Abstract
Western Amazonia's landscape and biota were shaped by an enormous wetland during the Miocene epoch. Among the most discussed topics of this ecosystem range the question on the transitory influx of marine waters. Inter alia the occurrence of typically brackish water associated ostracods is repeatedly consulted to infer elevated salinities or even marine ingressions. The taxonomical investigation of ostracod faunas derived from the upper part of the Solimões Formation (Eirunepé; W-Brazil) documents a moderately diverse assemblage (19 species). A wealth of freshwater ostracods (mainly Cytheridella, Penthesilenula) was found co-occurring with taxa (chiefly Cyprideis) usually related to marginal marine settings today. The observed faunal compositions as well as constantly very light δ18O- and δ13C-values obtained by measuring both, the freshwater and brackish water ostracod group, refer to entirely freshwater conditions. These results corroborate with previous sedimentological and palaeontological observations, which proposed a fluvial depositional system for this part of western Amazonia during the Late Miocene. We demonstrate that some endemic, “brackish” water ostracods (i.e., Cyprideis) have been effectively adapted to freshwater conditions. Thus, their occurrence is no univocal evidence for the influence of brackish or marine waters in western Amazonia during the Miocene.
Keywords: Western Amazonia, Late Miocene, Solimões Formation, Palaeoenvironments, Ostracoda
Highlights
► Miocene ostracod faunas from Amazonia (Solimões Fm.; Eirunepé/Brazil) were examined. ► Freshwater and “brackish” water elements are found regularly co-occurring. ► The isotopic composition of both groups indicates entirely freshwater conditions. ► The presence of “brackish” water taxa is no evidence for the influx of marine waters.
1. Introduction
Today, lowland Amazonia is famous for its biodiversity, which obviously root in pre-Quaternary times (e.g., Haffer, 2008; Hoorn et al., 2010a; Jaramillo et al., 2010; Wesselingh et al., 2010). The evolution of the modern Amazon system and its precursors is synoptically summarised recently (Hoorn and Wesselingh, 2010; Hoorn et al., 2010a). However, it remains heavily disputed by e.g., Latrubesse et al. (2007, 2010). Nevertheless, from a palaeobiological point of view the proposed Middle to (early) Late Miocene “Pebas system” (Hoorn et al., 2010a) is of outstanding interest. Albeit of its still debated nature (mega-lake, e.g., Wesselingh et al., 2002; mega-wetland, e.g., Hoorn et al., 2010b; mega-fan (partly), e.g., Latrubesse et al., 2010) and chronology, this vast wetland (∼1 million km2; Fig. 1a) shaped western Amazonia's landscapes and life for several millions of years. Among aquatic biota it holds a spectacularly diverse, largely endemic mollusc and ostracod fauna. Gastropods and bivalves have been studied intensively during the last years and revealed (aside essential palaeoenvironmental and biostratigraphical results) prime examples for speciation events related to a variety of ecological factors. Thus, fundamental questions of evolutionary biology are touched based on the fossil record (e.g., Wesselingh, 2007; Anderson et al., 2010). Comparably, ostracods experienced an extensive radiation during the “Pebas phase” (especially the genus Cyprideis; e.g., Whatley et al., 1998), which is, however, less well documented and understood.
Fig. 1.
Location of the study area around Eirunepé (western Amazonia). (a) Schematic illustration of the maximal extent of the “Pebas system” (= hatched area) during the Middle Miocene after Wesselingh (2008). (b) Location of investigated outcrops along the cut banks of the Juruá and Tarauacá River.
Studies on Neogene ostracods from the “Pebas system”, started with the fundamental work of Purper (1977, 1979), followed by contributions of Sheppard and Bate (1980), Purper and Pinto (1983, 1985), Purper and Ornellas (1991) and Swain (1998). Later, the comprehensive research of Muñoz-Torres et al. (1998), Whatley et al. (1998, 2000) significantly improved ostracod taxonomy and stimulated an initial ostracod based biozonation as well as phylogenetic hypotheses. Additional publications come from Ramos (2006), Celestino and Ramos (2007), Ramos et al. (2009). Lately, the state of the art in ostracodological research is reviewed by Wesselingh and Ramos (2010).
The current paper investigates ostracod faunas originating from a region, which is suggested to be situated at the edge of the “Pebas system” – both in terms of palaeogeography and time (Eirunepé area, western Brazil; Wesselingh et al., 2006a; Wesselingh and Ramos, 2010; Figueiredo, 2012; Fig. 1a, b). We aim to provide a profound taxonomical base, which is crucial for forthcoming palaeoecological, biostratigraphical and phylogenetic research. Our systematic evaluation is supplemented by compilations of the autecology as well as by detailed illustrations, inclusively of different sexes and ontogenetic stages. Ostracodological results of the present study are complementary to an earlier sedimentological analysis, which documented an aggrading fluvial system as depositional environment for the examined outcrops (Gross et al., 2011).
2. Geological setting
The sampled sections are situated along the river banks of the Juruá and Tarauacá River, NE respectively SE of Eirunepé (state of Amazonia; Solimões Basin (Jandiatuba Sub-Basin); e.g., Caputo, 1991; Wanderley-Filho et al., 2010; Fig. 1a, b). Aside Quaternary deposits (alluvium, terraces) the studied outcrops (Remanso, Aquidabã, Morada Nova, Pau D’Alho, Torre da Lua, Barro Branco) expose sediments of the upper part of the Solimões Formation (Del' Arco et al., 1977; Maia et al., 1977; Paz et al., in press). Detailed sedimentological descriptions of these locations and interpretations are already presented in Gross et al. (2011) to which we refer here.
The more than 1000 m thick Solimões Fm. covers most of western Amazonia and comprises pelitic–sandy alternations, lignitic intercalation as well as paleosols. Diverging views on in its definition, its stratigraphical and geographical range as well of its depositional environments clearly mirror the controversially debated history of Amazonia through Neogene times. In-depth reviews of the Solimões Fm. are provided by e.g., Del' Arco et al. (1977), Purper (1979), Hoorn (1994), Latrubesse et al. (1997, 2010), Hoorn et al. (2010b) and Silva-Caminha et al. (2010).
3. Materials and methods
For micropalaeontological investigations bulk samples (∼1–2 kg) were taken from all outcrops under investigation (Gross et al., 2011). 500 g of dried sediment (40 °C, 24 h) were washed by using diluted hydrogen superoxide for disintegration through standard sieves (H2O2:H2O = 1:5; 63/125/250/500 μm). Wet sieve residuals were washed with ethanol (70%) before drying (40 °C, 24 h).
Residuals ≥250 μm were picked out completely and subject of detailed taxonomic investigations. From the 125 μm sieve-residual 0.2 g/sample were picked, which contained mainly, hardly distinguishable juvenile and/or fragmented ostracod valves. Therefore the following taxa are not further differentiated within this fraction (for details see ESM 1): Cypria sp. and Physocypria sp. are counted as Cypria/Physocypria. Cyprideis graciosa, Cyprideis longispina, Cyprideis pebasae, C. aff. pebasae, Cyprideis sp. 1 and 2 are summarised under Cyprideis “ornate” as well as Cyprideis aff. machadoi and Cyprideis ?olivencai are subsumed under Cyprideis “smooth”.
Prior to stable isotope analyses (δ18O, δ13C; 48 measurements) ostracod valves were additionally washed with distilled water in an ultrasonic bath and rinsed in ethanol finally. Adults and juveniles (A-1 instars) of 5 species were measured: C. pebasae, C. graciosa, Cytheridella danielopoli, Penthesilenula olivencae (only adults), Rhadinocytherura amazonensis (only adults). The number of valves required for analyses (∼50 μg) varied between 2 and 7. For analyses a Thermo-Finnigan Kiel II automated reaction system and a Thermo-Finnigan Delta Plus isotope-ratio mass spectrometer were used (University of Graz; standard deviation = 0.1‰ relative to NBS-19; results in per mille relative to VPDB).
4. Results
4.1. Systematic palaeontology
All figured specimens are housed in the collection of the Museu Paraense Emílio Goeldi, Belém (Inv. No. MPEG-90-M to MPEG-193-M), additional material is stored at the Universalmuseum Joanneum, Department for Geology & Palaeontology, Graz (Inv. No. UMJG&P 210.903).
The suprageneric classification follows Meisch (2000) with some adaptations after Martens and Savatenalinton (2011). General descriptions of species are provided by Purper (1979), Sheppard and Bate (1980) and Muñoz-Torres et al. (1998; Whatley et al., 1998; Ramos, 2006). Further characteristics or deviations are discussed in the remarks.
Abbreviations: R = right valve; L = left valve; ♀ = female; ♂ = male; A-1, ... = juvenile stages; l = length, h = height (both in millimetres; length of spines not included in measurements), n = number of measured specimens.
Class Ostracoda Latreille, 1806
Order Podocopida Sars, 1866
Suborder Podocopina Sars, 1866
Superfamily Darwinuloidea Brady & Norman, 1889
Family Darwinulidae Brady & Norman, 1889
Genus Penthesilenula Rossetti & Martens, 1998
Penthesilenula olivencae (Purper, 1984) comb. nov.
- Pl. 1, figs. 1–10, 22–23
-
1977Darwinula sp. – Purper: 365, pl. 4, figs. 5–8.
-
∗ 1979Darwinula fragilis Purper, sp. nov. – Purper: 225, pl. 1, figs. 4–10.
-
? 1980Darwinula sp. – Sheppard and Bate: 117–118, pl. 13, fig. 7.
-
1984Darwinula olivencae – Purper: 1371.
-
1998D. fragilis Purper, 1979 – Muñoz-Torres et al.: 90, pl. 1, figs. 1–3.
-
2006D. fragilis Purper, 1979 – Ramos: 89–90, figs. 6a–c.
-
2009D. fragilis Purper, 1979 – Ramos et al.: 116, fig. 305-I.
-
2010Alicenula (Darwinula) fragilis Purper, 1979 – Wesselingh and Ramos: 309, figs. 18.7e–f.
-
1977
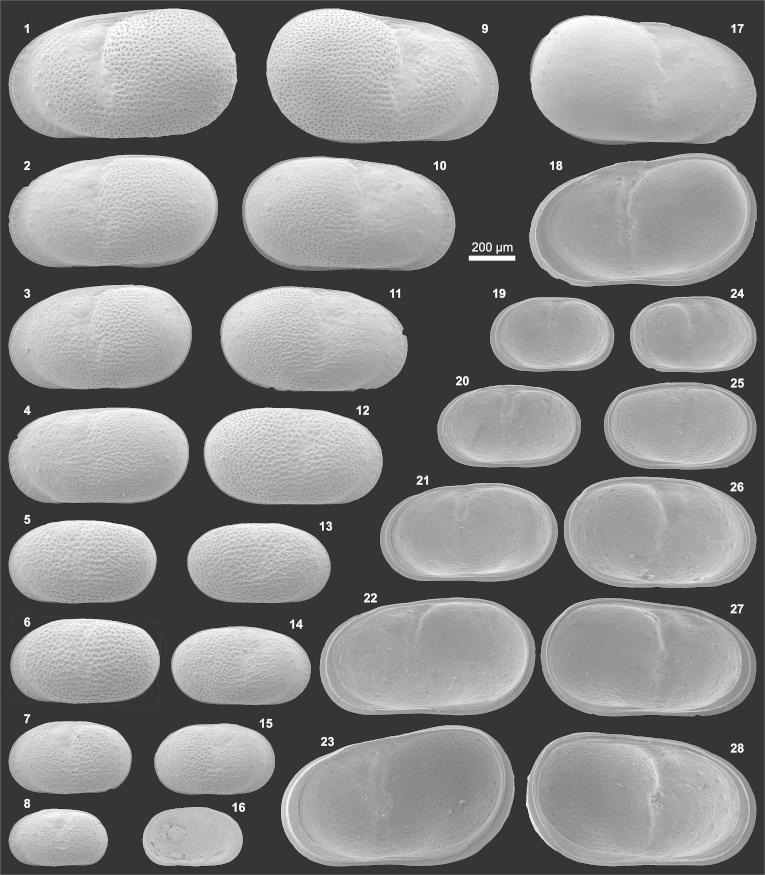
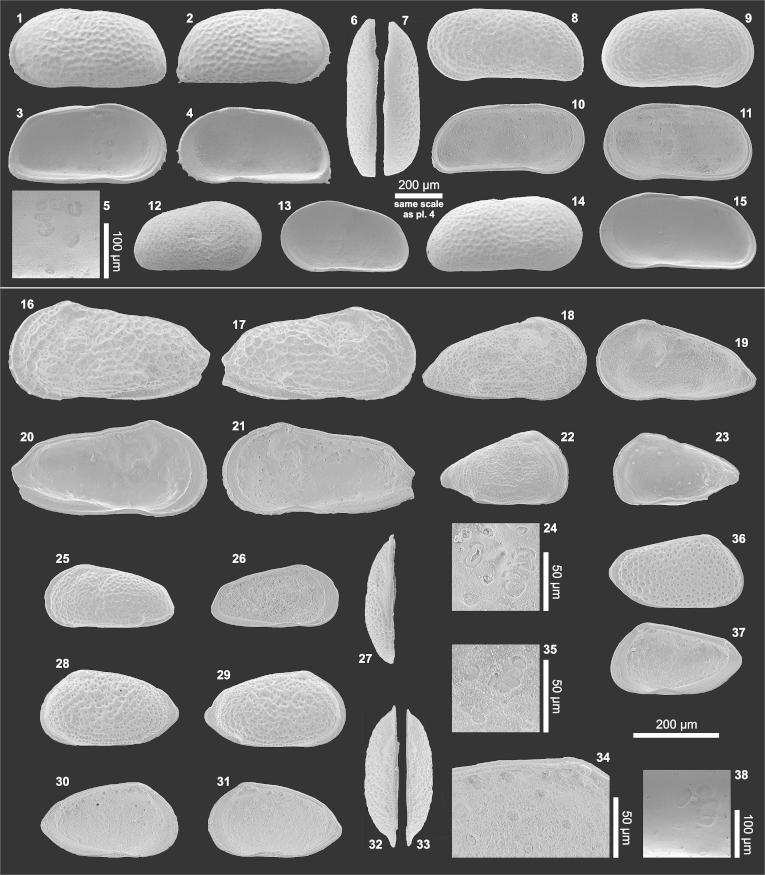
Plate1.
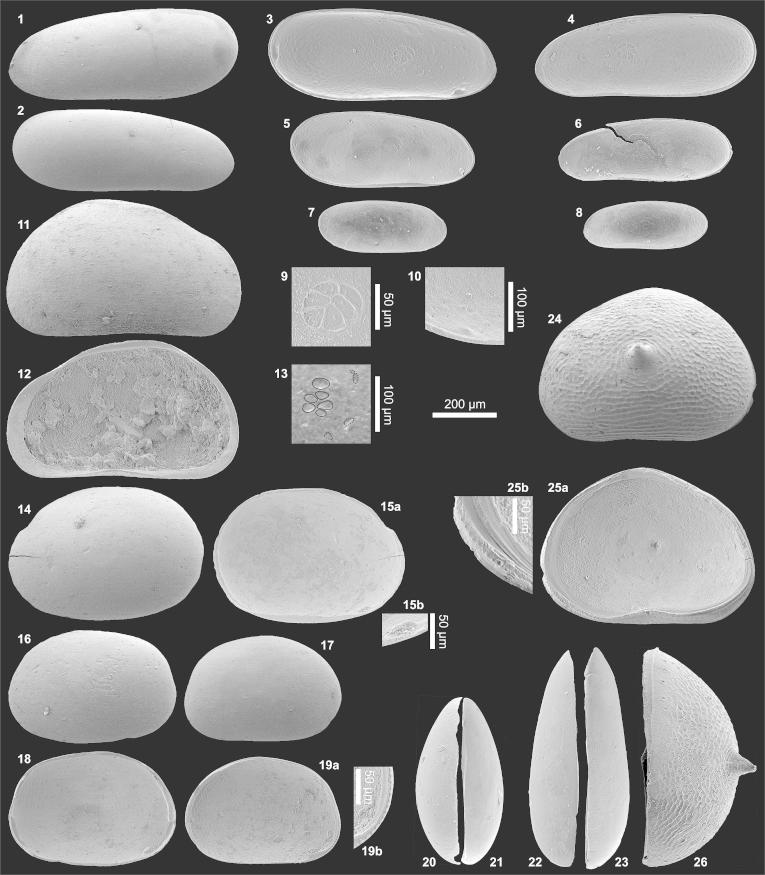
(1–10) Penthesilenula olivencae. (1) Le (0.74/0.30; AQ5-2_69), (2) Re (0.71/0.27; AQ6-2_24R), (3) Li (= fig. 1), (4) Ri (= fig. 2), (5) Li?A-1 (0.58/0.24; AQ19_46), (6) Ri?A-1 (0.54/0.20; AQ19_49), (7) Li?A-2 (0.40/0.17; AQ19_48), (8) Ri?A-2) (0.39/0.15; slightly deformed; AQ19_51), (9) central muscle scars of Li (detail of fig. 3), (10) anteroventral internal tooth in Ri (detail of fig. 4). (11–13) Pseudocandona sp. (11) Re (0.65/0.40; AQ16_02), (12) Ri (= fig. 11), (13) central muscle scars of Re fragment (transmitted light, redrawn; AQ16_03R). (14–15) Cypria sp. (14) Le (0.61/0.43; AQ6-2_25), (15) Li (= fig. 14; 15b: anteroventral “contact knob”. (16–21) Physocypria sp. (16) Le (0.53/0.36; AQ6-2_26), (17) Re (0.50/0.34; AQ8-2_06), (18) Li (= fig. 16), (19) Ri (= fig. 17; 19b: posteroventral marginal tubercles), (20) Ld (= fig. 16), (21) Rd (0.53/0.35; AQ8-2_05). (22–23) Penthesilenula olivencae (22) Ld (0.70/0.28; TO12_02), (23) Rd (0.71/0.27; TO12_04). (24–26) Cypretta sp. (24) Re (0.69/0.50; BA7a_01), (25) Ri (= fig. 24; 25b: nodes at the anteroventral margin), 26) Rd (= fig. 24). (Abbreviations: R = right valve; L = left valve; e = external view; d = dorsal view; i = internal view; ♀ = female; ♂ = male; A-1, … = juvenile stages. In brackets length (l) and height (h) are indicated in millimetres (e.g., 0.74/0.30 = length 0.74 mm, height 0.30 mm; the length of spines is not included) followed by a specimen code (sample number_specimen number; e.g., AQ5-2_69). Sample number = abbreviated location followed by the layer number; e.g., AQ5-2 = Aquidabã, layer number 5-2; AQ = Aquidabã, BA = Barro Branco, MN = Morada Nova, PD = Pau D'Alho, TO = Torre da Lua (Gross et al., 2011)).
Material: 271 adults, 7 juveniles (250 μm sieve fraction), 742 mainly juveniles and some adult fragments (125 μm sieve fraction).
Dimensions: R♀l = 0.67–0.73 (0.69), h = 0.26–0.28 (0.26; n = 7); L♀l = 0.68–0.74 (0.70), h = 0.27–0.30 (0.28; n = 10); R?A-1 l = 0.54, h = 0.20 (n = 1); L?A-1 l = 0.55–0.58, h = 0.23–0.24 (n = 2); R?A-2 l = 0.39, h = 0.15 (n = 1); L?A-2 l = 0.40, h = 0.17 (n = 1).
Remarks: Based on their similar valve morphology (outline, size) the present specimens are assigned to the species D. fragilis of Purper (1979) which she renamed 1984 into D. olivencae according to article 57.2 of ICZN. However, neither descriptions nor figures of the listed publications provide adequate details (especially of internal valve features) for a robust synonymisation. Left valves from Eirunepé exhibit prominent caudal- and anteroventral internal teeth (pl. 1, fig. 3). Such teeth can be anticipated in fig. 6 (plate 1) of Purper (1979) and support the assignment. In our material a very small anteroventral internal tooth is developed in right valves too (pl. 1, fig. 4, 10). There is no posteroventral external keel developed in right valves; a posterior brood pouch is visible.
