days included device-related infection and mechanical complications of the ICD requiring system revision and device reimplantation. Those patients with incomplete follow-up data for the complications end point (n=72) were excluded from the complications analysis.
Covariates
Patient characteristics included demographics (age, sex, and race or ethnicity), cardiovascular history (NYHA class, LVEF, ischemic heart disease, nonischemic cardiomyopathy, atrial fibrillation), noncardiovascular comorbidities, laboratory values (blood urea nitrogen, hemoglobin, and creatinine) at the time of implantation, electrocardiographic findings (QRS morphology and duration), and device type. For the death and hospitalization end points, we also considered the prescription of medications for heart failure known to influence heart failure outcomes (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta blockers, and aldosterone antagonists) at hospital discharge. To avoid case-wise deletion, missing values were imputed using approaches that varied based on the proportion of missing values. Variables were generally missing infrequently (<2.2%); in these cases, continuous variables were imputed using the median value of known measurements in the population, and categorical variables were imputed as the most common response in the population. Because QRS morphology and QRS duration were missing more frequently (12%), a dummy variable for missing status was used in structuring these variables as candidates for the multivariable models.18
Statistical Analysis
Baseline data are presented as proportions for categorical variables and as means with SDs for continuous variables. Comparisons of variables in sex and age strata were performed using chi-square tests for categorical variables and Student t tests for continuous variables. For the death and hospitalization outcomes, crude rates of events were calculated per 1000 person-years of follow-up with associated 95% CIs. Time-to-event analysis was performed with log-rank tests to compare actuarial survival rates between respective comparison groups.
Four separate multivariable models (1 per study outcome) were constructed. Bivariate associations with P<0.20 were included in multivariable analyses after removing any potentially collinear variables. In each case, collinearity among candidate variables was examined using eigenvalues obtained through principal component analysis. From the principal component results, conditional indices >3 were considered potentially collinear. The corresponding covariates were examined for collinearity in the multivariable model. If including both covariates resulted in instability in estimates or variance, the variable with the stronger bivariate association with the outcome was retained as a candidate. For death and the hospitalization outcomes, multivariable survival analysis was performed using Cox proportional hazards regression. In Cox models for death, patients were censored at the time of last documented encounter regardless of the continuity of health plan enrollment; for the hospitalization outcome, patients were censored at the time of first health plan disenrollment or death. All multivariable models accounted for the clustering of patients by site. For hospitalizations, a random-site effect was included in the multivariable Cox models. For mortality, a fixed-site effect was included in the multivariable Cox models because of lack of convergence of a random-effects model. For complications, generalized estimating equations were used in logistic models, with site treated as a random effect.
In all multivariable models, age, sex, LVEF, NYHA symptom status, and etiology of LVSD were included. To test for variability in relationships of age and sex to these outcomes, interaction terms between either age or sex with 3 dichotomous variables reflecting heart failure status (NYHA class I to II versus III to IV, LVEF <0.30 versus 0.30 to 0.35, and ischemic versus nonischemic etiology) were tested individually within the outcome models in which a significant primary age or sex association was identified. A 2-sided P value <0.05 was considered statistically significant. All tests were 2-sided. We used SAS version 9.2 and 9.3 (SAS Institute Inc) for all analyses.
Role of the Funding Source
This study was funded by the Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute of the NIH; and the American College of Cardiology Foundation. The study was reviewed and approved by the research and publications committee of the NCDR ICD Registry.
Results
Study Population
The study population consisted of 2954 patients receiving an ICD for primary prevention; 769 (26.0%) were women, and 1827 (61.8%) were aged >65 years (Table2). Compared with men, women were less frequently white (58.5% versus 64.3%, P<0.001), more likely to have advanced heart failure symptoms (48.0% with NYHA classification III or IV versus 35.2%, P<0.001), less likely to have a history of atrial fibrillation (23.5% versus 35.1%, P<0.001), and less likely to have ischemic cardiomyopathy (44.1% versus 68.5%, P<0.001).
Table 2.
