Abstract
Background
Isolated nocturnal hypertension (INH) has been studied among the general population and hypertensive patients. However, little insight is available on the prevalence of INH and its role in target-organ damage among patients with chronic kidney disease (CKD).
Methods and Results
We recruited 1282 CKD patients admitted to our hospital division. Patients were divided into 4 groups: INH; isolated daytime hypertension; day–night sustained; and ambulatory normotension. Multiple linear regression analyses were used to evaluate the correlation between INH and renal/cardiovascular parameters. A total of 262 (20.44%) CKD patients had isolated nocturnal hypertension and 651 (50.78%) had day–night sustained hypertension, whereas only 350 (27.30%) patients showed normotension and 19 (1.48%) had isolated daytime hypertension. Multivariate logistic regression analysis showed that INH was associated mainly with age, estimated glomerular filtration rate, clinic diastolic blood pressure, and that INH was determined only by age, estimated glomerular filtration rate, and clinic diastolic blood pressure. The prevalence of impaired renal function, left ventricular hypertrophy, and carotid intima-media thickness in patients with INH were higher than in normotensive patients (P<0.05), whereas impaired renal function and left ventricular hypertrophy in these patients were lower than patients in the day–night sustained hypertension group (P<0.05). INH was correlated with estimated glomerular filtration rate, left ventricular mass index, and carotid intima-media thickness according to multiple linear regression analyses.
Conclusions
The prevalence of INH in CKD patients was high, and INH was correlated with target-organ damage in CKD patients.
Keywords: ambulatory blood pressure monitoring, chronic kidney disease, isolated nocturnal hypertension
Chronic kidney disease (CKD) is a global public-health problem. Despite widespread use of interventions to slow the progression of CKD, the burden of end-stage renal disease in many industrialized countries remains significant.1,2 CKD has also become an important public-health problem in China. According to 1 survey, the prevalence of CKD in China is 10.8%, so the number of patients with CKD in China is ≈119.5 million.3 The prevalence of hypertension in patients with CKD is considerably higher than that in the normal, healthy population, and escalates with a decline in renal function. Elevated blood pressure (BP) causes injury to blood vessels in the kidney and other organs through excessive mechanical and oxidative stresses,4 which can lead to complications such as renal failure and cardiovascular disease.5 Hypertension is an important modifiable risk factor for such complications,6 so regulation of BP may substantially reduce the risk of cardiovascular events and slow the decline in kidney function.7
Recent evidence suggests that ambulatory blood pressure monitoring (ABPM) might be the best tool to classify hypertensive status and the risk of adverse events.8,9 ABPM has increased the ability to identify circadian variations in BP and identify types of hypertension, most notably isolated nocturnal hypertension (INH). The superiority of nighttime BP levels as captured by ABPM, rather than daytime or clinic values of BP, for predicting target-organ damage or cardiovascular disease development in hypertensive patients has been reported.10,11
INH was identified first in 2007 by Li et al. They reported a prevalence of 10.9% in a Chinese cohort of >600 participants, and these patients had more severe target-organ damage.12 Patients with INH had a higher risk of all-cause (hazard ratio, 1.29; P=0.045) and cardiovascular event (1.38; 0.037) in unadjusted analyses.8 INH was called “a masked disease in the dark” because it could be diagnosed only by ABPM, the use of which is limited.13
Compared with other patients, patients with CKD have special features. Firstly, they are the highest risk group for cardiovascular events.14 Secondly, the prevalence of hypertension and nocturnal hypertension is predominant and increases with decline in renal function based on the key role of the kidney in BP regulation.15 Thirdly, the primary cause of CKD in China is glomerular nephritis and not diabetes mellitus (DM), as is the case in Western countries16.
Previously, we reported that CKD patients had a higher prevalence of nondipper and reversed-dipper BP patterns, which suggested a higher prevalence of nocturnal hypertension in CKD patients.16 However, data on the “true” prevalence of INH in Chinese patients with CKD are lacking.
In the present study, we undertook 24-hour BP monitoring among CKD patients. Then, we comprehensively examined the prevalence of INH in CKD patients. We also explored with clinical parameters that were correlated with INH.
Materials and Methods
Study Population
The study protocol was approved by the ethics committee of the Third Hospital of Sun Yat-Sen University (Guangdong, China), and was approved by the Institutional Review Board. All of the study participants provided written informed consent to be included in the study.
Inclusion criteria were age 14 to 75 years and CKD patients. Exclusion criteria were the following: undergoing treatment with corticosteroids or hormones; acute changes in the estimated glomerular filtration rate (eGFR) >30% in the previous 3 months; pregnancy; history of abuse of drugs or alcohol; night work or shift-work employment; acquired immunodeficiency syndrome; cardiovascular disorders (unstable angina pectoris, heart failure, life-threatening arrhythmia, atrial fibrillation, and grade III to IV retinopathy); intolerance to ABPM; inability to communicate and comply with all of the study requirements; on maintenance dialysis; and being in receipt of any antihypertensive drug in the previous month.
From May 2010 to October 2014, 1740 consecutive CKD inpatients formed the cohort for this cross-sectional study. A total of 337 patients were ruled out because they had undergone some type of antihypertensive treatment. One hundred twenty-one patients were excluded due to deficiency of clinical or ultrasonographic data. Finally, 1282 CKD patients were enrolled in this study. In terms of causes of renal diseases, 723 patients had chronic glomerulonephritis; 165 cases had diabetic nephropathy; 65 subjects had hypertensive nephropathy; 95 individuals had lupus nephritis; and 234 patients had other causes of renal disease (Figure 1).
Figure 1.
