Abstract
Background
Night-shift work is suggested to be associated with an increased risk of breast cancer, but its association with prostate cancer is still controversial. We examined this association by conducting a systematic review and meta-analysis.
Methods
Studies were identified by searching PubMed, EMBASE, Ovid, Web of Science, the Cochrane register, and the China National Knowledge Infrastructure databases through December 25, 2014. Summary relative risks (SRRs) with their corresponding 95% confidence intervals (CIs) were calculated using a random effects or fixed effects model. Heterogeneity and publication bias were also evaluated.
Results
A total of 2,459,845 individuals from eight published studies were included in this meta-analysis. Analysis of all studies suggested that night-shift work was associated with a significantly increased risk of prostate cancer (RR: 1.24, 95% CI: 1.05–1.46; P=0.011). Sensitivity analysis showed that the association remained significant when repeating the analysis after removing one study each time. Dose–response meta-analysis suggested that an increase in night-shift work of 5 years duration was statistically significantly associated with a 2.8% (95% CI: 0.3, 5.4%, P=0.030) increase in the risk of prostate cancer. There was no significant publication bias.
Conclusion
Based on a meta-analysis, night-shift work is associated with an increased risk of prostate cancer. Because of the limited number of included studies and the large level of heterogeneity, further well-designed studies are still warranted to confirm the findings of our analysis.
Keywords: shift work, prostate cancer, risk, meta-analysis
Introduction
Prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men, with over 913,000 newly diagnosed cases and over 261,000 deaths in 2008.1 Apart from some established risk factors, including age, race/ethnicity, and family history,2 a growing number of other protective and risk factors have been examined. For example, certain vegetables, such as soy and carrot, have been linked to the reduction of prostate cancer risk.3,4 There is also some evidence that firefighters may have an increased risk of prostate cancer.5,6 In addition to the exposure to toxic combustion products, firefighters’ shift work schedule may partly explain this association.
In 2007, the International Agency for Research on Cancer designated shift work involving circadian disruption as a probable carcinogen in humans on the basis of sufficient evidence in vitro studies, but limited evidence in epidemiologic studies.7 In addition to extensive animal and in vitro studies, the main reason for this classification is evidence from data showing an increased risk of breast cancer among long-term female night-shift workers (eg, nurses and stewardess),8,9 compared with female non-night-shift workers, which was further confirmed by a recent updated meta-analysis.10 However, the meta-analyses performed by Kamdar et al11 and Ijaz et al12 indicated that there was weak or insufficient evidence to support previous reports that night-shift work was associated with an increased risk of breast cancer. It has been suggested that a common mechanism may be shared in hormone-dependent cancers in both men and women,13 and several studies have investigated the association between night-shift work and the risk of prostate cancer in men. However, the results of these studies were inconsistent. For example, one Japanese cohort study and two Canadian case–control studies have suggested a positive association between night-shift work and the risk of prostate cancer,14–16 while three cohort studies conducted in Europe did not support such an association.17–19
Given the importance of this potential association in both clinical practice and public health, we performed a meta-analysis of all eligible studies to derive a more precise estimation of the relationship between night-shift work and the risk of prostate cancer.
Methods
This systematic review and meta-analysis was performed according to meta-analysis of observation studies in epidemiology guidelines.20
Publication search
We searched EMBASE, PubMed, Ovid, Web of Science, the Cochrane register, and the China National Knowledge Infrastructure databases for studies published from January 1966 to December 25, 2014. The search strategy included terms for exposure (night shift or night work or shift work or work at night or shiftwork or light at night) and outcome (prostate neoplasm or prostate cancer or prostate tumor). No language restriction was applied. We also checked the cited references from retrieved articles and reviews for additional studies.
Inclusion criteria
Articles included in this meta-analysis had to meet all of the following criteria: 1) studies had a cohort or case–control design; 2) one of the exposures was night-shift work; 3) one of the outcomes was prostate cancer risk; and 4) studies provided effect estimates with their 95% confidence intervals (CIs), or data to calculate them. If multiple publications from the same or overlapping populations were available, the most recent or comprehensive information was included in this meta-analysis.
