Abstract
Background
During a myocardial infarction, no single best approach of systemic anticoagulation is recommended, likely due to a lack of comparative effectiveness studies and trade-offs between treatments.
Methods and Results
We investigated the patterns of use and site-level variability in anticoagulant strategies (unfractionated heparin [UFH] only, low-molecular-weight heparin [LMWH] only, UFH+LMWH, any bivalirudin) of 63 796 patients with a principal diagnosis of myocardial infarction treated with an early invasive strategy with percutaneous coronary intervention at 257 hospitals. About half (47%) of patients received UFH only, 6% UFH+LMWH, 7% LMWH only, and 40% bivalirudin. Compared with UFH, the median odds ratio was 2.90 for LMWH+UFH, 4.70 for LMWH only, and 3.09 for bivalirudin, indicating that 2 “identical” patients would have a 3- to 4-fold greater likelihood of being treated with anticoagulants other than UFH at one hospital compared with another. We then categorized hospitals as low- or high-users of LMWH and bivalirudin. Using hierarchical, multivariate regression models, we found that low bivalirudin-using hospitals had higher unadjusted bleeding rates, but the risk-adjusted and anticoagulant-adjusted bleeding rates did not differ across the hospital anticoagulation phenotypes. Risk-standardized mortality and risk-standardized length of stay also did not differ across hospital phenotypes.
Conclusions
We found substantial site-level variability in the choice of anticoagulants for invasively managed acute myocardial infarction patients, even after accounting for patient factors. No single hospital-use pattern was found to be clinically superior. More studies are needed to determine which patients would derive the greatest benefit from various anticoagulants and to support consistent treatment of patients with the optimal anticoagulant strategy.
Keywords: anticoagulation, bleeding, myocardial infarction, variation
Systemic anticoagulation is a core treatment for acute myocardial infarction (MI) that is supported by both European1,2 and American3,4 guidelines. Despite this strong recommendation for anticoagulation and many studies in this area, no single best approach has been endorsed.5–8 This is, in part, due to a limited number of comparative effectiveness studies, as new anticoagulant therapies are typically compared with unfractionated heparin (UFH). In addition, there are trade-offs between anticoagulants in terms of ischemic outcomes, bleeding, and costs. For example, bivalirudin reduces the risk of bleeding compared with UFH among invasively managed MI patients6,8 but at increased costs,9 although a recent clinical trial failed to find an advantage for bivalirudin for patients with ST-elevation MI undergoing primary percutaneous coronary intervention (PCI) using a primarily radial access.10 As such, the optimal strategy for anticoagulation across the population of patients with different risk profiles has not been firmly established.
Despite uncertainty concerning therapeutic options, practice patterns within hospitals sometimes coalesce around particular treatments. Accordingly, there may be consistency in local practice patterns that differs substantially from that of other hospitals based on physician preferences rather than patients’ characteristics or risk profiles.11–13 Understanding the patterns of care across institutions and their association with outcomes could provide the foundation for comparative effectiveness studies and targets for practice improvement where substantial deviation from existing evidence is observed. As such, we sought to better characterize practice patterns of use of anticoagulants (or hospital phenotypes) among patients with an MI who were treated with PCI across a broad sample of US hospitals; identify patient and practice factors associated with these patterns; and examine whether these patterns are associated with clinical outcomes. We used a database that has complete hospital-level resource utilization data that include drug information, and reflects real-world practice.
Methods
Data Source
We derived the study sample from a database maintained by Premier, Inc, Charlotte, NC.14 Premier is the nation’s largest hospital performance-improvement alliance. The Premier database contains administrative, operational, and limited clinical information that includes patient daily service records for 26% of hospital discharges in the United States. It includes all geographical areas of the United States and a broad range of hospital types in terms of bed size, teaching status, and urban or rural population served. In addition to the information available in the standard hospital discharge files, the database contains a date-stamped log of all billed items and related costs during hospitalizations at the patient level, including medications and laboratory, diagnostic, and therapeutic services. The database also contains information on patient age, sex, race, admission type, discharge status, and primary payer.
All data were de-identified in accordance with the Health Insurance Portability and Accountability Act. As a retrospective study using de-identified patient and provider data, the Yale University Human Investigation Committee exempted the protocol from review by the Office of Human Research Protections.
Study Cohort
We included hospitalizations from January 1, 2009 to December 31, 2011 for patients with a principal discharge diagnosis of MI (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 410.xx, excluding those with 410.x2). In accord with recent work by Yeh et al, a code of 410.7x was classified as a non-ST-elevation MI; all other codes were considered ST-elevation MI.15 To ensure a study group of patients with similar treatment goals, we further restricted the analytic population to those who were treated invasively within the first 2 days of hospitalization (ie, had coronary angiogram performed between hospital day 0 to 2), received PCI, and were treated with UFH (subcutaneous UFH was excluded), low-molecular-weight heparin (LMWH), or bivalirudin. Patients were excluded if they underwent coronary artery bypass graft surgery during the hospitalization, were <18 years of age, were assigned a pediatrician as the attending physician of record, or had a length of stay <1 day. As we were examining hospital-level variability in the use of anticoagulants and its associations with outcomes, we excluded patients who were transferred from another hospital (ie, the anticoagulation management was not solely under the control of the enrolling hospital) or admitted to a hospital with <25 cases during the study period (due to the inability to accurately categorize their anticoagulant practice patterns).
Patient comorbidities were defined using the Healthcare Cost and Utilization Project software, as described by Elixhauser et al.16 As per prior work,17 in-hospital bleeding events were identified using secondary ICD-9-CM diagnosis codes (Table1). PCIs performed during the MI hospitalization were identified using standard charge codes, and coronary artery bypass grafts were identified using ICD-9-CM procedure code 31.6x.
Table 1.
