Summary
Background
Bilirubin is a yellow breakdown product of heme catabolism. Increased serum levels of unconjugated bilirubin are conditions commonly seen in premature neonates and adults with acute hemolysis including thrombotic microangiopathy. Previous studies have shown that unconjugated bilirubin lowers plasma ADAMTS13 activity, but the mechanism is not fully understood.
Objectives
The study is to determine whether unconjugated bilirubin directly inhibits the cleavage of von Willebrand factor (VWF) and its analogs by ADAMTS13.
Methods
Fluorogenic, SELDI-TOF mass spectrometric assay, and Western blotting analyses were employed to address this question.
Results
Unconjugated bilirubin inhibits the cleavage of F485-rVWF73-H, D633-rVWF73-H, and GST-rVWF71-11K by ADAMTS13 in a concentration-dependent manner with a half-maximal inhibitory concentration (IC50) of ~13 μM, ~70 μM, and ~17 μM, respectively. Unconjugated bilirubin also dose-dependently inhibits the cleavage of multimeric VWF by ADAMTS13 under denaturing conditions. The inhibitory activity of bilirubin on the cleavage of D633-rVWF73-H and multimeric VWF, but not F485-rVWF73-H, was eliminated after incubation with bilirubin oxidase that converts bilirubin to biliverdin. Furthermore, plasma ADAMTS13 activity in patients with hyperbilirubinemia is lower prior to than after treatment with bilirubin oxidase.
Conclusions
unconjugated bilirubin directly inhibits ADAMTS13’s ability to cleave both peptidyl and native VWF substrates in addition to its interference with certain fluorogenic assays. Our findings may help proper interpretation of ADAMTS13 results under pathological conditions. Whether elevated serum unconjugated bilirubin has an adverse effect in vivo remains to be determined in our future study.
Introduction
ADAMTS13, a plasma metalloprotease, cleaves von Willebrand factor (VWF) specifically at the Tyr1605-Met1606 bond [1]. This proteolytic cleavage is essential for maintaining normal hemostasis. Excessive proteolysis of VWF by ADAMTS13 resulting from unwanted exposure of the central A2 domain where the cleavage bond resides, as seen in patients with aortic stenosis or type 2A von Willebrand disease, leads to bleeding diathesis [1]. Conversely, inability to cleave VWF anchored on endothelial membrane or in circulation, resulting from mutations in ADAMTS13 [3] or autoantibodies against ADAMTS13 [4], causes a potentially fatal syndrome thrombotic thrombocytopenic purpura (TTP). Moreover, reduced ratios of plasma ADAMTS13 activity to VWF concentrations are shown to be the risk factors for the development of other arterial thrombotic disorders, including myocardial infarction and cerebral ischemic stroke [5].
Plasma ADAMTS13 activity and inhibitors can be measured by a fluorescent energy resonance transfer (FRETS)-based assay [6], an immunoassay [7], a SELDI-TOF mass spectrometry-based assay [8], and Western blotting analysis [9,10]. The FRETS-based assay, first described by Kokame et al [6], appears to gain its popularity because of its rapid turn-around-time and relatively simple procedure. However, the first-generation FRETS-VWF73 assay is subjected to interference from plasma free hemoglobin [11] and unconjugated bilirubin [12] because the N-methyl anthranilate moiety in the FRETS-VWF73 peptide absorbs at 340 nm and emits at 450 nm, which are close to the absorbance of these plasma substances. Recently, a second-generation FRETS-based assay, FRETS-rVWF71, has been developed [13], in which a recombinant VWF71 peptide is labeled at Cys1610 with DyLight 633 (absorption at 638 nm, emission at 658 nm) and at N-terminus with IRDye QC-1 (absorption at 500–800 nm). This new assay is reported to suffer from no inference by unconjugated bilirubin [13]. We are encouraged by this report and decide to generate our own similar fluorogenic substrate using a previously described homo-quenching technology instead. This technology is relatively easier in term of labeling and purification than that reported [14].
In principal, our novel substrate would behave similarly to that reported [13] and expects little or no interference from unconjugated bilirubin because of its fluorescence detection at the excitation/emission of 638nm/658 nm. Unexpectedly, we find that the cleavage of this new substrate by ADAMTS13 is also inhibited by unconjugated bilirubin. Further analyses with SELDI-TOF mass spectrometry assay and Western blotting demonstrate the direct inhibition of ADAMTS13 activity by unconjugated bilirubin. Moreover, pretreatment of the reaction mixture or plasma samples containing unconjugated bilirubin with bilirubin oxidase, which converted bilirubin to biliverdin, essentially eliminates the inhibitory activity. Our findings may have clinical implications for proper interpretation of plasma ADAMTS13 activity in differential diagnosis of TTP from hemolytic uremic syndrome (HUS) or other pathological conditions. Whether elevated serum unconjugated bilirubin contributes to thrombosis under certain pathological conditions remains to be determined.