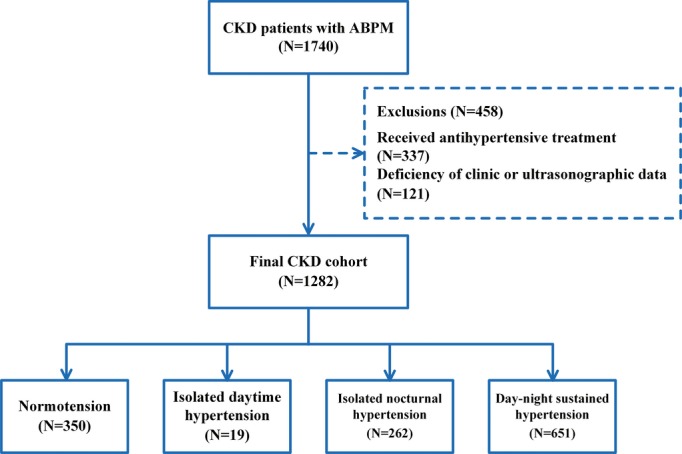
Patient selection and assignment to different ambulatory blood pressure status. ABPM indicates ambulatory blood pressure monitoring; CKD, chronic kidney disease.
Measurements
Ambulatory blood pressure monitoring
Patients underwent 24-hour ABPM using a TM-2430 Monitor (A&D, Tokyo, Japan). Cuff size was chosen based on arm circumference and was applied to the nondominant arm. Three clinic readings were collected. BP readings were obtained using a mercury sphygmomanometer by a physician who did not have access to ABP values, then BP was recorded every 15 minutes in daytime, and every 30 minutes in nighttime. Monitoring was done on a working day. Patients were asked to attend to their usual activities but to keep motionless at the time of measurement. Patients had no access to ABP values. Strenuous physical activity was discouraged in all patients during the monitoring period, and their daily activities were comparable. BP series were eliminated from the analyses if any of the following applied: >30% of the measurements were lacking; they had missing data for >3-hour spans; they were collected from subjects who were experiencing an irregular rest–activity schedule or a nighttime sleep span <6 or >12 hours during monitoring.
BP measurement in the clinic
BP was measured for each patient during a visit to the physician.17 Briefly, measurements were taken in a quiet environment using a mercury sphygmomanometer with the patient in a sitting position after 5 minutes of rest. BP was not measured if the patient had consumed tobacco, ingested caffeine, or eaten within the previous 30 minutes. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) values (Korotkoff’s phase I and phase V, respectively) at each visit enabled recording of a minimum of 3 BP measurements at intervals of ≥1 minutes. Reported values of clinic BP were the mean of values recorded during the 2 days in which the ABPM device was installed and removed. For all patients, sphygmomanometric measurements were recorded by the same physician, who was not aware of the results of ABP recordings.
Cardiac assessment
Cardiac structure was assessed by 2 investigators trained for this purpose before starting the study. Left ventricular mass (LVM), systolic function, and diastolic function were assessed using 2-dimensional echocardiography. Linear measurements of end-diastolic interventricular septal wall thickness (IVSd), end-diastolic left ventricular internal dimension (LVIDd), and end-diastolic posterior wall thickness (PWTd) were obtained from M-mode tracings. LVM was calculated using the following formula18:
The left ventricular mass index (LVMI) was obtained by calculating the ratio of LVM to body surface area.19
Carotid ultrasonography
Carotid intima-media thickness (cIMT) was assessed by 2 trained investigators before study commencement. A MicroMaxx Ultrasound system paired with a 5 to 10-MHz Multi-frequency High-resolution Linear Transducer (SonoSite, Bothell, WA) with Sono-Calc IMT software was used for taking automatic measurements of cIMT. This was achieved by averaging 3 measurements taken on each carotid artery (anterior, lateral, and posterior directions) and measuring the distance between the leading edge of the lumen–intima interface and the leading edge of the collagenous upper layer of the adventitia using high-resolution B-mode ultrasonography.
Renal assessment
Serum concentrations of creatinine (Scr) were measured by an enzymatic method traceable to isotope dilution mass spectrometry. The eGFR was calculated using a modified version of the Modification of Diet in Renal Disease equation based on data from Chinese CKD patients20 as follows:
where Scr is serum creatinine concentration (in mg/dL) and age is in years.
Collection of other data
We collected urine samples from 7 am to 7 am the next day to detect the extent of proteinuria and sodium levels over 24 hours. These patients were asked to void their bladders before the urine collection, then these patients did same thing after 24 hours. Proteinuria was measured by immunoturbidimetry. In addition, medical history, including demographic and laboratory data (hemoglobin, albumin, globulin, calcium, phosphorus, intact parathyroid hormone, serum fasting glucose, cholesterol, triglycerides, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein-cholesterol [LDL-C], homocysteine, uric acid, serum cystatin C, and blood urea nitrogen), were obtained at the initial study visit. All these experimental data were measured using a 7180 Biochemistry Auto-analyzer (Hitachi, Tokyo, Japan).
Definitions
“Clinic hypertension” was defined as clinic BP ≥140/90 mm Hg, and ambulatory hypertension was defined as average 24 hours BP ≥130/80 mm Hg.15
“ABPM daytime” and “ABPM nighttime” were defined as time intervals from 7 am to 10 pm and from 10 pm to 7 am, respectively. These definitions were based on patients’ schedule.
The definition of INH, isolated daytime hypertension, day–night sustained hypertension, and ambulatory normotension are summarized in Table1.
Table 1.