Study Population Characteristics and According to Sex and Age
| Characteristic | Total (n=2954) | Women (n=769) | Men (n=2185) | P Value | Age ≤65 Years (n=1127) | Age >65 Years (n=1827) | P Value |
|---|---|---|---|---|---|---|---|
| Aged >65 years | 1828 (61.9%) | 473 (61.5%) | 1354 (62.0%) | 0.82 | — | — | |
| Age, y, median (IQR) | 69.0 (60.0, 75.0) | 68.0 (60.0, 75.0) | 69.0 (60.0, 75.0) | 0.32 | 57.0 (51.0 to 62.0) | 74.0 (70.0 to 78.0) | NA |
| Female | 769 (26.0%) | — | — | — | 296 (26.3%) | 473 (25.9%) | 0.82 |
| Race/ethnicity | <0.001 | <0.001 | |||||
| White | 1856 (62.8%) | 450 (58.5%) | 1406 (64.3%) | 611 (54.2%) | 1245 (68.1%) | ||
| Black | 463 (15.7%) | 163 (21.2%) | 300 (13.7%) | 231 (20.5%) | 232 (12.7%) | ||
| Hispanic (not black) | 438 (14.8%) | 108 (14.0%) | 330 (15.1%) | 184 (16.3%) | 254 (13.9%) | ||
| Other | 197 (6.7%) | 48 (6.2%) | 149 (6.8%) | 101 (9.0%) | 96 (5.3%) | ||
| NYHA class | <0.001 | <0.001 | |||||
| I to II | 1809 (61.2%) | 397 (51.6%) | 1412 (64.6%) | 740 (65.7%) | 1069 (58.5%) | ||
| III to IV | 1139 (38.6%) | 369 (48.0%) | 770 (35.2%) | 386 (34.3%) | 753 (41.2%) | ||
| LVEF, % | 0.62 | 0.12 | |||||
| 31 to 35 | 431 (14.6%) | 108 (14.0%) | 323 (14.8%) | 150 (13.3%) | 281 (15.4%) | ||
| ≤30 | 2523 (85.4%) | 661 (86.0%) | 1862 (85.2%) | 977 (86.7%) | 1546 (84.6%) | ||
| Etiology of cardiomyopathy | <0.001 | <0.001 | |||||
| Ischemic | 1863 (62.2%) | 339 (44.1%) | 1497 (68.5%) | 556 (49.3%) | 1280 (70.1%) | ||
| Nonischemic | 1118 (37.8%) | 430 (55.9%) | 688 (31.5%) | 571 (50.7%) | 547 (29.9%) | ||
| Atrial fibrillation | 948 (32.1%) | 181 (23.5%) | 767 (35.1%) | <0.001 | 254 (22.5%) | 694 (38.0%) | <0.001 |
| Diabetes mellitus | 1246 (42.2%) | 312 (40.6%) | 934 (42.7%) | 0.40 | 459 (40.7%) | 787 (43.1%) | 0.43 |
| Hypertension | 2168 (73.4%) | 550 (71.5%) | 1618 (74.1%) | 0.39 | 713 (63.3%) | 1455 (79.6%) | <0.001 |
| Chronic lung disease | 590 (20.0%) | 172 (22.4%) | 418 (19.1%) | 0.13 | 191 (16.9%) | 399 (21.8%) | 0.004 |
| Left bundle branch block | 825 (27.9%) | 318 (41.4%) | 507 (23.2%) | <0.001 | 270 (24.0%) | 555 (30.4%) | <0.001 |
| QRS duration >0.12 s | 1450 (49.1%) | 419 (54.5%) | 1031 (47.2%) | <0.001 | 416 (36.9%) | 1034 (56.6%) | <0.001 |
| Creatinine, mg/dL | 1.4 (0.9) | 1.2 (0.7) | 1.4 (1.0) | <0.001 | 1.3 (1.03) | 1.4 (0.81) | 0.015 |
| Blood urea nitrogen, mg/dL | 24.9 (13.84) | 23.9 (13.65) | 25.2 (13.89) | 0.019 | 22.5 (12.99) | 26.3 (14.14) | <0.001 |
| Estimated GFR, mL/min per 1.72 m2 | 61.5 (22.5) | 59.3 (22.9) | 62.3 (22.3) | 0.001 | 71.6 (23.9) | 55.3 (19.1) | <0.001 |
| Hemoglobin, g/L | <0.001 | <0.001 | |||||
| >14.5 | 687 (23.3%) | 56 (7.3%) | 631 (28.9%) | 333 (29.5%) | 354 (19.4%) | ||
| 13.3 to 14.5 | 754 (25.5%) | 164 (21.3%) | 590 (27.0%) | 291 (25.8%) | 463 (25.3%) | ||
| 12.1 to 13.2 | 689 (23.3%) | 259 (33.7%) | 430 (19.7%) | 241 (21.4%) | 448 (24.5%) | ||
| <12.1 | 759 (25.7%) | 276 (35.9%) | 483 (22.1%) | 235 (20.9%) | 524 (28.7%) | ||
| Medications | |||||||
| ACE inhibitor or ARB | 2554 (86.5%) | 675 (87.8%) | 1879 (86.0%) | 0.21 | 989 (87.8%) | 1565 (85.7%) | 0.11 |
| Beta blocker | 2690 (91.1%) | 704 (91.5%) | 1986 (90.9%) | 0.48 | 1048 (93.0%) | 1642 (89.9%) | 0.02 |
| Aldosterone antagonist | 994 (33.6%) | 286 (37.2%) | 708 (32.4%) | 0.02 | 436 (38.7%) | 558 (30.5%) | <0.001 |
| Device type | <0.001 | <0.001 | |||||
| Single chamber | 955 (32.3%) | 220 (28.6%) | 735 (33.6%) | 494 (43.8%) | 461 (25.2%) | ||
| Dual chamber | 1062 (36.0%) | 235 (30.6%) | 827 (37.8%) | 352 (31.2%) | 710 (38.9%) | ||
| CRT-D | 937 (31.7%) | 314 (40.8%) | 623 (28.5%) | 281 (24.9%) | 656 (35.9%) |
ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CRT-D, cardiac resynchronization therapy with defibrillator; GFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Compared with younger patients, those aged >65 years were more likely to have advanced heart failure symptoms (41.2% versus 34.3%, P<0.001); were more frequently white (68.1% versus 54.2%, P<0.001); and had a higher prevalence of coexisting conditions, including atrial fibrillation (38.0% versus 22.5%, P<0.001), hypertension (79.6% versus 63.3%, P<0.001), and chronic lung disease (21.8% versus 16.9%, P<0.001). Older patients were more likely to have ischemic cardiomyopathy (70.1% versus 49.3%, P<0.001). There were no significant difference in age between men and women (Table2).