Materials and Methods
Materials
Unconjugated bilirubin and bilirubin oxidase were purchased from Sigma-Aldrich (St. Louis, MO). Fluorecene-5’-maleimide and DyLight633-maleimide were from Thermo Scientific (Waltham, MA). Metal ion chelating resin was purchased from GE Healthcare Life sciences (Mickleton, NJ). Pooled normal human plasma (NHP) was obtained from George King Biotechnology (Overland Park, KS). SeaKem HGT (P) agarose (Lonza, Rockland, ME), nitrocellulose membrane (Bio-Rad, Hercules, CA), Pefablock (Sigma-Aldrich), rabbit anti-human VWF antibody (Dako, Carpinteria, CA), and IRDye-800CW-labeled goat anti-rabbit IgG (LI-COR Biotech, Lincoln, NE) were all commercially available. Plasma VWF [15] and recombinant human ADAMTS13 (rA13) [16] were purified to homogeneity using the methods previously described. GST-VWF73-H [17] and GST-rVWF71-11K [8] were prepared according to methods described previously. Leftover de-identified plasma samples anti-coagulated with sodium heparin after chemistry tests were collected from hospitalized patients with significantly elevated serum levels of unconjugated bilirubin.
Preparation of fluorogenic substrates
Recombinant human VWF73 peptide (rVWF73) derived from the central A2 domain of VWF with cysteine substitution at residues Q1659 and N1610 was expressed in E. coli BL21 and labeled in column with either a fluorecene-5-maleimide (F485-rVWF73-H) or a DyLight633-maleimide dye (D633-rVWF73-H) as previously described [14]. The fluorescein-labeled peptides were purified to homogeneity with Ni-chelating affinity chromatography as previously described [14]. The final concentrations of fluorescein-labeled VWF73 peptides were determined using a NanoDrop spectrophotometer (Thermo Scientific) at absorbance of 280 nm, corrected with the absorbance of 495 or 638 nm, depending on the fluorescent dye used for labeling.
Cleavage of F485-rVWF73-H or D633-rVWF73-H by ADAMTS13
Purified F485-rVWF73-H (0.5 µM) or D633-rVWF73-H (2 µM) was incubated with either 2.5 µl or 20 µl of NHP in 50 mM HEPES, pH 7.4, 150 mM NaCl, 10 mM CaCl2, 0.05% Tween-20 at 25 °C. To test bilirubin effect, a fixed volume of NHP was incubated at 25 °C for 15 min with unconjugated bilirubin (0-125 µM). The residual ADAMTS13 activity in NHP was determined by its ability to cleave F485-rVWF73-H or D633-rVWF73-H according to methods described previously [13, 18]. The rate of proteolytic cleavage of the fluorogenic substrate was monitored every 2 min for 60 min by a Gemini XPS microplate reader (Molecular Devices, Sunnyvale CA) as previously described [13, 18]. Relative activity (%) was determined based on the standard curve generated with pooled NHP (defined as having 1 unit/ml of ADAMTS13 activity) and plotted against the concentrations of bilirubin.
To assess the assay specificity or to eliminate the bilirubin effect, F485-rVWF73-H (0.5 µM) or D633-rVWF73-H (2 µM) was incubated with 2.5 µl or 20 µl of NHP in the presence of various concentrations of bilirubin (0-125 μM) and bilirubin oxidase (0-8 units/ml) for 5 min at 37 °C. The residual ADAMTS13 activity in the reaction mixture was determined similarly as described above and plotted against the concentrations of unconjugated bilirubin and bilirubin oxidase.
Cleavage of GST-VWF71-11K by ADAMTS13
GST-VWF71-11K (~2 µg) was incubated at 37 °C for 60 min with ~2 μL of plasma samples or diluted NHP corresponding to 0, 2.5, 5, 10, 25, and 50% activity in 30 μL of 5 mM Tris HCl, 5 mM NaCl, 1 mM BaCl2, pH 7.5. The ADAMTS13 cleavage product was detected by SELDI-TOF mass spectrometry (ProteinChipSystem, Series 4000, Vermillion, Fremont, CA) as previously described [8].
Cleavage of multimeric VWF by ADAMTS13
Plasma-derived VWF (~50 nM in monomer) was incubated with rADAMTS13 (~10 nM) and various concentrations of bilirubin (0, 0.3125, 0.625, 1.25, 2.5, 5 mM) in 10mM Tris-HCl, pH 8.0, 150 mM NaCl, 10 mM BaCl2 at 37 ºC for 30 minutes. Alternatively, a fixed concentration (312 μM) was pre-incubated at 37 ºC for 10 min with increasing concentrations of bilirubin oxidase (0-8 U/ml, final) prior to being added to the reaction mixture containing VWF and rADAMTS13. The reaction mixtures were loaded on a nitrocellulose membrane floating on 50-ml dialysis buffer (10 mM Tris-HCl, pH 8.0 containing 1.5 M urea) as previously described [9, 19] and incubated at 37 ºC for 4 h. The cleavage of multimeric VWF was determined by Western blotting after being separated with 1% SDS-agarose [20-22] or 5% SDS-polyacrylamide gel [23]. Quantification of the cleavage product was performed with NIH ImageJ 1.48 software. The data shown are the means ± standard errors of the means from three independent experiments (n=3).