Currently, six Darwinulidae genera are described (Rossetti and Martens, 1998; Rossetti et al., 2011).
The genus Darwinula Brady & Robertson, 1885 lacks internal teeth in left valves. Microdarwinula Danielopol, 1968 is much smaller and its valves are rounded in lateral view and lack a brood pouch. Thus, both genera are not further considered for the present material.
Juveniles of Vestalenula Rossetti & Martens, 1998 have antero- and posteroventral teeth in left valves (which are absent in adults) and no external posteroventral keel in right valves (which is diagnostic for adults; e.g., Artheau, 2007; Minati et al., 2008; Smith and Kamiya, 2008). Due to the presence of a brooding cavity and the anterocentral position of the central muscle scars, here mentioned specimens represent adult (female) individuals, which are also larger than species of Vestalenula (Rossetti and Martens, 1998). Moreover, the posterior internal tooth of left juvenile valves of Vestalenula is situated posteroventrally and not caudal (Artheau, 2007; Smith and Kamiya, 2008; Minati et al., 2008).
The genus Isabenula Rossetti, Pinto & Martens, 2011 can be excluded due to the presence of a short, external posteroventral keel in right valves as well as it develops only an anteroventral, internal tooth (Rossetti et al., 2011).
The genus Alicenula Rossetti & Martens, 1998 with its elongated valves and internal teeth in left valves (Martens et al., 2003) is similar. Because it was initially described without possessing internal teeth (Ballent and Díaz, 2011), we follow the original diagnosis of Rossetti and Martens (1998), Pinto et al. (2004) and attribute our material to the genus Penthesilenula (diagnostic characters of valves: elongated to sub-squarish in lateral view, absence of an external keel in the right valve, internal teeth in the left valve).
Probably, within Penthesilenula the current specimens belong to the “incae”-group (more elongated valves than the “africana”-group; pointed caudal- and anteroventral internal tooth); the latter feature comparable with the teeth in the recent Penthesilenula aotearoa Rossetti, Eagar & Martens, 1998; see Rossetti and Martens (1998) and Pinto et al. (2004).
In the Eirunepé material, adult P. olivencae specimens are associated with smaller darwinulid valves with comparable outline and central muscle scar pattern. Although internal teeth are missing (a feature which can change during ontogeny; compare Vestalenula), the more postero(dorsal) position of central muscle scars clearly points to juvenile darwinulids, which we consider to represent juveniles of P. olivencae.
Ecology: Darwinulid ostracods live in various freshwater habitats such as lakes, ponds, springs, rivers, as well as in (semi-)terrestrial and interstitial habitats but also in mixohaline environments (e.g., Rossetti and Martens, 1998; Higuti et al., 2009a).
Extant species of Penthesilenula are found in lakes, rivers, swamps, in interstitial and terrestrial habitats (wet leaf litter or moss; Van Doninck et al., 2003; Pinto et al., 2004; Ballent and Díaz, 2011). It is demonstrated experimentally to tolerate a wide salinity range (distilled water to 25 PSU). However, in natural habitats Penthesilenula is usually restricted to salinities <3 PSU and is recorded only rarely in brackish, estuarine/lagoonal environments (e.g., Würdig et al., 1990; Ferrero, 1996; Van Doninck et al., 2003).
Species of the “incae”-group (i.e., Penthesilenula incae (Delachaux, 1928), P. aotearoa) are almost exclusively known from “warm” (>10 °C) freshwater habitats and are especially abundant in lotic habitats with sandy substrate (Van Doninck et al., 2003; Higuti et al., 2009a; Ballent and Díaz, 2011).
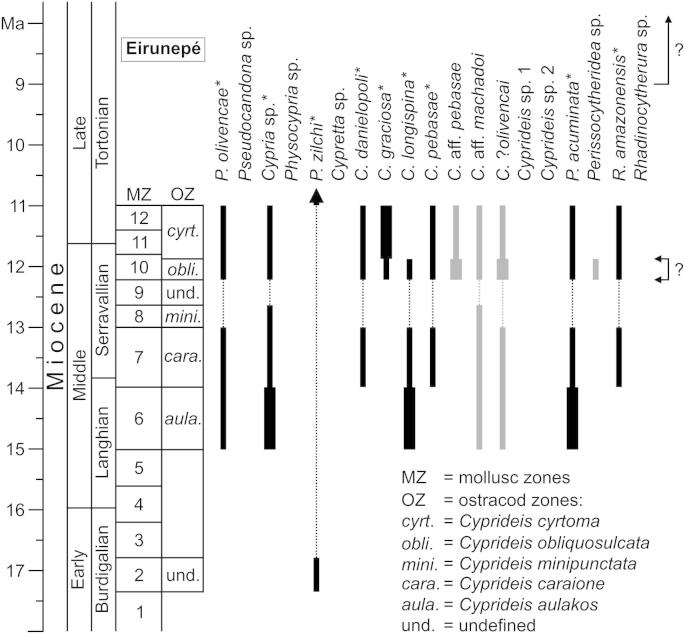
Occurrence: Western Amazonia, early Middle to early Late Miocene (Peru: Cyprideis aulakos–Cyprideis caraione zone; Colombia and Brazil: Cyprideis obliquosulcata–Cyprideis cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work: Aquidabã, Morada Nova, Pau D’Alho, Torre da Lua, Barro Branco.
Superfamily Cypridoidea Baird, 1845
Family Candonidae Kaufmann, 1900
Subfamily Candoninae Kaufmann, 1900
Genus Pseudocandona Kaufmann, 1900
Pseudocandona sp.
- Pl. 1, figs. 11–13
-
2010?Heterocypris sp. – Wesselingh and Ramos: 309, figs. 18.7g–h.
-
2010
Material: 1 right valve, 1 badly preserved carapace, 3 fragments (250 μm sieve fraction).
Dimensions: R l = 0.65, h = 0.40 (n = 1).
Remarks: Only a few specimens are on hand. Internal features are largely obscured by adhering sediment. However, one fragment displays central muscle scars (pl. 1, fig. 13) typical for candonid ostracods, which exclude their allocation to Heterocypris Claus, 1892 (Van Morkhoven, 1963; Meisch, 2000). Small pustules at the anterior and posterior margins of right valves are missing, which are usually developed in Heterocypris (e.g., Purper and Würdig-Maciel, 1974; Meisch, 2000).
Although an assignment to a specific Candoninae genus based solely on similarities of the valve morphology remains tentative, we attribute the current specimens to the candonid genus Pseudocandona. Perhaps, they represent a species of the “compressa”-group (short and stout in lateral view; dorsal margin straight, slightly sloping anteriorly; ventral margin almost straight; Meisch, 2000; Namiotko and Danielopol, 2004).
Ecology: Candonids are mainly found in freshwater habitats (e.g., pools, lakes, swampy habitats) but can tolerate slightly saline (brackish) waters (Van Morkhoven, 1963). Species of the Pseudocandona “compressa”-group prefer shallow freshwater environments but are recorded from oligo- to mesohaline settings too (Meisch, 2000).
Occurrence: Thus far, Candoninae are only recorded by Wesselingh and Ramos (2010) and Linhares et al. (2011) from the Neogene of western Amazonia. This work: Aquidabã.
Subfamily Cyclocypridinae Kaufmann, 1900
Genus Cypria Zenker, 1854
Cypria sp.
- Pl. 1, figs. 14, 15a–b
-
? 1998Cypria aqualica Sheppard & Bate, 1980 – Muñoz-Torres et al.: 91, pl. 1, figs. 4–6.
-
? 1998
Material: 33 mainly fragmented valves (250 μm sieve fraction).
Dimensions: L l = 0.61–0.67 (0.65), h = 0.43–0.46 (0.44; n = 3).
Remarks: These specimens are placed in Cypria due their laterally compressed, sub-circular valves and the presence of one anteroventral “contact-knob” in left valves (pl. 1, figs. 15a–b; Van Morkhoven, 1963; Malz, 1977; Meisch, 2000).
The only formally described species of Cypria in the Neogene of western Amazonia is C. aqualica, which differs due its almost straight ventral margin for the current form (Sheppard and Bate, 1980). Usually, the attribution of Cypria valves to that species is based on very few specimens (Purper, 1979; Sheppard and Bate, 1980; Ramos, 2006; Ramos et al., 2009; Wesselingh and Ramos, 2010). Thus, little is known about intraspecific variability or ontogenetic stages (note that Muñoz-Torres et al., 1998 suggested the type-material of Sheppard and Bate (1980) to represent juveniles). Equally, the present investigation is founded on scarce, largely fragmented and possibly juvenile valves, which hampers species identification. Although the specimens of Muñoz-Torres et al. (1998) are significantly larger, they are similar to the material from Eirunepé. With reservation we synonymise the herein mentioned specimens with the material from Muñoz-Torres et al. (1998).
Physocypria sp. (see below) differs in being smaller and having a more truncate posterior and almost straight dorsal margin. Moreover, right valves bear generotypical, marginal tubercles antero- and posteroventrally (pl. 1, figs. 19a–b; Van Morkhoven, 1963; Meisch, 2000).
Ecology: Cypria and Physocypria are active swimmers occurring in freshwater habitats like ponds and lakes but can be found also in oligo- to mesohaline waters (Van Morkhoven, 1963; Hartmann, 1989; Meisch, 2000).
Occurrence (of C. aqualica): Western Amazonia, early Middle to early Late Miocene (Peru: C. aulakos–Cyprideis minipunctata zone; Colombia and Brazil: C. obliquosulcata–C. cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work (Cypria sp.): Aquidabã, Morada Nova, Pau D’Alho, Torre da Lua, Barro Branco.
Genus Physocypria Vávra, 1897
Physocypria sp.
Pl. 1, figs. 16–21
Material: 11 mainly fragmented valves (250 μm sieve fraction).
Dimensions: R l = 0.50–0.54 (0.52), h = 0.32–0.35 (0.34; n = 5); L l = 0.53–0.54, h = 0.34–0.36 (n = 2).
Remarks: This is the first record of the genus Physocypria from the Solimões Formation. More material is necessary for further taxonomic analyses. Perhaps, the present material comprises juvenile valves only.
Ecology: see Cypria.
Occurrence (this work): Aquidabã, Torre da Lua.
Family Ilyocyprididae Kaufmann, 1900
Subfamily Pelocypridinae Triebel, 1962
Genus Pelocypris Klie, 1939
Pelocypris zilchi Triebel, 1953
- Pl. 2, figs. 1–13
-
∗ 1953P. zilchi n. sp. – Triebel: 2–4, pl. 1, figs. 1–8.
-
1980P. zilchi Triebel, 1953 – Sheppard and Bate: 104–106, text-fig. 3; pl. 10, figs. 8–13.
-
1980Pelocypris sp. – Sheppard and Bate: 108, pl. 10, fig. 14.
-
2010Ilyocypris (Pelocypris) zilchi (Triebel, 1953) – Wesselingh and Ramos: 309, fig. 18.7c.
-
∗ 1953
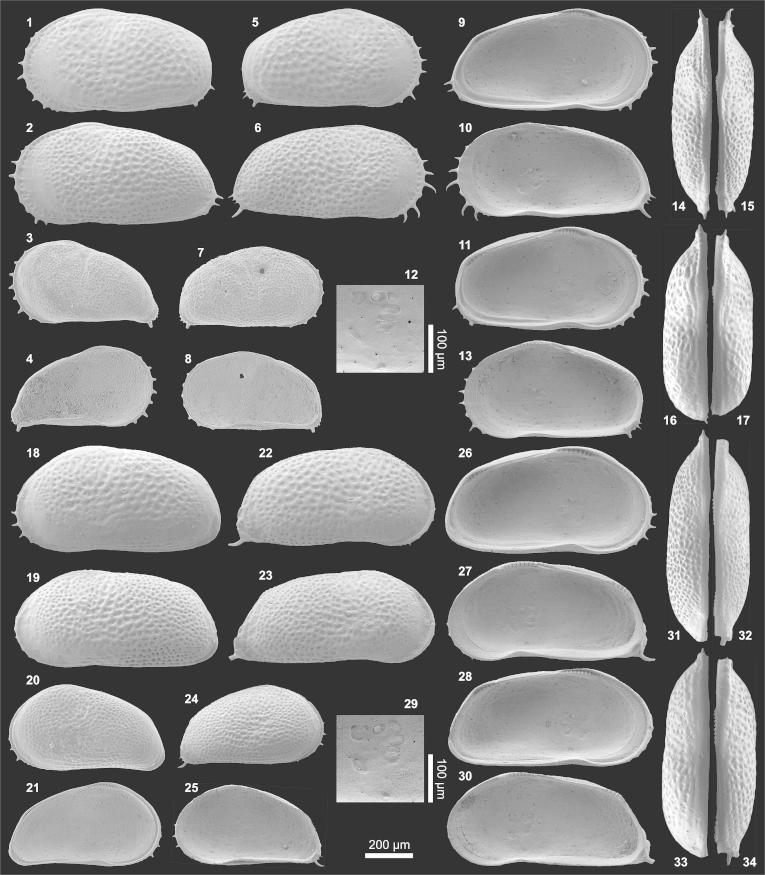
Plate 2.
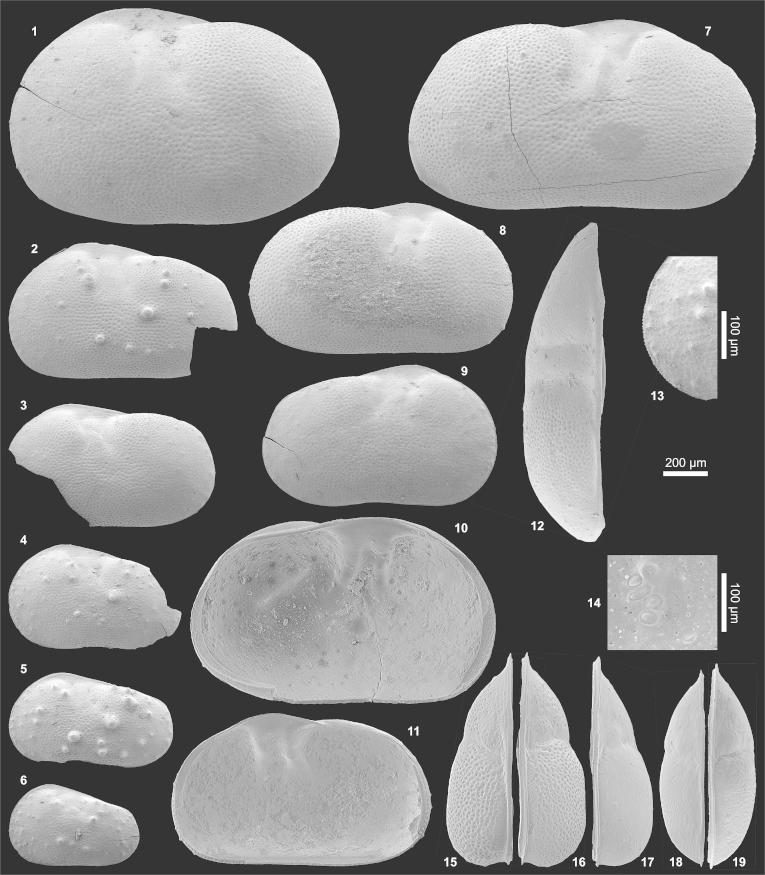
(1–13) Pelocypris zilchi (1) Le?♀ (1.55/1.03; AQ8-2_12), (2) Le?A-2 (1.07/0.64; AQ19_34), (3) Le?A-2 (0.97/0.57; AQ19_35), (4) Le?A-3 (0.80/0.49; AQ8-2_09), (5) Le?A-4 (0.76/0.42; AQ8-2_10), (6) Le?A-5 (0.60/0.38; AQ8-2_11), (7) Re?♂ (1.62/0.90; AQ19_42), (8) Re?A-1 (1.25/0.72; AQ19_43), (9) Re?A-2 (1.11/0.67; AQ19_45), (10) Li?♀ (1.45/0.88; AQ8-2_13), (11) Ri?A-1 (= fig. 8), (12) Ld?♀ (= fig. 1), (13) Le?A-4 (= fig. 5; anterior marginal denticles). (14–19) Cytheridella danielopoli (14) central muscle scars of Li♂ (0.88/0.47; TO12_05), (15) Ld♀ (1.01/0.59; AQ19_01), (16) Rd♀ (1.03/0.61; AQ19_37), (17) Rd♀ (1.00/0.58; AQ5-2_64), (18) Ld♂ (0.94/0.51; AQ19_09), (19) Rd♂ (0.95/0.51; AQ19_23). (For abbreviations see plate 1).
Material: 8 adults, 15 juveniles (both mainly fragmented; 250 μm sieve fraction).
Dimensions: R?♂ l = 1.62, h = 0.90 (n = 1); L?♀ l = 1.45–1.55 (n = 2), h = 0.88–1.03 (0.97; n = 3); R?A-1 l = 1.17–1.25, h = 0.72 (n = 2); R?A-2 l = 1.11, h = 0.67 (n = 1); R?A-3 l = 0.85 (broken), h = 0.56 (n = 1); L?A-2 l = 0.97–1.07 (all broken), h = 0.57–0.64 (0.61; n = 3); L?A-3 l = 0.80 (broken), h = 0.49 (n = 1); L?A-4 l = 0.76, h = 0.42 (n = 1); L?A-5 l = 0.58–0.60 (n = 2), h = 0.38 (n = 1).
Remarks: These ilyocypridid ostracods clearly belong to Pelocypris (generic characters of the valve: the bifurcate sulcus does not reach the central muscle scar area); it lacks central depressions corresponding to central muscle scars; at the anterior margin an inner list is missing (Tressler, 1949; Triebel, 1953; Lister, 1975; Hartmann, 1989).
Within the current material noded as well as un-noded instars co-occur, while this feature is not observed on adult valves. These tubercles correspond to normal pore canal openings. The position of the main tubuli respectively pores can be traced through different ontogenetic stages up to adult specimens with their only weakly developed pore tubuli. By considering the large ecophenotypic variability of tubercles in other ilyocypridids (Ilyocypris; Meisch, 2000; Yang et al., 2002), the noded specimen of Sheppard and Bate (1980: Pelocypris sp.) is included in the synonymy here. Different ecological parameters are discussed to be related to the presence/absence of nodes in Ilyocypris (e.g., temperature, salinity, locomotion; Van Harten, 1979; Yang et al., 2002) and might play a comparable role in Pelocypris.
So-called marginal denticles are well-developed along the free valve margin of right adult valves of P. zilchi but are lacking in left ones (Triebel, 1953; Sheppard and Bate, 1980). However, tiny denticles are present in left juvenile valves of the Eirunepé material too (pl. 2, fig. 5, 13). Due to limited material a discussion of the validity of the species-diagnostic criteria for P. zilchi and its delineation from the evidently related recent Pelocypris lenzi Klie, 1939 is left open for subsequent investigations (differential diagnosis to P. lenzi: P. zilchi is smaller, has a better developed ornamentation, shorter pore conuli and marginal denticles only in right valves; Triebel, 1953). The differentiation of different sexes and ontogenetic stages is provisional because of the lack of sufficient specimens (some are additionally fragmented).
Ecology: Pelocypris is considered to inhabit freshwater settings like streams and not saline playa lakes (Sheppard and Bate, 1980; Hartmann, 1989; Horne, 1996; Reheis et al., 2005).
Occurrence: Western Amazonia, Early Miocene (La Tagua; Sheppard and Bate, 1980; mollusc zone 2 according to Wesselingh et al. (2006b); chronostratigraphic correlation after Wesselingh and Ramos, 2010); El Salvador, Pleistocene (Barranca El Sisimico; Triebel, 1953; see Cisneros, 2005 for age). This work: Aquidabã.
Family Cyprididae Baird, 1845
Subfamily Cyprettinae Hartmann, 1971
Genus Cypretta Vávra, 1895
Cypretta sp.
Pl. 1, figs. 24–26
Material: 1 right valve (250 μm sieve fraction).
Dimensions: R l = 0.69, h = 0.50 (n = 1).
