Abstract
Aim
The aim of this study was to examine the relationship between sexual dysfunction, repeat biopsies and other demographic and clinical factors in men on active surveillance (AS).
Methods
Patient-reported outcomes (PROs) measures were administered at enrollment and every 6 months to assess quality of life (QOL), psychosocial and urological health outcomes. Using mixed-effects models, we examined the impact of repeat biopsies, total number of cores taken, anxiety, age, and comorbidity on sexual function over the first 24 months of enrolling in AS.
Main Outcome Measures
PROs included the Expanded Prostate Cancer Index Composite-26 (EPIC-26) Sexual Function (SF) subscale, the American Urological Association-Symptom Index (AUA-SI), and the Memorial Anxiety Scale for Prostate Cancer (MAX-PC).
Results
At enrollment (n = 195), mean age was 66.5 ± 6.8 with a mean EPIC-26 SF score of 61.4 ± 30.4. EPIC-26 SF scores steadily decreased to 53.9 ± 30.7 at 24 months (P < 0.01). MAX-PC scores also progressively decreased over time (P = 0.03). Factors associated with lower EPIC-26 scores over time included age, unemployed status, diabetes, coronary artery disease, and hypertension (all P < 0.05). Higher prostate-specific antigen (PSA) was associated with a more rapid decline in EPIC-26 SF over time (P = 0.03). In multivariable analysis, age, diabetes, and PSA × time interaction remained significant predictors of diminished sexual function. Anxiety, number of biopsies, and total cores taken did not predict sexual dysfunction or change over time in our cohort.
Conclusions
Men on AS experienced a gradual decline in sexual function during the first 24 months of enrollment. Older age, PSA × time, and diabetes were all independent predictors of diminished sexual function over time. Anxiety, AUA-SI, the number of cores and the number of biopsies were not predictors of reduced sexual function in men in AS.
Keywords: Prostate Cancer, Erectile Dysfunction, Sexual Dysfunction, Active Surveillance, Quality of Life
Introduction
Active surveillance (AS) has become a viable alternative to surgery and radiation in the management of men with low risk for prostate cancer (PCa). While it is currently underutilized, with only 9% of eligible men choosing AS [1], one would expect that AS will become a more popular treatment option as more long-term outcomes data become available. One clear advantage of AS is to minimize morbidity such as sexual dysfunction (SD) associated with radical prostatectomy (RP) and radiation therapy (RT). Nonetheless, it has been demonstrated that men on AS also experience some degree of SD 2–5.
As measured by the Sexual Health Inventory for Men (SHIM), 49% of men on AS for low-risk PCa experience erectile dysfunction (ED), the most common form of SD [2]. While not directly comparable with the AS population, the multicenter Prostate Cancer Intervention Versus Observation Trial (PIVOT) study reported SD in 44% of men being observed for PCa compared with 81% of patients undergoing RP at a median of 2 years after diagnosis [3]. It is not surprising that older men with PCa report SD, but 80% of men undergoing watchful waiting describe SD as a major issue compared with 46% of age-matched controls over an average follow-up of 12.2 years in a population-based Scandinavian study [4]. A recent study comparing men on AS with patients undergoing radical therapy (RT or RP) found that men on AS were more often sexually active compared with men who underwent local therapy. Among men who were sexually active, 44–51% of men on AS reported difficulty achieving or maintaining an erection compared with 84–85% of men in the treatment group [5]. Sexually inactive men on AS were also less likely to attribute their inactivity to ED [5]. Taken together, the data shown earlier indicate that while AS has a much more favorable risk profile compared with radical therapy, SD remains a significant problem for men being followed for low risk disease.
A thorough review of the existing literature has revealed a number of potential predictive factors for SD among men on AS including body mass index (BMI), prostate volume, number of cores taken at prostate biopsy (PB), frequency of PB, and psychosocial factors such as the anxiety [6]. However, examination of the association between PB and SD has yielded conflicting results with some data indicating no adverse effect of PB on erectile function 7–9, and other studies suggesting both short- and long-term SD as a result of PB 10–12. A retrospective study of men on AS found that increased biopsy number correlated with decreased SHIM scores, but age, prostate volume, and prostate-specific antigen (PSA) had no relation to decline in SHIM scores [12]. The psychologic interplay of AS and sexual function is highlighted by the finding that 6% of men experience some degree of SD in anticipation of a PB [13]. There is evidence that that men diagnosed with PCa after PB experience a greater decline in erectile function compared with men with benign biopsy results, indicating a potential psychologic effect of the cancer diagnosis on erectile function [14]. Additionally, there is evidence that increased levels of depression and anxiety are associated with decreased sexual function in men during the first 3 years after RP after controlling for age, cancer characteristics, and receipt of salvage treatment [15]. As most previous studies examining sexual function in men on AS have been cross-sectional, there is limited understanding of predictors of SD over time in this patient population. In order to effectively counsel patients regarding the risks of AS, we need a better understanding of factors that are predictive of SD among men on AS.