Surface plasmon resonance
The surface of a carboxymethylated dextran (CM5) chip was activated according to the method described previously [16]. Approximately 6000 response units (RU) of purified GST-rVWF73-H, ADAMTS13, and VWF proteins were covalently attached onto the activated CM5 chip surface. The control surface was activated similarly but not immobilized by protein. The reactive groups on the dextran surface were blocked by injection of 35 µL of 1 M ethanolamine (pH 8.5). Unconjugated bilirubin (Sigma, St. Louis, MO) at various concentrations (0 to 125 µM) in 10 mM HEPES, 150 mM NaCl, pH 7.5, containing 0.005% Tween 20 was injected and passed over the surface at injection rates of 20 µL/min for 3 minutes. Between each injection, bound bilirubin was removed by injection of 60 µL of 0.1 M glycine, pH 2.5, followed by re-equilibration. The kinetics of the interaction, the rates of complex formation (ka) and dissociation (kd), were determined from the sensorgrams by fitting the data globally. The equilibrium constant KD is the ratio (kd/ka) of the kinetic rate constants.
Statistical analysis
Student t-test, Wilcoxon matched-pairs signed rank test, ANOVA one-way analysis of variants were used to determine the significance of the difference between two groups and multiple groups. P values less than 0.05 and 0.01 are considered to be statistically significant and highly significant, respectively.
Results
Bilirubin directly inhibits the cleavage of peptidyl VWF substrates by ADAMTS13 in addition to its interference with fluorogenic assays
Unconjugated bilirubin is shown to falsely reduce plasma ADAMTS13 activity measured by commercially available fluorogenic assays [12, 24]. This is because bilirubin absorbs at the wavelength close to that used for the detection of the cleavage of fluorogenic substrates. To avoid such an interference, the novel fluorogenic rVWF73 substrate (D633-rVWF73-H) labeled at the modified Cys1599 and Cys1610 with a fluorescent dye DyLight633-maleimide as described [13] is prepared (Fig. 1A). The detection of cleavage of the D633-rVWF73-H substrate can be performed at the excitation/emission wavelength of 638nm/658 nm where all plasma constituents are essentially transparent (SI Fig. 1). It is therefore expected that this modified fluorogenic substrate would not be interfered by plasma bilirubin.
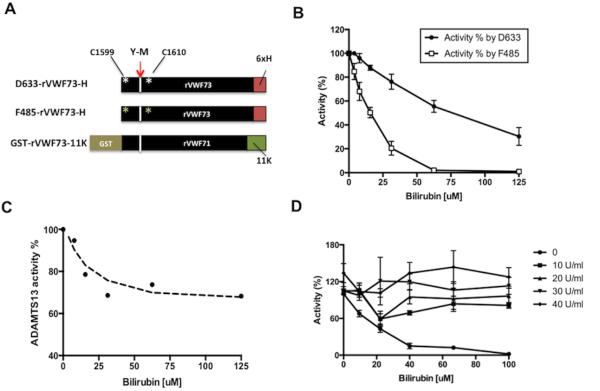
Fig. 1.
Bilirubin inhibits proteolytic cleavage of fluorogenic substrates by ADAMTS13 that is reversible by treatment with bilirubin oxidase. A. Constructs expressing rVWF73 contained Cys substitution at Q1599 and N1610 for labeling and a C-terminal 6xHis epitope and GST-rVWF71-11K peptide. B. Inhibition of plasma ADAMTS13 activity by unconjugated bilirubin at various concentrations (0-125 μM, final), assayed by the cleavage of F485-rVWF73-H or D633-rVWF73-H as indicated. The relative activity (%) is expressed as the means ± standard errors from three independent experiments. C. Inhibition of plasma ADAMTS13 activity by unconjugated bilirubin assayed by the cleavage of GST-rVWF71-11K using SELDI-TOF-MS. D. Bilirubin oxidase reverses the inhibitory activity of unconjugated bilirubin on the cleavage of D633-rVWF73-H by plasma ADAMTS13. The data represent the means ± standard errors from three independent experiments.
For a control, we also prepared F485-rVWF73-H labeled at residues Cys1599 and Cys1610 with fluorescein-5’-maleimide (Fig. 1A) [14, 18]. The cleavage of this substrate is detected at the excitation/emission of 485nm/530nm, which overlaps with the absorbance of unconjugated bilirubin (SI Fig. 1). As expected, unconjugated bilirubin significantly reduces the cleavage of F485-rVWF73-H by ADAMTS13 in a concentration-dependent manner. At the final concentration greater than 62.5 µM, unconjugated bilirubin nearly abolishes ADAMTS13 activity in human plasma (Fig. 1B). Unexpectedly, the cleavage of D633-rVWF73-H by ADAMTS13 is also significantly reduced in the presence of unconjugated bilirubin although the reduction of the cleavage of D633-rVWF73-H is less pronounced (nadir at 40% of residual activity) than that of F485-rVWF73-H even in the presence of 125 µM of unconjugated bilirubin (Fig. 1B). The estimated half maximal inhibitory concentrations (IC50’s) of unconjugated bilirubin toward the cleavage of F485-rVWF73-H and D633-rVWF73-H by ADAMTS13 are 17 µM and 70 µM, respectively. The difference in IC50’s between two peptides suggests that unconjugated bilirubin may not only interfere with the fluorescent detection but also directly inhibit plasma ADAMTS13 activity.