Diagnostic Criteria of Ambulatory Hypertension
| Daytime BP (mm Hg) | Nighttime BP (mm Hg) | |
|---|---|---|
| Ambulatory normotension | SBP<135 and DBP<85 | SBP<120 and DBP<70 |
| Isolated daytime hypertension | SBP≥135 or DBP≥85 | SBP<120 and DBP<70 |
| Isolated nocturnal hypertension | SBP<135 and DBP<85 | SBP≥120 or DBP≥70 |
| Day–night sustained hypertension | SBP≥135 or DBP≥85 | SBP≥120 or DBP≥70 |
DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
CKD was defined as the presence of kidney damage or decreased renal function (eGFR of <60 mL/min per 1.73 m2) for ≥3 months according to guidelines set by the Kidney Disease Outcomes Quality Initiative, and we divided these CKD patients into 5 stages (1, 2, 3, 4, 5) according to this guideline.21
Diabetes mellitus (DM) was defined as the need for antidiabetic drugs or meeting the diagnostic criteria for DM specified by Chinese Guidelines for Diabetes Prevention and Treatment: (1) symptoms of DM and casual blood glucose >11.1 mmol/L; (2) fasting blood glucose >7.0 mmol/L.22
Target-organ damage was defined as having any of 3 conditions. Firstly, in terms of heart disease, patients with a LVMI >115 g/m2 (man) and >95 g/m2 (woman) were diagnosed as having left ventricular hypertrophy.19 Secondly, with respect to large-vessel disease, cIMT >1 mm was regarded as an abnormal value.23 Thirdly, with regard to kidney disease, an eGFR <60 mL/min per 1.73 m2 was regarded as impaired renal function.24
Statistical Analyses
Descriptive statistics are the mean± SD for continuous variables and median values/interquartile range for nonparametric variables. Frequency and percentages are used for categorical variables. Log transformation for proteinuria and the eGFR in regression analyses were done in view of the skewed distribution of these data.
Comparisons of continuous variables between groups were evaluated by ANOVA, or nonparametric tests. Differences among categorical variables were analyzed using the χ2 test or 2-tailed Fisher exact test, as appropriate. P-values for multiple comparisons were corrected according to the Bonferroni method (6 comparisons).
Multivariable logistic regression analyses were used to explore factors associated with nocturnal hypertension and INH. Variables were the following: age; sex; disease course; diabetes mellitus; current smoking; alcohol intake; body mass index; urinary sodium excretion; eGFR, clinic SBP; clinic DBP; levels of hemoglobin, albumin, calcium×phosphorus, serum fasting glucose, cholesterol, triglyceride, HDL-C, LDL-C, Logarithm (proteinuria), urinary sodium excretion, eGFR, clinic SBP, and clinic DBP.
Clinic SBP was divided by 10-mm Hg portions into several segments to explore the prevalence of INH with elevation of clinic SBP.
Multiple linear regression models were employed to study the association of indices of renal function (Lg eGFR) and cardiovascular damage (LVMI and cIMT) with age, sex, ambulatory BP status, and other variables with P<0.05 explored in simple linear regression analysis.
All values were 2-tailed and P<0.05 was considered significant. Data were analyzed using IBM SPSS v20.0 (IBM, Armonk, NY).
Results
Demographic and Clinical Characteristics of the Study Population
Mean age of the study cohort was 43.96 years, and 59.13% of the cohort was male. A total of 723 patients had chronic glomerular nephritis (56.4%), whereas 234 patients suffered from DM (18.25%); 19.27% of patients were current smokers, and 9.28% consumed alcohol.
Compared with patients with normotension, patients with INH showed greater age; longer disease course; higher prevalence of DM; higher serum levels of globulin, calcium×phosphorus, intact parathyroid hormone, homocysteine, uric acid, cystatin C, blood urea nitrogen, and creatinine; and lower levels of hemoglobin, HDL-C, and LDL-C (P<0.05). When these patients were compared with those in the day–night sustained hypertension group, they showed younger age; lower prevalence of DM; lower levels of calcium×phosphorus, intact parathyroid hormone, homocysteine, uric acid, proteinuria, serum cystatin C, blood urea nitrogen, and serum creatinine; and higher levels of hemoglobin, HDL-C, and LDL-C (P<0.05) (Table2).
Table 2.
Differences of Demographic, Clinical, and ABPM Characteristics in Chinese CKD Patients With Different Ambulatory Blood Pressure Status
| Total (N=1282) | Normotension (N=350) | Isolated Daytime Hypertension (N=19) | Isolated Nocturnal Hypertension (N=262) | Day-Night Sustained Hypertension (N=651) | P Value | |
|---|---|---|---|---|---|---|
| Age, y | 43.96±16.61 | 34.87±14.95 | 43.79±16.76 | 44.21±15.58* | 48.74±15.85*‡ | <0.001 |