ICD-9-CM Codes Used to Define a Bleeding Event
| ICD-9-CM Code | Description |
|---|---|
| 285.1 | Anemia, acute posthemorrhagic |
| 456.0 | Varices, esophageal w/bleeding |
| 459.0 | Hemorrhage NOS |
| 530.7 | Mallory–Weiss syndrome |
| 530.82 | Hemorrhage, esophageal |
| 531.0 | Acute stomach ulcer with hemorrhage |
| 532.0 | Acute duodenal ulcer with hemorrhage |
| 532.2 | Acute duodenal ulcer with hemorrhage and perforation |
| 533.0 | Acute peptic ulcer with hemorrhage |
| 533.2 | Acute peptic ulcer with hemorrhage and perforation |
| 533.4 | Chronic peptic ulcer with hemorrhage |
| 533.6 | Chronic peptic ulcer with hemorrhage and perforation |
| 569.3 | Hemorrhage, rectal and anal |
| 569.85 | Angiodysplasia of intestine with hemorrhage |
| 578.0 | Hematemesis |
| 578.1 | Blood in stool |
| 578.9 | Hemorrhage, gastrointestinal NOS |
| 599.7 | Hematuria |
| 626.8 | Disorder, menstrual NEC |
| 626.9 | Disorder, menstrual NOS |
| 627.1 | Bleeding, postmenopausal |
| 729.92 | Nontraumatic hematoma soft tissue |
| 786.3 | Hemoptysis |
| 998.1 | Hemorrhage complicating a procedure |
ICD-9-CM indicates International Classification of Diseases, Ninth Revision, Clinical Modification; NEC, not elsewhere classifiable; NOS, not otherwise specified.
Anticoagulants and Hospital Phenotypes
Anticoagulant use was reviewed from admission day (day 0) to day 2. Anticoagulant use that did not meet MI treatment levels was excluded to avoid misclassification of doses given for other indications (eg, prophylaxis for deep vein thrombosis, catheter flushes). The minimum anticoagulant doses considered as treatment for the MI were 1000 U IV UFH (cumulative dose), 60 mg enoxaparin, 7200 IU of dalteparin, or 10 000 U of tinzaparin.18 As patients could receive >1 anticoagulant during their hospitalization, patients were categorized into the most common groups of cardiac anticoagulation use: any bivalirudin use, UFH only, LMWH only, and UFH+LMWH. Although patients who receive bivalirudin in the catheterization laboratory could receive another anticoagulant before the procedure, their clinical outcomes, in terms of bleeding and ischemic outcomes, have been previously determined to be similar to those of patients who received bivalirudin only.19–21
The frequency for each anticoagulant strategy was first calculated for each hospital. Hospitals were then categorized as being high versus low users of bivalirudin (based on above versus below the median of 37.4% (interquartile range 18.4% to 66.5%) and high versus low users of LMWH (based on above versus below the median of 9.8% (interquartile range 4.8% to 19.7%). We used these categories to construct 4 anticoagulant phenotypes: low bivalirudin/low LMWH (ie, predominate users of UFH), low bivalirudin/high LMWH, high bivalirudin/low LMWH, and high bivalirudin/high LMWH. We also performed a sensitivity analysis that reclassified hospitals using a threshold of above the third quartile of bivalirudin and LMWH use to define high users of these anticoagulants (ie, a high bivalirudin-using hospital was defined as use >66.5%; a high LMWH-using hospital was defined as use >19.7%).
Statistical Analysis
The variability in the use of different anticoagulant treatment strategies across sites was assessed using a hierarchical multinomial logistic regression model with anticoagulant choice as the dependent variable and adjusting for patient age, sex, ST-elevations at admission, select comorbidities (Table2), and use of concomitant glycoprotein IIb/IIIa inhibitors. All variables were first entered in a nonhierarchical multivariate model, which was then reduced using backward elimination. Covariates identified in this model as significantly associated with anticoagulant treatment strategy were then included in the final hierarchical model. Variability between hospitals was quantified using the median odds ratio (with UFH only as reference), which estimates the average difference in odds ratios of 2 hypothetical patients with identical clinical characteristics being treated with different anticoagulant strategies if they presented to 2 random hospitals in the dataset. Also, to estimate the contribution to variation by local practice patterns (hospital effect), we calculated the intraclass correlation coefficient for each anticoagulant strategy with UFH treatment (only) as reference.22
Table 2.
Demographic and Clinical Characteristics of Patients Treated With Different Anticoagulants
| Total (n=63 796) | UFH Only (n=30 230) | UFH+LMWH (n=3574) | LMWH Only (n=4406) | Any Bivalirudin (n=25 586) | |
|---|---|---|---|---|---|
| Age, median (IQR) | 61 (53 to 71) | 60 (52 to 70) | 61 (53 to 72) | 62 (53 to 72) | 62 (53 to 72) |
| Female, % | 31.0 | 29.9 | 31.5 | 33.4 | 31.8 |
| Hypertension, % | 67.2 | 65.5 | 68.9 | 70.8 | 68.3 |
| Congestive heart failure, % | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Peripheral vascular disease, % | 8.6 | 8.3 | 10.1 | 9.9 | 8.5 |
| Diabetes with complications, % | 3.5 | 3.4 | 3.7 | 3.5 | 3.7 |
| Renal failure, % | 9.6 | 9.3 | 9.7 | 8.9 | 10 |
| Chronic pulmonary disease, % | 14.7 | 14.5 | 14.3 | 16.9 | 14.6 |
| Liver disease, % | 1.0 | 1.0 | 0.9 | 1.0 | 0.9 |
| Weight loss, % | 1.3 | 1.3 | 0.9 | 1.3 | 1.2 |
| Deficiency anemia, % | 10.3 | 10.7 | 10.6 | 9.5 | 10.0 |
| Chronic blood loss anemia, % | 0.6 | 0.7 | 0.5 | 0.6 | 0.5 |
| Coagulopathy, % | 3.3 | 3.7 | 2.7 | 2.9 | 3.0 |
| Fluid and electrolyte disorders, % | 13.3 | 13.8 | 14.0 | 12.7 | 12.8 |
| Metastatic cancer, % | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 |
| Solid tumor without metastasis, % | 1.0 | 0.9 | 0.8 | 0.9 | 1.0 |
| Paralysis, % | 0.8 | 0.8 | 0.8 | 0.9 | 0.9 |
| ST-elevation MI, % | 57 | 67.3 | 36.6 | 32.1 | 52.1 |
| Glycoprotein IIb/IIIa inhibitor, % | 57.5 | 77.0 | 75.1 | 67.0 | 30.4 |
| Dual antiplatelet pretreatment, % | 28.0 | 25.2 | 27.0 | 21.2 | 32.7 |
IQR indicates interquartile range; LMWH indicates low-molecular-weight heparin; MI, myocardial infarction; UFH, unfractionated heparin.