To further investigate the inhibitory activity of unconjugated bilirubin on plasma ADAMTS13 activity, proteolytic cleavage of GST-rVWF73-11K by plasma ADAMTS13 in the presence of various concentrations (0-125 µM) of unconjugated bilirubin is determined by the SELDI-TOF-mass spectrometric assay. This method directly measures the amount of ADAMTS13 cleavage product and does not rely on the detection of fluorescence or optical density [8]. Consistent with the results obtained from the cleavage of D633-rVWF73-H, unconjugated bilirubin also inhibits the cleavage of GST-rVWF71-11K by ADAMTS13 in a concentration-dependent manner (nadir at 60% of residual activity) with an estimated IC50 of ~17 µM (Fig. 1C). Further increases in unconjugated bilirubin concentrations beyond 50 µM had no effect, suggesting that the binding site for unconjugated bilirubin on either GST-rVWF71-11K or ADAMTS13 is saturable.
Bilirubin oxidase treatment eliminates the inhibitory effects of unconjugated bilirubin on ADAMTS13 activity
Bilirubin oxidase is an enzyme that catalyzes the chemical reaction: 2 bilirubin (yellow) + O2 →2 biliverdin (brown) + 2H2O. This treatment was shown to increase ADAMTS13 activity in plasma samples obtained from TTP patients by 10-20% by the first generation commercial FRETS assay [24]. Here, we show that in the presence of bilirubin oxidase (0-40 U/ml) and various concentrations of unconjugated bilirubin (0-100 µM) ADAMTS13 activity assayed by D633-rVWF-H increases as a function of increasing concentrations of bilirubin oxidase (Fig. 1C). However, bilirubin oxidase treatment has little effect on ADAMTS13 activity by F485-rVWF73-H (data not shown). These results suggest the presence of brownish biliverdin product in the samples continues to interfere with the assay by F485-rVWF73-H, which partially overlaps with the absorbance of biliverdin, but has little effect on the assay by D633-rVWF73-H (SI Fig. 1). Our results are consistent with those reported [13] and clearly indicate that the modified fluorogenic substrate D633-rVWF73-H substrate is superior to F485-rVWF73-H and perhaps other first generation fluorogenic peptides [6, 14] in assessing plasma ADAMTS13 activity in patients with elevated serum levels of unconjugated bilirubin.
Bilirubin inhibits the cleavage of multimeric VWF by ADAMTS13
To determine the effect of bilirubin on proteolysis of native substrate, plasma-derived multimeric VWF is first denatured with 1.5 M urea in 5 mM Tris-HCl, pH 8.0 to expose the A2 domain and then incubated with increasing concentrations of unconjugated bilirubin (0-5 mM) for 30 min in the presence of recombinant ADAMTS13 [25]. The reaction mixture is further incubated on a dialysis membrane against 5 mM Tris-HCl, pH 8.0 at 37 ºC for 4 hours for proteolysis to occur. The loss of VWF multimers or increase in the cleavage products is determined by Western blotting after electrophoresis on SDS-agarose (1%) gel (Fig. 2A, top) or SDS-polyacrylamide (5%) gel (Fig. 2A, bottom) as indicated in the figure legends. As shown, unconjugated bilirubin dose-dependently inhibits the cleavage of multimeric VWF by ADAMTS13 under these conditions with an estimated IC50 of ~0.8 mM (Fig. 2B). This IC50 is ~50 times higher than that for inhibition of the cleavage of D633-rVWF73, likely due to the removal of unbound free bilirubin (molecular weight of 584.66 g mol−1) during the dialysis.
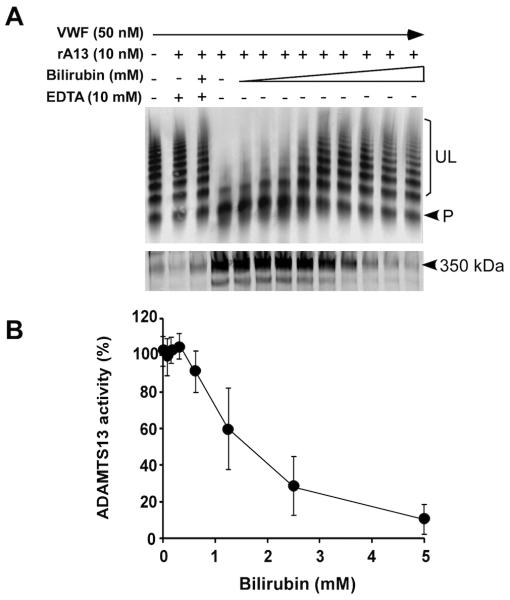
Fig. 2.
Bilirubin inhibits the proteolytic cleavage of plasma VWF by ADAMTS13. A. Purified plasma VWF (50 nM) was incubated with (+) or without (−) recombinant ADAMTS13 (rA13) (10 nM) in the absence (−) or presence (+) EDTA (20 mM) or increasing concentrations of unconjugated bilirubin (0-5 mM). The proteolytic cleavage of VWF was determined by Western blotting after being separated with 1% SDS-agarose (top) or 5% SDS-polyacrylamide (bottom) gel electrophoresis. UL and P indicate the uncleaved VWF multimers and the cleavage product (~350 kDa), respectively. B. The relative amount of cleavage product of VWF in A-bottom was quantified by densitometry using ImageJ and plotted against the concentrations of unconjugated bilirubin. The data indicate the means ± standard errors (SE) from three independent experiments (n=3).