Aims
SD over time in men on AS for PCa may be related to specific patient demographics, clinical characteristics and psychosocial parameters. We sought to examine the relationship between SD and demographic, clinical, and patient-reported outcome (PRO) data to determine predictive factors of SD in a longitudinal observation trial of men on AS.
Methods
Study Population
As part of a prospective, longitudinal, observational Institutional Review Board-approved AS protocol, several PROs measures were administered at enrollment and during follow-up. After enrollment a confirmatory biopsy was done on each participant, followed by a PSA and exam at regular 6-month intervals. Subsequent biopsies occur every 2 years unless otherwise indicated by changes in PSA or exam. PROs were used to assess how AS influences quality of life (QOL), psychosocial and urological health outcomes. Informed written consent was obtained from all participants. All men were diagnosed with PCa prior to enrollment. Men considered for AS under our study protocol met the following criteria based upon 12-core diagnostic transrectal ultrasound (TRUS)-biopsy and digital rectal exam: clinical stage ≤T2a, Gleason score ≤6, ≤3 cores positive, maximum involvement of any core <50%, and tumor volume ≤5% of total biopsy volume. Prior to enrollment, all men received consultation regarding the treatment options for early stage PCa and declined all of the other treatment options including surgery, external beam RT, brachytherapy, and hormonal therapy. In order to be eligible for enrollment, men must not had received any prior treatment for their PCa, other than medications that inhibit 5 alpha-reductase including finasteride and dutasteride.
Main Outcome Measures
Demographic and Clinical Information
Demographic and clinical information such as age, BMI, comorbidity, PSA, and prostate volume was attempted to be collected for all participants. Comorbid conditions such as diabetes, neurologic disorders (such as lumbar disc disease and stroke), coronary artery disease (CAD), hypertension, hyperlipidemia, and sleep apnea were obtained by diagnosis codes from the electronic medical record.
Expanded Prostate Cancer Index Composite-26 (EPIC-26)
EPIC-26 [16] is an abbreviated version of the original 50-item EPIC [17] and has previously undergone psychometric testing revealing adequate properties for clinical research purposes in measuring PCa-related QOL. The EPIC-26 contains subscales of sexual functioning, urinary incontinence, urinary irritation/obstruction, bowel function, and hormone therapy-related side effects. The subscales have shown high correlations (r's ≥ 0.95), as well as internal consistency and test–retest reliability (α ≥ 0.70; r ≥ 0.60). The EPIC-26 sexual function score ranges from 0 to 100, with higher scores indicating better sexual function. There are six items to address sexual function in the EPIC-26 including ratings of ability to have erections, quality of erections, frequency of erections, ability to orgasm, ability to function sexually and how big of a problem is the sexual function (or lack of function).
American Urological Association Symptom Index (AUA-SI)
The AUA-SI [18] is a brief, validated self-report measurement questionnaire that assesses the impact of lower urinary tract symptoms (LUTS) over the previous 4-week period. Previous psychometric testing revealed good internal consistency and test–retest reliability, and AUA scores distinguish individuals with benign prostate hyperplasia from healthy controls [19].
Memorial Anxiety Scale for Prostate Cancer (MAX-PC)
MAX-PC [20] is a self-reported 18-item measure to determine anxiety related to PCa. The MAX-PC scores range from 0 to 54 with higher scores indicating higher anxiety levels. The subscales include PCa anxiety (scores range from 0 to 33), PSA anxiety (0–9) and fear of recurrence (0–12). Previous psychometric testing revealed good internal consistency reliability (α ≥ 0.90) and high correlations with other common measures of anxiety, such as the Hospital Anxiety and Depression Score-Anxiety [21].