After phenotyping hospitals according to their unadjusted anticoagulant practice patterns (as described above), we used multiple logistic regression with multinomial outcomes to assess the association between hospital anticoagulant use phenotype and hospital characteristics. To do this, we calculated the odds ratio of a patient being treated at each anticoagulant phenotype hospital (reference: low bivalirudin/low LMWH) in relation to hospital characteristics (hospital population served [rural versus urban], teaching status, number of beds, and geographic region) after adjusting for other patient characteristics.
For clinical outcomes, we first used logistic regression to assess the patient-level association between bleeding events and different anticoagulant treatment strategies, adjusting for patient characteristics and percutaneous and surgical revascularization. Then, we assessed each hospital’s risk-standardized bleeding rates (RSBRs), risk-standardized in-hospital mortality rates (RSMRs), and risk-standardized lengths of stay (RSLOS). Similar to above, all variables were entered into a nonhierarchical multivariable model and the model was reduced using backward elimination. For each hospital, we calculated 2 RSBRs using hierarchical logistic regressions: first adjusting for patient characteristics, PCI, and coronary artery bypass grafts, then additionally adjusting for anticoagulant treatment strategies. All significant predictors of bleeding identified in the model above were included for risk standardization. RSMR was similarly calculated using hierarchical logistic regression. A hierarchical generalized linear model with a logarithmic link and Poisson distribution was used to derive RSLOS for each hospital, which was the product of national average LOS and the ratio of average predicted LOS to average expected LOS. The RSBRs, RSMRs, and RSLOS were then compared across the 4 hospital anticoagulant phenotypes using the Kruskal–Wallis test. These methods are consistent with those used by the Centers for Medicare & Medicaid Services to calculate risk-standardized outcomes.23
All statistical analyses were performed using SAS 9.3 (SAS Institute, Inc, Cary, NC) and a 2-sided P-value of <0.05 was used to determine statistical significance. Analyses were performed independently at the Yale Center for Outcomes Research and Evaluation.
Results
Study Population
During the study period, we identified 281 842 MI hospitalizations within the Premier database (Figure 1). We excluded 148 396 patients whose MIs were managed medically or surgically, 18 189 patients who were not treated with an early invasive strategy, and 26 237 patients who did not receive an anticoagulant during the first 2 days of hospitalization. After excluding an additional 25 194 patients who were <18 years of age, were treated by a pediatrician, were transferred from another hospital, had a length of stay <1 day, or were admitted by a hospital with <25 cases, our final analytic cohort totaled 63 796 patients treated invasively for an MI at 257 hospitals. The median age of the population was 61 years, one third were female, and 57% presented with ST-elevations. Of these patients, 47% were treated with UFH only, 6% were treated with a combination of UFH and LMWH, 7% received LMWH only, and 40% were treated with any bivalirudin. The demographic and clinical characteristics of patients treated with different anticoagulant strategies are shown in Table2.
Figure 1.
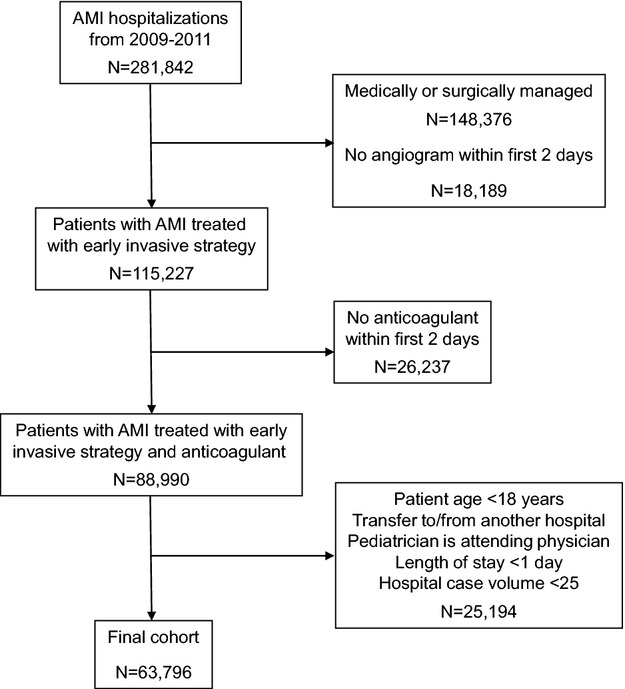
Flowchart of patients. AMI indicates acute myocardial infarction.