| Male:female ratio | 758:524 | 192:158 | 13:6 | 143:119 | 410:241 | 0.022 |
| Course, months | 6 (1 to 24) | 4 (1 to 12) | 4 (1 to 24) | 6 (1 to 36)* | 8 (1 to 32.5)* | <0.001 |
| Diabetes mellitus, N (%) | 234 (18.25) | 23 (6.57) | 3 (15.79) | 42 (16.03)* | 166 (25.50)*‡ | <0.001 |
| Current smoker, N (%) | 247 (19.27) | 58 (16.57) | 5 (26.32) | 41 (15.65) | 143 (21.97) | 0.056 |
| Alcohol intake, N (%) | 119 (9.28) | 24 (6.86) | 3 (15.79) | 24 (9.16) | 68 (10.45) | 0.206 |
| BMI, kg/m2 | 22.98±3.54 | 22.27±3.59 | 24.16±4.16 | 22.82±3.53 | 23.41±3.44* | <0.001 |
| Hemoglobin, g/L | 113.69±29.04 | 128.30±22.20 | 123.21±27.11 | 116.81±28.07* | 104.38±29.18*†‡ | <0.001 |
| Albumin, g/L | 33.83±8.33 | 33.04±9.63 | 33.71±11.13 | 34.58±7.88 | 33.96±7.63 | 0.158 |
| Globulin, g/L | 23.74±5.18 | 22.93±5.03 | 24.59±4.63 | 24.14±5.33* | 23.99±5.19* | 0.008 |
| Calcium×phosphorus, mg2/dL2 | 38.15±11.27 | 34.16±7.87 | 33.49±8.70 | 36.91±10.97* | 40.86±12.20*‡ | <0.001 |
| iPTH, pg/mL | 68.93 (36.78 to 219.25) | 38.19 (27.35 to 65.35) | 49.90 (26.88 to 188.96) | 60.35 (38.10 to 143.34)* | 120.70 (49.02 to 285.26)*†‡ | <0.001 |
| Serum fasting glucose, mmol/L | 5.23±1.59 | 4.92±1.25 | 5.20±1.22 | 5.20±1.57 | 5.40±1.75* | <0.001 |
| Cholesterol, mmol/L | 5.68±2.61 | 6.20±2.95 | 6.10±3.09 | 5.58±2.55* | 5.43±2.37* | <0.001 |
| Triglyceride, mmol/L | 1.94±1.34 | 1.72±1.19 | 2.50±2.15 | 1.93±1.30 | 2.05±1.39* | 0.002 |
| HDL-C, mmol/L | 1.20±0.44 | 1.35±0.45 | 1.23±0.47 | 1.17±0.43* | 1.12±0.41* | <0.001 |
| LDL-C, mmol/L | 3.73±2.13 | 4.16±2.41 | 3.95±1.64 | 3.54±1.86* | 3.56±2.05* | <0.001 |
| Homocysteine, μmol/L | 17.88±10.18 | 13.29±7.77 | 16.65±11.30 | 17.66±9.85* | 20.39±10.54*‡ | <0.001 |
| Uric acid, mmol/L | 460.36±137.52 | 399.72±119.88 | 407.17±114.01 | 454.90±134.15* | 496.04±136.36*†‡ | <0.001 |
| Proteinuria, g/24 h | 1.49 (0.45 to 3.90) | 0.90 (0.24 to 3.83) | 1.86 (0.42 to 7.96) | 1.03 (0.43 to 2.82) | 1.97 (0.80 to 4.41)*‡ | <0.001 |
| Urinary sodium excretion, mmol/24 h | 128.33±65.15 | 130.97±68.93 | 169.05±81.62 | 127.57±60.38 | 125.23±63.75 | 0.074 |
| Serum cystatin C, mg/L | 2.58±1.96 | 1.38±1.28 | 1.68±1.06 | 2.38±1.86* | 3.34±1.98*†‡ | <0.001 |
| Blood urea nitrogen, mmol/L | 8.86 (5.40 to 19.37) | 5.25 (3.95 to 7.07) | 6.79 (4.56 to 11.16) | 8.81 (5.36 to 17.10)* | 14.67 (7.66 to 24.25)*†‡ | <0.001 |
| Serum creatinine, μmol/L | 138.40 (78.85 to 466.00) | 79.00 (61.00 to 112.00) | 99.30 (69.20 to 214.00) | 125.40 (81.45 to 339.50)* | 291.20 (119.15 to 671.00)*†‡ | <0.001 |
| eGFR-MDRD, mL/min per 1.73 m2 | 47.46 (10.51 to 100.43) | 102.83 (65.15 to 135.29) | 80.80 (27.62 to 103.18) | 52.08 (14.99 to 96.71)* | 17.65 (6.32 to 57.54)*†‡ | <0.001 |
| LVMI, g/m2 | 108.13±32.76 | 83.80±21.75 | 91.77±12.81 | 101.56±23.13* | 121.54±33.05*†‡ | <0.001 |
| cIMT, mm | 0.72±0.25 | 0.60±0.19 | 0.69±0.30 | 0.74±0.24* | 0.77±0.26* | <0.001 |
| Clinic hypertension, N (%) | 757 (59.05) | 85 (24.29) | 12 (63.16)* | 132 (50.38)* | 528 (81.11)*‡ | <0.001 |
| Ambulatory hypertension, N (%) | 780 (60.84) | 0 (0) | 17 (89.47)* | 113 (43.13)*† | 650 (99.85)*†‡ | <0.001 |
| Clinic-SBP, mm Hg | 143.75±24.24 | 125.88±16.78 | 141.16±21.12* | 138.72±20.46* | 155.46±22.52*†‡ | <0.001 |
| Clinic-DBP, mm Hg | 86.48±14.09 | 78.45±10.62 | 87.32±12.31* | 86.18±13.13* | 90.89±14.25*‡ | <0.001 |
| 24 h-SBP, mm Hg | 133.59±18.66 | 113.31±7.98 | 131.42±3.50* | 124.43±7.02*† | 148.25±12.69*†‡ | <0.001 |
| 24 h-DBP, mm Hg | 79.61±10.58 | 68.54±4.98 | 78.89±4.23* | 76.15±4.85* | 86.97±8.49*†‡ | <0.001 |
| SBP-daytime, mm Hg | 135.39±18.31 | 116.29±8.73 | 137.89±3.84* | 124.99±6.90*† | 149.78±12.36*†‡ | <0.001 |
| DBP-daytime, mm Hg | 80.92±10.46 | 70.79±5.51 | 83.00±4.91* | 76.53±5.11*† | 88.06±8.60*†‡ | <0.001 |
| SBP-nighttime, mm Hg | 127.99±21.79 | 103.82±7.85 | 111.16±5.80 | 122.71±10.31*† | 143.59±16.76*†‡ | <0.001 |
| DBP-nighttime, mm Hg | 75.49±12.50 | 61.37±4.90 | 64.95±2.70 | 74.88±6.28*† | 83.64±10.10*†‡ | <0.001 |
ABPM indicates ambulatory blood pressure monitoring; BMI, body mass index; cIMT, carotid intima-media thickness; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; iPTH, intact parathyroid hormone; LDL-C, low-density lipoprotein cholesterol; LVMI, left ventricular mass index; MDRD, Modification of Diet in Renal Disease; SBP, systolic blood pressure.
P-value for analysis of variance or χ2 test between these 4 groups; P-value for multiple comparisons was corrected according to the Bonferroni method (6 comparisons). *Comparison with the normotension group P<0.05
comparison with the isolated daytime hypertension group P<0.05
comparison with the isolated nocturnal hypertension group P<0.05.