Similarly, pre-treatment of the reaction mixtures containing bilirubin (312 μM) with increasing concentrations of bilirubin oxidase (0-8 U/ml) dose-dependently reverses the inhibitory effect of bilirubin on VWF proteolysis by ADAMTS13 (Fig. 3). In the presence of ~2 U/ml of bilirubin oxidase, the cleavage of VWF by ADAMTS13 in the presence of 312 µM of bilirubin in the samples is nearly completely reversed (Fig. 3). Together, these data demonstrate that unconjugated bilirubin can directly inhibit the proteolytic cleavage of VWF by ADAMTS13 in addition to its known interference with the measurement of ADAMTS13 activity by the conventional fluorogenic assays, which use the fluorescence detection wavelengths overlapping with the absorbance of bilirubin and its oxidized product biliverdin.
Fig. 3.
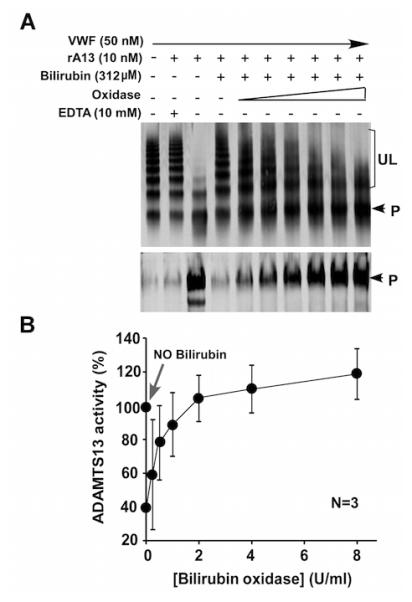
Bilirubin oxidase reverses the inhibitory activity of bilirubin on the cleavage of multimeric VWF by recombinant ADAMTS13. A. Pretreatment of the reaction mixture containing plamsa VWF, rA13, and unconjugated bilirubin in the absence (−) or presence (+) of increasing concentrations of bilirubin oxidase (0-8 U/ml) reversed the inhibitory activity on the cleavage of multimeric VWF in a concentration-dependent manner, determined by Western blot after separation with either 1% agarose gel electrophoresis (top) or 5% SDS-polyacrylamide gel electrophoresis (bottom). B. The quantitative data of the cleavage product (~350 kDa) shown in the bottom of panel A represent the means ± standard errors from three independent experiments. The arrow indicates 100% of ADAMTS13 activity in the absence of bilirubin and bilirubin oxidase.
Unconjugated bilirubin binds both VWF and ADAMTS13
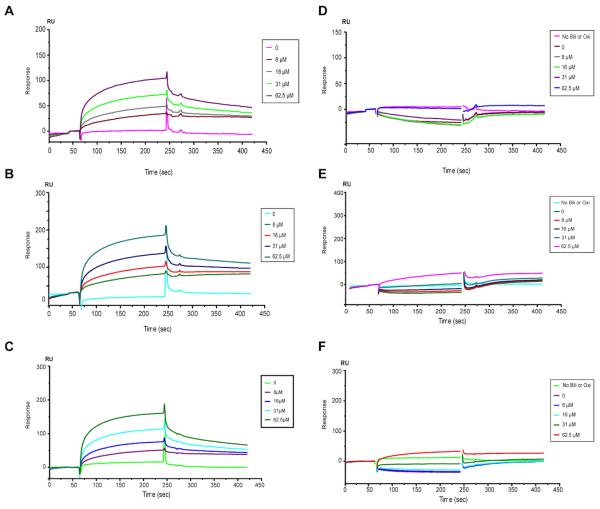
To elucidate the molecular mechanism underlying bilirubin-mediated inhibition of ADAMTS13 activity, we determined the binding interactions between bilirubin and rVWF73, VVWF and ADAMTS13 using the SPR assay. Unconjugated bilirubin at various concentrations (0-62.5 µM) in the absence or presence of bilirubin oxidase is flown at 20 µL/min over each channel immobilized with nothing (blank control), GST-VWF73-H, plasma-derived VWF, and rADAMTS13, respectively. The binding sensorgrams for each ligand-ligand binding interaction are recorded (Fig. 4) and analyzed. The kinetics parameters i.e. the rates of complex formation (ka) and dissociation (kd) are determined by fitting the data (1:1 binding) using BIAcore evaluation system and presented in Table 1. Unconjugated bilirubin binds to GST-VWF73-H peptide (Fig. 4A), multimeric VWF (Fig. 4B), and rADAMTS13 (Fig. 4C) in a concentration-dependent manner with a dissociation constants (KD) at equilibrium of 57.5 ± 80.7, 17.5 ± 4.0, and 29.6 ± 40.1 µM, respectively (Table 1). Pretreatment of unconjugated bilirubin with bilirubin oxidase nearly abolished the binding of bilirubin to GST-VWF73-H (Fig. 4D), VWF (Fig. 4E), and rADAMTS13 (Fig. 4F). These results suggest that bilirubin binds directly to both VWF and ADAMTS13 to mediate its inhibitory activity.
Fig. 4.