Statistic Analysis
Patient characteristics were summarized using descriptive statistics (frequency counts and percentages for categorical variables; means and standard deviations for continuous variables). EPIC-26, AUA-SI, and MAX-PC scores were compared across time (at baseline, and 6, 12, 18, and 24 months) using mixed-effects models. The effect size between baseline and subsequent follow-up time points was computed using Cohen's d [22]. Both univariate and multivariable mixed-effects models (using baseline demographics and clinical characteristics as fixed effects and individual intercepts as random effects) were constructed for the EPIC-26 sexual scale to determine the predictors of SD in men on AS. A time interaction effect was also examined in each mixed-effects model to compare the rate of change in sexual function over time among various subgroups. For example, PSA × time represents the impact of PSA on the rate of change in EPIC-26 over time. Significant changes in EPIC-26 sexual scores over time were determined using maximum likelihood estimating methods with unstructured covariance controlling for random effects of individual intercept. Only predictors that were significantly associated with EPIC-26 sexual score in the univariate analysis were included in the multivariable model. Statistic testing was two-sided with a threshold of statistic significance at P < 0.05. All statistic analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline Patient Characteristics
Among the entire study cohort (n = 195), the mean age was 66.5 ± 6.8 years and mean BMI was 28.2 ± 4.3 kg/m2 (Table 1). The majority were white (84.6%), married (84.6%), employed (45.1%), and had a college education or higher (54.9%). PSA at enrollment was 5.2 ± 3.9 ng/mL with a PSA density of 0.12 ± 0.09. The most common comorbidity was hypertension (48.7%), followed by hyperlipidemia (27.2%) and diabetes (16.4%). At the time of enrollment into AS, the majority of men had one to two prostate biopsies (96.5%) and the mean number of cores taken was 12.4 ± 1.5.
Table 1.
Patient characteristics
| Variables | All patients (N = 195) | |
|---|---|---|
| n | % | |
| Age (year), mean ± SD | 66.5 ± 6.8 | |
| 45–59 | 29 | 14.9 |
| 60–70 | 114 | 58.4 |
| 70+ | 52 | 26.7 |
| BMI (kg/m2), mean ± SD | 28.2 ± 4.3 | |
| <25 | 37 | 19.0 |
| 25–30 | 97 | 49.7 |
| 30+ | 47 | 24.1 |
| Missing | 14 | 7.2 |
| Race (n, %) | ||
| White | 165 | 84.6 |
| African American | 13 | 6.7 |
| Other | 17 | 8.7 |
| Marital status | ||
| Married | 165 | 84.6 |
| Not married | 26 | 13.3 |
| Missing | 4 | 2.1 |
| Employment | ||
| Work | 88 | 45.1 |
| Not work | 80 | 41.0 |
| Missing | 27 | 13.8 |
| Education | ||
| Less than college | 49 | 25.1 |
| College | 40 | 20.5 |
| Graduate degree | 67 | 34.4 |
| Missing | 36 | 18.5 |
| Prostate volume (g), mean ± SD | 46.9 ± 24.7 | |
| PSA (ng/mL), mean ± SD | 5.2 ± 3.9 | |
| PSA density, mean ± SD | 0.12 ± 0.09 | |
| Comorbidity | ||
| Diabetes | 32 | 16.4 |
| Neurologic | 13 | 6.7 |
| Sleep disorder | 22 | 11.3 |
| CAD | 26 | 13.3 |
| Hyperlipidemia | 53 | 27.2 |
| Hypertension | 95 | 48.7 |
| Total number of cores taken, mean ± SD | 12.4 ± 1.5 | |
| Number of biopsies | ||
| 1 | 129 | 66.2 |
| 2 | 59 | 30.3 |
| 3 | 6 | 3.1 |
| 4 | 1 | 0.5 |
BMI = body mass index; CAD = coronary artery disease; PSA = prostate-specific antigen; SD = standard deviation.
Sexual Function, Anxiety and Urinary Symptoms Over Time
The mean EPIC-26 sexual score was 61.4 ± 30.4 at baseline and decreased to 53.9 ± 30.7 at 24 months (Table 2). There was a gradual decline in EPIC-26 sexual score over time at −3.73 points per year (P = 0.0014). While statistically significant, the effect size was small between baseline and 18 months (d = 0.23), and approached a medium effect by 24 months (d = 0.35). Over time, there was also a decline in total MAX-PC score (P = 0.0287) and the PCa anxiety subscale score (P < .0001). However, similar to declines in sexual function, the magnitude of the decline in the MAX-PC score and PCa anxiety subscore was small between baseline and 24 months (d = 0.28 and d = 0.30, respectively). There were no significant trends in PSA anxiety (P = 0.8), fear of recurrence subscale scores (P = 0.6), and AUA-SI urological symptom scores (P = 0.3) over time.
Table 2.