Outcomes
The crude incidence rates, unadjusted risks, and adjusted risks for adverse outcomes in the total cohort and in patients further stratified by age and sex are shown in Table3. The median duration of enrollment was 2.4 years (interquartile range 1.3 to 3.8 years). The incidence rate of death was 110 per 1000 patient-years, which was lower in women compared with men (crude incidence 86 versus 118, adjusted hazard ratio [HR] 0.67, 95% CI 0.56 to 0.80) and higher in patients aged >65 years compared with younger patients (crude incidence 133 versus 67, adjusted HR 1.55, 95% CI 1.30 to 1.86). The incidence rate of all-cause hospitalization was 438 per 1000 patient-years and did not differ significantly between men and women or between younger and older patients. The incidence rate of hospitalization for heart failure was 111 per 1000 patient-years, which was lower in women compared with men (crude incidence rates 102 versus 115, adjusted HR 0.82, 95% CI 0.68 to 0.98) and higher in older compared with younger patients (crude incidence rates 127 versus 84, adjusted HR 1.25, 95% CI 1.05 to 1.49). The overall proportion of patients who developed any device-related complication was 8.36% and was higher in women than in men (crude proportions 10.74% versus 7.52%, adjusted odds ratio 1.38, 95% CI 1.01 to 1.90). With respect to individual complications, women had a significantly higher risk of tamponade (1.89% versus 0.47%, P<0.001); the sex differences in risks of other complications were not statistically significant (Table4). Older patients had a significantly higher risk of hematoma requiring evacuation or transfusion compared with younger patients (1.01% versus 0.27%, P=0.03). The rates of complications varied by device type (single lead 4.63%, dual lead 8.34%, and cardiac resynchronization therapy–defibrillator 12.20%; P<0.001) and were generally higher in women than in men across device types, although the device-specific differences in complications by sex were statistically significant among single-lead devices (Table5). None of the comparisons of the differences in complication rates by device were statistically significant between age groups. The rates of individual complications by device type are presented in Table6.
Table 3.
Event Rates and Risk Ratios According to Age and Sex
| Event | All | Sex | Age, y | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Risk Ratio Crude | Risk Ratio Adjusted | <65 | ≥65 | Risk Ratio Crude | Risk Ratio Adjusted | ||
| Death* | 110 | 86 | 118 | 0.73 (0.61 to 0.86) | 0.67 (0.56 to 0.80) | 67 | 133 | 1.91 (1.62 to 2.26) | 1.55 (1.30 to 1.86) |
| Hospitalization for all causes* | 438 | 421 | 445 | 0.95 (0.86 to 1.06) | 0.93 (0.83 to 1.05) | 370 | 480 | 1.25 (1.13 to 1.38) | 1.07 (0.96 to 1.18) |
| Hospitalization for heart failure* | 111 | 102 | 114 | 0.91 (0.77 to 1.08) | 0.82 (0.68 to 0.98) | 84 | 127 | 1.41 (1.19 to 1.66) | 1.25 (1.05 to 1.49) |
| Complications† | 8.36% | 10.74% | 7.52% | 1.43 (1.12 to 1.96) | 1.38 (1.01 to 1.90) | 7.15% | 9.10% | 1.30 (0.98 to 1.72) | 1.17 (0.86 to 1.59) |
Fully adjusted multivariable models included the following variables for each outcome in addition to accounting for clustering of patients by Cardiovascular Research Network site. Death: left ventricular ejection fraction, etiology of cardiomyopathy (ischemic vs non-ischemic), New York Heart Association symptom classification, blood urea nitrogen, atrial fibrillation, diabetes, hypertension, chronic lung disease, hemoglobin, QRS duration, ICD device type, ACE/ARB therapy, and beta blocker. All-cause hospitalization: left ventricular ejection fraction, etiology of cardiomyopathy (ischemic vs nonischemic), New York Heart Association symptom classification, race, atrial fibrillation, diabetes, hypertension, lung disease, estimated glomerular filtration rate, blood urea nitrogen, hemoglobin, ICD device type, QRS duration, ACE/ARB therapy, and beta blocker. Heart failure hospitalization: left ventricular ejection fraction, etiology of cardiomyopathy (ischemic vs nonischemic), New York Heart Association symptom classification, race, atrial fibrillation, diabetes, hypertension, lung disease, estimated glomerular filtration rate, blood urea nitrogen, hemoglobin, ICD device type, QRS duration, ACE/ARB therapy, and aldosterone antagonist. Complications: left ventricular ejection fraction, etiology of cardiomyopathy (ischemic vs non-ischemic), New York Heart Association symptom classification, atrial fibrillation, diabetes, blood urea nitrogen, hemoglobin, ICD device type, left bundle branch block, and QRS duration. ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ICD, implantable cardioverter-defibrillator.
Rates expressed as incidence per 1000 patient-years; ratios expressed as hazard ratios with 95% CIs.
Rates expressed as proportions; ratios expressed as odds ratios with 95% CIs.
Table 4.