Direct binding of bilirubin to GST-VWF73-H, VWF, and rADAMTS13, which is eliminated by bilirubin oxidase treatment. A, B, and C show the binding of unconjugated bilirubin at various concentrations (0-62.5 μM) to immobilized GST-rVWF73-H, plasma VWF, and rADAMTS13, respectively. D, E, and F show the binding of unconjugated bilirubin that was pretreated for 15 min with bilirubin oxidase (2 U/ml) to GST-VWF73-H, plasma VWF, and rADAMTS13, respectively. All data shown were the net response units after subtracting the response units in the control cell that was activated and blocked with 1 M ethanolamine (pH 8.5) without being immobilized with a protein of interest.
Table 1.
Kinetics of binding of unconjugated bilirubin to GST-rVWF73-H, rADAMTS13, and VWF
| ka (1/Ms) | kd (1/s) ×10−3 | Rmax (RU) | KD (μM) | |
|---|---|---|---|---|
| GST-rVWF73-H | 159.8 ± 86.0 | 4.2 ± 2.1 | 216.0 ± 200.0 | 57.5 ± 80.7 |
| rADAMTS13 | 208.8 ± 25.2 | 3.6 ± 0.61 | 189.3 ± 69.3 | 17.5 ± 4.0 |
| VWF | 169.9 ± 84.9 | 2.4 ± 1.0 | 301.0 ± 264.7 | 29.6 ± 40.1 |
The kinetic parameters were determined using 1:1 Langmuir fit model. ka and kd respresent the rates of association and dissociation; KD is the ratio of kd/ka, the association rate constant. The data are the means ± standard deviation (SD) of 4 repeated experiments
Bilirubin oxidase treatment increases plasma ADAMTS13 activity obtained from patients with hyperbilirubinemia
Elevated serum or plasma levels of unconjugated bilirubin are common in newborns. To determine the physiological consequence of elevated bilirubin on plasma ADAMTS13 activity, we determined plasma ADAMTS13 activity in patients with hyperbilirubinemia with two different fluorogenic substrates before and after treatment with bilirubin oxidase. As shown, plasma ADAMTS13 activity in all patient samples is significantly lower when assessed by F485-rVWF73-H than by D633-rVWF73-H (Fig. 5A) (p<0.001). Treatment of patient plasma with bilirubin oxidase (2 U/ml) at 25 °C for 15 min results in little or no change of plasma ADAMTS13 activity by F485-rVWF73-H (Fig. 5B) (p>0.05), but dramatic increase by D633-rVWF73-H (Fig. 5C) (p<0.001). On average, plasma ADAMTS13 activity measured by D633-rVWF73-H increases by ~3-fold after treatment with bilirubin oxidase. The net increase in plasma ADAMTS13 activity by D633-rVWF73-H (r2=0.49), but not by F485-rVWF73-H (r2=0.02) after bilirubin oxidase appears to be proportional to the serum concentrations of unconjugated bilirubin. These results further support the hypothesis that bilirubin directly inhibits ADAMTS13 activity in addition to its interference with certain fluorogenic assays.
Fig. 5.
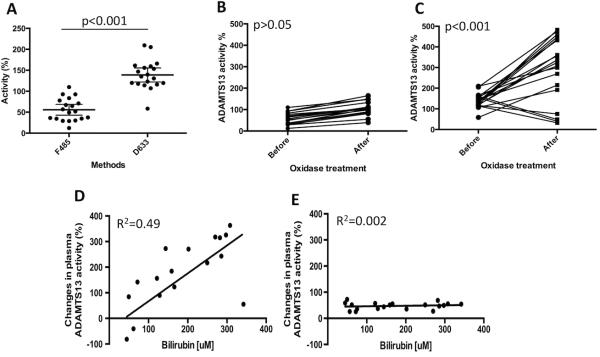
Plasma ADAMTS13 activity in patients with hyperbilirubinemia before and after bilirubin oxidase treatment. A. Plasma ADAMTS13 activity in patients with elevated serum levels of unconjugated bilirubin determined by the cleavage of F485-rVWF73-H and D633-rVWF73-H as indicated. An unpaired student t-test was performed to demonstrate that the difference is statistically highly significant, p<0.001. B and C are plasma ADAMTS13 activity before and after being treated with bilirubin oxidase (2 U/ml) that was determined by the cleavage of F485-rVWF73-H and D633-rVWF73-H, respectively. Paired student t-test was performed to demonstrate the difference before and after bilirubin oxidase treatment was not statistically significant in B, but highly significant in C with p<0.001. D and E show the linear correlations between the net increase of plasma ADAMTS13 activity after being treated with bilirubin oxidase and serum bilirubin concentrations determined by D633-rVWF73-H (r2=0.49) and by F485-rVWF73-H (r2=0.002), respectively.
Discussion
The present study demonstrates for the first time that unconjugated bilirubin directly inhibits the proteolytic cleavage of both peptidyl and multimeric VWF substrates by ADAMTS13 (Figs. 1-3). The findings extend beyond the observations made previously by others [12, 24], in which unconjugated bilirubin is shown to only interfere with certain fluorogenic assays such as the FRETS-VWF73 assay for plasma ADAMTS13 activity. The reason is that the detection wavelength (excitation/emission: 340nm/450 nm) for fluorescence overlaps the absorbance of bilirubin (SI Fig. 1), resulting in falsely low ADAMTS13 activity and false positive anti-ADAMTS13 autoantibodies. The inhibitory activity of unconjugated bilirubin on the cleavage of VWF by ADAMTS13 appears to be mediated by its direct binding to both VWF (or A2 domain) substrate and ADAMTS13 enzyme (Fig. 4), although the exact binding sites on VWF and ADAMTS13 remain to be identified. Conversion of bilirubin to biliverdin by bilirubin oxidase nearly abolishes the binding of bilirubin to VWF and ADAMTS13 and its inhibition of plasma ADAMTS13 activity by D633-rVWF73-H. These results suggest that the reduced form of open chain of four pyrrole-like rings in unconjugated bilirubin is necessary for its inhibitory activity, although the exact molecular mechanism is yet to be determined in the future study.