Memorial anxiety, EPIC-26 sexual function, and AUA-SI symptom index scores over time
| Variables | Time | Time effect* | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | 18 months | 24 months | Coefficient | P value | |
| EPIC-26 sexual score (0–100)† | 61.4 ± 30.4 | 59.8 ± 33.0 | 60.2 ± 30.9 | 57.7 ± 31.4 | 53.9 ± 30.7 | −0.311 | 0.0014 |
| MAX-PC total score (0–54)‡ | 9.8 ± 8.0 | 8.0 ± 8.2 | 7.9 ± 7.6 | 8.0 ± 8.2 | 7.6 ± 7.1 | −0.053 | 0.0287 |
| Prostate cancer anxiety (0–33)§ | 5.6 ± 6.0 | 4.7 ± 6.0 | 3.9 ± 5.3 | 3.9 ± 5.9 | 3.8 ± 4.9 | −0.067 | <0.0001 |
| PSA anxiety (0–9)¶ | 0.27 ± 0.72 | 0.33 ± 0.95 | 0.31 ± 0.87 | 0.34 ± 1.06 | 0.29 ± 0.85 | 0.001 | 0.8348 |
| Fear of recurrence (0–12)** | 3.8 ± 3.1 | 2.9 ± 2.9 | 3.7 ± 3.8 | 3.7 ± 3.7 | 3.6 ± 3.8 | 0.008 | 0.6169 |
| AUA-SI symptom score (0–35)†† | 6.9 ± 5.5 | 7.5 ± 5.6 | 7.3 ± 5.1 | 7.3 ± 5.5 | 7.7 ± 5.4 | 0.016 | 0.3183 |
Mixed-effects models.
Higher score indicates higher sexual function.
,†,‡,§,¶Higher score indicates greater anxiety.
Higher score indicates worse AUA-SI urological symptom.
EPIC-26 = Expanded Prostate Cancer Index Composite-26; MAX-PC = Memorial Anxiety Scale for Prostate Cancer; PSA = prostate-specific antigen; AUA-SI = American Urological Association-Symptom Index.
Predictors of SD
Men had significantly lower EPIC-26 sexual scores over time if they were older (P < 0.01), not employed (P < 0.01), had a history of diabetes (P = 0.01), CAD (P < 0.01), or hypertension (P = 0.03) (Table 3). Men with higher baseline PSA had a more rapid decline in sexual function over time (P = 0.03), while no other patient characteristics had significant time interaction effects. Anxiety, number of biopsies, and total cores taken did not predict SD over time in our cohort (all P > 0.6). In the multivariable analysis, time on AS (P < 0.01), older age (P < 0.01), diabetes (P = 0.04), and PSA × time interaction (P = 0.04) remained significant predictors of reduced sexual function (Table 4).
Table 3.
Univariate analysis for EPIC-26 sexual function score over time
| Variable | Fixed effect | Time interaction effect* | ||
|---|---|---|---|---|
| Coefficient | P value | Coefficient | P value | |
| Age | −2.0979 | <.0001 | −0.0160 | 0.1134 |
| BMI | −0.5005 | 0.3677 | −0.0154 | 0.3410 |
| Race (white) | 7.3861 | 0.2906 | −0.2670 | 0.2566 |
| Marital status (married) | 1.7483 | 0.7995 | −0.1109 | 0.5831 |
| Employment (employed) | 18.0325 | 0.0002 | 0.1335 | 0.3498 |
| Education (college or above) | 5.0022 | 0.3595 | −0.0188 | 0.9056 |
| Prostate volume | −0.1647 | 0.1080 | −0.0041 | 0.2111 |
| PSA | −0.7686 | 0.2018 | −0.0447 | 0.0292 |
| PSA density | −35.5273 | 0.2719 | −0.5670 | 0.5374 |
| Comorbidity | ||||
| Diabetes | −15.7067 | 0.0108 | 0.0913 | 0.6102 |
| Neurologic | −0.1463 | 0.1298 | 0.0326 | 0.9154 |
| Sleep disorder | −9.2508 | 0.1884 | 0.1658 | 0.4602 |
| CAD | −19.2249 | 0.0035 | 0.0343 | 0.8491 |
| Hyperlipidemia | 4.7292 | 0.3739 | 0.0642 | 0.6755 |
| Hypertension | −10.1293 | 0.0322 | 0.2048 | 0.1383 |
| Total number of cores taken | 0.0180 | 0.9910 | 0.0151 | 0.7701 |
| Number of biopsies | 0.9068 | 0.8203 | 0.0267 | 0.8031 |
| MAX-PC total score | −0.0238 | 0.8842 | −0.0027 | 0.7766 |
| Prostate cancer anxiety | −0.1073 | 0.6354 | −0.0040 | 0.7626 |
| PSA anxiety | 0.4702 | 0.7308 | −0.1386 | 0.1249 |
| Fear of recurrence | 0.1264 | 0.7309 | −0.0009 | 0.9681 |
| AUA-SI symptom score | −0.4468 | 0.0750 | −0.0028 | 0.8276 |
Time was significant in all univariate models.