Rates of Individual Device-Related Complications by Sex and Age
| Complication | Total (n=2882) | Women (n=754) | Men (n=2128) | P Value | Age ≤65 Years (n=1091) | Age >65 Years (n=1791) | P Value |
|---|---|---|---|---|---|---|---|
| Hematoma requiring evacuation/transfusion* | 21 (0.73%) | 7 (0.93%) | 14 (0.66%) | 0.45 | 3 (0.27%) | 18 (1.01%) | 0.03 |
| Tamponade* | 24 (0.83%) | 14 (1.89%) | 10 (0.47%) | <0.001 | 11 (1.01%) | 13 (0.73%) | 0.42 |
| Death* | 21 (0.73%) | 6 (0.80%) | 15 (0.70%) | 0.80 | 4 (0.37%) | 17 (0.95%) | 0.07 |
| Device reimplantation† | 68 (2.36%) | 17 (2.25%) | 51 (2.40%) | 0.83 | 23 (2.11%) | 45 (2.51%) | 0.49 |
| Device-related infection† | 36 (1.25%) | 10 (1.33%) | 26 (1.22%) | 0.82 | 16 (1.47%) | 20 (1.12%) | 0.41 |
| Mechanical complication requiring revision† | 150 (5.2%) | 46 (6.10%) | 104 (4.89%) | 0.20 | 51 (4.67%) | 99 (5.53%) | 0.32 |
| Any nonfatal complication | 225 (7.81%) | 79 (10.48%) | 146 (6.86%) | 0.001 | 75 (6.87%) | 150 (8.38%) | 0.15 |
| Any complication (including death within 30 days) | 241 (8.36%) | 81 (10.74%) | 160 (7.52%) | 0.006 | 78 (7.15%) | 163 (9.10%) | 0.07 |
Ascertained over 30 days after ICD implantation.
Ascertained over 90 days after ICD implantation.
Table 5.
Rates of Device-Related Complications by Sex and Age Stratified by Device Type
| Device Type | Total (n=2882) | Women (n=754) | Men (n=2128) | P Value | Age ≤65 Years (n=1091) | Age >65 Years (n=1791) | P Value |
|---|---|---|---|---|---|---|---|
| All devices | 241 (8.36%) | 81 (10.74%) | 160 (7.52%) | 0.006 | 78 (7.15%) | 163 (9.10%) | 0.07 |
| Single lead | 43 (4.63%) | 15 (6.91%) | 28 (3.93%) | 0.07 | 18 (3.79%) | 25 (5.51%) | 0.21 |
| Dual lead | 87 (8.34%) | 23 (10.00%) | 64 (7.87%) | 0.30 | 26 (7.58%) | 61 (8.71%) | 0.53 |
| CRT-D | 111 (12.20%) | 43 (14.01%) | 68 (11.28%) | 0.23 | 34 (12.45%) | 77 (12.09%) | 0.88 |
CRT-D indicates cardiac resynchronization therapy with defibrillator.
Table 6.
Rates of Individual Device-Related Complications Stratified by Device Type
| Complication | Total (n=2882) | Single Lead (n=929) | Dual Lead (n=1043) | CRT-D (n=1005) | P Value |
|---|---|---|---|---|---|
| Hematoma requiring evacuation/transfusion* | 21 (0.73%) | 4 (0.43%) | 8 (0.77%) | 9 (0.99%) | 0.37 |
| Tamponade* | 24 (0.83%) | 1 (0.11%) | 11 (1.05%) | 12 (1.32%) | 0.01 |
| Death* | 21 (0.73%) | 1 (0.11%) | 9 (0.86%) | 11 (1.21%) | 0.02 |
| Device reimplantation† | 68 (2.36%) | 12 (1.29%) | 19 (1.82%) | 37 (4.07%) | <0.001 |
| Device-related infection† | 36 (1.25%) | 9 (0.97%) | 16 (1.53%) | 11 (1.21%) | 0.54 |
| Mechanical complication requiring revision† | 150 (5.2%) | 26 (2.80%) | 48 (4.60%) | 76 (8.35%) | <0.001 |
| Any nonfatal complication | 225 (7.81%) | 43 (4.63%) | 78 (7.48%) | 104 (11.43%) | <0.001 |
| Any complication (including death within 30 days) | 241 (8.36%) | 43 (4.63%) | 87 (8.34%) | 111 (12.20%) | <0.001 |
CRT-D indicates cardiac resynchronization therapy with defibrillator.
Ascertained over 30 days after ICD implantation.
Ascertained over 90 days after ICD implantation.
Age and Sex Interactions With Measures of Heart Failure Status
Differences in study outcomes were stratified by NYHA symptom status, LVEF, and etiology of LVSD when the principal age or sex relationship was significant (Figures1A through 1C and 2A and 2B). The interactions between sex and these heart failure characteristics were not statistically significant, with 2 exceptions. First, women had a higher risk of complications compared with men in the stratum of patients with NYHA I to II symptoms (women versus men: HR 1.94, 95% CI 1.26 to 3.01) versus those with III to IV symptoms (HR 1.03, 95% CI 0.68 to 1.57; sex–NYHA interaction, P=0.03) (Figure 1C). Second, older patients had a higher risk of heart failure hospitalizations compared with younger patients in the stratum of patients with ischemic cardiomyopathy (aged >65 versus ≤65 years: HR 1.46, 95% CI 1.15 to 1.85) versus those with nonischemic cardiomyopathy (HR 1.03, 95% CI 0.80 to 1.34; age–etiology interaction, P=0.05) (Figure 2B).
Figure 1.