Hospital Variability and Phenotypes
The patterns of use of the different anticoagulants varied substantially across the sample of hospitals (Figure 2). The median (interquartile range) use of UFH among the hospitals was 45.8% (19.0% to 65.0%), UFH+LMWH 4.2% (1.0% to 10.8%), LMWH only 4.2% (1.0% to 10.8%), and any bivalirudin 37.4% (18.4% to 66.5%). In the hierarchical, multivariate model adjusted for patient characteristics, there was substantial site-level variability in the anticoagulant strategy. For example, compared with a strategy of UFH, the median odds ratio was 2.90 for LMWH+UFH, indicating that 2 “identical” patients would have an almost 3-fold greater likelihood of being treated with LMWH+UFH at 1 hospital as compared with another. Similarly, the median odds ratio for LMWH only and bivalirudin were 4.70 and 3.09, respectively. Furthermore, the amount of variability in the use of anticoagulants that could be attributed to hospital-level variation (as opposed to patient-level differences), as measured with the intraclass correlation coefficient with UFH only as the reference group, was 28% for UFH+LMWH, 45% for LMWH only, and 30% for bivalirudin.
Figure 2.
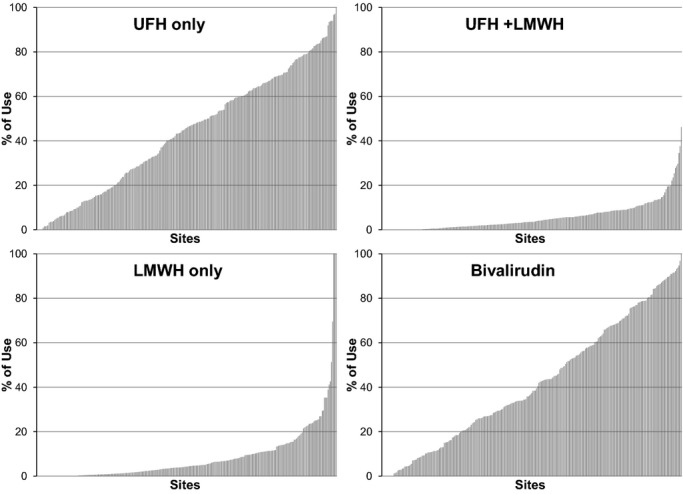
Unadjusted use of each anticoagulation strategy across the 262 hospitals. LMWH indicates low-molecular-weight heparin; UFH, unfractionated heparin.
We then categorized hospitals based on their observed practice patterns: high bivalirudin/high LMWH (n=54), high bivalirudin/low LMWH (n=75), low bivalirudin/high LMWH (n=74), and low bivalirudin/low LMWH (n=54). The median use of each of the 4 anticoagulant strategies among the hospitals in the different anticoagulant use phenotypes is shown in Figure 3. As expected, the anticoagulant treatment strategies varied substantially among different anticoagulant use phenotypes; for example, the median proportion of UFH only ranged from 18% in high bivalirudin/high LMWH hospitals to 77% in low bivalirudin/low LMWH hospitals. In the multivariate model assessing institutional factors with a hospital’s anticoagulant phenotype, rural hospitals were more likely to be high users of bivalirudin (Table3), while teaching hospitals and hospitals with >400 beds were more likely to be low bivalirudin/low LMWH (ie, UFH only) hospitals. Finally, hospitals in the West census region were more likely to be higher users of bivalirudin, particularly in comparison with hospitals in the Northeast. Hospitals in the South and Midwest census regions were higher users of LMWH.
Figure 3.
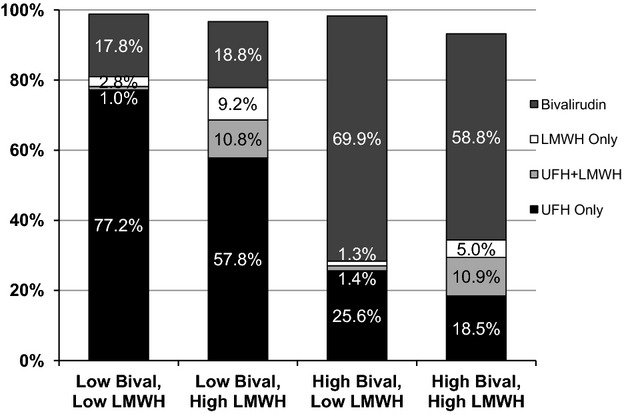
Median use of each anticoagulant strategy by hospital anticoagulant phenotype (percentages do not add up to 100% due to variability around each median estimate). Bival indicates bivalirudin; LMWH, low-molecular-weight heparin; UFH, unfractionated heparin.
Table 3.
Association of Hospital Characteristics and Anticoagulant Use Phenotypes
| OR (95% CI) (REF: Low Bival, Low LMWH) | |||
|---|---|---|---|
| High Bival, High LMWH | High Bival, Low LMWH | Low Bival, High LMWH | |
| Rural population (vs urban) | 1.1 (1.0 to 1.2) | 1.1 (1.0 to 1.2) | 0.7 (0.7 to 0.8) |
| Nonteaching status (vs teaching) | 0.6 (0.5 to 0.6) | 0.6 (0.6 to 0.6) | 0.4 (0.4 to 0.5) |
| Number of beds (REF: >400) | |||
| <200 beds | 1.5 (1.5 to 1.6) | 1.8 (1.7 to 1.9) | 1.8 (1.7 to 1.9) |
| 200 to 400 beds | 9.5 (8.3 to 11.0) | 5.3 (4.7 to 6.1) | 14.8 (12.9 to 16.9) |
| Census region (REF: West) | |||
| Midwest | 1.4 (1.3 to 1.5) | 0.5 (0.5 to 0.5) | 3.6 (3.3 to 3.9) |
| Northeast | 0.1 (0.1 to 0.1) | 0.4 (0.4 to 0.4) | 1.8 (1.6 to 2) |
| South | 3.2 (3 to 3.4) | 0.7 (0.7 to 0.8) | 9.7 (8.9 to 10.5) |
Adjusting for patient age group, gender, STEMI, and comorbidities. High/low users of bivalirudin and LMWH based on above/below median use in the study cohort (37.4.1% and 9.8%, respectively). Bival indicates bivalirudin; LMWH, low-molecular-weight heparin; OR, odds ratio; STEMI, ST-elevation myocardial infarction.