BMI = body mass index; CAD = coronary artery disease; EPIC-26 = Expanded Prostate Cancer Index Composite-26;MAX-PC = Memorial Anxiety Scale for Prostate Cancer; PSA = prostate-specific antigen; AUA-SI = American Urological Association-Symptom Index.
Table 4.
Multivariable analysis for EPIC-26 sexual functioning score
| Variable* | Coefficient | P value |
|---|---|---|
| Age | −1.99 | <.0001 |
| Employed | 7.84 | 0.0752 |
| Diabetes | −11.84 | 0.0414 |
| CAD | −5.02 | 0.3994 |
| Hypertension | −3.56 | 0.4153 |
| PSA | 0.11 | 0.8457 |
| Time | −0.19 | 0.0045 |
| PSA × time | −0.044 | 0.0381 |
Only variables that were found significant in the univariate analysis were included in the multivariable mixed-effects model.
CAD = coronary artery disease; EPIC-26 = Expanded Prostate Cancer Index Composite-26; PSA = prostate-specific antigen.
In order to compare changes in SD among men undergoing AS to a similar cohort of men without PCa, we obtained results from a previous study reporting EPIC-26 sexual scores amongst a group of community dwelling men [23]. Our cohort exhibited a exhibited a similar EPIC-26 sexual function score at baseline compared with men of similar age without PCa [23], but after 24 months of surveillance, the EPIC-26 sexual function score was lower among men on AS (P < 0.05) (Figure 1).
Figure 1.
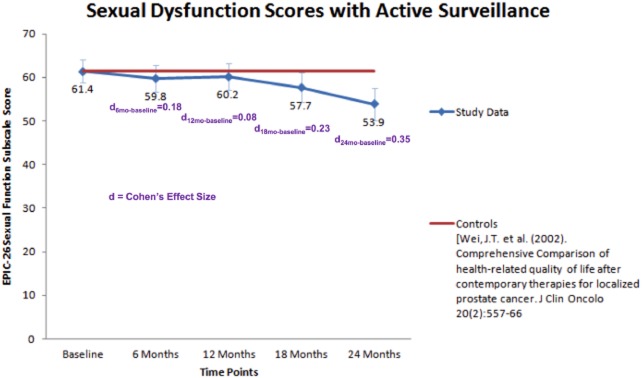
Sexual function score over time for men in active surveillance.
Discussion
Longitudinal data on the sexual function of men on AS are scarce, and there is conflicting evidence regarding the association between prostate biopsies and erectile function. Our study has several interesting findings in this unique study population. We found that sexual function and PCa anxiety gradually decreased over the first 24 months of enrollment among men on AS for PCa. We identified length of time on surveillance, older age at enrollment, and diabetes as independent risk factors for reduced sexual function over time. Higher baseline PSA also resulted in a more rapid decline in sexual function over time. Anxiety, BMI, race, number of biopsies, and total cores taken did not predict SD in our cohort.
Fujita et al. evaluated the erectile function of 152 men on an AS protocol by comparing baseline 5-item SHIM scores to scores obtained at a single cross-sectional time point [12]. With a mean follow-up of 3.2 years and mean number of biopsies of 2.3, they found that only the number of biopsies was independently associated with decreased SHIM scores. The change in SHIM score in men who underwent 0 to 2 PBs was −2.3 ± 0.7 compared with −5.7 ± 1.1 in men who underwent 3 + PBs. Multivariable analysis also indicated that age, prostate volume and PSA had no association with SHIM changes; however, this study did not analyze the impact of ED-related comorbidities in their multivariable analysis. It must be noted that men who underwent more prostate biopsies were on AS longer; therefore, the observed reductions in erectile function must be interpreted with caution as it is well established that erectile function declines over time in men.