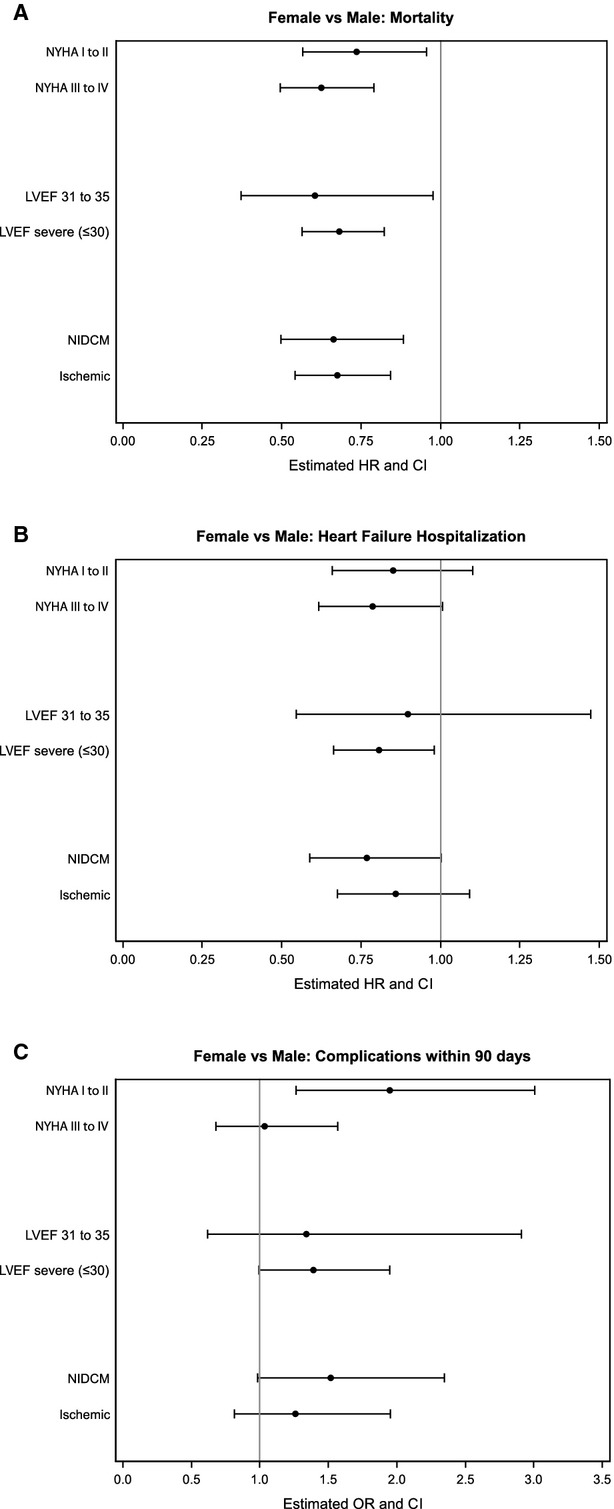
Outcomes comparing women with men in strata according to NYHA symptom status, LVEF, and etiology of left ventricular systolic dysfunction (A, mortality; B, heart failure hospitalization; C, complications). HR <1 indicates lower risk in women compared with men. All interaction terms are not significant except for the interaction between sex and NYHA in the complications model (C, P=03). HR indicates hazard ratio; LVEF, left ventricular ejection fraction; NIDCM, nonischemic dilated cardiomyopathy; NYHA, New York Heart Association; OR, odds ratio.
Figure 2.
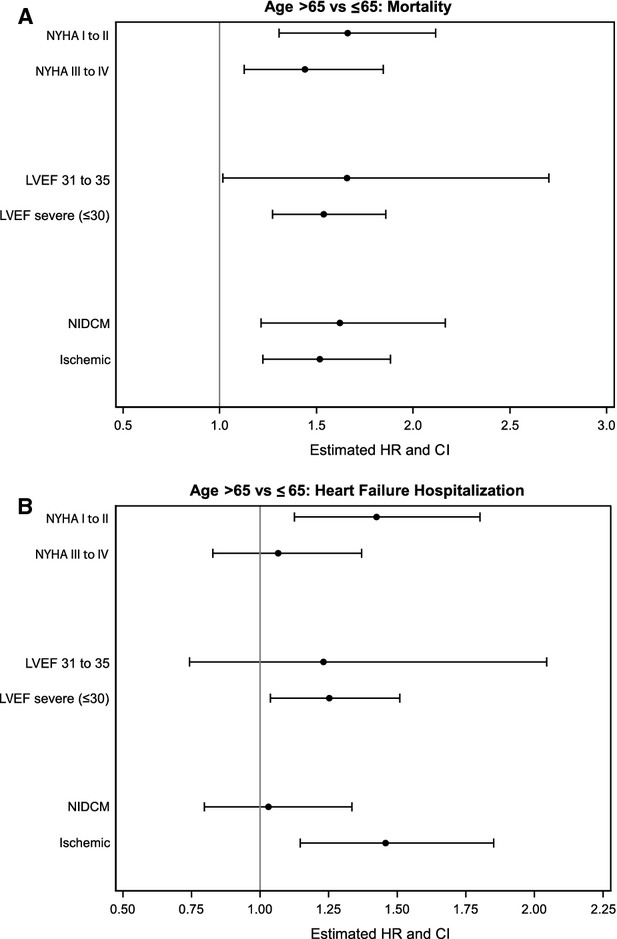
Outcomes comparing patients aged >65 years compared with those aged ≤65 years in strata according to NYHA symptom status, LVEF, and etiology of left ventricular systolic dysfunction (A, mortality; B, heart failure hospitalization). HR >1 indicates a higher risk in patients aged >65 years compared with younger patients. All interaction terms are not significant except for the interaction between age and etiology (nonischemic vs ischemic) in the hospitalizations model (B, P=0.05). HR indicates hazard ratio; LVEF, left ventricular ejection fraction; NIDCM, nonischemic dilated cardiomyopathy; NYHA, New York Heart Association.