Outcomes
The demographic and clinical factors associated with increased risk of in-hospital bleeding are shown in Table4 and include factors such as older age, heart failure, lung disease, weight loss, anemia, and coagulopathy. At the patient level, bivalirudin (any) was associated with a lower risk of in-hospital bleeding compared with UFH, while patients treated with LMWH or a combination of UFH and LMWH had similar bleeding risk compared with UFH only.
Table 4.
Association of Anticoagulant Treatment Strategies and Patient Characteristics With In-Hospital Bleeding Events
| OR (95% CI) | |
|---|---|
| Anticoagulants (REF: UFH only) | |
| UFH+LMWH | 0.98 (0.82 to 1.17) |
| LMWH only | 0.84 (0.71 to 1.00) |
| Bivalirudin (any) | 0.84 (0.76 to 0.93) |
| Patient factors | |
| Age (REF ≥75 years) | |
| 18 to <55 | 0.45 (0.40 to 0.51) |
| 55 to <65 | 0.56 (0.50 to 0.63) |
| 65 to <75 | 0.78 (0.70 to 0.87) |
| Female | 1.51 (1.39 to 1.65) |
| Hypertension | 0.87 (0.79 to 0.95) |
| Congestive heart failure | 3.77 (2.46 to 5.77) |
| Peripheral vascular disease | 1.37 (1.22 to 1.55) |
| Diabetes with complications | 1.22 (1.02 to 1.45) |
| Renal failure | 1.64 (1.46 to 1.84) |
| Chronic pulmonary disease | 1.53 (1.39 to 1.69) |
| Liver disease | 1.81 (1.34 to 2.44) |
| Weight loss | 2.48 (2.04 to 3.01) |
| Deficiency anemia | 1.17 (1.04 to 1.30) |
| Chronic blood loss anemia | 6.64 (5.25 to 8.40) |
| Coagulopathy | 2.35 (2.04 to 2.71) |
| Fluid and electrolyte disorder | 2.43 (2.22 to 2.67) |
| Metastatic cancer | 1.81 (1.20 to 2.72) |
| Solid tumor without metastasis | 1.88 (1.42 to 2.49) |
| Paralysis | 1.64 (1.22 to 2.20) |
| ST-elevation MI | 1.24 (1.14 to 1.36) |
| Glycoprotein IIb/IIIa inhibitor | 1.60 (1.45 to 1.76) |
c-index=0.76. LMWH indicates low-molecular-weight heparin; MI, myocardial infarction; OR, odds ratio; UFH, unfractionated heparin.
We then examined the outcomes according to the hospital’s anticoagulant use phenotype. In unadjusted analysis, there was a difference in the rate of bleeding events across the 4 phenotypes, with the 2 high bivalirudin phenotypes having the lowest reported rates of bleeding at 3.4%, the low bivalirudin/high LMWH 4.0%, and low bivalirudin/low LMWH 4.4% (Table5; P=0.033). However, there was no significant difference in RSBRs after adjusting for patient characteristics or anticoagulant treatment. In addition, there were no significant differences in RSMR or RSLOS across the 4 phenotypes (Table5).
Table 5.
Anticoagulant Use Phenotypes and Bleeding, Mortality, and Length of Stay
| Anticoagulant Use Phenotypes | High Bival, High LMWH (n=54) | High Bival, Low LMWH (n=75) | Low Bival, High LMWH (n=74) | Low Bival, Low LMWH (n=54) | P Value |
|---|---|---|---|---|---|
| Observed bleeding rate, % | 3.4 (2.3 to 5.4) | 3.4 (2.3 to 4.9) | 4.0 (2.7 to 5.6) | 4.4 (3.4 to 5.6) | 0.033 |
| RS-bleeding rate, adjusted for patient factors, % | 4.1 (3.7 to 4.5) | 4 (3.7 to 4.4) | 4.1 (3.8 to 4.6) | 4.2 (3.9 to 4.6) | 0.338 |
| RS-bleeding rate, also adjusted for anticoagulant, % | 4.1 (3.7 to 4.5) | 4.1 (3.8 to 4.4) | 4.1 (3.8 to 4.6) | 4.2 (3.8 to 4.5) | 0.845 |
| RS-mortality rate, % | 2.3 (2.1 to 2.6) | 2.2 (2 to 2.6) | 2.3 (2 to 2.5) | 2.2 (2 to 2.5) | 0.377 |
| RS-length of stay, days | 3.0 (2.7 to 3.2) | 2.9 (2.7 to 3.2) | 3.0 (2.9 to 3.4) | 3.1 (2.7 to 3.3) | 0.066 |
All values are expressed as median (IQR). High/low users of bivalirudin and LMWH based on above/below median use in the study cohort (37.4% and 9.8%, respectively). Bival indicates bivalirudin; IQR, interquartile range; LMWH, low-molecular-weight heparin; RS, risk standardized.
In the sensitivity analysis using the upper quartile of use to define high-users of bivalirudin and LMWH, there were no differences in unadjusted, risk-adjusted, or anticoagulant-adjusted bleeding rates, and RSMRs across the 4 hospital phenotypes (Table6). RSLOS was slightly shorter at high bivalirudin-using hospitals.
Table 6.