Characteristics of ABPM in CKD Patients According to Different Ambulatory BP Status
The prevalence of clinic hypertension in patients with INH was 50.38% patients, which was higher than that in patients with normotension (24.29%), but lower than in patients with day–night sustained hypertension (81.11%) (P<0.05). The prevalence of ambulatory hypertension in patients with INH was 43.13%, which was higher than that in patients with normotension (0%), but lower than that in patients with day–night sustained hypertension (99.85%) (P<0.05). Patients with INH had a higher clinic SBP/DBP, 24-hour SBP/DBP, daytime SBP/DBP and nighttime SBP/DBP, as well as a lower rate of nocturnal decline of SBP/DBP, compared with normotensive patients (P<0.05), whereas opposite results were obtained upon comparison with the day–night sustained hypertension group (P<0.05) (Table2).
Prevalence of INH in CKD Patients at Different Stages
A total of 262 (20.44%) CKD patients had INH and 651 (50.78%) had day–night sustained hypertension, whereas only 350 (27.30%) patients showed normotension and 19 (1.48%) had isolated daytime hypertension. The prevalence of INH in patients with CKD types 1, 2, 3, 4, and 5 was 21.18%, 21.88%, 24.23%, 21.21%, and 16.88%, respectively, and no significant difference was found among patients at different CKD stages. The prevalence of day–night sustained hypertension in patients with CKD types 1, 2, 3, 4, and 5 was 21.45%, 39.06%, 54.12%, 68.18%, and 79.68%, respectively, and patients with worse renal function had a higher prevalence of day–night sustained hypertension than patients with better renal function (P<0.05) (Figures2 and 3).
Figure 2.
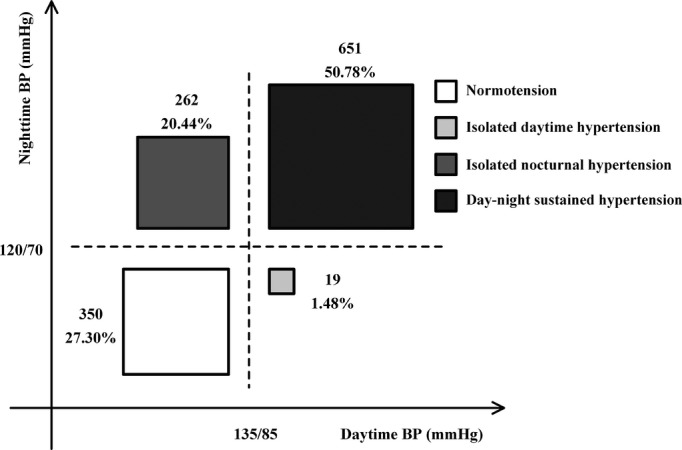
Prevalence of different ambulatory blood pressure status in Chinese CKD patients. BP indicates blood pressure; CKD, chronic kidney disease.
Figure 3.
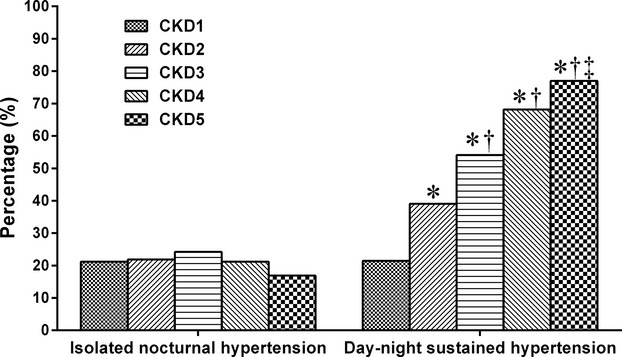
Prevalence of isolated nocturnal hypertension and day–night sustained hypertension in different chronic kidney disease (CKD) stages (P-value for multiple comparisons was corrected according to the Bonferroni method. *Comparison with CKD1 stage P<0.05, †comparison with CKD2 stage P<0.05, ‡comparison with CKD3 stage P<0.05.
Factors Associated With INH
Age, course, DM, eGFR, clinic SBP, DBP as well as levels of hemoglobin, serum albumin, calcium×phosphorus, intact parathyroid hormone, fasting glucose, cholesterol, LDL-C, HDL-C, and uric acid were correlated with INH onset according to univariate logistic regression analysis. Multivariate logistic regression analysis showed that INH was associated with age, eGFR, and clinic DBP (Table3).
Table 3.
Univariate and Multivariable Logistic Regression Analysis for Isolated Nocturnal Hypertension (1=Normotension; 2=Isolated Nocturnal Hypertension) in Chinese CKD Patients
| Univariate Regression Analysis | Multivariable Regression Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (per 1 y) | 1.040 (1.028 to 1.051) | <0.001 | 1.021 (1.007 to 1.036) | 0.003 |
| Gender (male=1; female=2) | 1.011 (0.733 to 1.395) | 0.946 | ||
| Course (per 1 month) | 1.006 (1.002 to 1.011) | 0.003 | ||
| Diabetes mellitus (no=0, yes=1) | 2.714 (1.587 to 4.641) | <0.001 | ||
| Hemoglobin (per 1 g/L) | 0.982 (0.975 to 0.988) | <0.001 | ||
| Albumin (per 1 g/L) | 1.020 (1.001 to 1.039) | 0.040 | ||
| Calcium×phosphorus (per 1 mg2/dL2) | 1.032 (1.014 to 1.051) | 0.001 | ||
| iPTH (per 1 pg/mL) | 1.003 (1.001 to 1.004) | <0.001 | ||
| Serum fasting glucose (per 1 mmol/L) | 1.152 (1.020 to 1.302) | 0.022 | ||
| Cholesterol (per 1 mmol/L) | 0.920 (0.864 to 0.980) | 0.010 | ||
| HDL-C (per 1 mmol/L) | 0.382 (0.253 to 0.576) | <0.001 | ||
| LDL-C (per 1 mmol/L) | 0.874 (0.805 to 0.949) | 0.001 | ||
| Uric acid (per 1 mmol/L) | 1.003 (1.002 to 1.005) | <0.001 | ||
| Urinary sodium excretion (per 1 mmol/24 h) | 0.999 (0.996 to 1.003) | 0.636 | ||
| eGFR-MDRD (per 1 mL/min per 1.73 m2) | 0.985 (0.981 to 0.988) | <0.001 | 0.989 (0.984 to 0.993) | <0.001 |
| Clinic-SBP (per 1 mm Hg) | 1.039 (1.029 to 1.049) | <0.001 | ||
| Clinic-DBP (per 1 mm Hg) | 1.059 (1.043 to 1.076) | <0.001 | 1.060 (1.041 to 1.079) | <0.001 |
Adjusted variables: age, gender (male=1, female=2). Variables of univariate regression analysis include course, diabetes mellitus (no=0, yes=1), current smoker (no=0, yes=1), alcohol intake (no=0, yes=1), BMI, hemoglobin, albumin, calcium×phosphorus, iPTH, serum fasting glucose, cholesterol, triglyceride, HDL-C, LDL-C, uric acid, Lg(proteinuria), urinary sodium excretion, eGFR, clinic-SBP, and clinic-DBP. All variables with significant associations were included in multivariable regression analysis. BMI indicates body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; iPTH, intact parathyroid hormone; LDL-C, low-density lipoprotein cholesterol; Lg, Logarithm; MDRD, Modification of Diet in Renal Disease; OR, odds ratio; SBP: systolic blood pressure.