Braun et al. performed a retrospective review of 342 patients enrolled on an AS protocol between 2000 and 2011 [8]. At each clinic visit, patients were asked to complete the Prostate Health-Related Quality of Life (PHRQoL), which includes six questions about erectile function similar to the International Index of Erectile Function (IIEF). The median age in this cohort was 64 years, median number of biopsies was 5, and the median follow-up was 3.5 years. During the first 4 years of AS, erectile function decreased by 1.0 point per year, and similar declines were seen when stratified by number of biopsies. Because their AS protocol mandated annual PB, analysis of the effect of multiple biopsies was limited, but their results suggest that number of biopsies does not have a large effect on erectile function. Another recent study comprising 427 men on AS used an adjusted SHIM score accounting for sexual activity level to examine the impact of PB on erectile function [9]. Median follow-up was 3.2 years from PCa diagnosis, 69% of patients had one prior biopsy, 16% had two biopsies, and the remainder had three or more. This study examined the association between PB and erectile function between men and longitudinally in the same patients. Neither erectile function nor sexual activity status was associated with number of PBs. Our study confirms these findings that the number of prostate biopsies and number of cores taken are not associated with significant reductions in sexual function [8],[9].
PROs were collected every 6 months starting at enrollment allowing for detailed longitudinal evaluation of sexual function and identification of predictors of reduced sexual function over time. This information may prove valuable for counseling patients who are considering AS. An overall decline in sexual function was observed during the 24-month study period, with older age at enrollment and longer time on surveillance as independent predictors for reduced sexual function over time. The observed small magnitude of decline is confirmed by recently published data from the prospective Comparative Effectiveness Analysis of Surgery and Radiation study of 3,691 men with newly diagnosed PCa [24]. Pretreatment EPIC-26 SF scores decreased by 1.24 points with every 1 year increase in age. The Massachusetts Male Aging Study also demonstrated an increased incidence of ED with advancing age [25]. These findings further support our assertion that the decline in sexual function observed in our AS cohort is related to aging. In our AS cohort, total MAX-PC scores and the PCa anxiety sub-scores also saw a gradual, small decline over time. Our results support data from a recent study of 150 Dutch men with low-risk PCa managed with AS [26]. This study found that general anxiety and fear of progression significantly decreased during the first 18 months of enrollment on AS. Reductions in anxiety could be the result of increased patient acceptance of AS as a safe management strategy for PCa and better tolerance of the repeated PB and PSA testing required for compliance with AS protocols. These findings are potentially important for clinicians attempting to manage patient expectations and may provide reassurance for patients considering AS. While we did not identify a correlation between MAX-PC scores and SD, studies in men after surgical treatment for PC have found that increased PCa anxiety is associated with poor sexual satisfaction and function [27]. Taken together, men entering AS can anticipate an age related decline in sexual function, but may benefit from reduced disease-related anxiety over time.
General comorbidity scores have been associated with reduced overall sexual function in men on AS; however, to our knowledge, individual comorbidities as risk factors for decreased sexual function have not been examined. We identify diabetes as an independent predictor of reduced sexual function over time in men on AS. This adds to the extensive body of literature supporting diabetes as a risk factor for SD, with rates of ED among diabetics ranging from 32% to 90% depending on the patient population, diabetes type, severity, and duration of disease [28]. We also found that increased baseline PSA predicted a more rapid decline in sexual function over time. Considering previous epidemiological studies, which have found strong associations between benign prostatic hyperplasia (BPH), LUTS and SD [29],[30], it is possible that the association between PSA and declining SF in our study is related to the presence of BPH in patients with higher PSA. However, we did not identify LUTS or prostate volume as predictors of SD in our analysis, therefore, no definitive conclusions can be made.
The study had several limitations. We do not have data on patient sexual function prior to diagnosis with PCa. The magnitude of change for sexual function during the study period was relatively small (−7.5 points from baseline to 24 months), which should be taken into consideration when interpreting these data. The EPIC-26 looks at erectile function (three out of the six items focusing on erectile function) as part of overall sexual function subscale and does not provide a validated, focused assessment of erectile function domain specifically in comparison with IIEF. The IIEF-5 has been added to the questionnaires given to participants more recently to capture more specific erectile function information from participants. As ED is the most common sexual complaint, it will be helpful to have a validated assessment erectile function as a specific domain within overall sexual function. Comorbid conditions, which impact sexual function, such as diabetes, hypertension and CAD, were obtained from the medical record, which may result in underreporting of comorbid disease prevalence. Unfortunately, we are unable to examine more informative disease-specific measures such as glycemic control and their role in SD among men in our AS cohort. This would help differentiate the impact of aging vs. the impact of comorbid disease progression on erectile function. Longer follow-up could also allow us to examine the effect of lifestyle changes such as weight loss on sexual function over time. Additionally, our study is limited by the lack of an age-matched control group without PC, which could better delineate the specific effect of age and aging on sexual function. Because we limited analysis to the first 24 months of enrollment on AS, relatively few biopsies were performed and follow-up was limited. Over 60% of patients in our study had only one biopsy during the study period, which limits our ability assess the impact of multiple prostate biopsies on sexual function. Future studies will need to evaluate sexual function over a longer period of time because most men will be maintained on an AS protocol for more than 2 years.