Our results on the bilirubin interference with the first-generation fluorogenic assays are consistent with those described in the literature [12, 13, 24]. However, Muia et al did not observe the significant inhibitory activity of unconjugated bilirubin at the final concentration of 20 mg/dL (or ≈140 μM) on the cleavage of FRETS-rVWF71. The FRETS-rVWF71 is labeled at Cys1610 with DyLight 633 (abs./emi. 638/658 nm) and at the N-terminus with IRDye QC-1 (abs. of 500–800 nm) [13]. The reason for the discrepancy is not known. We speculate that the preparation, quality, and solubility of unconjugated bilirubin, as well as the property of substrate may affect the results. Considering the results that bilirubin inhibits the cleavage of both GST-VWF71-11K, which is detected by SELDI-TOF-mass spectrometry (Fig. 1C) and multimeric VWF, which is detected by Western blotting (Figs. 2 & 3), we propose that unconjugated bilirubin may be a direct inhibitor of ADAMTS13.
Our findings may have important clinical implications in two fold: 1) helping proper interpretations of plasma ADAMTS13 activity and inhibitors for differential diagnosis of thrombotic microangiopathy; 2) suggesting a potential novel contribution of hyperbilirubinemia to thrombosis under certain pathological conditions such as in sickle cell disease, paroxysmal nocturnal hemoglobinuria, and autoimmune hemolytic anemia (see the review) [26]. It has been postulated that the released free hemoglobin from lyses of red blood cells scavenges nitric oxide, triggers the release of proinflammatory cytokines and reactive oxygen species, and inhibits ADAMTS13 activity (see the review) [26]. The data from current study suggest that unconjugated bilirubin may further lower plasma ADAMTS13 activity. This can be detrimental if patients have low ADAMTS13 activity to begin with. Consistent with this hypothesis is that plasma ADAMTS13 activity increases by ~3 fold after treatment with bilirubin oxidase (Fig. 5). However, the in vivo effect of unconjugated bilirubin on VWF proteolysis remains speculative at this point and needs further investigation.
We conclude that elevated serum unconjugated bilirubin may not only interfere with certain fluorogenic assays for assessing plasma ADAMTS13 activity and inhibitors, but also directly inhibit plasma ADAMTS13 activity, which may contribute in part to the adverse events in patients with preexisting partial deficiency of plasma ADAMTS13 activity such as in the cases of ADAMTS13 mutations or anti-ADAMTS13 autoantibodies by further lowering their plasma ADAMTS13 activity below the threshold.
Supplementary Material
SI Fig. 1. Wavelength scanning. 0.5 ml solution containing PBS (A), 20% of diluted plasma (B), 5 μM F485-rVWF73-H (C), 5 μM D633-rVWF73-H (D), phosphate buffered saline (PBS) plus 100 μM unconjugated bilirubin (E, G) or 20% diluted plasma plus 100 μM unconjugated bilirubin (F, H) in the absence (E, F) or presence of 1.2 U/ml bilirubin oxidase (G, H). The scanning was performed on a NanoDrop 2000 UV-Vis spectrophotometer (190-840nm) (Thermo Scientific).
Acknowledgement
This study was supported in part by grants of R01HL115187-01A1 and the Bridge fund from American Society of Hematology to XLZ, R01FD003932 to HMW, and Jiangsu government scholarship for overseas studies to RL.
Footnotes
Authorship Statement
RNL, SY, HMW, and XLZ designed and performed experiments, interpreted results, and wrote manuscript.
Conflict of interest disclosure
Authors declare no conflict of interest relevant to this study.