Discussion
In this study of a community-based cohort of patients with LVSD receiving an ICD for primary prevention, the burden of adverse outcomes, including death and hospitalization for heart failure over 3 years, was substantial and varied by age and sex. After accounting for differences in other characteristics, women had a lower risk of death and hospitalization for heart failure than men and higher risks of device-related complications. Older patients had higher risks of death and heart failure–related hospitalizations but not for all-cause hospitalizations. The age and sex differences in these outcomes generally did not vary by severity of heart failure symptoms, etiology of systolic dysfunction, or LVEF. The results of this study provide estimates of the risks of several clinically important adverse outcomes in contemporary practice. These data can inform the decision-making process and the design of trials of therapies in the growing population of patients receiving ICD therapy. Furthermore, the results of this multisite longitudinal study provide greater context for understanding sex- and age-related differences in this high-risk patient population.
The results of this observational study provide estimates of the risks of adverse outcomes in a demographically diverse population with high rates of guideline-recommended medical therapies for LVSD receiving an ICD for primary prevention with long-term follow-up for a wide variety of outcomes important to patients. Although several prior studies have focused on outcomes in this population, these studies have been limited by the lack of heterogeneity of the study population with respect to age or sex, the lack of follow-up beyond 1 year, and the availability of follow-up in patients across a limited age spectrum or by the inability to characterize outcomes other than death.6–11 The results of this study suggest that in this diverse population of patients with systolic dysfunction who are treated with an ICD, the risks of adverse outcomes other than death are high, including a substantial burden of hospitalization across patient strata during long-term follow-up after device implantation.
The randomized trials of ICD therapy for primary prevention that underlie current guideline recommendations enrolled younger patients and few women.2–4 Moreover, because a number of these trials were published >2 decades ago, therapy for patients with heart failure has evolved substantially, the estimates of outcomes from these trials are of limited value, particularly in these important demographic groups. Although a number of observational studies have focused on outcomes after ICD implantation, in some cases these studies are also limited because of a lack of contemporary data, relatively limited follow-up, or a primary focus on death. Because of the diversity of the CVRN cohort, we were able to characterize sex- and age-specific risks of death, hospitalization, and device-related complications.
Studies of sex differences in clinically meaningful long-term outcomes after ICD placement have been conflicting, perhaps in part due to differences in study design, population, time period, and length of follow-up. Post hoc analyses of individual randomized trials have shown variable differences in mortality based on patient sex19–21; a meta-analysis of the major primary prevention trials found no sex-based differences in mortality between men and women.22 Because of the differences between the populations enrolled in trials and those receiving ICD therapy in practice,5 observational studies provide complementary information. Although the finding of a lower risk of death among women in our study is similar to that of a prior single-center study of women receiving primary-prevention devices, other studies have found no mortality differences.7,8,12,23 Prior studies, however, have been heterogeneous and have included patients receiving ICDs for both primary and secondary prevention or focused on older patients with a shorter time frame of follow-up, possibly influencing the frequency and correlates of adverse outcomes. The relationship between sex and hospitalizations has not been the frequent focus of other investigations; although a recent study from the NCDR identified a higher risk of hospitalizations for women, this study was conducted in an older cohort followed for 6 months and did not include cardiac resynchronization therapy devices.12 Women have been noted to be at consistently higher risk for ICD-related complications.12,23,24 The current study provides an estimate of the magnitude of these differences in a contemporary population, including the differences across the spectrum of the devices commonly used in clinical practice.
Not surprisingly, age has been identified as an important determinant of adverse outcomes in a wide variety of cardiovascular conditions; however, the data generated in this study are important because they provide contemporary estimates of the magnitude of the age-associated risks for a broad variety of outcomes with long-term follow-up. In a cohort from Ontario, Canada, including patients receiving both primary and secondary prevention ICD therapy, advanced age was an important risk factor for death and hospitalization.25 The death rates in that cohort were somewhat lower than in this study. Although the cardiovascular characteristics of the populations of our study and that from Ontario were similar, the burden of comorbidities was greater in the CVRN cohort. In addition, the follow-up in the current study was, on average >1 year longer. Rates of all-cause and heart failure–related hospitalizations were not reported in the Ontario study, limiting comparisons with our study. Despite observed increases in the risk of death with older age, analyses of data from published randomized trials have not identified clear evidence that effectiveness of ICD therapy among eligible patients is sensitive to age.26 Nonetheless, the rates of adverse outcomes in the demographically diverse population of this study provide a perspective in terms of what patients might expect after ICD implantation and emphasize the need for additional effective therapies and treatment strategies to reduce the longer term risks of death or hospitalization.
Reasons for age and sex differences in outcomes after ICD implantation are not clear. With regard to sex, proposed mechanisms range from inherent biological differences to differences in the severity of heart failure in patients receiving this therapy. Some data suggest, for example, important biological differences between men and women that could result in lower risks of death associated with LVSD in women.27 This sex variability could also reflect differences in heart failure severity or the extent and etiology of LVSD of patients who are selected for ICD therapy. Women, for example, might have better survival if they have less advanced heart failure than men when they are identified for ICD therapy; however, in our patient cohort, this was not the case because a higher proportion of women in the CVRN cohort had advanced heart failure symptoms. With regard to age, older patients may have competing causes of death that would render ICDs less effective, especially in those with less severe heart failure. The age and sex differences observed in this study persisted after accounting for a wide variety of factors and did not vary according to the burden of heart failure symptoms, severity of LVSD, or etiology of heart failure. Consequently, alternative explanations for these demographic differences in outcomes warrant exploration. Better understanding of the underlying reasons for these differences could refine the identification of patients who might derive maximal benefit from ICD therapy.