Remarks: One single, subovate, finely reticulated right valve with a remarkable central tubercle was found. It largely coincides with the diagnostic features of the genus Cypretta (e.g., Furtos, 1934; Sohn and Kornicker, 1973; Victor and Fernando, 1981). Unfortunately, the valve is not translucent enough to proof the presence of diagnostic septa along the anterior margin. Because only one valve is available, we avoided to fracture the valve for studying the marginal valve structure. However, small nodes are developed between the flange and the selvage along the anterior and posteroventral margin.
This specimen resembles extant Cypretta brevisaepta Furtos, 1934, a species with reverse valve overlap (left over right overlap), which is visible here likewise (e.g., Keyser, 1976; Smith and Delorme, 2010; for discussions on the taxonomical value of this feature see: Sohn and Kornicker, 1973; Keyser, 1976; Victor and Fernando, 1981; Holmes, 1998).
Ecology: Cypretta is an actively swimming, freshwater ostracod, which is rarely found in oligohaline environments. It is recorded mainly from tropical and subtropical climates (Sohn and Kornicker, 1973; Keyser, 1976; Holmes, 1998; Pérez et al., 2010a,b).
Occurrence (this work): Barro Branco.
Superfamily Cytheroidea Baird, 1850
Family Limnocytheridae Klie, 1938
Subfamily Timiriaseviinae Mandelstam, 1960
Genus Cytheridella Daday, 1905
Cytheridella danielopoli Purper, 1979
- Fig. 2; pl. 2, figs. 14–19; pl. 3, figs. 1–28
-
1977Cytheridella sp.nov. A – Purper: 365, pl. 4, figs. 1–4.
-
* 1979C. danielopoli Purper, sp. nov. – Purper: 243–244, pl. 7, figs. 21–27.
-
1980Cytheridella postornata sp. nov. – Sheppard and Bate: 108–110, pl. 10, figs. 1–7.
-
1998C. danielopoli Purper, 1979 – Muñoz-Torres et al.: 104, pl. 6, figs. 13–14.
-
2006Cytheridella purperae sp. nov. – Ramos: 92–93, figs. 7o–v.
-
2006Cytheridella sp. – Ramos: 93–94, figs. 7x–y.
-
2009Cytheridella sp. – Ramos et al.: 116, figs. 299–300-I.
-
2009C. purperae Ramos, 2006 – Ramos et al.: 116, figs. 301–304-I.
-
2010C. purperai [sic!] Ramos, 2006 – Wesselingh and Ramos: 309, figs. 18.7a–b.
-
1977
Fig. 2.
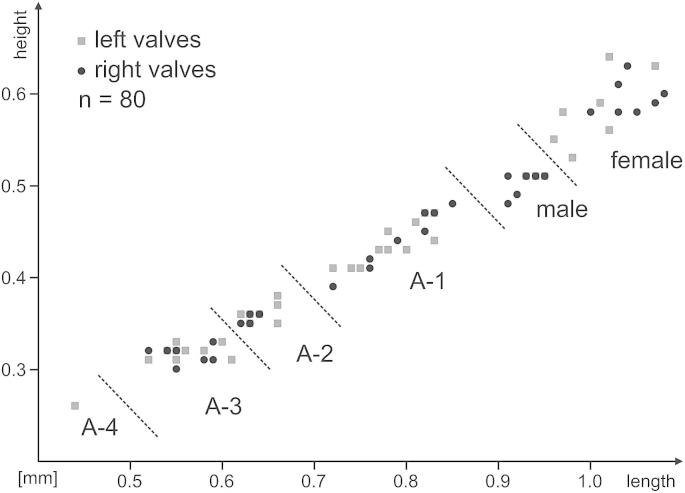
Length/height diagram for Cytheridella danielopoli.
Plate 3.
(1–28) Cytheridella danielopoli (1) Le♀ (1.01/0.59; AQ19_01 = pl. 2, fig. 15), (2) Le♂ (0.93/0.51AQ19_07), (3) LeA-1 (0.81/0.46; AQ5-2_51), (4) LeA-1 (0.80/0.43; AQ5-2_50), (5) LeA-2 (0.66/0.37; TO12_11; forma purperae), (6) LeA-2 (0.66/0.38; AQ5-2_53L), (7) LeA-3 (0.55/0.33; AQ19_14), (8) LeA-4 (0.44/0.26; AQ5-2_58), (9) Re♀ (1.03/0.61; AQ19_37 = pl. 2, fig. 16), (10) Re♂ (0.95/0.51; AQ19_23 = pl. 2, fig. 19), (11) ReA-1 (0.82/0.47; AQ5-2_59), (12) ReA-1 (0.79/0.44; AQ5-2_60; forma purperae), (13) ReA-2 (0.64/0.36; AQ19_25; forma purperae), (14) ReA-2 (0.62/0.35; TO12_21), (15) ReA-3 (0.54/0.32; AQ19_29), (16) LiA-4 (= fig. 8), (17) Re♀ (1.00/0.58; AQ5-2_64 = pl. 2, fig. 17), (18) Ri♀ (= fig. 17), (19) RiA-3 (= fig. 15), (20) RiA-2 (= fig. 14), (21) RiA-1 (0.76/0.42; AQ5-2_62), (22) Ri♂ (= fig. 10), (23) Ri♀ (= fig. 9), (24) LiA-3 (= fig. 7), (25) LiA-2 (= fig. 5), (26) LiA-1 (0.82/0.47; AQ5-2_49), (27) Li♂ (0.94/0.51; AQ19_09 = pl. 2, fig. 18), (28) Li♀ (= fig. 1). (For abbreviations see plate 1).
Material: 131 adults, 1863 juveniles (250 μm sieve fraction), 176 juveniles and fragments (125 μm sieve fraction).
Dimensions: R♀l = 1.00–1.08 (1,00), h = 0.58–0.63 (0,57; n = 7); L♀l = 0.96–1.07 (1.01), h = 0.53–0.64 (0.58; n = 7); R♂l = 0.91–0.95 (0.92), h = 0.48–0.51 (0.50; n = 7); L♂l = 0.93–0.95 (0.94), h = 0.51–0.53 (0.51; n = 4); RA-1 l = 0.72–0.85 (0.79), h = 0.39–0.48 (0.44; n = 10); LA-1 l = 0.72–0.83 (0.78), h = 0.41–0.47 (0.44; n = 11); RA-2 l = 0.62–0.64 (0.63), h = 0.35–0.36 (0.35; n = 7); LA-2 l = 0.62–0.66 (0.64), h = 0.35–0.38 (0.36; n = 9); RA-3 l = 0.52–0.59 (0.56), h = 0.30–0.33 (0.32; n = 7); LA-3 l = 0.52–0.61 (0.56), h = 0.31–0.33 (0.32; n = 11); LA-4 l = 0.44, h = 0.26 (n = 1).
Remarks: Females of C. danielopoli differ from males due to their larger size and their well expressed brood pouch (compare Purper, 1974; Pérez et al., 2010a; pl. 2, figs. 15–19). The valve surface is usually pitted, which is less pronounced along the anterior margin and at the dorsomedian sulcus. Nevertheless, some adult specimens are almost smooth and display a faint reticulation only (pl. 3, fig. 17). Beside ornamentation there are no additional differences, neither in shape nor in internal features; the position of pore tubuli is strikingly equivalent. Also within instars the degree of ornamentation and the expression of sulci vary (pl. 3, figs. 5, 12, 13).
The current material almost match with the type-specimens of Purper (1979), which are somewhat smaller, a little bit stronger punctated and their dorsal margin is slightly less inclined towards anterior. Based on the observed variability in ornamentation and outline the Eirunepé material is conspecific with C. danielopoli. C. postornata of Sheppard and Bate (1980), which has a smooth valve surface anterior and a pitted ornamentation posterior of the sulcus, is here considered to be a junior synonym of that species (compare van den Bold, 1986). C. danielopoli of Muñoz-Torres et al. (1998) belongs to A-1 instars as already suggested by these authors. Further, C. purperae of Ramos (2006; compare Ramos et al., 2009; Wesselingh and Ramos, 2010) is based on juvenile valves (probably A-2) and range within the intraspecific variability of C. danielopoli (pl. 3, figs. 5, 12, 13). Likewise, Cytheridella sp. from Ramos (2006), Ramos et al. (2009) represents the smooth morphotype of C. danielopoli.
The type-species of Cytheridella, Cytheridella ilosvayi Daday, 1905, is very similar to C. danielopoli and belongs to the same lineage (for C. ilosvayi see: Löffler, 1961; Purper, 1974; Pérez et al., 2010a; Smith and Delorme, 2010). Differences to other species are discussed in Purper (1979) and Sheppard and Bate (1980).
Ecology: Cytheridella is almost exclusively restricted to freshwater environments (Purper, 1974; Colin et al., 1997). Extant C. ilosvayi is found in shallow (<40 m), permanent, freshwater to slightly saline (<3.2 PSU) waters in subtropical/tropical (>20 °C) climates (Alvarez Zarikian et al., 2005; Pérez et al., 2010a,b).
Occurrence: Western Amazonia, late Middle to early Late Miocene (Peru: C. caraione zone; Colombia and Brazil: C. obliquosulcata–C. cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work: Aquidabã, Pau D’Alho, Torre da Lua, Barro Branco.
Family Cytherideidae Sars, 1925
Subfamily Cytherideinae Sars, 1925
Genus Cyprideis Jones, 1857
Cyprideis graciosa (Purper, 1979)
- Pl. 4, figs. 1–17
-
1977Cytheridea sp. nov. D – Purper: 363, pl. 3, figs. 5–6.
-
∗ 1979C. graciosa Purper, sp. nov. – Purper: 229–230, pl. 3, figs. 1–9.
-
1991C. graciosa Purper, 1979 – Purper and Ornellas: 26–28, pl. 1, figs. 10–15.
-
? 1998C. graciosa (Purper, 1979) – Muñoz-Torres et al.: 96, pl. 3, figs. 1–3.
-
? 1998C. graciosa (Purper, 1979) – Whatley et al.: 234, text-fig. 2; pl. 1, figs. 11–15.
-
2006C. graciosa Purper, 1979 – Ramos: 92, figs. 7d–h.
-
2009C. graciosa Purper, 1979 – Ramos et al.: 114, fig. 289-I.
-
2010C. graciosa Purper, 1979 – Wesselingh and Ramos: 308, figs. 18.5e–f.
-
? 2011C. graciosa – Linhares et al: 96, fig. 3/9–10.
-
1977
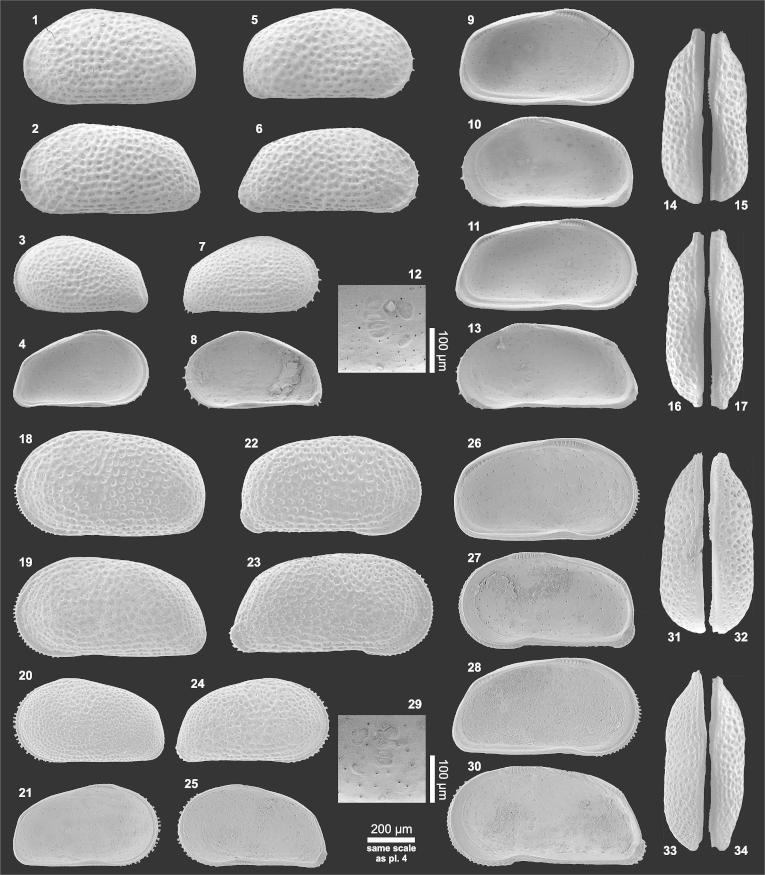
Plate 4.
(1–17) Cyprideis graciosa (1) Le♀ (0.82/0.46; MN14_08), (2) Le♂ (0.88/0.45; MN14_06), (3) LeA-1 (0.61/0.35; PD25_04), (4) LiA-1 (= fig. 3), (5) Re♀ (0.79/0.44; MN14_04), (6) Re♂ (0.84/0.41; MN14_02), (7) ReA-1 (0.61/0.34; PD25_07), (8) RiA-1 (= fig. 7), (9) Li♂ (= fig. 2), (10) Ri♂ (= fig. 6), (11) Li♀ (= fig. 1), (12) central muscle scars of Ri (detail of fig. 13), (13) Ri♀ (= fig. 5), (14) Ld♂ (0.86/0.44; MN14_07), (15) Rd♂ (= fig. 6), (16) Ld♀ (= fig. 1), (17) Rd♀ (= fig. 5). (18–34) Cyprideis longispina (18) Le♀ (0.90/0.48; AQ5-2_01), (19) Le♂ (0.89/0.43; AQ5-2_06), (20) LeA-1 (0.66/0.36; TO12_37), (21) LiA-1 (= fig. 20), (22) Re♀ (0.87/0.44; AQ5-2_12), (23) Re♂ (0.89/0.41; AQ5-2_17), (24) ReA-1 (0.64/0.35; AQ5-2_21), (25) RiA-1 (= fig. 24), (26) Li♀ (= fig. 1), (27) Ri♀ (= fig. 22), (28) Li♂ (= fig. 19), (29) central muscle scars of Ri♂ (0.87/0.40; AQ5-2_16), (30) Ri♂ (= fig. 23), (31) Ld♂ (0.89/0.42; AQ6-2_02), (32) Rd♂ (= fig. 23), (33) Ld♀ (= fig. 18), (34) Rd♀ (= fig. 22). (For abbreviations see plate 1).
Material: 149 adults, 84 juveniles (250 μm sieve fraction).
Dimensions: R♀l = 0.77–0.79 (0.78), h = 0.42–0.44 (0.43; n = 5); L♀l = 0.81–0.83 (0.82), h = 0.46–0.47 (0.46; n = 6); R♂l = 0.82–0.84 (0.83), h = 0.41–0.42 (0.41; n = 5); L♂l = 0.86–0.88 (0.87), h = 0.44–0.45 (0.45; n = 5); RA-1 l = 0.60–0.61, h = 0.34–0.35 (n = 2); LA-1 l = 0.61–0.62 (0.61), h = 0.34–0.35 (0.35; n = 3).
Remarks: The present material matches with the original description of Purper (1979; Purper, 1977) as well as with the specimens documented by Purper and Ornellas (1991) and Wesselingh and Ramos (2010). C. graciosa of Muñoz-Torres et al. (1998; Whatley et al., 1998) is considerably larger. The posterior margin of males in these publications is more rounded and females are posterior higher. Posteroventral spines are not visible in left valves of these specimens, which are a characteristic feature according to Purper (1979). However, such spines were mentioned in the diagnosis of Whatley et al. (1998). To date, it is not clear if these differences range within the intraspecific variability of this species. C. graciosa, discussed by Ramos (2006; Ramos et al., 2009), is noticeably smaller. The figured carapace of a female (Ramos, 2006, figs. 7f–h) has a triangular shape and possibly represents a juvenile individual.
C. graciosa is similar to C. longispina (pl. 4, figs. 18–34). Nonetheless, adults of C. longispina bear posteroventral spines (generally one spine) in the right valves only. The most ventrally located and strongest one of these spines originates higher up on the flange and is more horizontally directed than in C. graciosa. Posteroventrally, C. longispina develops a significantly extended flange in right valves, which is missing in C. graciosa. Anterior spines are restricted to the lower half of the anterior margin and the anterior hinge element is longer and narrower than in C. graciosa. Whereas in C. graciosa the anteroventral and central anterior surface of the valve is usually smooth, in C. longispina the ornament is reduced in the anteroventral area only. In dorsal view the anterior portion is slightly more tapered in C. graciosa.
Juveniles of C. graciosa and C. longispina can be distinguished by the development of marginal spines, which is equivalent to adult valves (note: in juveniles of C. longispina up to three posteroventral spines are observed in right valves). Similar to adult specimens the anterior hinge element is more elongated in C. longispina-instars than in juveniles of C. graciosa.
Ecology: The genus Cyprideis is a holoeuryhaline (freshwater–hypersaline), pandemic ostracod genus; however, most commonly found in shallow, oligo- and mesohaline (brackish) water environments (e.g., lakes, lagoons, estuaries, salt marshes). It is able to cope with highly fluctuating salinities, aberrant water chemistries, variable temperatures and oxygenation. Cyprideis is a bisexually reproducing taxon with internal brood-care, which makes it susceptible to withstand unstable conditions and to passive dispersal (e.g., Sandberg, 1964; Jahn et al., 1996; De Deckker et al., 1999; Meisch, 2000; Frenzel and Boomer, 2005; Gross et al., 2008; and references therein).
Occurrence: Western Amazonia, latest Middle to early Late Miocene (Colombia and Brazil: C. obliquosulcata–C. cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). Linhares et al. (2011) recorded C. graciosa also in the caraione zone. This work: Remanso, Morada Nova, Pau D'Alho, Torre da Lua, Barro Branco.
Cyprideis longispina (Purper, 1979)
- Pl. 4, figs. 18–34
-
∗ 1979C. longispina Purper, sp. nov. – Purper: 230–231, pl. 3, figs. 10–21.
-
1998C. longispina (Purper, 1979) – Muñoz-Torres et al.: 96–98, pl. 3, figs. 12–14.
-
1998C. longispina (Purper, 1979) – Whatley et al.: 235, text-fig. 2; pl. 1, figs. 21–25.
-
2006C. longispina (Purper, 1979) Whatley et al., 1998 – Ramos: 92, figs. 7i–n.
-
2009C. longispina (Purper, 1979) – Ramos et al.: 114, figs. 291–294-I.
-
2010C. longispina (Purper, 1979) – Wesselingh and Ramos: 308, figs. 18.5a, b.
-
∗ 1979
Material: 133 adults, 117 juveniles (250 μm sieve fraction).
Dimensions: R♀l = 0.84–0.88 (0.86), h = 0.43–0.45 (0.44; n = 7); L♀l = 0.87–0.90 (0.88), h = 0.46–0.48 (0.47; n = 5); R♂ l = 0.85–0.89 (0.87), h = 0.40–0.41 (0.41; n = 4); L♂l = 0.89–0.91 (0.89), h = 0.42–0.44 (0.43; n = 5); RA-1 l = 0.61–0.65 (0.63), h = 0.34–0.36 (0.35; n = 10); LA-1 l = 0.63–0.68 (0.66), h = 0.35–0.39 (0.36; n = 5).
Remarks: The present material largely coincides with the given synonyms. Purper (1979) mentioned that males differ from females by the presence of a set of four posteroventral spines in right valves. In our adult specimens, regularly we found one spine occurring equally in both sexes. Nevertheless, right juvenile valves with up to three posteroventral spines were observed. C. longispina of Muñoz-Torres et al. (1998; compare Whatley et al., 1998) is somewhat larger and females are less elongated in outline in lateral view than the material from the Eirunepé region. The specimens of Ramos (2006) are slightly more elongated. Differences to C. graciosa are discussed above.
Ecology: see C. graciosa.
Occurrence: Western Amazonia, early to latest Middle Miocene (Peru: C. aulakos–C. caraione zone; Colombia and Brazil: C. obliquosulcata zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work: Aquidabã, Morada Nova, Torre da Lua, Barro Branco.
Cyprideis pebasae (Purper, 1979)
- Pl. 5, figs. 1–17
-
1977Cytheridea sp. nov. C – Purper: 363, pl. 3, figs. 1–4.