Target-Organ Damage According to Different Ambulatory BP Status
Patients with INH had lower eGFR as well as higher LVMI and cIMT compared with normotensive patients (P<0.05), whereas patients with INH had higher eGFR and lower LVMI compared with the day–night sustained hypertensive group (P<0.05). The similar results on the prevalence of impaired renal function, left ventricular hypertrophy, and abnormal cIMT could be found in these patients with different groups (P<0.05) (Figure 4 and Table2).
Figure 4.
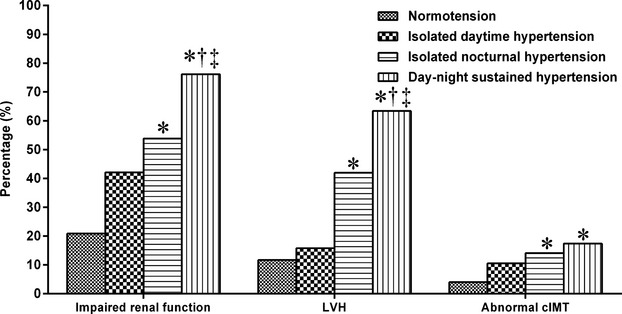
Comparison of target-organ damages in different ambulatory blood pressure status (P-value for multiple comparisons was corrected according to the Bonferroni method (6 comparisons). *Comparison with the normotension group P<0.05, †comparison with the isolated daytime hypertension group P<0.05, ‡comparison with the isolated nocturnal hypertension group P<0.05. cIMT indicates carotid intima-media thickness; LVH, left ventricular hypertrophy.
Factors Associated With Target-Organ Damage
Multiple linear regression analyses were carried out to clarify factors associated with target-organ damage. Age, sex, INH, day–night sustained hypertension (versus normotension), as well as levels of hemoglobin, calcium×phosphorus, and uric acid, were correlated with Lg(eGFR). Also, age, sex, hemoglobin level, eGFR, INH, and day–night sustained hypertension (versus normotension) were related to LVMI. Age, sex, DM, INH, and day–night sustained hypertension (versus normotension) independently determined cIMT (Table4).
Table 4.
Multiple Linear Regression Analysis: Relationship Between Lg(eGFR), LVMI, and cIMT With Different Ambulatory Blood Pressure Status in Chinese CKD Patients
| Variables | Unstandardized Coefficients Beta (95% CI) | Standardized Coefficients Beta | P Value |
|---|---|---|---|
| Dependent variable: Lg(eGFR by MDRD formula) (adjusted R2=0.683) | |||
| Age (per 1 y) | −0.005 (−0.006 to −0.004) | −0.155 | <0.001 |
| Gender (male=1; female=2) | 0.120 (0.076 to 0.163) | 0.109 | <0.001 |
| Hemoglobin (per 1 g/L) | 0.008 (0.007 to 0.009) | 0.427 | <0.001 |
| Calcium×phosphorus (per 1 mg2/dL2) | −0.019 (−0.021 to −0.017) | −0.338 | <0.001 |
| Uric acid (per 1 mmol/L) | −0.001 (−0.001 to −0.000) | −0.149 | 0.001 |
| IDH (vs NT) | −0.122 (−0.282 to 0.039) | −0.028 | 0.137 |
| INH (vs NT) | −0.127 (−0.187 to −0.067) | −0.091 | <0.001 |
| DNH (vs NT) | −0.197 (−0.250 to −0.144) | −0.181 | <0.001 |
| Dependent variable: LVMI (kg/m2) (adjusted R2=0.443) | |||
| Age (per 1 y) | 0.149 (0.018 to 0.280) | 0.075 | 0.026 |
| Gender (male=1; female=2) | −13.868 (−18.004 to −9.733) | −0.210 | <0.001 |
| Hemoglobin (per 1 g/L) | −0.374 (−0.463 to −0.285) | −0.326 | <0.001 |
| eGFR (per 1 mL/min per 1.73 m2) | −0.131 (−0.186 to −0.076) | −0.203 | <0.001 |
| IDH (vs NT) | 1.909 (−11.560 to 15.378) | 0.009 | 0.781 |
| INH (vs NT) | 7.731 (1.587 to 13.876) | 0.093 | 0.014 |
| DNH (vs NT) | 17.656 (12.190 to 23.123) | 0.269 | <0.001 |
| Dependent variable: cIMT (mm) (adjusted R2=0.423) | |||
| Age (per 1 y) | 0.008 (0.007 to 0.010) | 0.539 | <0.001 |
| Gender (male=1; female=2) | −0.066 (−0.104 to −0.028) | −0.129 | 0.001 |
| Diabetes mellitus (no=0, yes=1) | 0.079 (0.030 to 0.129) | 0.131 | 0.002 |
| IDH (vs NT) | −0.022 (−0.195 to 0.150) | −0.010 | 0.798 |
| INH (vs NT) | 0.077 (0.018 to 0.137) | 0.121 | 0.011 |
| DNH (vs NT) | 0.050 (0.001 to 0.099) | 0.099 | 0.045 |
Adjusted variables: age, gender (male=1, female=2). Variables of simple regression analysis for Lg(eGFR) include course, diabetes mellitus (no=0, yes=1), current smoker (no=0, yes=1), alcohol intake (no=0, yes=1), BMI, hemoglobin, albumin, calcium×phosphorus, iPTH, uric acid, cholesterol, triglyceride, HDL-C, LDL-C, urinary sodium excretion, Lg(proteinuria) and isolated daytime hypertension, isolated nocturnal hypertension, day–night sustained hypertension (no=0, yes=1) (vs normotension). Variables of simple regression analysis for LVMI and cIMT include course, diabetes mellitus (no=0, yes=1), current smoker (no=0, yes=1), alcohol intake (no=0, yes=1), BMI, hemoglobin, albumin, cholesterol, triglyceride, HDL-C, LDL-C, urinary sodium excretion, Lg(proteinuria), eGFR and isolated daytime hypertension, isolated nocturnal hypertension, day–night sustained hypertension (no=0, yes=1) (vs normotension). Significant variables of simple regression analysis for Lg(eGFR) include age, gender (male=1, female=2), course, diabetes mellitus (no=0, yes=1), current smoker (no=0, yes=1), hemoglobin, albumin, calcium×phosphorus, iPTH, LDL-C, urinary