Anticoagulant Use Phenotypes and Bleeding, Mortality, and Length of Stay, With High Use Defined as in the Top Quartile of Utilization
| Anticoagulant Use Phenotypes | High Bival, High LMWH (n=5) | High Bival, Low LMWH (n=60) | Low Bival, High LMWH (n=59) | Low Bival, Low LMWH (n=133) | P Value |
|---|---|---|---|---|---|
| Observed bleeding rate, % | 3.4 (1.2 to 6.8) | 3.5 (2.3 to 5.0) | 4.1 (3.1 to 5.6) | 3.8 (2.7 to 5.4) | 0.345 |
| RS-bleeding rate, adjusted for patient factors, % | 4.0 (3.9 to 4.4) | 4.2 (3.8 to 4.4) | 4.1 (3.8 to 4.6) | 4.0 (3.8 to 4.5) | 0.827 |
| RS-bleeding rate, also adjusted for anticoagulant, % | 4.1 (3.9 to 4.4) | 4.2 (3.8 to 4.4) | 4.1 (3.8 to 4.7) | 4.0 (3.8 to 4.4) | 0.464 |
| RS-mortality rate, % | 2.3 (2.3 to 2.4) | 2.2 (2.0 to 2.5) | 2.4 (2.1 to 2.6) | 2.2 (2.0 to 2.5) | 0.123 |
| RS-length of stay, days | 2.6 (2.5 to 3.1) | 2.8 (2.6 to 3.1) | 3.2 (2.9 to 3.5) | 3.0 (2.7 to 3.2) | <0.001 |
All values are expressed as median (IQR). High users of bivalirudin and LMWH based on the highest quartile of use in the study cohort (66.5% and 19.7%, respectively). Bival indicates bivalirudin; IQR, interquartile range; LMWH, low-molecular-weight heparin; RS, risk standardized.
Discussion
In a study of 63 796 MI patients from 257 US hospitals, we found substantial site-level variability with regard to choice of anticoagulant strategy, even after accounting for patient factors, with >25% of the variability in anticoagulant choice attributable just to local practice patterns. This indicates that the preferences of the treating hospital (presumably through its physicians) play a substantial role in the anticoagulant strategy selected for patients, rather than patients’ individual characteristics. The degree of variability in the use of anticoagulants that was independent of patient characteristics highlights the need for greater guidance on how to most effectively and efficiently use these medicines.
At the core of this issue is the need for more comparative effectiveness studies, ideally with a focus on the heterogeneity of treatment benefit among patients, so that all anticoagulant decisions can be evidence based and patient tailored. If these medicines were truly interchangeable in terms of benefits, risks, and costs, then finding such variation in practice patterns would be inconsequential. However, available studies suggest otherwise,6,8 and the most recent studies only serve to create more controversy and confusion as to the optimal strategy.10,24 Our hospital-based analysis did not find 1 particular anticoagulation phenotype that was superior in terms of patient outcomes. Consequently, more work is needed to define the optimal way to minimize site-level variability and improve patient outcomes.
Potential Explanations
The discrepancy between the patient- and hospital-level analyses could indicate that anticoagulants are not being applied in a manner consistent with the risk profile of the patient. For example, if a high bivalirudin-using hospital uses bivalirudin more often in patients with low bleeding risk (a documented risk-treatment paradox25), then bleeding could be reduced at the patient level but the overall bleeding rate for the hospital would be minimally affected (as the patients in whom bivalirudin is used have an overall low risk of bleeding). However, hospitals that use bivalirudin less often, but more selectively in high bleeding-risk patients, may have lower overall bleeding rates. Nevertheless, this is predicated on an assumption that bivalirudin reduces bleeding risk in patients with MI, an assumption that a recent trial has questioned in patients undergoing primary PCI.10
However, the disconnect between the patient- and hospital-level outcomes analyses may also indicate that other processes of care employed at these hospitals that may be associated with bleeding risk (eg, access site management, postprocedure monitoring) could account for the observed differences in RSBRs beyond anticoagulation management. This finding is supported by recent work in the National Cardiovascular Data Registry, where greater than two thirds of the variation in hospitals’ bleeding rates in the setting of an MI were found to persist after accounting for case mix and treatment strategies, with anticoagulant and antiplatelet therapies explaining only 16% of variation in hospital-adjusted bleeding rates.26
Further work is needed both to understand how to apply these anticoagulants most effectively and efficiently and to identify other aspects of care that contribute to bleeding in order to improve the outcomes of invasively managed MI patients. Preliminary work has demonstrated that simply providing estimates of bleeding risk at the point of care resulted in more targeted bivalirudin use in high-risk patients and reductions in bleeding.27 However, it is unclear whether the improvement in outcomes is mediated through the specific anticoagulant used versus the application of ancillary strategies such as access site management, when the bleeding risk of the patient is known. More studies are needed to better understand the specifics of this patient-directed treatment strategy and whether or not it can be applied on a larger scale.
Limitations
There are potential limitations to consider when interpreting our study. First, while we were able to establish that there was substantial variability in the use of different anticoagulants that was based purely on local practice patterns, the ideal pattern of use, which minimizes ischemic and bleeding complications while also minimizing costs, is currently unknown. In addition, we defined high and low users of LMWH and bivalirudin based on median levels of use. While we did perform a sensitivity analysis defining the upper quartile of use as a high user, we were unable to determine the appropriateness of use—either overuse or underuse—among hospitals, and it is possible that there is an ideal pattern of use that our approach was unable to identify. Thus, further work is needed to determine the ideal balance of anticoagulants in various patient populations.
Second, we did not limit or stratify our analyses based on ST-elevation. While this certainly affects both the choice of anticoagulant and also the risk of bleeding, we wished to conduct a broader analysis of MI patients and thus elected to adjust for this important factor in the multivariate models instead of dividing the analytic population. Third, given the complexity of the multivariate models and the need to identify meaningful hospital phenotypes, we did not include all possible combinations of anticoagulants and instead used 4 broad categories, as has been done previously.28 In addition, we did not further stratify by glycoprotein IIb/IIIa use, which was used less commonly in patients who received bivalirudin. However, we did adjust for the use of these medications in all of our models, in order to account for these differences across the anticoagulants. Fourth, we were unable to determine the access site for the coronary angiogram, which could impact bleeding. While it is possible that physicians who use more femoral access also use more bivalirudin to compensate for the increased risk of bleeding, this could not explain why the patient-level results showed benefit with bivalirudin that was not observed in the hospital-level results. Fifth, our patient-level results agree with those of many,6,8 but not all,10 anticoagulant trials. Importantly, our work was designed as an investigation of patterns of care and the association of these patterns with outcomes rather than as a comparative effectiveness study.