-
∗ 1979C. pebasae Purper, sp. nov. – Purper: 228–229, pl. 2, figs. 11–23.
-
? 1980Cyprideis purperi colombiaensis subsp. nov. – Sheppard and Bate: 101, pl. 8, figs. 3–9.
-
non 1998C. pebasae (Purper, 1979) – Muñoz-Torres et al.: 100, pl. 4, figs. 8–10.
-
non 1998C. pebasae (Purper, 1979) – Whatley et al.: 236, text-fig. 2; pl. 2, figs. 16–20.
-
? 1998Cyprideis lacrimata sp. nov. – Muñoz-Torres et al.: 96, text-fig. 2; pl. 3, figs. 7–11.
-
pars 2006C. pebasae (Purper, 1979) Whatley et al., 1998 emend. – Ramos: 90–91, figs. 6i–y. [non figs. 6e–h]
-
2010C. pebasae (Purper, 1979) – Wesselingh and Ramos: 308, figs. 18.5c, d.
-
1977
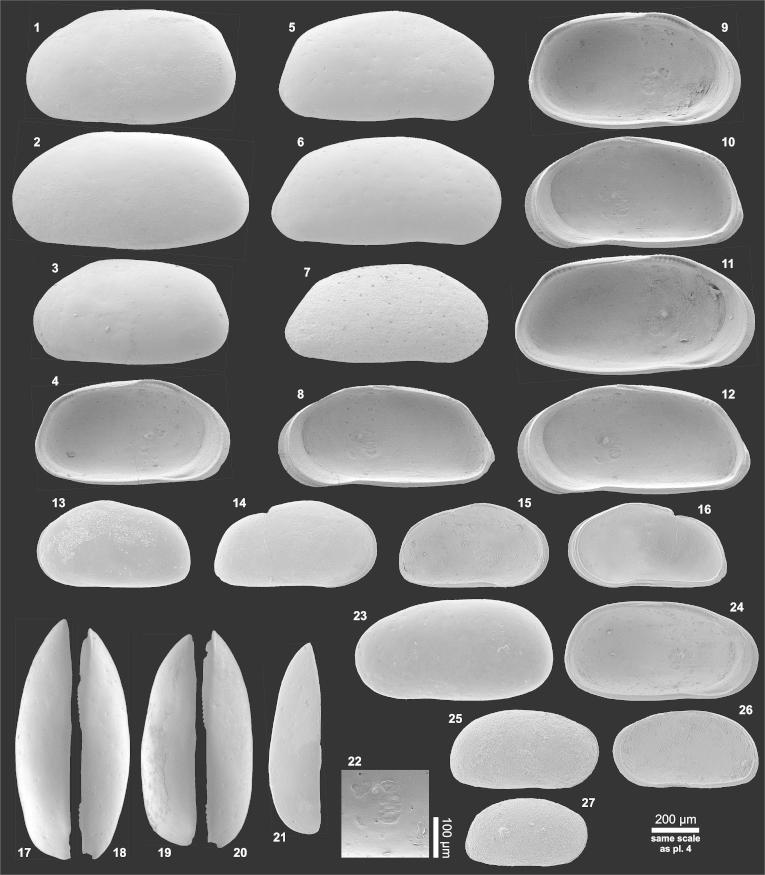
Plate 5.
(1–17) Cyprideis pebasae (1) Le♀ (0.75/0.43; AQ5-2_26), (2) Le♂ (0.78/0.40; AQ5-2_30), (3) LeA-1 (0.59/0.34; AQ5-2_32), (4) LiA-1 (= fig. 3), (5) Re♀ (0.73/0.41; AQ5-2_37), (6) Re♂ (0.78/0.37; AQ5-2_41), (7) ReA-1 (0.60/0.34; AQ5-2_44), (8) RiA-1 (= fig. 7), (9) Li♀ (= fig. 1), (10) Ri♀ (= fig. 5), (11) Li♂ (= fig. 2), (12) central muscle scars of Li♂ (0.82/0.42; MN14_10), (13) Ri♂ (= fig. 6), (14) Ld♀ (= fig. 1), (15) Rd♀ (= fig. 5), (16) Ld♂ (= fig. 2), (17) Rd♂ (= fig. 6). (18–34) Cyprideis aff. pebasae (18) Le♀ (0.80/0.44; TO12_29), (19) Le♂ (0.81/0.42; TO10_09), (20) LeA-1 (0.63/0.35; TO12_30), (21) LiA-1 (= fig. 20), (22) Re♀ (0.76/0.42; TO12_32), (23) Re♂ (0.86/0.43; TO12_33), (24) ReA-1 (0.64/0.36; TO12_35), (25) RiA-1 (= fig. 24), (26) Li♀ (= fig. 18), (27) Ri♀ (= fig. 22), (28) Li♂ (= fig. 19), (29) central muscle scars of Ri♂ (0.77/0.39; MN9_11), (30) Ri♂ (= fig. 23), (31) Ld♀ (= fig. 18), (32) Rd♀ (= fig. 22), (33) Ld♂ (= fig. 19), (34) Rd♂ (= fig. 29). (For abbreviations see plate 1).
Material: 521 adults, 260 juveniles (250 μm sieve fraction).
Dimensions: R♀l = 0.66–0.74 (0.71), h = 0.36–0.41 (0.38; n = 11); L♀l = 0.71–0.80 (0.75), h = 0.39–0.43 (0.41; n = 7); R♂l = 0.75–0.79 (0.77), h = 0.36–0.38 (0.37; n = 8); L♂l = 0.74–0.84 (0.79), h = 0.38–0.44 (0.40; n = 15); RA-1 l = 0.57–0.60 (0.58), h = 0.33–0.34 (0.34; n = 5); LA-1 l = 0.55–0.61 (0.59), h = 0.33–0.36 (0.34; n = 12).
Remarks: C. pebasae of Muñoz-Torres et al. (1998), compare Whatley et al. (1998) is obviously closely related to the original material of Purper (1977, 1979). However, those specimens exhibit numerous, small denticles along the anterior margin, which is in contrast to the species description of Purper (1979: “about seven spines”). Further, the flange along the anterior margin and at the posteroventral corner is thinner than in C. pebasae (Purper, 1979). For these reasons we believe that C. pebasae of Muñoz-Torres et al. (1998; see Whatley et al., 1998) is not identical with C. pebasae (Purper, 1979). Consequently, only the specimens with a “well pronounced anterior and ventroposterior marginal border” as well as with a “coarsely spinose” flange of Ramos (2006: figs. 6i–y) are supposed to belong to C. pebasae.
C. lacrimata of Muñoz-Torres et al. (1998) is very similar to the present species but differs by its more rectangular outline (“truncated posterior margin”) and its slightly coarser ornamentation along the free valve margin. Apparently, C. lacrimata of Muñoz-Torres et al. (1998) and C. pebasae (Purper, 1979) are very closely related or are even synonyms.
C. purperi colombiaensisSheppard and Bate, 1980 resembles C. pebasae from the Eirunepé region. It is slightly larger, the median sulcus is more pronounced, its ornamentation is faintly finer and the anterior rim is less well expressed. However, based on the given documentation an assured synonymisation is not possible to date (compare also Swain, 1998).
Ecology: see C. graciosa.
Occurrence (of C. lacrimata Muñoz-Torres et al., 1998): Western Amazonia, (early) late Middle to early Late Miocene (Peru: C. caraione zone; Colombia and Brazil: C. obliquosulcata–C. cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work (C. pebasae): Remanso, Aquidabã, Morada Nova, Pau D’Alho, Torre da Lua, Barro Branco.
Cyprideis aff. pebasae (Purper, 1979)
- Pl. 5, figs. 18–34
-
non 1979Cytheridea pebasae Purper, sp. nov. – Purper: 228–229, pl. 2, figs. 11–23.
-
1979Hulingsina sp. – Purper: 239–240, pl. 7, figs. 1–5.
-
non 1998C. lacrimata sp. nov. – Muñoz-Torres et al.: 96, text-fig. 2; pl. 3, figs. 7–11.
-
? 1998C. pebasae (Purper, 1979) – Muñoz-Torres et al.: 100, pl. 4, figs. 8–10.
-
?1998C. pebasae (Purper, 1979) – Whatley et al.: 236, text-fig. 2; pl. 2, figs. 16–20.
-
pars 2006C. pebasae (Purper, 1979) Whatley et al., 1998 emend. – Ramos: 90–91, figs. 6e–h. [non figs. 6i–y]
-
2006C. lacrimata Muñoz-Torres et al., 1998 – Ramos: 92, figs. 7a–c.
-
2009C. lacrimata Muñoz-Torres et al., 1998 – Ramos et al.: 114, fig. 290-I.
-
2010C. lacrimata Muñoz-Torres et al., 1998 – Wesselingh and Ramos: 308, figs. 18.5g, h.
-
? 2011Cyprideis sp. 4 – Linhares et al.: 97, fig. 4/12.
-
non 1979
Material: 54 adults, 52 juveniles (250 μm sieve fraction).
Dimensions: R♀l = 0.72–0.76, h = 0.38–0.42 (n = 2); L♀l = 0.75–0.80 (0.78), h = 0.40–0.44 (0.43; n = 3); R♂l = 0.77–0.86 (0.81), h = 0.39–0.44 (0.41; n = 5); L♂l = 0.81–0.85, h = 0.42–0.46 (n = 2); RA-1 l = 0.60–0.65 (0.62), h = 0.34–0.37 (0.36; n = 5); LA-1 l = 0.61–0.65 (0.63), h = 0.34–0.37 (0.35; n = 3).
Remarks: These valves are identical with C. lacrimata of Ramos (2006), Ramos et al. (2009), Wesselingh and Ramos (2010). Also the specimens of C. pebasae in Ramos (2006: figs. e–h) with a less “pronounced anterior and ventroposterior marginal border” and numerous, small denticles at the anterior margin match with the present specimens. The one valve described by Purper (1979) as Hulingsina? sp. is also very similar. C. pebasae in Muñoz-Torres et al. (1998), Whatley et al. (1998), which differs particularly due its infracurvate anterior margin and Cyprideis sp. 4 of Linhares et al. (2011), which is more elongated, are possibly synonymous.
Nonetheless, we consider C. lacrimata of Ramos (2006), Wesselingh and Ramos (2010) to be not equivalent to C. lacrimata of Muñoz-Torres et al. (1998) (see also Whatley et al., 1998 and remarks to C. pebasae above). Although the current specimens belong to the species group around C. pebasae, they cannot be ascribed to C. pebasae of Purper (1979).
Whether C. lacrimata Muñoz-Torres et al., 1998 is a junior synonym of C. pebasae (Purper, 1979) or not, a new species has to be established for the Cyprideis species under discussion here. Since the aim of this paper is beyond describing a new species, it is left in open nomenclature and recorded under C. aff. pebasae at this time.
Ecology: see C. graciosa.
Occurrence (of C. pebasae sensu Muñoz-Torres et al., 1998): Western Amazonia, latest Middle to early Late Miocene (Colombia and Brazil: C. obliquosulcata–C. cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work (C. aff. pebasae): Aquidabã, Morada Nova, Pau D’Alho, Torre da Lua, Barro Branco.
Cyprideis aff. machadoi (Purper, 1979)
- Pl. 6, figs. 1–20, 22
-
non 1979Chlamydocytheridea machadoi Purper, gen. et sp. nov. – Purper: 237–238, pl. 6, figs. 1–6.
-
? 1979Cyprideis truncata Purper, sp. nov. – Purper: 232–233, pl. 4, figs. 12–22.
-
2010Cyprideis machadoi (Purper, 1979) – Wesselingh and Ramos: 308, figs. 18.5m, n.
-
2011Cyprideis machadoi – Linhares et al.: 96, fig. 3/13–14.
-
non 1979
Plate 6.
(1–20) Cyprideis aff. machadoi (1) Le♀ (0.92/0.51; MN14_12), (2) Le♂ (1.03/0.52; MN14_11), (3) Le♀ (0.86/0.46; MN9_28), (4) Li♀ (= fig. 3), (5) Re♀ (0.94/0.49; MN14_14), (6) Re♂ (1.01/0.48; MN14_13), (7) Re♂ (0.89/0.44; MN9_33), (8) Ri♂ (0.96/0.46; MN9_31), (9) Li♀ (= fig. 1), (10) Ri♀ (= fig. 5), (11) Li♂ (= fig. 2), (12) Ri♂ (= fig. 6), (13) LeA-1 (0.65/0.37; PD20_01), (14) ReA-1 (0.69/0.37; MN14_17), (15) LiA-1 (= fig. 13), (16) RiA-1 (= fig. 14), (17) Ld♂ (= fig. 2), (18) Rd♂ (= fig. 6), (19) Ld♀ (= fig. 1), (20) Rd♀ (= fig. 5). (21) Cyprideis ?olivencai, Ld♀ (0.84/0.43; PD20_03). (22) Cyprideis aff. machadoi, central muscle scars of Ri♀ (= fig. 10). (23–27) Cyprideis ?olivencai (23) Le♀ (= fig. 21), (24) Li♀ (= fig. 23), (25) ReA-1 (0.63/0.33; PD25_01), (26) RiA-1 (= fig. 25), (27) ReA-2 (0.51/0.28; PD25_03). (For abbreviations see plate 1).
Material: 51 adults, 4 juveniles (250 μm sieve fraction).
Dimensions: R♀l = 0.85–0.94, h = 0.44–0.49 (n = 2); L♀l = 0.85–0.93 (0.89), h = 0.46–0.51 (0.49; n = 4); R♂l = 0.89–1.01 (0.95), h = 0.44–0.48 (0.46; n = 4); L♂l = 0.91–1.03, h = 0.47–0.52 (n = 2); RA-1 l = 0.69, h = 0.37 (n = 1); LA-1 l = 0.65, h = 0.37 (n = 1).
Remarks: Due to the well developed hinge and inner lamella as well as a pronounced sexual dimorphism, these specimens are regarded to represent adults, which show a considerable variation in size (e.g., pl. 6, figs. 1 and 3, figs. 6 and 7).
The present species clearly belongs to the “smooth lineage” of Amazonian Cyprideis (Whatley et al., 1998, p. 237). But it seems not to be identical with the similar C. machadoi, which is much larger, has stronger terminal hinge elements and differs noticeably in outline, especially due to the development of a very wide area between the flange and the selvage anteriorly (Purper, 1979; Muñoz-Torres et al., 1998; Whatley et al., 1998: pl. 2, figs. 6–10 [sic!]).
However, Muñoz-Torres et al. (1998), Whatley et al. (1998) consider C. machadoi as a variable species in relation to the shape and development of the anterior margin and its flange. Those authors placed Paulacoutoia kroemmelbeini Purper, 1979, Otarocyprideis elegans Sheppard & Bate, 1980 and Chlamydocytheridea kotzianae Purper & Ornellas, 1991 in the synonymy of C. machadoi. Further, they suggest the “scattered punctate” Cyprideis truncata Purper, 1979 to be a juvenile of C. machadoi.
The present material of adult valves largely matches with C. truncata aside the punctate surface. Slight punctae are observed on all valves, which correspond to normal pore canals. On somewhat corroded specimens these depressions become more prominent and look like punctate (pl. 6, fig. 7). Nevertheless, further investigations of the type material are needed to clarify the status of C. truncata. Here, we assign the present specimens to C. aff. machadoi.
Ecology: see C. graciosa.
Occurrence (of C. machadoi): Western Amazonia, early Middle to early Late Miocene (Peru: C. aulakos–C. minipunctata zone; Colombia and Brazil: C. obliquosulcata–C. cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work (C. aff. machadoi): Morada Nova, Pau D'Alho.
Cyprideis ?olivencai (Purper, 1979)
- Pl. 6, figs. 21, 23–27
-
? 1979Paulacoutoia olivençai Purper, gen. et sp. nov. – Purper: 235–236, pl. 5, figs. 10–17.
-
? 1998C. olivencai (Purper, 1979) – Muñoz-Torres et al.: 100, pl. 4, figs. 5–7.
-
? 1998C. olivencai (Purper, 1979) – Whatley et al.: 236, text-fig. 2; pl. 2; figs. 1–5 [sic!].
-
2010C. olivencai (Purper, 1979) – Wesselingh and Ramos: 308, fig. 18.5o, p.
-
? 2011C. olivencai – Linhares et al.: 97, fig. 4/1–2.
-
? 1979
Material: 6 adults, 3 juveniles (250 μm sieve fraction).
Dimensions: L♀l = 0.84–0.87, h = 0.43–0.47 (n = 2); R?♂l = 0.85, h = 0,39 (n = 1); RA-1 l = 0.63–0.64, h = 0.32–0.35 (n = 2); RA-2 l = 0.51, h = 0.28 (n = 1).
Remarks: Only few specimens were found which resemble in lateral view C. olivencai of Muñoz-Torres et al. (1998), Whatley et al. (1998), Linhares et al. (2011) and, especially, of Wesselingh and Ramos (2010). For the later no inner view is figured, which hampers further comparisons. Because the valves of Wesselingh and Ramos (2010) originate from Morada Nova, probably they are synonymous with our material.
The specimens of Muñoz-Torres et al. (1998) and Whatley et al. (1998) diverge due to the posteroventrally much wider inner lamella and in having only a narrow anterior vestibulum (the material of Eirunepé displays a wide anterior and a narrow posterior vestibulum). The most striking difference is, however, the dentate anterior and posterior hinge elements, which are smooth in here studied valves.
Although C. olivencai seems to be a rather variable taxon (e.g., valve size, width of the inner lamella, development of vestibuli, ornamentation) by considering the given synonyms in Muñoz-Torres et al. (1998), Whatley et al. (1998), compare Purper (1979), Sheppard and Bate (1980), Purper and Pinto (1983), an assured identification is not possible based on the available material.
A few, smooth-shelled, juvenile valves resemble in outline adults of C. olivencai sensu Muñoz-Torres et al. (1998) and Whatley et al. (1998). They can be distinguished due to their less inclined dorsal margin and the narrower anterior inner lamella from juveniles of C. aff. machadoi and are allocated to C. ?olivencai here.
Ecology: see C. graciosa.
Occurrence (of C. olivencai): Western Amazonia, early Middle to early Late Miocene (Peru: C. aulakos–C. caraione zone; Colombia and Brazil: C. obliquosulcata–C. cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work (C. ?olivencai): Morada Nova, Pau D’Alho, Torre da Lua.
Cyprideis sp. 1
Pl. 7, figs. 1–5
Plate 7.
(1–5) Cyprideis sp. 1 (1) Le?♂ (0.69/0.35; BA7b_03), (2) Re?♂ (0.66/0.33; BA7b_18), (3) Li?♂ (= fig. 1), (4) Ri?♂ (= fig. 2), (5) central muscle scars of Li?♂ (= fig. 3). (6–15) Cyprideis sp. 2 (6) Ld♂ (0.66/0.30; TO6_01), (7) Rd♀ (0.64/0.33; BA7a_02), (8) Le♂ (= fig. 6), (9) Re♀ (= fig. 7), (10) Li♂ (= fig. 6), (11) Ri♀ (= fig. 7), (12) ReA-1 (0.56/0.31; BA7b_26), (13) RiA-1 (= fig. 12), (14) Re♂ (0.68/0.33; MN9_51), (15) Ri♂ (= fig. 14). (16–24) Perissocytheridea acuminata (16) Le (0.48/0.24; PD20_04), (17) Re (0.47/0.22; PD20_05), (18) ReA-1 (0.39/0.20; BA7b_34), (19) RiA-1 (= fig. 18), (20) Li (= fig. 16), (21) Ri (= fig. 17), (22) Re?A-2 (0.31/0.18; MN8b_01), (23) Ri?A-2 (= fig. 22), (24) central muscle scars of Ri (= fig. 21). (25–27) Perissocytheridea sp. (25) Le (0.31/0.15; PD20_06), (26) Li (= fig. 25), (27) Ld (= fig. 25). (28–34) Rhadinocytherura amazonensis (28) Le (0.33/0.18; BA7a_06), (29) Re (0.33/0.18; BA7a_07), (30) Li (= fig. 28), (31) Ri (0.32/0.18; BA7a_08), (32) Ld (0.32/0.18; BA7a_03), (33) Rd (0.33/0.17; BA7a_10), (34) detail of Ri (anterodorsal area with sieve pores; 0.31/0.17; BA7a_04). (35–37) Rhadinocytherura sp. (35) central muscle scars of Ri (0.32/0.17; AQ6-2_27), (36) Re (= fig. 35), (37) Ri (= fig. 35). (38) Cyprideis sp. 2, central muscle scars of Ri♂ (= fig. 14). (For abbreviations see plate 1).