Certain factors should be considered in the interpretation of the results of this study. Because all patients received an ICD, it was not possible to estimate differences in outcomes associated with the ICD therapy itself. In an observational context, identifying suitable untreated control groups is challenging because of the risk of substantial confounding by indication. Nonetheless, characterizing the risks of adverse outcomes is pertinent to the growing population of patients receiving ICD therapy for primary prevention in contemporary practice. In addition, the study sample was identified from integrated health plans and thus may not be representative of some patient populations such as the uninsured; however, the population was demographically diverse, with robust representation of women, racial and ethnic minorities, and elderly patients and similar to larger US populations of patients receiving an ICD for primary prevention.5
In this cohort study of a diverse population of patients with LVSD receiving an ICD for primary prevention, the burden of adverse outcomes in the 3 years following device implantation was substantial and varied importantly by age and sex. Observed age and sex differences were generally consistent across the spectrum of heart failure symptoms, left ventricular systolic function, and heart failure etiology. The risks of death, hospitalization, and device-related complications delineated in this study are important to the large population of patients with LVSD who are considering ICD therapy for primary prevention, to the clinicians caring for them, and to investigators interested in understanding the risks of clinically meaningful outcomes in the growing population of patients receiving this potentially life-saving therapy.
Sources of Funding
This project was funded under Contract No. 290-05-0033 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program; and by the American College of Cardiology Foundation; with support from the National Heart, Lung, and Blood Institute (U19HL091179). Although the sponsoring organizations have been involved in discussions of this research as it has progressed and have provided oversight and guidance, the authors of this report are solely responsible for its content. Sponsorship may not be construed as an endorsement of all statements in the report by the Agency for Healthcare Research and Quality, the National Heart, Lung, and Blood Institute, the US Department of Health and Human Services or the American College of Cardiology Foundation. Dr Peterson is supported by funding from Agency for Healthcare Research and Quality (K08HS019814).
Disclosures
Dr Masoudi has a contract with the American College of Cardiology for his role as the Senior Medical Officer of the National Cardiovascular Data Registries. Dr Gupta serves as local site PI on multi-center clinical trials sponsored by St. Jude Medical, Boston Scientific, and Medtronic. The other authors have no relevant disclosures to report.
References
- Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA, III, Ferguson TB, Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol. 2012;60:1297–1313. doi: 10.1016/j.jacc.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- Masoudi FA, Go AS, Magid DJ, Cassidy-Bushrow AE, Doris JM, Fiocchi F, Garcia-Montilla R, Glenn KA, Goldberg RJ, Gupta N, Gurwitz JH, Hammill SC, Hayes JJ, Jackson N, Kadish A, Lauer M, Miller AW, Multerer D, Peterson PN, Reifler LM, Reynolds K, Saczynski JS, Schuger C, Sharma PP, Smith DH, Suits M, Sung SH, Varosy PD, Vidaillet HJ, Greenlee RT. Longitudinal study of implantable cardioverter-defibrillators: methods and clinical characteristics of patients receiving implantable cardioverter-defibrillators for primary prevention in contemporary practice. Circ Cardiovasc Qual Outcomes. 2012;5:e78–e85. doi: 10.1161/CIRCOUTCOMES.112.965368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden AC, Thijssen J, Borleffs CJ, van Rees JB, Hoke U, van der Velde ET, van Erven L, Schalij MJ. Gender-specific differences in clinical outcome of primary prevention implantable cardioverter defibrillator recipients. Heart. 2013;99:1244–1249. doi: 10.1136/heartjnl-2013-304013. [DOI] [PubMed] [Google Scholar]
- Amit G, Suleiman M, Konstantino Y, Luria D, Kazatsker M, Chetboun I, Haim M, Gavrielov-Yusim N, Goldenberg I, Glikson M. Sex differences in implantable cardioverter-defibrillator implantation indications and outcomes: lessons from the nationwide Israeli-ICD registry. Europace. 2014;16:1175–1180. doi: 10.1093/europace/euu015. [DOI] [PubMed] [Google Scholar]
- Bhavnani SP, Pavuluri V, Coleman CI, Guertin D, Yarlagadda RK, Clyne CA, Kluger J. The gender-paradox among patients with implantable cardioverter-defibrillators: a propensity-matched study. Pacing Clin Electrophysiol. 2013;36:878–884. doi: 10.1111/pace.12141. [DOI] [PubMed] [Google Scholar]
- Duray G, Richter S, Manegold J, Israel CW, Gronefeld G, Hohnloser SH. Efficacy and safety of ICD therapy in a population of elderly patients treated with optimal background medication. J Interv Card Electrophysiol. 2005;14:169–173. doi: 10.1007/s10840-006-5200-y. [DOI] [PubMed] [Google Scholar]