References
- 1.Zheng X, Majerus EM, Sadler JE. ADAMTS13 and TTP. Curr Opin Hematol. 2002;9:389–94. doi: 10.1097/00062752-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Sadler JE. von Willebrand factor: two sides of a coin. J Thromb Haemost. 2005;3:1702–09. doi: 10.1111/j.1538-7836.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 3.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr., Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 4.Tsai HM, Rice L, Sarode R, Chow TW, Moake JL. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Annals of internal medicine. 2000;132:794–99. doi: 10.7326/0003-4819-132-10-200005160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng XL. ADAMTS13, TTP and Beyond. Hereditary Genet. 2013;2:e104. doi: 10.4172/2161-1041.1000e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fujimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46:1444–52. doi: 10.1111/j.1537-2995.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 8.Jin M, Cataland S, Bissell M, Wu HM. A rapid test for the diagnosis of thrombotic thrombocytopenic purpura using surface enhanced laser desorption/ionization time-of-flight (SELDI-TOF)-mass spectrometry. J Thromb Haemost. 2006;4:333–38. doi: 10.1111/j.1538-7836.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- 9.Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, Lammle B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood. 1997;89:3097–03. [PubMed] [Google Scholar]
- 10.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Eng J Med. 1998;339:1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Studt JD, Kremer Hovinga JA, Antoine G, Hermann M, Rieger M, Scheiflinger F, Lammle B. Fatal congenital thrombotic thrombocytopenic purpura with apparent ADAMTS13 inhibitor: in vitro inhibition of ADAMTS13 activity by hemoglobin. Blood. 2005;105:542–24. doi: 10.1182/blood-2004-06-2096. [DOI] [PubMed] [Google Scholar]
- 12.Meyer SC, Sulzer I, Lammle B, Kremer Hovinga JA. Hyperbilirubinemia interferes with ADAMTS-13 activity measurement by FRETS-VWF73 assay: diagnostic relevance in patients suffering from acute thrombotic microangiopathies. J Thromb Haemost. 2007;5:866–67. doi: 10.1111/j.1538-7836.2007.02438.x. [DOI] [PubMed] [Google Scholar]
- 13.Muia J, Gao W, Haberichter SL, Dolatshahi L, Zhu J, Westfield LA, Covill SC, Friedman KD, Sadler JE. An optimized fluorogenic ADAMTS13 assay with increased sensitivity for the investigation of patients with thrombotic thrombocytopenic purpura. J Thromb Haemost. 2013;11:1511–18. doi: 10.1111/jth.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Lawson HL, Harish VC, Huff JD, Knovich MA, Owen J. Creation of a recombinant peptide substrate for fluorescence resonance energy transfer-based protease assays. Anal Biochem. 2006;358:298–300. doi: 10.1016/j.ab.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Skipwith CG, Cao W, Zheng XL. Factor VIII and platelets synergistically accelerate cleavage of von Willebrand factor by ADAMTS13 under fluid shear stress. J Biol Chem. 2010;285:28596–03. doi: 10.1074/jbc.M110.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Pan W, Rux AH, Sachais BS, Zheng XL. The cooperative activity between the carboxyl-terminal TSP1 repeats and the CUB domains of ADAMTS13 is crucial for recognition of von Willebrand factor under flow. Blood. 2007;110:1887–94. doi: 10.1182/blood-2007-04-083329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai J, Smith P, Wang S, Zhang P, Zheng XL. The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor. J Biol Chem. 2005;280:29428–34. doi: 10.1074/jbc.M505513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raife TJ, Cao W, Atkinson BS, Bedell B, Montgomery RR, Lentz SR, Johnson GF, Zheng XL. Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood. 2009;114:1666–74. doi: 10.1182/blood-2009-01-195461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furlan M, Lammle B. Deficiency of von Willebrand factor-cleaving protease in familial and acquired thrombotic thrombocytopenic purpura. Bailliere's clinical haematology. 1998;11:509–14. doi: 10.1016/s0950-3536(98)80064-4. [DOI] [PubMed] [Google Scholar]
- 20.Cao W, Krishnaswamy S, Camire RM, Lenting PJ, Zheng XL. Factor VIII accelerates proteolytic cleavage of von Willebrand factor by ADAMTS13. Proc Natl Acad Sci U S A. 2008;105:7416–21. doi: 10.1073/pnas.0801735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin SY, Tohyama J, Bauer RC, Cao NN, Rader DJ, Zheng XL. Genetic ablation of Adamts13 gene dramatically accelerates the formation of early atherosclerosis in a murine model. Arterioscler Thromb Vasc Biol. 2012;32:1817–23. doi: 10.1161/ATVBAHA.112.247262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao J, Jin SY, Xue J, Sorvillo N, Voorberg J, Zheng XL. Essential domains of a disintegrin and metalloprotease with thrombospondin type 1 repeats-13 metalloprotease required for modulation of arterial thrombosis. Arterioscler Thromb Vasc Biol. 2011;31:2261–69. doi: 10.1161/ATVBAHA.111.229609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin SY, Skipwith CG, Zheng XL. Amino acid residues Arg(659), Arg(660), and Tyr(661) in the spacer domain of ADAMTS13 are critical for cleavage of von Willebrand factor. Blood. 2010;115:2300–10. doi: 10.1182/blood-2009-07-235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckmann CM, De Laaf RT, Van Keulen JM, Van Mourik JA, De Laat B. Bilirubin oxidase as a solution for the interference of hyperbilirubinemia with ADAMTS-13 activity measurement by FRETS-VWF73 assay. J Thromb Haemost. 2007;5:1330–31. doi: 10.1111/j.1538-7836.2007.02510.x. [DOI] [PubMed] [Google Scholar]
- 25.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lammle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578–84. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 26.L'Acqua C, Hod E. New perspectives on the thrombotic complications of haemolysis. Br J Haematol. 2014;168:175–85. doi: 10.1111/bjh.13183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SI Fig. 1. Wavelength scanning. 0.5 ml solution containing PBS (A), 20% of diluted plasma (B), 5 μM F485-rVWF73-H (C), 5 μM D633-rVWF73-H (D), phosphate buffered saline (PBS) plus 100 μM unconjugated bilirubin (E, G) or 20% diluted plasma plus 100 μM unconjugated bilirubin (F, H) in the absence (E, F) or presence of 1.2 U/ml bilirubin oxidase (G, H). The scanning was performed on a NanoDrop 2000 UV-Vis spectrophotometer (190-840nm) (Thermo Scientific).