Finally, we relied on ICD-9-CM codes, not adjudication of hospital records, both to identify MI patients and the incidence of clinically relevant bleeding. Our rate of MI patients not treated with an anticoagulant was ≈13% compared with ≈6% found in the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With the Guidelines.28 While our study design of analyzing only patients who received an anticoagulant somewhat accounts for this first point, it is possible that some patients included in our analyses were miscoded as having had MIs. In addition, using administrative coding to base our categorization of MIs as non-ST-elevation or ST-elevation likely resulted in some misclassification. However, as this was only used for adjustment in the models and was not a key variable in the results, we believe the results are unlikely to have been materially affected. For the bleeding events, while we are likely underestimating the true rate of bleeding in this population (≈4% in our study versus ≈10% in the national registry28), it is unlikely that this undercoding was biased among hospitals and thus should not have affected our observed associations between treatments and hospital phenotypes with bleeding outcomes. Furthermore, analyses in warfarin-treated patients have shown ICD-9-CM codes to be reasonably reliable for identifying clinically relevant bleeding events.29
Conclusions
In a large database of US hospitals, we found substantial site-level variability in the choice of anticoagulants for patients with an acute MI who were invasively managed, even after accounting for patient factors. When we phenotyped hospitals by their pattern of anticoagulant use, no single pattern was found to be clinically superior. While the ideal pattern of use of anticoagulants—one that minimizes ischemic and bleeding complications as well as costs—is not known, it is important to understand the current patterns of use and the clinical outcomes associated with them. The amount of variability that we identified that was based on local practice patterns and not patient characteristics highlights the need for more studies to determine which patients would benefit most from the anticoagulant choices. Such studies are needed to help inform our guidelines and clinical practice such that these therapies can be applied effectively and efficiently, and individualized to patients’ needs.
Sources of Funding
Dr Krumholz is supported by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. This work was supported by grant DF10-301 from the Catherine and Patrick Weldon Donaghue Medical Research Foundation in West Hartford, Connecticut.
Disclosures
Wang: Research grants to the Duke Clinical Research Institute from Eli Lilly, Daiichi Sankyo, Gilead Sciences, GlaxoSmith Kline, American College of Cardiology, American Society of Nuclear Cardiology; Consultant honoraria: Astra Zeneca, American College of Cardiology Foundation. Spertus: Research grants: National Heart, Lung, and Blood Institute, American Heart Association, American College of Cardiology Foundation, Gilead Sciences, Eli Lilly, EvaHeart, Amorcyte. Consultant honoraria: United Healthcare, Genentech, Amgen. Kosiborod: Research grants: American Heart Association, Genentech, Sanofi-Aventis, Gilead Sciences, Medtronic Minimed, Glumetrics, Maquet, Eisai; Consultant honoraria: Genentech, Gilead Sciences, F. Hoffmann-La Roche, Medtronic Minimed, AstraZeneca, Abbvie, Regeneron, Edwards Lifesciences, Eli Lilly. Krumholz: Research agreements to Yale University from Medtronic and from Johnson & Johnson to develop methods of clinical trial data sharing; Chair of a cardiac scientific advisory board for UnitedHealth. Arnold, Li, Alexander, Nallamothu, Curtis, Gupta, Lin, Dharmarajan, Strait, and Lowe report no conflicts of interest.
References
- Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van‘t Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Jacobs AK. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:2022–2060. doi: 10.1161/CIR.0b013e31820f2f3e. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Anand SS, Malmberg K, Weitz JI, Ginsberg JS, Yusuf S. Unfractionated heparin and low-molecular-weight heparin in acute coronary syndrome without ST elevation: a meta-analysis. Lancet. 2000;355:1936–1942. doi: 10.1016/S0140-6736(00)02324-2. [DOI] [PubMed] [Google Scholar]
- Sinnaeve PR, Simes J, Yusuf S, Garg J, Mehta S, Eikelboom J, Bittl JA, Serruys P, Topol EJ, Granger CB. Direct thrombin inhibitors in acute coronary syndromes: effect in patients undergoing early percutaneous coronary intervention. Eur Heart J. 2005;26:2396–2403. doi: 10.1093/eurheartj/ehi590. [DOI] [PubMed] [Google Scholar]
- Mahaffey KW, Cohen M, Garg J, Antman E, Kleiman NS, Goodman SG, Berdan LG, Reist CJ, Langer A, White HD, Aylward PE, Col JJ, Ferguson JJ, III, Califf RM. High-risk patients with acute coronary syndromes treated with low-molecular-weight or unfractionated heparin: outcomes at 6 months and 1 year in the SYNERGY trial. JAMA. 2005;294:2594–2600. doi: 10.1001/jama.294.20.2594. [DOI] [PubMed] [Google Scholar]
- Stone GW, White HD, Ohman EM, Bertrand ME, Lincoff AM, McLaurin BT, Cox DA, Pocock SJ, Ware JH, Feit F, Colombo A, Manoukian SV, Lansky AJ, Mehran R, Moses JW. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the acute catheterization and urgent intervention triage strategy (ACUITY) trial. Lancet. 2007;369:907–919. doi: 10.1016/S0140-6736(07)60450-4. [DOI] [PubMed] [Google Scholar]