Material: 3 adults (250 μm sieve fraction).
Dimensions: R♀l = 0.74, h = 0.40; R?♂l = 0.66, h = 0.33; L?♂l = 0.69, h = 0.35.
Remarks: These specimens are related to C. longispina but seem not to range within the variability of that species. Cyprideis sp. 1 is noticeably smaller, has a coarse reticulate ornamentation and the flange is less pronounced posteroventrally. Because only three adult valves are available to date, no further species identification is attempted.
Ecology: see C. graciosa. Occurrence (this work): Barro Branco.
Cyprideis sp. 2
Pl. 7, figs. 6–15, 38
Material: 17 adults, 3 juveniles (250 μm sieve fraction).
Dimensions: R♀l = 0.64–0.68, h = 0.33–0.35 (n = 2); R♂l = 0.64–0.69 (0.67), h = 0.32–0.33 (0.33; n = 4); L♂l = 0.62–0.67 (0.65), h = 0.30–0.32 (0.31; n = 3); RA-1 l = 0.55–0.56 (0.55), h = 0.30–0.31 (0.30; n = 3).
Remarks: Cyprideis sp. 2 is close to Pseudoparakrithella paralela Purper, 1979 (compare also Purper and Pinto, 1983). Size, outline in lateral and dorsal view, development of the inner lamella as well as the central muscle scars are equivalent. Both, Cyprideis sp. 2 and P. paralela, exhibit an inverse hinge (and inverse valve size) with crenulated anterior and posterior bars and a short median groove in left valves.
However, the specimens from the Eirunepé area are stronger ornamented (coarsely punctate to reticulate, whereas P. paralela is punctate), marginal pore canals are either simple or branched (in P. paralela they are simple) and two to three, very inconspicuous posteroventral spines on some left valves are observed, which are not mentioned by Purper (1979).
Pseudoparakrithella Purper, 1979 is considered by Whatley et al. (1998); see also Muñoz-Torres et al. (1998) to be a synonym of the genus Cyprideis (here we follow that assumption). Moreover, these authors place P. paralela into the synonymy of the “very variable species” C. olivencai (Purper, 1979) (Whatley et al., 1998, p. 236). Neither the reversed hinge nor differences in ornamentation (olivencai was originally described to be punctate; in the diagnosis given by Whatley et al. (1998) it is characterised to be smooth), the development of the inner lamella (fused zone narrower in paralela of Purper (1979) than in olivencai of Whatley et al. (1998)) and differences in marginal pore canals (simple in paralela; simple, bifurcating or polyfurcating in olivencai sensu Whatley et al., 1998) are supposed to justify a species delineation.
Based on the scarce material, we cannot attest if Cyprideis sp. 2 belongs to C. paralela (Purper, 1979) or represents a new Cyprideis-species. Nevertheless, we consider Cyprideis sp. 2 not to be synonymous with C. olivencai sensu Whatley et al. (1998), Muñoz-Torres et al. (1998).
Ecology: see C. graciosa. Occurrence (this work): Aquidabã, Morada Nova, Torre da Lua, Barro Branco.
Family Cytheridae Baird, 1850
Genus Perissocytheridea Stephenson, 1938
Perissocytheridea acuminata (Purper, 1979)
- Pl. 7, figs. 16–24
-
1977Ostracoda C n.g.,n.sp. – Purper: 365, pl. 4, figs. 15–16.
-
∗ 1979P. acuminata Purper gen et sp.nov. – Purper: 241–242, pl. 7, figs. 15–20.
-
1998P. acuminata (Purper, 1979) – Muñoz-Torres et al.: 102, pl. 5, figs. 5–8.
-
1977
Material: 2 adults, 2 juveniles (125 μm sieve fraction).
Dimensions: R♀l = 0.47, h = 0.22 (n = 1); L♀l = 0.48, h = 0.24 (n = 1); RA-1 l = 0.39, h = 0.20 (n = 1); ?RA-2: l = 0.31, h = 0.18 (n = 1).
Remarks: In lateral view, the rare specimens from Eirunepé are slightly less acuminate and display a small convexity at the centre of the dorsal margin. Nonetheless, they match well with the documentation given by Purper (1977, 1979) and Muñoz-Torres et al. (1998).
Ecology: Perissocytheridea is considered a euryhaline (mainly mesohaline) form, typical for lagoonal environments (e.g., Keyser, 1977; Colin et al., 1996; Muñoz-Torres et al., 2006; Mebrouk et al., 2011).
Occurrence: Western Amazonia, early Middle to early Late Miocene (Peru: C. aulakos–C. caraione zone; Colombia and Brazil: C. obliquosulcata–C. cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work: Morada Nova, Pau D'Alho, Barro Branco.
Perissocytheridea sp.
- Pl. 7, figs. 25–27
-
? 1998Perissocytheridea sp. 1 – Muñoz-Torres et al.: 104, pl. 6, figs. 1–6.
-
? 1998
Material: 1 adult valve (125 μm sieve fraction).
Dimensions: L l = 0.31, h = 0.15 (n = 1).
Remarks: One left, due to its well developed hinge and inner lamella clearly adult valve is available, which we place in Perissocytheridea (compare generic diagnosis emended by Pinto and Ornellas (1970)).
This specimen comes close to Perissocytheridea sp. 1 of Muñoz-Torres et al. (1998), which is: somewhat larger; its posterior margin is more rounded; it has a better expressed bifurcate sulcus that borders a node (below an additional small node is developed); and, its inner lamella is much narrower. Because our material consists of only one specimen, it is left in open nomenclature.
Ecology: see P. acuminata.
Occurrence (of Perissocytheridea sp. 1 of Muñoz-Torres et al. (1998)): Western Amazonia, latest Middle Miocene (Colombia and Brazil: C. obliquosulcata zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work: Pau D'Alho.
Family Cytheruridae Müller, 1894
Genus Rhadinocytherura Sheppard & Bate, 1980
Rhadinocytherura amazonensis Sheppard & Bate, 1980
- Pl. 7, figs. 28–34
-
∗ 1980R. amazonensis sp. nov. – Sheppard and Bate: 112–113, pl. 11, figs. 10–16.
-
1998R. amazonensis Sheppard & Bate, 1980 – Muñoz-Torres et al.: 104, pl. 6, figs. 7–12.
-
∗ 1980
Material: 18 adults (including one fragmented carapace), 1 juvenile, 3 fragments (125 μm sieve fraction).
Dimensions: R l = 0.31–0.35 (0.33), h = 0.17–0.20 (0.18; n = 8); L l = 0.31–0.33 (0.32), h = 0.17–0.19 (0.18; n = 6); L?A-1 l = 0.27, h = 0.15 (n = 1).
Remarks: Size, shape as well as the development of the inner lamella and the characteristic hinge (left valve: dentate anterior tooth, smooth median bar, loculate posterior socket; right valve complementary; Sheppard and Bate, 1980) largely coincide with the given synonyms.
Muñoz-Torres et al. (1998) noticed variations in ornamentation of the valves. Most specimens from the Eirunepé area bear a posteroventral spine in both valves, which is not reported by Sheppard and Bate (1980) and Muñoz-Torres et al. (1998). Here, we consider that feature to range within the variability of the species.
Originally, normal pores are mentioned to be simple by Sheppard and Bate (1980). In contrast, large sieve-plates are evident in the current material. Nevertheless, we believe our specimens to be conspecific with R. amazonensis.
Ecology: Sheppard and Bate (1980) consider R. amazonensis as a marine to brackish water taxon.
Occurrence: Western Amazonia, early Middle to early Late Miocene (Peru: C. caraione zone; Colombia and Brazil: C. obliquosulcata–C. cyrtoma zone; Muñoz-Torres et al., 2006; chronostratigraphic correlation after Wesselingh and Ramos, 2010). This work: Aquidabã, Morada Nova, Pau D'Alho, Torre da Lua, Barro Branco.
Rhadinocytherura sp.
Pl. 7, figs. 35–37
Material: 4 adult valves (125 μm sieve fraction).
Dimensions: R l = 0.32, h = 0.17 (n = 2); L l = 0.30, h = 0.17 (n = 1).
Remarks: Four valves were found, which resemble R. amazonensis (Sheppard and Bate, 1980; Muñoz-Torres et al., 1998; and above). However, these specimens differ significantly due to their entirely straight dorsal margin, the caudal process is situated at the half of the valves' height, the posterior tooth in the left valve is more robust and the ornament is regularly pitted.
The hinge is developed as characteristic for the genus. Again, normal pores of sieve type are observed (compare remarks to R. amazonensis above).
Ecology: see R. amazonensis. Occurrence (this work): Aquidabã, Torre da Lua.
4.2. Ostracod occurrence and geochemical results
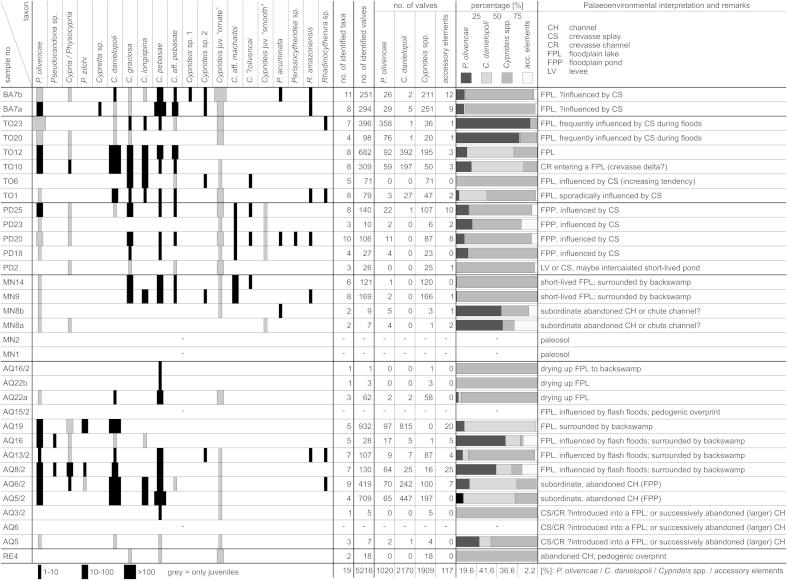
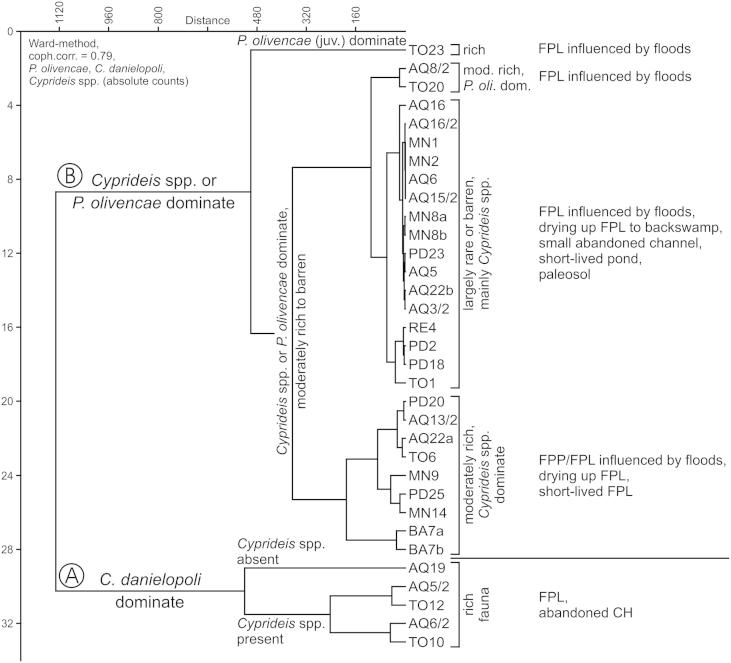
Thirty-three bulk samples delivered a moderately diverse fauna with 19 species, belonging to 10 genera and 8 families. Four samples were barren of ostracods (AQ6, AQ15/2, MN1, MN2).
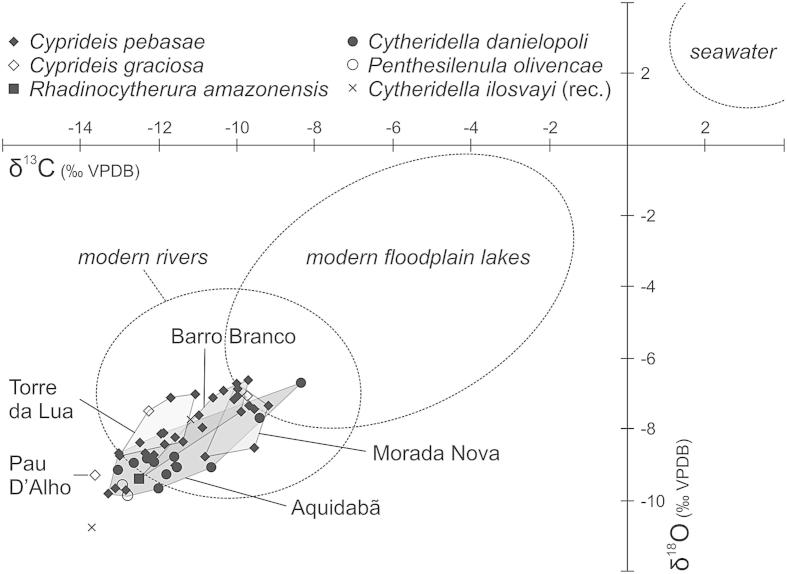
Overall, the fauna is dominated by the limnocytherid Cytheridella danielopoli (41.6%), the cytherideid Cyprideis (36.6%; 8 species) and the darwinulid Penthesilenula olivencae (19.6%; Fig. 3; ESM 1). The other 9 taxa just account with 2.2% to the total ostracod assemblage. Among Cyprideis the “ornate” group (Whatley et al., 1998) predominates with ∼35% (C. pebasae ∼15%; C. longispina ∼4.8%; C. graciosa ∼4.5%; C. aff. pebasae ∼2%), whereas the “smooth” group (C. aff. machadoi, C. ?olivencai) yields ∼1.6% only (ESM 1). All isotopic analyses provided very light values with a range for δ18O from −6.62 to −9.89‰ and for δ13C with −8.36 to −13.63‰ (Fig. 4; Table 1).
Fig. 3.
Distribution of ostracods in the samples (∼arranged in stratigraphical order; palaeoenvironmental interpretation after Gross et al., 2011; note: Cyprideis juv. “ornate” and Cyprideis juv. “smooth” refer to specimens from the 125 μm sieve-residual; AQ = Aquidabã, BA = Barro Branco, MN = Morada Nova, PD = Pau D’Alho, RE = Remanso, TO = Torre da Lua).
Fig. 4.
δ18O- and δ13C-isotopic ratios of ostracod valves from the Eirunepé area (freshwater taxa: Cytheridella, Penthesilenula; “brackish/marine” taxa: Cyprideis, Rhadinocytherura; note: Cytheridella ilosvayi = recent material from two oxbow lakes: Lago Barro Branco: S 06°50′18.3″/W 069°45′37.0″, Lago Comprido: S 06°43′52.7″/W 069°44′33.9″; Fig. 1b). The indicated range for modern rivers and floodplain lakes is based on aragonitic mollusc shells (after Wesselingh et al., 2006c), which give somewhat heavier values for the same environmental parameters compared to ostracod calcite (Grossman and Ku, 1986).
Table 1.
Isotope data of Eirunepé ostracod valves (juv. = juvenile, ad. = adult; no.v. = number of valves used for analysis; B. Branco, Comprido = Lago Barro Branco, Lago Comprido, both recent oxbow lakes; for species abbreviations see Fig. 4).
| Sample no. | Species | no. v. | δ13C | δ18O | Sample no. | Species | no. v. | δ13C | δ18O |
|---|---|---|---|---|---|---|---|---|---|
| BA7b | C. pebasae juv. | 3 | −10.59 | −7.11 | AQ19 | C. danielopoli juv. | 4 | −13.04 | −9.15 |
| BA7b | C. pebasae juv. | 3 | −9.87 | −7.49 | AQ19 | C. danielopoli ad. | 2 | −8.36 | −6.68 |
| BA7b | C. pebasae juv. | 3 | −10.87 | −7.94 | AQ6/2 | C. pebasae juv. | 4 | −11.59 | −9.01 |
| BA7b | C. pebasae ad. | 2 | −10.08 | −7.17 | AQ6/2 | C. pebasae juv. | 4 | −11.56 | −8.24 |
| BA7b | C. pebasae ad. | 2 | −10.97 | −7.62 | AQ6/2 | C. pebasae ad. | 2 | −12.49 | −8.38 |
| BA7b | R. amazonensis ad. | 7 | −12.50 | −9.38 | AQ6/2 | C. pebasae ad. | 2 | −13.09 | −9.66 |
| BA7a | C. pebasae juv. | 4 | −9.98 | −7.07 | AQ6/2 | C. pebasae ad. | 2 | −12.10 | −8.72 |
| BA7a | C. pebasae ad. | 2 | −9.69 | −6.62 | AQ6/2 | C. pebasae ad. | 2 | −13.27 | −9.82 |
| BA7a | C. pebasae ad. | 2 | −10.33 | −6.94 | AQ6/2 | C. pebasae ad. | 2 | −11.95 | −8.14 |
| BA7a | C. pebasae ad. | 2 | −9.96 | −6.86 | AQ6/2 | C. danielopoli juv. | 3 | −11.81 | −9.29 |
| TO12 | C. pebasae juv. | 3 | −11.04 | −7.03 | AQ6/2 | C. danielopoli juv. | 3 | −12.01 | −9.66 |
| TO12 | C. pebasae ad. | 2 | −11.69 | −7.12 | AQ6/2 | C. danielopoli juv. | 3 | −11.54 | −9.09 |
| TO12 | C. pebasae ad. | 2 | −11.88 | −8.09 | AQ6/2 | C. danielopoli ad. | 2 | −9.43 | −7.69 |
| TO12 | C. pebasae ad. | 2 | −13.00 | −8.73 | AQ6/2 | P. olivencae ad. | 6 | −12.95 | −9.55 |
| TO12 | C. pebasae ad. | 2 | −11.38 | −8.38 | AQ6/2 | P. olivencae ad. | 6 | −12.81 | −9.89 |
| TO12 | C. graciosa ad. | 4 | −12.26 | −7.49 | AQ5/2 | C. pebasae ad. | 2 | −12.36 | −8.70 |
| PD20 | C. graciosa ad. | 3 | −13.63 | −9.31 | AQ5/2 | C. pebasae ad. | 2 | −13.03 | −8.66 |
| MN14 | C. pebasae ad. | 2 | −10.16 | −6.72 | AQ5/2 | C. pebasae ad. | 2 | −11.83 | −8.41 |
| MN14 | C. pebasae ad. | 3 | −10.95 | −8.78 | AQ5/2 | C. pebasae ad. | 2 | −12.83 | −9.70 |
| MN14 | C. pebasae ad. | 3 | −9.82 | −7.34 | AQ5/2 | C. danielopoli juv. | 3 | −11.60 | −8.79 |
| MN9 | C. pebasae ad. | 2 | −9.67 | −8.52 | AQ5/2 | C. danielopoli juv. | 3 | −12.13 | −8.92 |
| MN9 | C. pebasae ad. | 3 | −9.68 | −7.45 | AQ5/2 | C. danielopoli juv. | 3 | −12.64 | −8.95 |
| MN9 | C. pebasae ad. | 3 | −9.34 | −7.34 | AQ5/2 | C. danielopoli ad. | 2 | −10.66 | −9.06 |
| MN9 | C. graciosa ad. | 3 | −9.74 | −7.06 | B. Branco | C. ilosvayi | 1 | −13.69 | −10.73 |
| AQ19 | C. danielopoli juv. | 4 | −12.29 | −8.84 | Comprido | C. ilosvayi | 1 | −11.34 | −7.84 |
4.2.1. Remanso
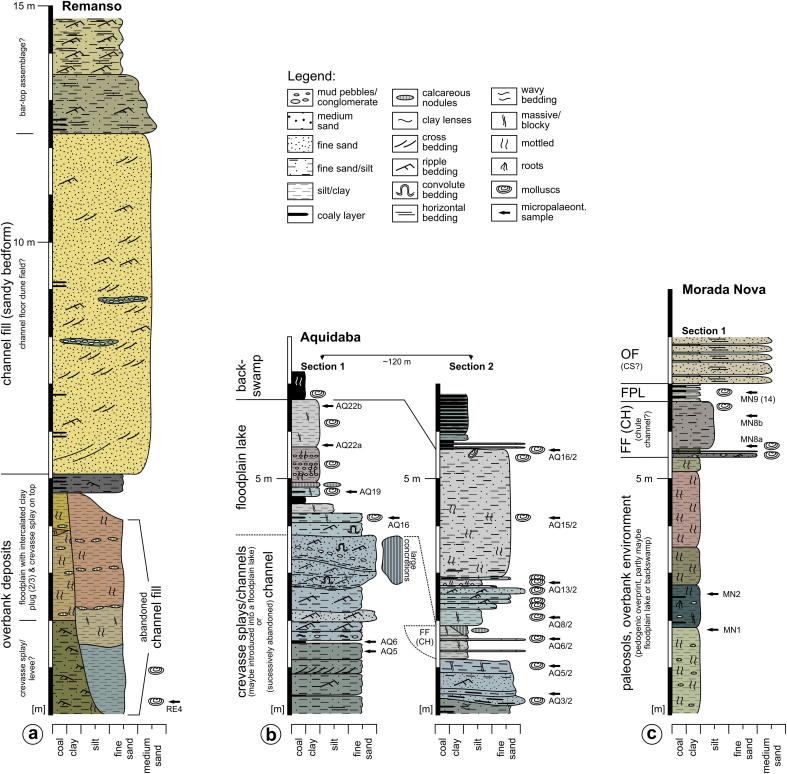
Location
27.4 km NE Eirunepé (S 06°31′22.0″/W 069°35′42.8″; altitude: ∼105 m; section thickness: ∼15 m), left cutbank of the Juruá River (Figs. 1b, 5).