Quality assessment
The quality of each included study was assessed by two investigators using the Newcastle–Ottawa Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). This scale is an 8-item instrument used to assess the selection of study population, study comparability, and ascertainment of the exposure or outcome for case–control or cohort studies, respectively. The total score ranged from 0 to 9, and higher scores reflected better methodological quality.
Data extraction
Data were extracted independently by two authors using a predefined data collection form, with areas of disagreement or uncertainty resolved by consensus. For each study, the following information was collected: first author’s surname, publication year, the country where the study was conducted, study design, source of participants, definition of exposure, the method of exposure assessment, the number of incident cases, effect size with 95% CI, and covariates adjusted for in the analysis. For studies that reported several multivariable-adjusted relative risks (RRs), we extracted the RR estimate that was maximally adjusted for potential confounders.
Statistical methods
RR was used to assess the relationship between night-shift work and prostate cancer risk. Studies that reported measures of odds ratio and hazard ratio were pooled as RRs because the absolute risk of prostate cancer is low.21 Similarly, standardized incidence ratio was also regarded as RR based on the assumption that the person-time of the at-risk population (night-shift workers) was substantially lower compared to the general population.11,22 In night-shift work versus daytime work meta-analyses, pooled RRs and 95% CIs were used to assess the strength of the association between night-shift work and prostate cancer with a fixed model (P≥0.10 for heterogeneity)23 or a random model (P<0.10 for heterogeneity).24 The significance of the pooled RRs was assessed by the Z-test.
Linear dose–response meta-analyses were also conducted via the method described by Greenland and Longnecker25 and Orsini et al26 to calculate study-specific slopes (linear trends) and 95% CIs. Of note, the dose–response meta-analysis required that studies reported at least three categories of night-shift work. For each study, we assigned the midpoint of the upper and lower boundaries in each category as the average of night-shift work exposure. If the upper boundary of the highest category was not provided, it was assigned to be 25% higher than the lower boundary.10,27
Heterogeneity across studies was evaluated by the Q-statistic (significance level at P<0.10) and the I2 score.28 Additionally, the Galbraith plot was used to detect the studies that contribute to heterogeneity,29 and reanalysis was performed by eliminating these studies. To evaluate the robustness of combined estimates, sensitivity analysis was performed by removing one study at a time and recalculating the remaining studies. Cumulative meta-analysis was also conducted through the assortment of studies by publication year. Publication bias was evaluated by Begg’s test (rank correlation method)30 and Egger’s test (linear regression method).31 The trim-and-fill method was also used to address publication bias.32 All statistical analyses were performed using STATA 11.0 (StataCorp, College Station, TX, USA) and a two-sided P<0.05 was considered significant, except for those specifically indicated.
Results
Literature search and study characteristics
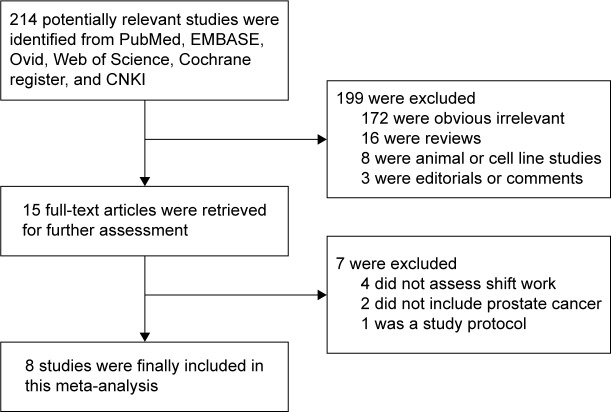
We identified eight eligible articles14–19,33,34 from the databases (Figure 1), and these included five cohort studies14,17–19,34 and three case–control studies.15,16,33 Combined, these studies included 9,669 prostate cancer cases and 2,459,845 participants. These studies were conducted in the following regions: Japan (n=2),14,34 Canada (n=2),15,16 Germany (n=1),19 US (n=1),18 Spain (n=1),33 and Sweden (n=1).17 Studies were published between 2006 and 2014. The points of study quality assessed by Newcastle–Ottawa Scale ranged from 6 to 8 (with a mean of 7.25). The characteristics of the eight eligible studies are presented in Table 1.