- Amin AP, Marso SP, Rao SV, Messenger J, Chan PS, House J, Kennedy K, Robertus K, Cohen DJ, Mahoney EM. Cost-effectiveness of targeting patients undergoing percutaneous coronary intervention for therapy with bivalirudin versus heparin monotherapy according to predicted risk of bleeding. Circ Cardiovasc Qual Outcomes. 2010;3:358–365. doi: 10.1161/CIRCOUTCOMES.110.957290. [DOI] [PubMed] [Google Scholar]
- Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, Andron M, Appleby C, Fisher M, Khand A, Kunadian B, Mills JD, Morris JL, Morrison WL, Munir S, Palmer ND, Perry RA, Ramsdale DR, Velavan P, Stables RH. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet. 2014;384:1849–1858. doi: 10.1016/S0140-6736(14)60924-7. [DOI] [PubMed] [Google Scholar]
- Burwen DR, Galusha DH, Lewis JM, Bedinger MR, Radford MJ, Krumholz HM, Foody JM. National and state trends in quality of care for acute myocardial infarction between 1994–1995 and 1998–1999: the Medicare health care quality improvement program. Arch Intern Med. 2003;163:1430–1439. doi: 10.1001/archinte.163.12.1430. [DOI] [PubMed] [Google Scholar]
- Partovian C, Gleim SR, Mody PS, Li SX, Wang H, Strait KM, Allen LA, Lagu T, Normand SL, Krumholz HM. Hospital patterns of use of positive inotropic agents in patients with heart failure. J Am Coll Cardiol. 2012;60:1402–1409. doi: 10.1016/j.jacc.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi KC, Dharmarajan K, Kim N, Strait KM, Li SX, Chen SI, Lagu T, Krumholz HM. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation. 2013;127:923–929. doi: 10.1161/CIRCULATIONAHA.112.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premier Inc. Mission/Vision: What Premier Does. 2015. . Retrieved from https://www.premierinc.com/about-premier/mission-and-vision. [Google Scholar]
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Pinto DS, Ogbonnaya A, Sherman SA, Tung P, Normand SL. Bivalirudin therapy is associated with improved clinical and economic outcomes in ST-elevation myocardial infarction patients undergoing percutaneous coronary intervention: results from an observational database. Circ Cardiovasc Qual Outcomes. 2012;5:52–61. doi: 10.1161/CIRCOUTCOMES.111.961938. [DOI] [PubMed] [Google Scholar]
- Rassen JA, Mittleman MA, Glynn RJ, Alan Brookhart M, Schneeweiss S. Safety and effectiveness of bivalirudin in routine care of patients undergoing percutaneous coronary intervention. Eur Heart J. 2010;31:561–572. doi: 10.1093/eurheartj/ehp437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangas GD, Mehran R, Nikolsky E, Claessen BE, Lansky AJ, Brodie BR, Witzenbichler B, Guagliumi G, Peruga JZ, Dudek D, Mockel M, Caixeta A, Parise H, White H, Stone GW. Effect of switching antithrombin agents for primary angioplasty in acute myocardial infarction: the HORIZONS-SWITCH analysis. J Am Coll Cardiol. 2011;57:2309–2316. doi: 10.1016/j.jacc.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Astroulakis Z, Hill JM. Does ‘switching’ from heparin to bivalirudin monotherapy result in a bleeding benefit? Eur Heart J Suppl. 2009;11:C13–C18. [Google Scholar]
- Stone GW, Mehran R, Goldstein P, Witzenbichler B, Van’t Hof A, Guagliumi G, Hamm CW, Genereux P, Clemmensen P, Pocock SJ, Gersh BJ, Bernstein D, Deliargyris EN, Steg PG. Bivalirudin versus heparin with or without glycoprotein IIb/IIIa inhibitors in patients with STEMI undergoing primary percutaneous coronary intervention: pooled patient-level analysis from the HORIZONS-AMI and EUROMAX trials. J Am Coll Cardiol. 2015;65:27–38. doi: 10.1016/j.jacc.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Grilli L, Rampichini C. A multilevel multinomial logit model for the analysis of graduates’ skills. Stat Methods Appl. 2007;16:381–393. [Google Scholar]
- Krumholz HM, Wang Y, Mattera JA, Han LF, Ingber MJ, Roman S, Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- Briguori C, Visconti G, Focaccio A, Donahue M, Golia B, Selvetella L, Ricciardelli B. Novel approaches for preventing or limiting events (Naples) III trial: randomized comparison of bivalirudin versus unfractionated heparin in patients at increased risk of bleeding undergoing transfemoral elective coronary stenting. JACC Cardiovasc Interv. 2015;8:414–423. doi: 10.1016/j.jcin.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- Xian Y, Chen AY, Thomas L, Roe MT, Subherwal S, Cannon CP, Pollack CV, Jr, Fonarow GC, Kosiborod M, Peterson ED, Alexander KP. Sources of hospital-level variation in major bleeding among patients with non-ST-segment elevation myocardial infarction: a report from the national cardiovascular data registry (NCDR) Circ Cardiovasc Qual Outcomes. 2014;7:236–243. doi: 10.1161/CIRCOUTCOMES.113.000715. [DOI] [PubMed] [Google Scholar]
- Rao SC, Chhatriwalla AK, Kennedy KF, Decker CJ, Gialde E, Spertus JA, Marso SP. Pre-procedural estimate of individualized bleeding risk impacts physicians’ utilization of bivalirudin during percutaneous coronary intervention. J Am Coll Cardiol. 2013;61:1847–1852. doi: 10.1016/j.jacc.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Kadakia MB, Desai NR, Alexander KP, Chen AY, Foody JM, Cannon CP, Wiviott SD, Scirica BM. Use of anticoagulant agents and risk of bleeding among patients admitted with myocardial infarction: a report from the NCDR Action Registry—GWTG (national cardiovascular data registry acute coronary treatment and intervention outcomes network registry–get with the guidelines) JACC Cardiovasc Interv. 2010;3:1166–1177. doi: 10.1016/j.jcin.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]