Fig. 5.
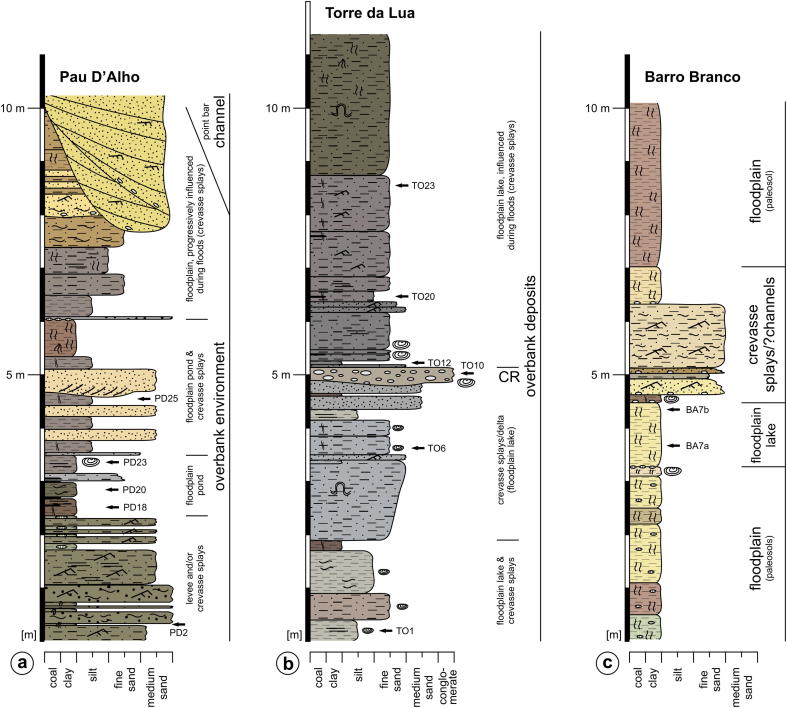
Lithological sections of (a) Remanso, (b) Aquidabã and (c) Morada Nova as well as interpretation of depositional environment (after Gross et al., 2011, where more detailed information is presented; CS = crevasse splay, FF (CH) = abandoned channel, FPL = floodplain lake, OF = overbank fines; legend also used in Fig. 6).
Sedimentological interpretation (Gross et al., 2011)
Overbank deposits at the base of the section are cut by a channel, which became filled by pelitic, afterwards pedogenically overprinted sediments (sample RE4). It is overlain by crevasse splay deposits, sandy bedforms as well as possible bar-top assemblages.
Ostracods
Sample RE4 delivered a very poor fauna with a few juveniles of C. graciosa, C. pebasae and some juveniles of the “ornate” Cyprideis group.
4.2.2. Aquidabã
Location
22.0 km NE Eirunepé (S 06°31′40.8″/W 069°39′52.0″; altitude: ∼106 m; section thickness: ∼8 m), left cutbank of the Juruá River (Figs. 1b, 5).
Sedimentological interpretation (Gross et al., 2011)
The basal layers are referred to crevasse splay and/or crevasse channel sediments, introduced into a floodplain lake or, alternatively, are channel deposits of an abandoned avulsive river arm (samples AQ5, 6, 3/2). Subordinately, a small channel fill (floodplain pond) is intercalated (AQ5/2, 6/2). Up-section, the influx of crevassing respectively the active channel decreased and led to the formation of a successively drying up floodplain lake within a densely vegetated backswamp (AQ8/2, 13/2, 16, 19, 15/2, 22a, 22b). Sporadically, it was influenced by flash floods. Finally, a swampy environment established (AQ16/2).
Ostracods
Samples from Aquidabã yielded a rich ostracod fauna (2403 determined specimens; 12 taxa). In total, C. danielopoli (64.3%) dominates the assemblage. Cyprideis accounts with 19.6% (4 species; only of the “ornate” group) and Pelocypris olivencae with 13.6% to the fauna. Pseudocandona sp. and Pelocypris zilchi are exclusive for Aquidabã.
Samples from the base of the outcrop (AQ5, 6, 3/2) and from the upper part (AQ15/2, 22a, 22b AQ16/2) yielded rather poor faunas (predominantly Cyprideis spp., only rarely C. danielopoli and P. olivencae; other taxa are lacking). In contrast, rich and diverse faunas were found in the samples in between (AQ5/2–AQ19). In the three most productive samples C. danielopoli is the dominant species (AQ5/2, 6/2, 19). Remarkably, Cyprideis spp. is completely absent in AQ19.
Geochemistry
Juveniles and adults of C. pebasae (AQ5/2, 6/2) range within −8.14 to −9.82‰ in respect to δ18O (δ13C: −11.56 to −13.27‰). Similar are the results for adults of P. olivencae (AQ6/2; δ18O: −9.55 to −9.89‰; δ13C: −12.81 to −12.95‰) and for juveniles of C. danielopoli (AQ5/2, 6/2, 19; δ18O: −8.79 to −9.66‰; δ13C: −11.54 to −13.04‰). Only adults of C. danielopoli (AQ19) yielded somewhat heavier values (δ18O: −6.68 to −9.06‰; δ13C: −8.36 to −10.66‰).
4.2.3. Morada Nova
Location
17.9 km NE Eirunepé (S 06°32′51.1″/W 069°42′39.4″; altitude: ∼107 m; section thickness: ∼8 m), left cutbank of the Juruá River (Figs. 1b, 5).
Sedimentological interpretation (Gross et al., 2011)
The lower part of the outcrop is formed by a succession of paleosols (samples MN1, 2). The paleosols are overlain by point bar deposits (only present in Sections 2, 3, 4 as shown in Gross et al., 2011) in which a subordinate channel (?chute channel fill) is intersected in the NW (MN8a, 8b). Up-section, the influx of the active channel decreased and a short-lived floodplain lake developed within a richly vegetated backswamp (MN9, 14).
Ostracods
The small ostracod fauna of Morada Nova (306 determined specimens; 11 taxa) comprises 94.8% Cyprideis spp. (7 species). P. olivencae is present with 3.9%, C. danielopoli is lacking. Samples MN1 and MN2 were barren of ostracods; MN8a and MN8b contained only a few ostracod remains. In samples MN9 and MN14 a moderately rich fauna was found, which is highly dominated by Cyprideis spp.
Geochemistry
Values obtained from adults of C. pebasae (MN9, 14) range between −6.72 and −8.78‰ for δ18O and −9.34 to −10.95‰ for δ13C; the result for C. graciosa adult (MN9) is comparable (δ18O: −7.06‰; δ13C = −9.74‰).
4.2.4. Pau D'Alho
Location
12.6 km NE Eirunepé (S 06°33′55.5″W 069°46′11.7″; altitude: ∼108 m; section thickness: ∼10 m), left cutbank of the Juruá River (Figs. 1b, 6).
Fig. 6.
Lithological sections of (a) Pau D'Alho, (b) Torre da Lua and (c) Barro Branco as well as interpretation of depositional environment (after Gross et al., 2011; where more detailed information is presented; CR = crevasse channel).
Sedimentological interpretation (Gross et al., 2011)
The section starts with fluvial overbank deposits, probably related to levee and/or crevasse splay sedimentation with possibly intercalated short-lived ponds (sample PD2). The layers up-section (samples PD18, 20, 23, 25) are assigned to the formation of a shallow floodplain pond, which was episodically influenced by crevasse splays. Above, floodplain pond conditions ended due to enhanced clastic input from an approaching channel, which finally intersected the succession and became filled up by point bar deposits.
Ostracods
The limited ostracod fauna of Pau D'Alho (309 determined specimens; 11 taxa) is largely dominated by Cyprideis spp. (80.3%; 5 species), followed by the darwinulid P. olivencae (12.6%). C. danielopoli is virtually absent (Fig. 3).
Geochemistry
The isotopic analysis of four adults of C. graciosa (PD20) resulted in light δ18O (−9.31‰) and δ13C (−13.63‰) values.
4.2.5. Torre da Lua
Location
17.5 km SE Eirunepé (S 06°49′23″/W 069°47′04″; altitude: ∼117 m; section thickness: ∼12 m), left cutbank of the Tarauacá River (Figs. 1b, 6).
Sedimentological interpretation (Gross et al., 2011)
The basal layers are interpreted to be deposited in a floodplain lake, which was influenced by crevasse splays (with increasing tendency up-section; samples TO1, 6). In layer 10 (sample TO10) a crevasse channel reached the floodplain lake via a crevasse delta. Up-section, the crevasse channel became abandoned and floodplain lake conditions were reinstalled (TO12), which were, however, frequently influenced by crevasse splays during floods (TO20, 23).
Ostracods
The rich ostracod fauna of Torre da Lua (1635 determined specimens; 12 taxa) is dominated by C. danielopoli (37.8%), followed by P. olivencae (36.0%) and Cyprideis spp. (25.6%; 6 species). Whereas the sample from the base of the section (TO1) contained a moderately abundant and diverse fauna, TO6 yielded only Cyprideis spp. Up-section, samples were rich in ostracods with C. danielopoli as the dominant taxon in TO10 and TO12. Above (TO20, 23), C. danielopoli almost vanished and the spectrum is largely dominated by P. olivencae (mainly juveniles), accompanied by Cyprideis spp.
Geochemistry
C. pebasae (adults and juveniles) and C. graciosa (adult) range within −7.03 to −8.73‰ in δ18O and between −11.04 and −13.00‰ in δ13C.
4.2.6. Barro Branco
Location
22.1 km SSE Eirunepé (S 06°52′18.3″/W 069°47′05.1″; altitude: ∼120 m; section thickness: ∼10 m), left cutbank of the Tarauacá River (Figs. 1b, 6).
Sedimentological interpretation (Gross et al., 2011)
The lower part of the section comprises a succession of paleosols, which is overlain by floodplain lake sediments (BA7a, 7b). Afterwards, a series of crevasse splay deposits follows, which is topped by pedogenically overprinted floodplain fines.
Ostracods
At Barro Branco Cyprideis spp. dominates the fauna (84.8%); P. olivencae is the second most abundant taxon (10.1%) and C. danielopoli occurs only in a low amount (1.3%). Cyprideis sp. 1 and Cypretta sp. are exclusive for Barro Branco.
Geochemistry
Values for adults and juveniles of C. pebasae (BA7a, 7b) range within: δ18O: −6.62 to −7.94‰; δ13C: −9.69 to −10.97‰. The analysis of adults of R. amazonensis (BA7b) resulted in light values (δ18O: −9.38‰; δ13C: −12.50‰).
5. Discussion
5.1. Palaeoecological indication of the Eirunepé ostracod fauna
5.1.1. Freshwater versus brackish or marine waters
One of the most controversially discussed issues in western Amazonia's history is the influence of marine incursions during Neogene times. Their existence, chronology, origin as well as their spatial extent is still disputed (e.g., Hoorn et al., 2010a,b; Hovikoski et al., 2010; Latrubesse et al., 2010; Ruskin et al., 2011). Aside sedimentological and ichnological indications (e.g., Gingras et al., 2002; Hovikoski et al., 2007, 2010), palaeontological evidences (i.e., mangrove pollen, foraminifers, specific molluscs, barnacles) were used to infer transitorily marine influences (e.g., Hoorn, 1993, 2006; Wesselingh et al., 2002; Vonhof et al., 2003; Linhares et al., 2011). Additionally, the occurrence of brackish water ostracods (particularly Cyprideis) and some rarely recorded marine species, motivated several authors to propose brackish (mixohaline) waters (Purper, 1979; Purper and Pinto, 1983, 1985; Purper and Ornellas, 1991; Whatley et al., 1998) or marine transgressions (Sheppard and Bate, 1980; Swain, 1998).
In total the ostracod fauna of the Eirunepé region comprise about 62.8% freshwater ostracods (Penthesilenula, Pseudocandona, Cypria, Physocypria, Pelocypris, Cypretta, Cytheridella). Cyprideis (36.6%) is associated with them, accompanied by Perissocytheridea and Rhadinocytherura (0.6%). Cyprideis and Perissocytheridea are euryhaline taxa, today typically occurring in marginal marine settings (for palaeoecology of the taxa see 4.1.). Rhadinocytherura is considered as a marine to brackish water form (Sheppard and Bate, 1980); however, it is endemic for western Amazonia.
In our samples fresh- and brackish water elements co-occur (Fig. 3). Usually various ontogenetic stages of the taxa were found and valves are well preserved (translucent, no signs of corrosion), which excludes a significant relocation (Sheppard and Bate, 1980; Whatley, 1988; Purper and Ornellas, 1991; Whatley et al., 1998; Ramos, 2006).
Stable isotope analyses (δ18O, δ13C; 137 valves in total) performed on both groups (freshwater: Penthesilenula olivencae, Cytheridella danielopoli; “brackish/marine”: Cyprideis graciosa, C. pebasae, Rhadinocytherura amazonensis) yielded constantly very light values (Fig. 4, Table 1). No clear trends between different taxa, adult and juvenile valves, localities or layers could be observed. However, such depleted δ18O and δ13C are compatible with a freshwater system (Leng and Marshall, 2004) and does not permit to conjecture about brackish waters or marine influences here. Our results are consistent with previous isotopic investigations (mainly molluscs; Vonhof et al., 2003; Kaandorp et al., 2006; Ramos, 2006; Wesselingh et al., 2006c; Wesselingh, 2008; Wesselingh and Ramos, 2010). Moreover, pilot measurements on recent material (Cytheridella ilosvayi) from two oxbow lakes along the Tarauacá River show comparable values (δ18O: −7.84 to −10.73‰; δ13C: −11.34 to −13.69‰).
Consequently, based on the faunal composition as well as on stable isotope results, there is no hint that Cyprideis and Rhadinocytherura (most likely also Perissocytheridea) indicate brackish waters or a marine influx in the Eirunepé outcrops. Apparently these taxa have been successfully adapted to freshwater settings, which is also well documented for Cyprideis today (Lake Tanganyika; Wouters and Martens, 2001, 2007) and proposed by Ramos (2006) and Latrubesse et al. (2010) earlier. Hence, palaeosalinity reconstructions of western Amazonia's Neogene environments based on the occurrence of highly endemic ostracod taxa should be used with some concern.
5.1.2. Ostracod distribution
Earlier sedimentological analyses (Gross et al., 2011) referred the deposits of the sections around Eirunepé to a suspended load river system (possibly to an anastomosing system) and demonstrated the inexistence of a long-lived lake s.str. there (“Lake Pebas”; Wesselingh, 2007). Aside sandy channel deposits, the bulk of the sediments belongs to a variety of overbank environments (4.2.). Lacustrine sediments are related to abandoned channels and short-lived floodplain ponds/lakes, which were frequently affected by flooding events. The herein presented freshwater ostracod assemblages corroborate well with this interpretation. Probably, this wetland is comparable to the Quaternary mega-fans of the Chaco plain and the modern Pantanal as suggested by Latrubesse et al. (2007, 2010) for the uppermost Solimões Fm. (Late Miocene) in the south-western Brazil (Acre).
Thus, and in accordance with this continental sedimentation model, we can assume a highly structured fluvial landscape with diverse habitats and substrates. An array of regional (e.g., flood pulses, seasonality) and local (e.g., physicochemical properties of the water, nutrient supply) parameters may have affected ostracod assemblages in such a setting (Kaandorp et al., 2005; Higuti et al., 2010). Since we suppose to deal with an avulsive river system, flooding events might have played an important role in ostracod distribution (e.g., mixing of riverine and floodplain lake faunas, inclusively due to floating macrophytes; sudden anoxia due to water level rise in floodplain lakes; Higuti et al., 2007, 2010).
Despite all these virtually unpredictable variables, a rough differentiation is signalised by the ostracod distribution. Based on a cluster analysis (Q-mode; software package PAST 1.92; Hammer and Harper, 2008, Fig. 7) the most ostracod rich samples are related to floodplain lakes or abandoned channels, which seem to be less affected by the hydrodynamics of the river. These faunas are dominated by C. danielopoli (cluster A). More fluvially characterised layers display lower ostracod abundances and are dominated by Cyprideis spp. and/or P. olivencae (cluster B). Extant C. ilosvayi, which is closely related to the C. danielopoli, prefers permanent, lentic environments (Pérez et al., 2010a,b), while species of the “incae” group of Penthesilenula are especially abundant in lotic habitats (inclusively floating macrophytes; Higuti et al., 2009a). Hence, a coarse, probably hydrodynamically controlled differentiation is suggested. Cyprideis is a euryoecious taxon, usually common in stressful environments. Based on the observed ostracod distribution, Cyprideis seems to be better predisposed for unstable settings like short-lived ponds and more fluvially affected environments. A further differentiation – as indicated by the cluster analysis – is tentative because the sub environments cannot be characterised in more detail.
Fig. 7.
Dendrogram resulting from cluster analysis of ostracod data and generalised facies interpretation (FPL = floodplain lake, FPP = floodplain pond, CH = channel).
One sample from Torre da Lua (TO23; possibly also TO20) is highly dominated by juveniles of P. olivencae, associated by few, mainly juveniles of Cyprideis spp. This association could be an example for an allochthonous fauna (?related to floating meadows; Higuti et al., 2010). Remarkably, among almost thousand specimens (largely C. danielopoli) not even one fragment of Cyprideis was recovered from sample AQ19. To date this observation remains unexplained (ecological parameters?, simply patchiness of occurrence?). The absence of ostracods in some samples is clearly linked to (palaeo-)pedogenic alteration (MN1, MN2 and AQ15/2).