Figure 1.
Flowchart of study assessment and selection.
Abbreviation: CNKI, China National Knowledge Infrastructure.
Table 1.
Main characteristics of published studies on night-shift work and prostate cancer risk
| Study, region | Source of participants | Definition of exposure | NOS score | Night-shift work
|
Adjusted variables | ||
|---|---|---|---|---|---|---|---|
| Exposure | Events | RR (95% CI) | |||||
| Kubo et al,14 Japan | JACC study | Rotating-shift work, or fixed-night work is their most regular work schedules | 7 | Daytime | 21 | 1.00 | Age, study area, family history of prostate cancer, BMI, smoking, alcohol drinking, job type, physical activity at work, workplace, perceived stress, educational level, and marriage status |
| Rotating shift | 7 | 3.0 (1.2–7.7) | |||||
| Conlon et al,15 Canada | Population-based study | Rotating full-time work and other quantitative metrics related to full-time rotating-shift work | 6 | No | 391 | 1.00 | Age and family history |
| ≤7 y | 115 | 1.44 (1.10–1.87) | |||||
| 7.1–22.0 y | 87 | 1.14 (0.86–1.52) | |||||
| 22.1–34.0 y | 81 | 0.93 (0.70–1.23) | |||||
| >34.0 y | 86 | 1.30 (0.97–1.74) | |||||
| Schwartzbaum et al,17 Sweden | Population-based study | Rotating schedule with three or more possible shifts per day or had work hours during the night at least 1 day during the week | 7 | Daytime | NA | 1.04 (0.99–1.10) | Age, socioeconomic status, occupational position, and county of residence |
| Shift | NA | ||||||
| Kubo et al,34 Japan | Health-care database of a Japanese corporation | Rotating three-shift work for >80% of their career | 8 | Daytime | 13 | 1.00 | Age, BMI, alcohol intake, smoking, exercise, and marital status |
| Shift | 4 | 1.79 (0.57–5.68) | |||||
| Parent et al,16 Canada | Population-based study | Included working between 1 am and 2 am for at least 6 months | 8 | Never | 268 | 1.00 | Age, ancestry, educational level, family income, respondent status, smoking, alcohol, BMI, farming, occupational physical activity |
| 5 y | 68 | 3.13 (1.98–4.95) | |||||
| 5–10 y | 27 | 2.11 (1.11–3.99) | |||||
| >10 y | 36 | 2.68 (1.45–4.95) | |||||
| Gapstur et al,18 US | Cancer prevention study-II | Usually worked alternate shifts that fell at least partially outside the daytime shift range | 8 | Fixed day | 4,497 | 1.00 | Age, race, education, BMI, smoking status, family history of prostate cancer, and painful/frequent urination |
| Rotating | 268 | 1.08 (0.95–1.22) | |||||
| Yong et al,19 Germany | Male production workers in a German chemical company | Fast forward-rotating 12-hour shift schedules | 7 | Day work | NA | 1.00 | Age, job level, cigarette smoking, and employment duration in categories |
| Rotating shift | NA | 0.93 (0.71–1.21) | |||||
| Papantoniou et al,33 Spain | The MCC-Spain study | Work partly or entirely between 12 am and 6 am, at least three times per month | 7 | Never | 733 | 1.00 | Age, center, educational level, family history of prostate cancer, physical activity over the past decade, smoking status, past sun exposure, and daily meat consumption |
| ≤10 y | 128 | 1.10 (0.83–1.45) | |||||
| 11–27 y | 92 | 0.94 (0.69–1.27) | |||||
| ≥28 y | 138 | 1.38 (1.05–1.81) | |||||
Abbreviations: JACC, Japan Collaborative Cohort; NOS, Newcastle–Ottawa Scale; RR, relative risk; CI, confidence interval; y, year; BMI, body mass index; NA, not available; MCC, multicase-control.