Conclusions
Men experienced decreased sexual function and a decline in PCa anxiety over the first 24 months of enrollment on an AS protocol. Older age, time on AS, and diabetes were all associated with declining sexual function over time. Increased baseline PSA was associated with a more rapid decline in sexual function as well. Anxiety, AUA-SI, other comorbid conditions, the number of cores, and the number of biopsies were not associated with declining sexual function in our study; however, longer-follow-up with more patients is needed to draw any definitive conclusions. Sexual function in men undergoing AS is multifactorial and is influenced by many factors including age, aging over time and comorbid conditions rather than increased biopsy exposure. Larger prospective multicenter clinical trials using varied approaches to examine the various factors that may impact sexual function are needed to further determine the etiology of SD in men in AS.
Conflict of Interest
The author(s) report no conflicts of interest.
References
- Barocas DA, Cowan JE, Smith JA, Carroll PR CaPSURE Investigators. What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database. J Urol. 2008;180:1330–1334. doi: 10.1016/j.juro.2008.06.019. , discussion 1334–5. [DOI] [PubMed] [Google Scholar]
- Soloway MS, Soloway CT, Eldefrawy A, Acosta K, Kava B, Manoharan M. Careful selection and close monitoring of low-risk prostate cancer patients on active surveillance minimizes the need for treatment. Eur Urol. 2010;58:831–835. doi: 10.1016/j.eururo.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, Gingrich JR, Wei JT, Gilhooly P, Grob BM, Nsouli I, Iyer P, Cartagena R, Snider G, Roehrborn C, Sharifi R, Blank W, Pandya P, Andriole GL, Culkin D, Wheeler T Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E, Steineck G, Holmberg L, Johansson J-E, Nyberg T, Ruutu M, Bill-Axelson A SPCG-4 Investigators. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: The Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891–899. doi: 10.1016/S1470-2045(11)70162-0. [DOI] [PubMed] [Google Scholar]
- Van den Bergh RCN, Korfage IJ, Roobol MJ, Bangma CH, de Koning HJ, Steyerberg EW, Essink-Bot ML. Sexual function with localized prostate cancer: Active surveillance vs radical therapy. BJU Int. 2012;110:1032–1039. doi: 10.1111/j.1464-410X.2011.10846.x. [DOI] [PubMed] [Google Scholar]
- Glaser AP, Novakovic K, Helfand BT. The impact of prostate biopsy on urinary symptoms, erectile function, and anxiety. Curr Urol Rep. 2012;13:447–454. doi: 10.1007/s11934-012-0277-6. [DOI] [PubMed] [Google Scholar]
- Chrisofos M, Papatsoris AG, Dellis A, Varkarakis IM, Skolarikos A, Deliveliotis C. Can prostate biopsies affect erectile function? Andrologia. 2006;38:79–83. doi: 10.1111/j.1439-0272.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- Braun K, Ahallal Y, Sjoberg DD, Ghoneim T, Dominguez Esteban M, Mulhall J, Vickers A, Eastham J, Scardino PT, Touijer KA. Effect of repeated prostate biopsies on erectile function in men on active surveillance for prostate cancer. J Urol. 2014;191:744–749. doi: 10.1016/j.juro.2013.08.054. [DOI] [PubMed] [Google Scholar]
- Hilton JF, Blaschko SD, Whitson JM, Cowan JE, Carroll PR. The impact of serial prostate biopsies on sexual function in men on active surveillance for prostate cancer. J Urol. 2012;188:1252–1258. doi: 10.1016/j.juro.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Zisman A, Leibovici D, Kleinmann J, Siegel YI, Lindner A. The impact of prostate biopsy on patient well-being: A prospective study of pain, anxiety and erectile dysfunction. J Urol. 2001;165:445–454. doi: 10.1097/00005392-200102000-00023. [DOI] [PubMed] [Google Scholar]
- Tuncel A, Kirilmaz U, Nalcacioglu V, Aslan Y, Polat F, Atan A. The impact of transrectal prostate needle biopsy on sexuality in men and their female partners. Urology. 2008;71:1128–1131. doi: 10.1016/j.urology.2008.01.055. [DOI] [PubMed] [Google Scholar]
- Fujita K, Landis P, McNeil BK, Pavlovich CP. Serial prostate biopsies are associated with an increased risk of erectile dysfunction in men with prostate cancer on active surveillance. J Urol. 2009;182:2664–2669. doi: 10.1016/j.juro.2009.08.044. [DOI] [PubMed] [Google Scholar]