- Kraaier K, Scholten MF, Tijssen JG, Theuns DA, Jordaens LJ, Wilde AA, van Dessel PF. Early mortality in prophylactic implantable cardioverter-defibrillator recipients: development and validation of a clinical risk score. Europace. 2014;16:40–46. doi: 10.1093/europace/eut223. [DOI] [PubMed] [Google Scholar]
- Pires LA, Sethuraman B, Guduguntla VD, Todd KM, Yamasaki H, Ravi S. Outcome of women versus men with ventricular tachyarrhythmias treated with the implantable cardioverter defibrillator. J Cardiovas Electrophysiol. 2002;13:563–568. doi: 10.1046/j.1540-8167.2002.00563.x. [DOI] [PubMed] [Google Scholar]
- Russo AM, Daugherty SL, Masoudi FA, Wang Y, Curtis J, Lampert R. Gender and outcomes following primary prevention ICD implantation: findings from the NCDR. Am Heart J. 2015 doi: 10.1016/j.ahj.2015.02.025. ; doi: 10.1016/j.ahj.2015.02.025 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammill SC, Stevenson LW, Kadish AH, Kremers MS, Heidenreich P, Lindsay BD, Mirro MJ, Radford MJ, Wang Y, Lang CM, Harder JC, Brindis RG. Review of the registry’s first year, data collected, and future plans. Heart Rhythm. 2007;4:1260–1263. doi: 10.1016/j.hrthm.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA. The National Cardiovascular Data Registry (NCDR) data quality brief: the NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Go AS, Magid DJ, Wells B, Sung SH, Cassidy-Bushrow AE, Greenlee RT, Langer RD, Lieu TA, Margolis KL, Masoudi FA, McNeal CJ, Murata GH, Newton KM, Novotny R, Reynolds K, Roblin DW, Smith DH, Vupputuri S, White RE, Olson J, Rumsfeld JS, Gurwitz JH. The Cardiovascular Research Network (CVRN): a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1:10. doi: 10.1161/CIRCOUTCOMES.108.801654. [DOI] [PubMed] [Google Scholar]
- Masoudi FA, Rathore SS, Wang Y, Havranek EP, Curtis JP, Foody JM, Krumholz HM. National patterns of use and effectiveness of angiotensin-converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation. 2004;110:724–731. doi: 10.1161/01.CIR.0000138934.28340.ED. [DOI] [PubMed] [Google Scholar]
- 2010. National quality forum endorsed standards. Available at: http://www.qualityforum.org/Measures_List.aspx. Accessed December 30, 2014.
- Harrell FE., Jr . Regression Modeling Strategies With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer-Verlag New York, Inc; 2001. [Google Scholar]
- Albert CM, Quigg R, Saba S, Estes NA, III, Shaechter A, Subacius H, Howard A, Levine J, Kadish A. Sex differences in outcome after implantable cardioverter defibrillator implantation in non-ischemic cardiomyopathy. Am Heart J. 2008;156:367–372. doi: 10.1016/j.ahj.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Russo AM, Poole JE, Mark DB, Anderson J, Hellkamp AS, Lee KL, Johnson GW, Domanski M, Bardy GH. Primary prevention with defibrillator therapy in women: results from the sudden cardiac death in heart failure trial. J Cardiovasc Electrophysiol. 2008;19:720–724. doi: 10.1111/j.1540-8167.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- Zareba W, Moss AJ, Jackson Hall W, Wilber DJ, Ruskin JN, McNitt S, Brown M, Wang H. Clinical course and implantable cardioverter defibrillator therapy in post-infarction women with severe left ventricular dysfunction. J Cardiovasc Electrophysiol. 2005;16:1265–1270. doi: 10.1111/j.1540-8167.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- Santangeli P, Pelargonio G, Dello Russo A, Casella M, Bisceglia C, Bartoletti S, Santarelli P, Di Biase L, Natale A. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta-analysis. Heart Rhythm. 2010;7:876–882. doi: 10.1016/j.hrthm.2010.03.042. [DOI] [PubMed] [Google Scholar]
- MacFadden DR, Crystal E, Krahn AD, Mangat I, Healey JS, Dorian P, Birnie D, Simpson CS, Khaykin Y, Pinter A, Nanthakumar K, Calzavara AJ, Austin PC, Tu JV, Lee DS. Sex differences in implantable cardioverter-defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med. 2012;156:195–203. doi: 10.7326/0003-4819-156-3-201202070-00007. [DOI] [PubMed] [Google Scholar]
- Peterson PN, Daugherty SL, Wang Y, Vidaillet HJ, Heidenreich PA, Curtis JP, Masoudi FA. Gender differences in procedure-related adverse events in patients receiving implantable cardioverter-defibrillator therapy. Circulation. 2009;119:1078–1084. doi: 10.1161/CIRCULATIONAHA.108.793463. [DOI] [PubMed] [Google Scholar]
- Yung D, Birnie D, Dorian P, Healey JS, Simpson CS, Crystal E, Krahn AD, Khaykin Y, Cameron D, Chen Z, Lee DS. Survival after implantable cardioverter-defibrillator implantation in the elderly. Circulation. 2013;127:2383–2392. doi: 10.1161/CIRCULATIONAHA.113.001442. [DOI] [PubMed] [Google Scholar]
- Earley A, Persson R, Garlitski AC, Balk EM, Uhlig K. Effectiveness of implantable cardioverter defibrillators for primary prevention of sudden cardiac death in subgroups a systematic review. Ann Intern Med. 2014;160:111–121. doi: 10.7326/M13-1787. [DOI] [PubMed] [Google Scholar]
- Rho RW, Patton KK, Poole JE, Cleland JG, Shadman R, Anand I, Maggioni AP, Carson PE, Swedberg K, Levy WC. Important differences in mode of death between men and women with heart failure who would qualify for a primary prevention implantable cardioverter-defibrillator. Circulation. 2012;126:2402–2407. doi: 10.1161/CIRCULATIONAHA.111.069245. [DOI] [PubMed] [Google Scholar]