sodium excretion and isolated daytime hypertension, isolated nocturnal hypertension, and day–night sustained hypertension (no=0, yes=1) (vs normotension). Significant variables of simple regression analysis for LVMI include age, gender (male=1, female=2), course, diabetes mellitus (no=0, yes=1), current smoker (no=0, yes=1), hemoglobin, albumin, LDL-C, eGFR and isolated daytime hypertension, isolated nocturnal hypertension, and day–night sustained hypertension (no=0, yes=1) (vs normotension). Significant variables of simple regression analysis for cIMT include age, gender (male=1, female=2), course, diabetes mellitus (no=0, yes=1), current smoker (male=1, female=2), BMI, hemoglobin, LDL-C, eGFR and isolated daytime hypertension, isolated nocturnal hypertension, and day–night sustained hypertension (no=0, yes=1) (vs normotension). All variables with significant associations were included in multiple regression analysis. BMI indicates body mass index; cIMT, carotid intima-media thickness; CKD, chronic kidney disease; DNH, day–night sustained hypertension; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IDH, isolated daytime hypertension; INH, isolated nocturnal hypertension; iPTH, intact parathyroid hormone; LDL-C, low-density lipoprotein cholesterol; Lg, Logarithm; LVMI, left ventricular mass index; MDRD, Modification of Diet in Renal Disease; NT, normotension.
Discussion
We investigated the prevalence of INH in Chinese CKD patients, its correlation with target-organ damage, and the main factors that determine INH. The prevalence of INH in Chinese patients with CKD was 20.44%. INH was associated mainly with age, eGFR, and clinic DBP. Patients with INH had higher clinic, daytime, and nighttime BP, and more severe target organ damage compared with normotensive subjects. Patients with INH showed lower clinic, daytime and nighttime BP, and less target-organ damage compared with day–night sustained hypertensive subjects. INH was correlated with eGFR, LVMI, and cIMT by linear regression analyses. Taken together, these data suggested that INH played a part in target-organ damage, and that we should identify CKD patients with INH.
This is the first report on the prevalence of INH in CKD patients. Our value (20.44%) differs greatly from that in other reports: Chinese (10.9%), Japanese (10.2%), South Africans (10.5%), Western Europeans (6.0%), and Eastern Europeans (7.9%).10 We also found that patients with poorer renal function had the similar prevalence of INH, while higher prevalence of day–night sustained hypertension than patients with better renal function. Since volume retention and sympathovagal imbalance were the main factors for nocturnal BP elevation,11 and kidneys play an important role in the regulation of fluid volume, these patients with worse renal function had not only nocturnal hypertension but also daytime hypertension (termed “day–night sustained hypertension”), which could explain the stable prevalence of INH, yet increasing prevalence of nocturnal hypertension, in patients at different stages of CKD. Patients with INH showed only nocturnal hypertension without daytime hypertension, and these are good subjects to explore the role of nocturnal BP in hypertension-induced organ damage. Patients with INH had more severe target-organ damage compared with patients with normotensive subjects, whereas these patients showed slightly more target-organ damage compared with day–night sustained hypertensive subjects; also, INH was correlated with renal/cardiovascular parameters by linear regression analyses. Above all, we established that INH has an important role in CKD patients. We should pay special attention to INH in CKD patients based on its higher prevalence and correlation with renal/cardiovascular parameters.
Definition of INH might be clinically relevant for control of hypertension and cardiovascular prevention. This would be achieved by early identification and care of those people with normal clinic and daytime ambulatory BP but who are at high cardiovascular risk.13 Only half of patients with INH showed clinic hypertension in our study. Hence, half of the patients would remain undetected and untreated because INH can be diagnosed only by ABPM, which might lead to a poor prognosis. Finding clinical parameters to predict INH onset is essential. INH was associated mainly with age, eGFR, and clinic DBP. These findings suggest that we should pay more attention to older patients with worse renal function and higher clinic DBP, and undertake ABPM to detect INH.