- Stravodimos KG, Haritopoulos KN, Alamanis C, Anastasiou I, Constantinides C. Local anesthesia during transrectal ultrasonography-guided prostate biopsy: Does it have any effect on sexual function? Int Urol Nephrol. 2007;39:893–896. doi: 10.1007/s11255-006-9063-z. [DOI] [PubMed] [Google Scholar]
- Helfand BT, Glaser AP, Rimar K, Zargaroff S, Hedges J, McGuire BB, Catalona WJ, McVary KT. Prostate cancer diagnosis is associated with an increased risk of erectile dysfunction after prostate biopsy. BJU Int. 2013;111:38–43. doi: 10.1111/j.1464-410X.2012.11268.x. [DOI] [PubMed] [Google Scholar]
- Punnen S, Cowan JE, Dunn LB, Shumay DM, Carroll PR, Cooperberg MR. A longitudinal study of anxiety, depression and distress as predictors of sexual and urinary quality of life in men with prostate cancer. BJU Int. 2013;112:E67–75. doi: 10.1111/bju.12209. [DOI] [PubMed] [Google Scholar]
- Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76:1245–1250. doi: 10.1016/j.urology.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- Barry MJ, Fowler FJ, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association Symptom Index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. , discussion 1564. [DOI] [PubMed] [Google Scholar]
- Cook DA, Beckman TJ. Current concepts in validity and reliability for psychometric instruments: Theory and application. Am J Med. 2006;119:166. doi: 10.1016/j.amjmed.2005.10.036. , e7–16. [DOI] [PubMed] [Google Scholar]
- Roth AJ, Rosenfeld B, Kornblith AB, Gibson C, Scher HI, Curley-Smart T, Holland JC, Breitbart W. The memorial anxiety scale for prostate cancer: Validation of a new scale to measure anxiety in men with prostate cancer. Cancer. 2003;97:2910–2918. doi: 10.1002/cncr.11386. [DOI] [PubMed] [Google Scholar]
- Dale W, Hemmerich J, Meltzer D. Extending the validity of the Memorial Anxiety Scale for Prostate Cancer (MAX-PC) at the time of prostate biopsy in a racially-mixed population. Psychooncology. 2007;16:493–498. doi: 10.1002/pon.1107. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. Mahwah, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, Nyquist L, Sanda MG. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- Resnick MJ, Barocas DA, Morgans AK, Phillips SE, Chen VW, Cooperberg MR, Goodman M, Greenfield S, Hamilton AS, Hoffman KE, Kaplan SH, Paddock LE, Stroup AM, Wu XC, Koyama T, Penson DF. Contemporary prevalence of pretreatment urinary, sexual, hormonal, and bowel dysfunction: Defining the population at risk for harms of prostate cancer treatment. Cancer. 2014;120:1263–1271. doi: 10.1002/cncr.28563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts Male Aging Study. J Urol. 2000;163:460–463. [PubMed] [Google Scholar]
- Venderbos LDF, van den Bergh RCN, Roobol MJ, Schröder FH, Essink-Bot M-L, Bangma CH, Steyerberg EW, Korfage IJ. A longitudinal study on the impact of active surveillance for prostate cancer on anxiety and distress levels. Psychooncology. 2015;24:348–354. doi: 10.1002/pon.3657. [DOI] [PubMed] [Google Scholar]
- Tavlarides AM, Ames SC, Diehl NN, Joseph RW, Castle EP, Thiel DD, Broderick GA, Parker AS. Evaluation of the association of prostate cancer-specific anxiety with sexual function, depression and cancer aggressiveness in men 1 year following surgical treatment for localized prostate cancer. Psychooncology. 2013;22:1328–1335. doi: 10.1002/pon.3138. [DOI] [PubMed] [Google Scholar]
- Kamenov ZAA. Comprehensive review of erectile dysfunction in men with diabetes. Exp Clin Endocrinol Diabetes. 2015;123:141–158. doi: 10.1055/s-0034-1394383. [DOI] [PubMed] [Google Scholar]
- Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: Results of the “Cologne Male Survey”. Int J Impot Res. 2000;12:305–311. doi: 10.1038/sj.ijir.3900622. [DOI] [PubMed] [Google Scholar]
- Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, O'Leary MP, Puppo P, Robertson C, Giuliano F. Lower urinary tract symptoms and male sexual dysfunction: The Multinational Survey of the Aging Male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]