5.2. Relation of the Eirunepé ostracod fauna to Pebasian faunas and remarks to the western Amazonian Cyprideis flock
Except a few works (e.g., Ramos, 2006), investigations of fossil ostracods from western Amazonia deal with material originating from the Brazilian–Colombian–Peruvian borderland, roughly centred around the small town Pebas (∼165 km ENE of Iquitos; e.g., Purper, 1979; Muñoz-Torres et al., 1998, 2006; Whatley et al., 1998). The ostracod composition of these “classical” Pebasian fauna, where Cyprideis totally dominates the assemblages with up to 93%, differs notably from the Eirunepé fauna (only 36.6% Cyprideis). Nonetheless, out of the 19 species recorded in Eirunepé, 9 species are shared with the Pebasian fauna. Seven additional species – left in open nomenclature – show some similarity and only 3 taxa are new records for the Neogene of western Amazonia (Fig. 8). Thus, from taxonomical point of view, large affinities to the Pebasian fauna are evident. Depending on the dating of the Eirunepé fauna (late Middle Miocene or Late Miocene; see below, 5.3.), these relations can be referred to a marginal position to the “Pebas wetland” (Ramos, 2006; Celestino and Ramos, 2007; Wesselingh and Ramos, 2010) or, more likely, to the fade out of the Pebasian fauna before the onset of the Amazon system (Wesselingh et al., 2006a; Hoorn et al., 2010a; Gross et al., 2011).
Fig. 8.
Stratigraphic range of ostracod taxa based on Muñoz-Torres et al. (2006; chronostratigraphic correlation of mollusc and ostracod zones after Wesselingh and Ramos, 2010). For discussion on the estimated age of Eirunepé outcrops see text (thick lines = characteristic faunal element according to Muñoz-Torres et al. (2006); stippled lines = not recorded; grey lines = uncertain taxonomic equivalence; asterisks mark taxa shared with Pebasian faunas).
Interestingly, 97.8% of the ostracod fauna of the Eirunepé sections is composed of only 3 genera, the darwinuloid genus Penthesilenula and the cytheroid genera Cytheridella and Cyprideis. Taxa of the Cypridoidea (e.g., candonids, ilyocyprinids, cypridids) were found only subordinately, which are otherwise common in modern freshwater settings (Martens et al., 2008; Higuti et al., 2009b). Whatley et al. (1998) argued for an elevated (brackish) salinity to be responsible for their rare occurrence. In Eirunepé that could not be the case due to exclusively freshwater conditions. Extant Penthesilenula, Cytheridella and Cyprideis exhibit brood care, whereas cypridoid ostracods to not brood (Smith and Delorme, 2010). Hypothetically, this mode of reproduction inherits a reproductive advantage and/or facilitates dispersal (?rivers, ?birds) and colonisation of this herein treated, patchy structured environment (Sandberg, 1964; Van Harten, 1990; Higuti et al., 2010).
Within the Eirunepé ostracod fauna Cyprideis is the most specious taxon and clearly related to the Cyprideis species flock of Neogene western Amazonia (Whatley et al., 1998). Comparable radiation events are documented from modern freshwater Lake Tanganyika (Wouters and Martens, 2001, 2007) as well as from the brackish, Late Miocene Lake Pannon (Central Europe; Krstić, 1968a,b; Van Harten, 1990), both famous examples for long-lived lakes. Muñoz-Torres et al. (2006), Whatley et al. (1998) regarded the palaeoenvironmental stability of a large, brackish, lacustrine habitat to be the trigger for this extensive radiation. At least on a local scale, stable palaeoenvironments are missing in the Eirunepé region (Gross et al., 2011). The combination of Cyprideis' euryoecious biology, its passive dispersal capacity and its sexual reproduction mode, possibly contributed significantly to this speciation event within a locally unstable but on a regional scale long-lived wetland (Cohen and Johnston, 1987; Wouters and Martens, 1994; Wilkinson et al., 2006).
5.3. Biostratigraphical remarks
“One of the frustrating problems for a palaeontologist is the dating of Cenozoic fresh and brackish water deposits. They often contain only endemic, and specialised, environmentally controlled faunas.” (van den Bold, 1986, p. 141). Large progress is achieved during the last two decades but – at least in detail – this statement still characterises the “assumption-laden” (Wesselingh, 2008, p. 15) situation of western Amazonia's stratigraphy.
Until now, palynological biozonations are still used for correlations across Amazonia and provide a rough temporal framework (Hoorn, 1993, 1994). Nevertheless, definitions of pollen zones and their chronostratigraphic allocation are far beyond from settled down (Jaramillo et al., 2010; Latrubesse et al., 2010; Silva-Caminha et al., 2010; Dino et al., 2012; see also Jaramillo et al., 2011 for detailed palynological zonations of the Colombian Llanos Basin). An in-depth appraisal of the mollusc contents resulted in a detailed mollusc biozonation, which is, however, tightly linked to the palynological concept (Wesselingh et al., 2006b). Similarly, Muñoz-Torres et al. (2006) proposed an ostracod biozonation for the Pebas/Solimões Formation, based on a comprehensive analysis of Peruvian, Colombian and Brazilian faunas. Again, the chronostratigraphic calibration of the zones follows palynostratigraphy.
Recently, Wesselingh and Ramos (2010; Wesselingh, 2008) adjusted the chronostratigraphic framework of mollusc and ostracod zones by placing the end of the “Pebas system” to ∼11 Ma in accordance to the onset of the modern Amazon system as estimated by Figueiredo et al. (2009; note that other authors argue for a latter onset; e.g., Wesselingh et al., 2006b; Hoorn et al., 2010a (Late Miocene); Latrubesse et al., 2010 (Latest Miocene–Early Pliocene); Campbell, 2010 (Late Pliocene); Fig. 8).
The herein reported ostracod taxa exhibit long ranges based on the available literature data (Muñoz-Torres et al., 2006; mainly early Middle to early Late Miocene according to the proposed chronology of Wesselingh and Ramos, 2010) and do not permit a more precise biostratigraphical dating today. Key taxa sensu Muñoz-Torres et al. (2006) are missing in the Eirunepé material. Only the co-occurrence of C. graciosa and C. longispina may hint to the C. obliquosulcata zone (latest Middle Miocene after Wesselingh and Ramos, 2010; but: early Late Miocene after Wesselingh et al., 2006a,b; Fig. 8).
The mollusc faunas of Aquidabã and Torre da Lua are considered by Wesselingh et al. (2006a,b) to slightly post-date the Pebas fauna and to be of Late Miocene origin (possibly ∼9 Ma; but Wesselingh and Ramos, 2010: late Middle Miocene). However, based on the occurrence of some palynological index taxa a Late Miocene age (probably Asteraceae zone sensu Lorente, 1986; Jaramillo et al., 2010) is proposed (Gross et al., 2011; Paz et al., in press). This allocation is strongly supported by the vertebrate fauna of Torre da Lua (Ramos, 2006), which belongs to the well documented “Acre fauna” and is correlated to the Late Miocene (Huayquerian South American Land Mammal Age; ∼9.0–6.5 Ma; Cozzuol, 2006; Latrubesse et al., 2007, 2010; Negri et al., 2010). In view of these palaeontological indications as well as the palaeogeographic background of this region (Latrubesse et al., 1997, 2007, 2010; Cozzuol, 2006) a Late Miocene age (<9 Ma?) seems most plausible to date (Fig. 8).
6. Conclusions
The taxonomical analysis of ostracod faunas from the upper part of the Solimões Formation (Eirunepé area) documents a moderately diverse assemblage (19 species). Freshwater genera (particularly Cytheridella and Penthesilenula; ∼3/2 of the total fauna) were found co-occurring with usually brackish water indicating taxa (especially Cyprideis; ∼1/3). Stable isotope analyses (δ18O, δ13C), performed on both groups, furnished for all taxa very negative values throughout and refer to exclusively freshwater conditions. This is in concert with the fluvial sedimentation model presented earlier (Gross et al., 2011). Consequently, the occurrence of Cyprideis and probably of some other “brackish/marine” taxa (Perissocytheridea, Rhadinocytherura) is per se no general indicator for the presence brackish waters or the influx of marine waters in this region as mentioned previously (e.g., Purper, 1979; Whatley et al., 1998) but already challenged by Ramos (2006) and Latrubesse et al. (2010). In combination with sedimentological interpretations a rough, hydrodynamically controlled differentiation of the faunas is suggested (more fluvially influenced: Cyprideis–Penthesilenula-dominated; less fluvially characterised: Cytheridella-dominated).
Based on the taxonomical composition, a relation to the Middle–(early) Late Miocene faunas of the “Pebas system” is obvious. Eight species of Cyprideis were found, which are clearly related to the Pebasian Cyprideis species flock. However, due to the lack of index taxa sensu Muñoz-Torres et al. (2006) among ostracods, we consider the fauna of Eirunepé to post-date the “Pebas system” as signified by other palaeontolgical evidences. We propose a Late Miocene age (<9 Ma?), somewhat prior to the effective installation of the modern Amazon system.
Acknowledgements
This study was financially supported by the Austrian Science Fund project P21748-N21. We acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico/Ministério da Ciência e Tecnologia (CNPq/MCT; process number EXC 010389/2009-1) as well as the Departamento Nacional de Produção Mineral (DNPM/Manaus, especially Gert Woeltje) for sampling and shipping licences. The authors are grateful to Jackson Douglas da Silva Paz (Universidade Federal de Mato Grosso), Miguel Sombra (Eirunepé) and Norbert Winkler (Joanneum, Graz) for their assistance during fieldwork. Finally, we would like to thank the two reviewers of this paper – Elsa Gliozzi and Edgardo M. Latrubesse – for their constructive comments.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jsames.2012.10.002.
Contributor Information
Martin Gross, Email: martin.gross@museum-joanneum.at.
Maria Ines Ramos, Email: mramos@museu-goeldi.br.
Marco Caporaletti, Email: marco.caporaletti@uni-graz.at.
Werner E. Piller, Email: werner.piller@uni-graz.at.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alvarez Zarikian C.A., Swart P.K., Gifford J.A., Blackwelder P.L. Holocene paleohydrology of Little Salt Spring, Florida, based on ostracod assemblages and stable isotopes. Palaeogeography, Palaeoclimatology, Palaeoecology. 2005;225:134–156. [Google Scholar]
- Anderson L.C., Wesselingh F.P., Hartman J.H. A phylogenetic and morphologic context for the radiation of an endemic fauna in a long-lived lake: Corbulidae (Bivalvia; Myoidea) in the Miocene Pebas Formation of western Amazonia. Paleobiology. 2010;36:534–554. [Google Scholar]
- Artheau M. Geographical review of the ostracod genus Vestalenula (Darwinulidae) and a new subterranean species from southern France. Invertebrate Systematics. 2007;21:471–486. [Google Scholar]
- Ballent S.C., Díaz A.R. Contribution to the taxonomy, distribution and paleoecology of the early representatives of Penthesilenula Rossetti & Martens, 1998 (Crustacea, Ostracoda, Darwinulidae) from Argentina, with the description of a new species. Hydrobiologia. 2011 doi: 10.1007/s10750-011-0658-8. [DOI] [Google Scholar]
- Campbell K.E. Late Miocene onset of the Amazon River and the Amazon deep-sea fan: evidence from the Foz do Amazonas Basin: Comment. Geology. 2010;38:212. [Google Scholar]
- Caputo M.V. Solimões megashear: intraplate tectonics in northwestern Brazil. Geology. 1991;19:246–249. [Google Scholar]
- Celestino, E.A., Ramos, M.I.F., 2007. Sistemática de ostracodes e reconstruçã paleoambiental no Neógeno da bacia do Solimões, Formação Solimões, sudoeste do estada do Amazonas. In: XII Congresso Latino-Americano de Ciências do Mar, Florianópolis, Associação Brasileira de Oceanografia, pp. 1–3.
- Cisneros J.C. New Pleistocene vertebrate fauna from El Salvador. Revista Brasileira de Paleontologia. 2005;8:239–255. [Google Scholar]
- Cohen A.S., Johnston M.R. Speciation in brooding and poorly dispersing lacustrine organisms. Palaios. 1987;2:426–435. [Google Scholar]
- Colin J.-P., Tambareau Y., Krasheninikov V.A. Ostracodes limniques et lagunaires dans le Crétacé supérieur du Mali (Afrique de l'Ouest): systématique, paléoécologie et affinités paléobiogéographiques. Revue de Micropaléontologie. 1996;39:211–222. [Google Scholar]
- Colin J.-P., Tambareau Y., Krasheninikov V.A. An early record of the genus Cytheridella Daday, 1905 (Ostracoda, Limnocytheridae, Timiriaseviinae) from the Upper Cretaceous of Mali, West Africa: palaeobiogeographical and palaeoecological considerations. Journal of Micropalaeontology. 1997;16:91–95. [Google Scholar]
- Cozzuol M.A. The Acre vertebrate fauna: age, diversity, and geography. Journal of South American Earth Sciences. 2006;21:185–203. [Google Scholar]
- De Deckker P., Chivas A.R., Shelley J.M.G. Uptake of Mg and Sr in the euryhaline ostracod Cyprideis determined from in vitro experiments. Palaeogeography, Palaeoclimatology, Palaeoecology. 1999;148:105–116. [Google Scholar]
- Del' Arco J.O., Santos R.O.B., Rivetti M., Olivera Alves E.D., Fernandes C.A.C., Silva L.L. Folha SB. 19 Juruá. I–Geologia. Projeto Radambrasil. Levantamento de Recursos Naturais. 1977;15:19–88. [Google Scholar]
- Dino R., Soares E.A.A., Antonioli L., Riccomini C., Nogueira A.C.R. Palynostratigraphy and sedimentary facies of Middle Miocene fluvial deposits of the Amazonas Basin, Brazil. Journal of South American Earth Sciences. 2012;34:61–80. [Google Scholar]
- Ferrero L. Paleoecologia de ostrácodos holocenos del estuario del Rio Quequén Grande (Provincia de Buenos Aires) Ameghiniana. 1996;33:209–222. [Google Scholar]
- Figueiredo J.J.P. Comment by J.P. Figueiredo, & Hoorn, C. on ‘Late Miocene sedimentary environments in south-western Amazonia (Solimões Formation; Brazil)’ by Martin Gross, Werner E. Piller, Maria Ines Ramos, Jackson Douglas da Silva Paz. Journal of South American Earth Sciences. 2012;35:74–75. doi: 10.1016/j.jsames.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo J., Hoorn C., Ven van der P., Soares E. Late Miocene onset of the Amazon River and the Amazon deep-sea fan: evidence from the Foz do Amazonas Basin. Geology. 2009;37:619–622. [Google Scholar]
- Frenzel P., Boomer I. The use of ostracods from marginal marine, brackish waters as bioindicators of modern and Quaternary environmental change. Palaeogeography, Palaeoclimatology, Palaeoecology. 2005;225:68–92. [Google Scholar]
- Furtos N.C. Two new species of Cypretta (Ostracoda) from the Marquesas Island and Florida with notes on the distribution of the genus. Bernice P. Bishop Museum Bulletin. 1934;114:279–286. [Preprint 1934, Pacific Entomological Survey, article 21] [Google Scholar]
- Gingras M.J., Räsänen M.E., Pemberton S.G., Romero L.P. Ichnology and sedimentology reveal depositional characteristics of bay-margin parasequences in the Miocene Amazonian foreland basin. Journal of Sedimentary Research. 2002;72:871–883. [Google Scholar]
- Gross M., Minati K., Danielopol D.L., Piller W.E. Environmental changes and diversification of Cyprideis in the Late Miocene of the Styrian Basin (Lake Pannon, Austria) Senckenbergiana Lethaea. 2008;88:161–181. [Google Scholar]
- Gross M., Piller W.E., Ramos M.I., Paz J.D.S. Late Miocene sedimentary environments in south-western Amazonia (Solimões Formation; Brazil) Journal of South American Earth Sciences. 2011;32:169–181. doi: 10.1016/j.jsames.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman E.L., Ku T.-L. Oxygen and carbon isotope fractionation in biogenic aragonite: temperature effects. Chemical Geology. 1986;59:59–74. [Google Scholar]
- Haffer J. Hypotheses to explain the origin of species in Amazonia. Brazilian Journal of Biology. 2008;68:917–947. doi: 10.1590/s1519-69842008000500003. Supplement. [DOI] [PubMed] [Google Scholar]
- Hammer Ø., Harper D. Blackwell; Oxford: 2008. Paleontological Data Analysis. 351 pp. [Google Scholar]
- Hartmann, G., 1989. Ostracoda. Dr. H.G. Bronns Klassen und Ordnungen des Tierreichs, 5, 1. Abteilung, 2. Buch, 4. Teil, 5. Lieferung, VEB Gustav Fischer Verlag, Jena, pp. 787–1067.
- Higuti J., Velho L.F.M., Lansac-Tôha F.A., Martens K. Pleuston communities are buffered from regional flood pulses: the example of ostracods in the Paraná River floodplain, Brazil. Freshwater Biology. 2007;52:1930–1943. [Google Scholar]
- Higuti J., Lansac-Tôha F.A., Velho L.F.M., Pinto R.L., Vieira L.C.G., Martens K. Composition and distribution of Darwinulidae (Crustacea, Ostracoda) in the alluvial valley of the upper Paraná River, Brazil. Brazilian Journal of Biology. 2009;69:253–262. doi: 10.1590/s1519-69842009000200004. [DOI] [PubMed] [Google Scholar]
- Higuti J., Lansac-Tôha F.A., Velho L.F.M., Martens K. Biodiversity of non-marine ostracods (Crustacea, Ostracoda) in the alluvial valley of the upper Paraná River, Brazil. Brazilian Journal of Biology. 2009;69:661–668. [PubMed] [Google Scholar]
- Higuti J., Declerck S.A.J., Lansac-Tôha F.A., Velho L.F.M., Martens K. Variation in ostracod (Crustacea, Ostracoda) communities in the alluvial valley of the upper Paraná River (Brazil) in relation to substrate. Hydrobiologia. 2010;644:261–278. [Google Scholar]
- Holmes J.A. A late Quaternary ostracod record from Wallywash Great Pond, a Jamaican marl lake. Journal of Paleolimnology. 1998;19:115–128. [Google Scholar]
- Hoorn C. Marine incursions and the influence of Andean tectonics on the Miocene depositional history of northwestern Amazonia: results of a palynostratigraphic study. Palaeogeography, Palaeoclimatology, Palaeoecology. 1993;105:267–309. [Google Scholar]
- Hoorn C. An environmental reconstruction of the palaeo-Amazon River system (Middle–Late Miocene, NW Amazonia) Palaeogeography, Palaeoclimatology, Palaeoecology. 1994;112:187–238. [Google Scholar]
- Hoorn C. Mangrove forests and marine incursions in Neogene Amazonia (Lower Apaporis River, Colombia) Palaios. 2006;21:197–209. [Google Scholar]
- Hoorn C., Wesselingh F.P., editors. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. p. 464. [Google Scholar]
- Hoorn C., Wesselingh F.P., ter Steege H., Bermudez M.A., Mora A., Sevink J., Sanmartín I., Sanchez-Meseguer A., Anderson C.L., Figueiredo J.P., Jaramillo C., Riff D., Negri F.R., Hooghiemstra H., Lundberg J., Stadler T., Särkinen T., Antonelli A. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science. 2010;330:927–931. doi: 10.1126/science.1194585. [DOI] [PubMed] [Google Scholar]
- Hoorn C., Wesselingh F.P., Hovikoski J., Guerrero J. The development of the Amazonian mega-wetland (Miocene; Brazil, Colombia, Peru, Bolivia) In: Hoorn C., Wesselingh F.P., editors. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 123–142. [Google Scholar]
- Horne F.R. Ostracods of Texas playas. The Southwestern Naturalist. 1996;41:450–455. [Google Scholar]
- Hovikoski J., Gingras M., Räsänen M., Rebata L.A., Guerrero J., Ranzi A., Melo J., Romero L., Nuñez del Prado H., Jaimes F., Lopez S. The nature of Miocene Amazonian epicontinental embayment: high-frequency shifts of the low-gradient coastline. Geological Society of America Bulletin. 2007;119:1506–1520. [Google Scholar]
- Hovikoski J., Wesselingh F.P., Räsänen M., Gingras M., Vonhof H.B. Marine influence in Amazonia: evidence from the geological record. In: Hoorn C., Wesselingh F.P., editors. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 143–161. [Google Scholar]
- Jahn A., Gamenick I., Theede H. Physiological adaptations of Cyprideis torosa (Crustacea, Ostracoda) to hydrogen sulphide. Marine Ecology Progress Series. 1996;142:215–223. [Google Scholar]
- Jaramillo C., Hoorn C., Silva S.A.F., Leite F., Herrera F., Quiroz L., Dino R., Antonioli L. The origin of the modern Amazon rainforest: implications of the palynological and palaeobotanical record. In: Hoorn C., Wesselingh F.P., editors. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 317–334. [Google Scholar]
- Jaramillo C.A., Rueda M., Torres V. A palynological zonation for the Cenozoic of the Llanos and Llanos Foothills of Colombia. Palynology. 2011;35:46–84. [Google Scholar]
- Kaandorp R.J.G., Vonhof H.B., Wesselingh F.P., Romero Pittman L., Kroon D., Van Hinte J.E. Seasonal Amazonian rainfall variation in the Miocene climate optimum. Palaeogeography, Palaeoclimatology, Palaeoecology. 2005;221:1–6. [Google Scholar]
- Kaandorp R.J.G., Wesselingh F.P., Vonhof H.B. Ecological implications from geochemical records of Miocene Western Amazonian bivalves. Journal of South American Earth Sciences. 2006;21:54–74. [Google Scholar]
- Keyser, D., 1976. Zur Kenntnis der brackigen mangrovenbewachsenen Weichböden Südwest-Floridas unter besonderer Berücksichtigung ihrer Ostracodenfauna thesis., University of Hamburg, Hamburg, 142 pp.