Prospective observational studies have (for the most part) concluded that nocturnal BP is a better predictor of a worse prognosis in comparison with 24-hour BP or daytime BP.10,25 Our results showed that patients with INH had more severe target-organ damage compared with patients with normotension. Linear regression analysis showed that INH was independently correlated with renal and cardiovascular damage, which suggested that CKD patients with INH might have a worse prognosis than normotensive CKD subjects. This finding was related to BP alone (nighttime BP better represents the baseline BP of a patient) or with aspects regarding its measurement (nighttime BP is usually measured while the patient is in the supine position and is subjected to less variability). Nocturnal hypertension is opposite to the physiologic rhythm of BP. Nocturnal BP represents the minimal BP needed for adequate organ perfusion in healthy subjects.26 Maintaining a high BP at night, however, overloads the cardiovascular system, with a consequent negative impact on the heart and vascular structures. In the present study, it was not surprising to find that patients with INH had more severe renal and cardiovascular injuries among CKD patients, and that INH was closely related to target-organ damage. Therefore, lowering nocturnal BP might help to reduce cardiovascular and renal risks for such CKD patients. Antihypertensive chronotherapy could be used to lower nocturnal BP.27 Our pilot study showed the advantages of awakening versus bedtime scheduling of valsartan (80 to 320 mg once daily for 1 year) on 60 nondipper Chinese patients with CKD; bedtime treatment was significantly more effective in reducing nocturnal BP, albuminuria, and left ventricular mass.28
The present study had strengths and limitations. Firstly, CKD patients who received any antihypertensive drug in the previous month were excluded, so drug use would not have affected analyses. However, we could not rule out the effect of Chinese medicines. Secondly, all our patients had comprehensive assessments, and the cohort size was large. Thirdly, all CKD patients were admitted to our hospital division. Actually, these patients had severe proteinuria or severe renal damage, so some CKD patients with nonsevere proteinuria or nonsevere renal damage might have been omitted. Fourthly, we arranged the same schedule for all patients, which helped to expedite BP monitoring, but this arrangement might have led to different results from monitoring in the outpatient setting. Fifthly, the prevalence of INH might be different from studies in Western countries because most patients suffered from primary kidney disease, and fewer patients had DM. Finally, we cannot infer a cause–effect relationship based on our cross-sectional data.
In conclusion, in a study of CKD patients, we provide the first evidence of a high prevalence of INH and its correlation with target-organ damage. Further studies are needed to ascertain whether treatment of this disease reduces the risk of cardiovascular disease and mortality, and which strategy would be effective in treating this form of high BP.
Acknowledgments
We would like to thank all patients and their families for participating in this study.
Sources of Funding
This work was supported by a training project for excellent younger scholars of 3rd Hospital of Sun Yat-sen University.
Disclosures
None.
References
- Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med. 2010;268:456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Boari B, Manfredini R. Oxidative stress in essential hypertension. Curr Pharm Des. 2004;10:1695–1698. doi: 10.2174/1381612043384619. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- Drawz PE, Abdalla M, Rahman M. Blood pressure measurement: clinic, home, ambulatory, and beyond. Am J Kidney Dis. 2012;60:449–462. doi: 10.1053/j.ajkd.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafidis PA, Khosla N, Bakris GL. Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis. 2007;49:12–26. doi: 10.1053/j.ajkd.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Fan HQ, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, Bjorklund-Bodegard K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Ibsen H, O’Brien E, Wang J, Staessen JA. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036–2045. doi: 10.1097/HJH.0b013e32833b49fe. [DOI] [PubMed] [Google Scholar]
- Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O’Brien E. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900. [DOI] [PubMed] [Google Scholar]
- Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res. 2012;35:695–701. doi: 10.1038/hr.2012.26. [DOI] [PubMed] [Google Scholar]
- Li Y, Staessen JA, Lu L, Li LH, Wang GL, Wang JG. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension. 2007;50:333–339. doi: 10.1161/HYPERTENSIONAHA.107.087767. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang JG. Isolated nocturnal hypertension: a disease masked in the dark. Hypertension. 2013;61:278–283. doi: 10.1161/HYPERTENSIONAHA.111.00217. [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20–27. doi: 10.1161/HYPERTENSIONAHA.108.115154. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang J, Liu X, Li C, Ye Z, Peng H, Chen Z, Lou T. Reversed dipper blood-pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PLoS One. 2013;8:e55419. doi: 10.1371/journal.pone.0055419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeja SK, Dudeja RK. Blood-pressure measurement. N Engl J Med. 2009;360:2034–2035. [PubMed] [Google Scholar]
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- The Department of Disease Control, Ministry of Health, China, the Chinese Diabetes Society. The Chinese guideline of diabetes prevention and treatment. Chin J Prev Contr Chron Non Commun Dis. 2004;12:283–285. [Google Scholar]
- Simon A, Megnien JL, Chironi G. The value of carotid intima-media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol. 2010;30:182–185. doi: 10.1161/ATVBAHA.109.196980. [DOI] [PubMed] [Google Scholar]
- Bidani AK, Griffin KA, Epstein M. Hypertension and chronic kidney disease progression: why the suboptimal outcomes? Am J Med. 2012;125:1057–1062. doi: 10.1016/j.amjmed.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- Gonzalez RE, Hernandez A, Dibner C, Koehler BB, Pechere-Bertschi A. Arterial blood pressure circadian rhythm: significance and clinical implications. Rev Med Suisse. 2012;8:1709–1712. 1714–1715. [PubMed] [Google Scholar]
- Hermida RC, Smolensky MH, Ayala DE, Fernandez JR, Moya A, Crespo JJ, Mojon A, Rios MT, Fabbian F, Portaluppi F. Abnormalities in chronic kidney disease of ambulatory blood pressure 24 h patterning and normalization by bedtime hypertension chronotherapy. Nephrol Dial Transplant. 2014;29:1160–1167. doi: 10.1093/ndt/gft285. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang J, Liu X, Li CC, Ye ZC, Peng H, Chen Z, Lou T. Effect of valsartan with bedtime dosing on chronic kidney disease patients with nondipping blood pressure pattern. J Clin Hypertens (Greenwich) 2013;15:48–54. doi: 10.1111/jch.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]