- Keyser, D., 1977. Brackwasser-Cytheracea aus Süd-Florida. Abhandlungen und Verhandlungen des naturwissenschaftlichen Vereins in Hamburg, Neue Folge, 20, pp. 43–85.
- Krstić N. Ostracodes des couches congeriennes: 1. Cyprideis I. Bulletin du Museum d’Histoire Naturelle, A. 1968;23:107–151. [Google Scholar]
- Krstić N. Ostracodes des couches congeriennes: 3. Cyprideis II. Bulletin du Museum d’Histoire Naturelle, A. 1968;23:153–183. [Google Scholar]
- Latrubesse E.M., Bocquentin J., Santos J.C.R., Ramonell C.G. Paleoenvironmental model for the late Cenozoic of southwestern Amazonia: paleontology and geology. Acta Amazonica. 1997;27:103–118. [Google Scholar]
- Latrubesse E., Silva S.A.F., Cozzuol M., Absy M.L. Late Miocene continental sedimentation in the southwestern Amazonia and its regional significance: biotic and geological evidence. Journal of South American Earth Sciences. 2007;23:61–80. [Google Scholar]
- Latrubesse E.M., Cozzuol M., Silva-Caminha S.A.F., Rigsby C.A., Absy M.L., Jaramillo C. The Late Miocene paleogeography of the Amazon Basin and the evolution of the Amazon River system. Earth-Science Reviews. 2010;99:99–124. [Google Scholar]
- Leng M.J., Marshall J.D. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quaternary Science Reviews. 2004;23:811–831. [Google Scholar]
- Linhares A.P., Ramos M.I.F., Gross M., Piller W.E. Evidence for marine influx during the Miocene in southwestern Amazonia, Brazil. Geología Colombiana. 2011;36:91–103. [Google Scholar]
- Lister K.H. The University of Kansas Paleontological contributions; Utah: 1975. Quaternary Freshwater Ostracoda from the Great Salt Lake Basin. Paper 78, 1–34. [Google Scholar]
- Löffler H. Zur Systematik und Ökologie der chilenischen Süßwasserentomostraken. Beiträge zur neotropischen Fauna. 1961;2:197–206. [Google Scholar]
- Lorente, M.A., 1986. Palynology and Palynofacies of the Upper Tertiary in Venezuela. Dissertationes Botanicae, vol. 99, pp. 1–225.
- Maia, R.G.N., Godoy, H.K., Yamaguti, H.S., Moura, P.A., Costa, F.S.F., Holanda, M.A., Costa, J.A., 1977. Projeto Carvão no Alto Solimões. Relatório Final. Companhia de Pesquisa de Recursos Minerais-Departamento Nacional da Produçáo Mineral, Manaus, 142 pp.
- Malz H. Cypridopsine Ostracoden aus dem Tertiär des Mainzer Beckens. Senckenbergiana Lethaea. 1977;58:219–261. [Google Scholar]
- Martens K., Savatenalinton S. A subjective checklist of the Recent, free-living non-marine Ostracoda (Crustacea) Zootaxa. 2011;2855:1–79. [Google Scholar]
- Martens K., Rossetti G., Horne D.J. How ancient are ancient asexuals? Proceedings of the Royal Society of London, B. 2003;270:723–729. doi: 10.1098/rspb.2002.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens K., Schön I., Meisch C., Horne D.J. Global diversity of ostracods (Ostracoda, Crustacea) in freshwater. Hydrobiologia. 2008;595:185–193. [Google Scholar]
- Mebrouk F., Colin J.-P., Hennache F. Un gisement d'ostracodes non-marins dans l'Éocène inférieur du Djebel Amour, Atlas saharien central, Algérie: taxonomie, paléoécologie et paléobiogéographie. Carnets de Géologie (Notebooks on Geology) 2011;2011/04:83–97. [Google Scholar]
- Meisch C. Freshwater ostracoda of western and central Europe. In: Schwoerbel J., Zwick P., editors. vol. 8/3. Spektrum Akademischer Verlag; Heidelberg/Berlin: 2000. (Süßwasserfauna von Mitteleuropa). 522 pp. [Google Scholar]
- Minati K., Cabral M.C., Pipík R., Danielopol D.L., Linhart J., Neubauer W. Morphological variability among European populations of Vestalenula cylindrica (Straub) (Crustacea, Ostracoda) Palaeogeography, Palaeoclimatology, Palaeoecology. 2008;264:296–305. [Google Scholar]
- Muñoz-Torres F., Whatley R.C., Van Harten D. The endemic non-marine Miocene ostracod fauna of the Upper Amazon Basin. Revista Española de Micropaleontología. 1998;30:89–105. [Google Scholar]
- Muñoz-Torres F.A., Whatley R.C., Van Harten D. Miocene ostracod (Crustacea) biostratigraphy of the upper Amazon Basin and evolution of the genus Cyprideis. Journal of South American Earth Sciences. 2006;21:75–86. [Google Scholar]
- Namiotko T., Danielopol D.L. Review of the eremita species-group of the Pseudocandona Kaufmann (Ostracoda, Crustacea), with the description of a new species. Revista Española de Micropaleontología. 2004;36:109–125. [Google Scholar]
- Negri F.R., Bocquentin-Villanueva J., Ferigolo J., Antoine P.-O. A review of Tertiary mammal faunas and birds from western Amazonia. In: Hoorn C., Wesselingh F.P., editors. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 245–258. [Google Scholar]
- Paz, J.D.S., Ramos, M.I.F., Moraes-Santos, H.M., Silva, S. Depósitos fluviais da formação Solimões, Bacia do Solimões, Eirunepé (AM), Brasil. Latin American Journal of Sedimentology and Basin Analysis, in press.
- Pérez L., Lorenschat J., Brenner M., Scharf B., Schwalb A. Extant freshwater ostracodes (Crustacea: Ostracoda) from Lago Petén Itzá, Guatemala. Revista de Biologia Tropical. 2010;58:871–895. doi: 10.15517/rbt.v58i2.5252. [DOI] [PubMed] [Google Scholar]
- Pérez L., Lorenschat J., Bugja R., Brenner M., Scharf B., Schwalb A. Distribution, diversity and ecology of modern freshwater ostracodes (Crustacea), and hydrochemical characteristics of Lago Petén Itzá, Guatemala. Journal of Limnology. 2010;69:146–159. [Google Scholar]
- Pinto I., Ornellas L.P. A new brackishwater ostracode Perissocytheridea krömmelbeini Pinto & Ornellas, sp. nov., from southern Brazil. Escola de Geologia de Porto Alegre, Publicação Especial. 1970;20:1–19. [Google Scholar]
- Pinto R.L., Rocha C.E.F., Martens K. On the genus Penthesilenula Rossetti and Martens, 1998 (Crustacea, Ostracoda, Darwinulidae) from (semi-) terrestrial habitats in São Paulo State (Brazil), with the description of a new species. Journal of Natural History. 2004;38:2567–2589. [Google Scholar]
- Purper I. Cytheridella boldii Purper, sp. nov. (Ostracoda) from Venezuela and a Revision of the Genus Cytheridella Daday, 1905. Anais da Academia Brasileira de Ciências. 1974;46:635–662. [Google Scholar]
- Purper I. Some ostracodes from the Upper Amazon Basin. Brazil. Environment and age. In: Löffler H., Danielopol D.L., editors. Aspects of Ecology and Zoogeography of Recent and Fossil Ostracoda. W. Junk Publishers; The Hague: 1977. pp. 353–367. [Google Scholar]
- Purper I. Cenozoic Ostracodes of the upper Amazon Basin, Brazil. Pesquisas. 1979;12:209–281. [Google Scholar]
- Purper I., Ornellas L.P. New Ostracodes of the endemic fauna of the Pebas Formation, Upper Amazon Basin, Brazil. Pesquisas. 1991;18:25–30. [Google Scholar]
- Purper I., Pinto I.D. New genera and species of ostracodes of the Upper Amazon Basin, Brasil. Pesquisas. 1983;15:113–126. [Google Scholar]
- Purper, I., Pinto, I.D., 1985. New data and new ostracods from Pebas Formation – Upper Amazon Basin. In: 8° Congresso Brasileiro de Paleontologia, Rio de Janeiro, 1985, Seria Geologia, Paleontologia/Estratigrafia, DNPM vol.27, pp. 427–441.
- Purper I., Würdig-Maciel N.L. Occurrence of Heterocypris incongruens (Ramdohr), 1808 – Ostracoda – in Rio Grande do Sul, Brazil. Discussion on the allied genera: Cyprinotus, Hemicypris, Homocypris and Eucypris. Pesquisas. 1974;3:69–91. [Google Scholar]
- Ramos M.I.F. Ostracods from the Neogene Solimões Formation (Amazonas, Brazil) Journal of South American Earth Sciences. 2006;21:87–95. [Google Scholar]
- Ramos M.I.F., Santos H.M.M., Costa S.A.R.F., Toledo P.M. Museu Paranese Emilio Goeldi; Belém: 2009. CatáIogo de Fósseis. (CoIeção Paleontológica do Museu Paraense Emilio Goeldi). 172 pp. [Google Scholar]
- Reheis, M.C., Laabs, B.J.C., Forester, R.M., McGeehin, J.P., Kaufman, D.S., Bright, J., 2005. Surficial Deposits in the Bear Lake Basin. U.S. Geological Survey, Open-File Report 2005-1088, pp. 1–30.
- Rossetti G., Martens K. Taxonomic revision of the Recent and Holocene representatives of the family Darwinulidae (Crustacea, Ostracoda), with a description of three new genera. Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen, Biologie. 1998;68:55–110. [Google Scholar]
- Rossetti G., Pinto R.L., Martens K. Description of a new genus and two new species of Darwinulidae (Crustacea, Ostracoda), from Christmas Island (Indian Ocean) with some considerations on the morphological evolution of ancient asexuals. Belgian Journal of Zoology. 2011;141:55–74. [Google Scholar]
- Ruskin B.G., Dávila F.M., Hoke G.D., Jordan T.E., Astini R.A., Alonso R. Stable isotope composition of middle Miocene carbonates of the Frontal Cordillera and Sierras Pampeanas: did the Paranaense seaway flood western and central Argentina? Palaeogeography, Palaeoclimatology, Palaeoecology. 2011;308:293–303. [Google Scholar]
- Sandberg P.A. The ostracod genus Cyprideis in the Americas. Acta Universitatis Stockholmiensis. 1964;12:1–178. [Google Scholar]
- Sheppard L.M., Bate R.H. Plio-Pleistocene ostracods from the Upper Amazon of Colombia and Peru. Palaeontology. 1980;23:97–124. [Google Scholar]
- Silva-Caminha S.A.F., Jaramillo C.A., Absy M.L. Neogene palynology of the Solimões Basin, Brazilian Amazonia. Palaeontographica, B. 2010;284:13–79. [Google Scholar]
- Smith A.J., Delorme L.D. Chapter 19. Ostracoda. In: Thorp J.H., Covich A.L., editors. Ecology and Classification of North American Freshwater Invertebrates. Academic Press; London: 2010. pp. 725–772. [Google Scholar]
- Smith R.J., Kamiya T. The ontogeny of two species of Darwinuloidea (Ostracoda, Crustacea) Zoologischer Anzeiger. 2008;247:275–302. [Google Scholar]
- Sohn I.G., Kornicker L.S. Morphology of Cypretta kawatai Sohn and Kornicker, 1972 (Crustacea, Ostracoda), with a discussion of the genus. Smithsonian Contributions to Zoology. 1973;141:1–28. [Google Scholar]
- Swain F.M. 1998. Ostracoda from the Pliocene? Pebas Formation at Iquitos, Peru.http://www.geo.umn.edu/people/profs/swain/iquitos_Peru.pdf [Google Scholar]
- Tressler W.L. Fresh-water Ostracoda from Brazil. Proceedings of the United States National Museum. 1949;100:61–83. [Google Scholar]
- Triebel E. Eine fossile Pelocypris (Crust., Ostr.) aus El Salvador. Senckenbergiana Lethaea. 1953;34:1–4. [Google Scholar]
- Van Doninck K., Schön I., Maes F., Bruyn L., Martens K. Ecological strategies in the ancient asexual animal group Darwinulidae (Crustacea, Ostracoda) Freshwater Biology. 2003;48:1285–1294. [Google Scholar]
- van den Bold W.A. Fresh and brackish water ostracoda from the Neogene of northern Venezuela. Tulane Studies in Geology and Paleontology. 1986;19:141–157. [Google Scholar]
- Van Harten D. Some new shell characters to diagnose the species of the Ilyocypris gibba-biplicata-bradyi group and their ecological significance. In: Krstić N., editor. Taxonomy, Biostratigraphy and Distribution of Ostracods. Serbian Geological Society; Beograd: 1979. pp. 71–76. [Google Scholar]
- Van Harten D. The Neogene evolutionary radiation in Cyprideis Jones (Ostracoda: Cytheracea) in the Mediterranean area and the Paratethys. Courier Forschungsinstitut Senckenberg. 1990;123:191–198. [Google Scholar]
- Van Morkhoven F.P.C.M. vol 2. Elsevier Publishing Company; Amsterdam/London/New York: 1963. Post-palaeozoic Ostracoda, Their Morphology, Taxonomy, and Economic Use. (Generic Descriptions). 478 pp. [Google Scholar]
- Victor R., Fernando C.H. Freshwater Ostracods (Crustacea: Ostracoda) of the genus Cypretta Vavra, 1895 from Malaysia, Indonesia and the Philippines. Internationale Revue der gesamten Hydrobiologie und Hydrographie. 1981;66:415–433. [Google Scholar]
- Vonhof H.B., Wesselingh F.P., Kaandorp R.J.G., Davies G.R., Van Hinte J.E., Guerrero J., Räsänen M., Romero-Pittman L., Ranzi A. Paleogeography of Miocene Western Amazonia: Isotopic composition of molluscan shells constrains the influence of marine incursions. Geological Society of America Bulletin. 2003;115:983–993. [Google Scholar]
- Wanderley-Filho J.R., Eiras J.F., Cunha P.R.C., Ven van der P.H. The Paleozoic Solimões and Amazonas basins and the Acre foreland basin of Brazil. In: Hoorn C., Wesselingh F.P., editors. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 29–37. [Google Scholar]
- Wesselingh F.P. Long-lived lake molluscs as island faunas: a bivalve perspective. In: Renema W., editor. Biogeography, Time, and Place: Distributions, Barriers, and Islands. Springer; Dordrecht: 2007. pp. 275–314. [Google Scholar]
- Wesselingh F.P. Molluscan radiations and landscape evolution in Miocene Amazonia. Annales Universitates Turkuensis, Biologica-Geographica-Geologica. 2008;232:1–41. [Google Scholar]
- Wesselingh F.P., Ramos M.I.F. Amazonian aquatic invertebrate faunas (Mollusca, Ostracoda) and their development over the past 30 million years. In: Hoorn C., Wesselingh F.P., editors. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 302–316. [Google Scholar]
- Wesselingh F.P., Räsänen M.E., Irion G., Vonhof H.B., Kaandorp R., Renema W., Romero Pittman L., Gingras M. Lake Pebas: a palaeoecological reconstruction of a Miocene, long-lived lake complex in western Amazonia. Cainozoic Research. 2002;1:35–81. [Google Scholar]
- Wesselingh F.P., Ranzi A., Räsänen M.E. Miocene freshwater Mollusca from western Brazilian Amazonia. Scripta Geologica. 2006;133:419–437. [Google Scholar]
- Wesselingh F.P., Hoorn M.C., Guerrero J., Räsänen M.E., Romero Pittmann L., Salo J. The stratigraphy and regional structure of Miocene deposits in western Amazonia (Peru, Colombia and Brazil), with implications for late Neogene landscape evolution. Scripta Geologica. 2006;133:291–322. [Google Scholar]
- Wesselingh F.P., Kaandorp R.J.G., Vonhof H.B., Räsänen M.E., Renema W., Gingras M. The nature of aquatic landscapes in the Miocene of western Amazonia: an integrated palaeontological and geochemical approach. Scripta Geologica. 2006;133:363–393. [Google Scholar]
- Wesselingh F.P., Hoorn C., Kroonenberg S.B., Antonelli A., Lundberg J.G., Vonhof H.B., Hooghiemstra H. On the origin of Amazonian landscapes and biodiversity: a synthesis. In: Hoorn C., Wesselingh F.P., editors. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 421–431. [Google Scholar]
- Whatley R.C. Population structure of ostracods: some general principles for the recognition of palaeoenvironments. In: De Deckker P., Colin J.P., Peypouquet J.P., editors. Ostracoda in the Earth Sciences. Elsevier; Amsterdam: 1988. pp. 245–256. [Google Scholar]
- Whatley R.C., Muñoz-Torres F., Van Harten D. The Ostracoda of an isolated Neogene saline lake in the western Amazon Basin, Peru. Bulletin du Centres de Recherches Elf Exploration-Production, Memoires. 1998;20:231–245. [Google Scholar]
- Whatley R.C., Muñoz-Torres F., Van Harten D. Skopaeocythere: a minute new limnocytherid (Crustacea, Ostracoda) from the Neogene of the Amazon Basin. Ameghiniana. 2000;37:163–167. [Google Scholar]
- Wilkinson M.J., Marshall L.G., Lundberg J.G. River behavior on megafans and potential influences on diversification and distribution of aquatic organisms. Journal of South American Earth Sciences. 2006;21:151–172. [Google Scholar]
- Wouters K., Martens K. Contribution to the knowledge of the Cyprideis species flock (Crustacea: Ostracoda) of Lake Tanganyika, with the description of three new species. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie. 1994;64:111–128. [Google Scholar]
- Wouters K., Martens K. On the Cyprideis species flock (Crustacea, Ostracoda) in Lake Tanganyika, with the description of four new species. Hydrobiologia. 2001;450:111–127. [Google Scholar]
- Wouters K., Martens K. Three new species of the Cyprideis species flock (Crustacea, Ostracoda) of Lake Tanganyika (East Africa) Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie. 2007;77:147–160. [Google Scholar]
- Würdig N.L., Freitas S.F., Fausto I.V. Comunidade de ostracods associada ao bentos e nacrofitas aquatica da lagoa do Gentil, Tramandai, Rio Grande do Sul. Acta Limnologica Brasiliensia. 1990;3:807–828. [Google Scholar]
- Yang F., Sun Z., Zhang Y., Qiao Z. Taxonomic significance of nodal ornamentation of Quaternary genus Ilyocypris (Ostracoda) from Qaidam Basin, Qinghai province. Acta Micropalaeontologica Sinica. 2002;19:15–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.