Overall and subgroup analyses
Figure 2 shows the plots of the pooled risk estimates for night-shift work. We found a significantly increased risk of prostate cancer for night-shift work (RR =1.24, 95% CI: 1.05–1.46, P=0.011). In the stratified analyses, significant associations were observed for the following subgroups: studies conducted in Asia (RR =2.45, 95% CI: 1.19–5.04, P=0.015), population-based studies (RR =1.29, 95% CI: 1.07–1.55, P=0.007), number of cases >1,000 (RR =1.05, 95% CI: 1.00–1.10, P=0.049), number of cases ≤1,000 (RR =1.98, 95% CI: 1.07–3.65, P=0.030), studies controlling family history (RR =1.13, 95% CI: 1.03–1.24, P=0.007), studies controlling smoking (RR =1.41, 95% CI: 1.03–1.92, P=0.030), studies controlling alcohol (RR =2.71, 95% CI: 1.98–3.70, P<0.001), studies not controlling alcohol (RR =1.06, 95% CI: 1.01–1.10, P=0.016), studies not controlling body mass index (BMI) (RR =1.05, 95% CI: 1.00–1.10, P=0.035), and studies controlling factors >6 (RR =1.59, 95% CI: 1.06–2.37, P=0.024) (Table 2).
Figure 2.
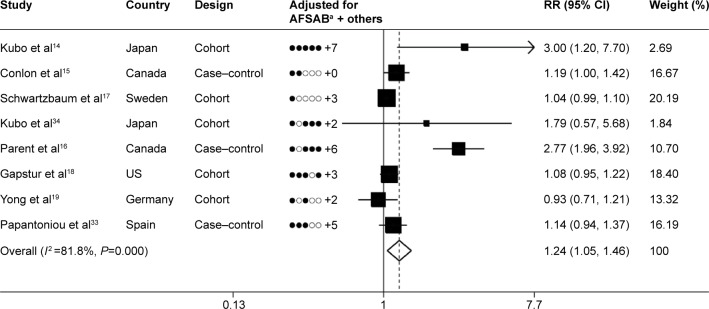
Summary risk estimates and 95% CIs for night-shift work and prostate cancer.
Notes: aAdjusted variables (AFSAB): A, age; F, family history of prostate cancer; S, smoking; A, alcohol; B, body mass index. For example, Kubo et al14 adjusted for age, family history of prostate cancer, smoking, alcohol, body mass index, and seven other factors. Weights are from random effect analysis.
Abbreviations: RR, relative risk; CI, confidence interval.
Table 2.
Subgroup analyses of relative risk for the association between night-shift work and prostate cancer risk
| Variables | Number | Events | Participants | RR (95% CI) | P-valuea | P-valueb | P-valuec | I2 (%) |
|---|---|---|---|---|---|---|---|---|
| Total | 8 | 9,669 | 2,459,845 | 1.24 (1.05–1.46) | 0.011 | <0.001 | 81.8 | |
| Geographical region | 0.004 | |||||||
| Europe | 3 | 3,487 | 2,132,437 | 1.04 (0.99–1.10) | 0.101 | 0.454 | 0.0 | |
| North America | 3 | 6,134 | 308,361 | 1.47 (0.99–2.18) | 0.057 | <0.001 | 92.0 | |
| Asia | 2 | 48 | 19,047 | 2.45 (1.19–5.04) | 0.015 | 0.494 | 0.0 | |
| Study quality | 0.036 | |||||||
| >7 | 3 | 5,391 | 310,964 | 1.72 (0.79–3.73) | 0.170 | <0.001 | 92.2 | |
| ≤7 | 5 | 4,278 | 2,148,881 | 1.10 (0.97–1.24) | 0.140 | 0.076 | 52.7 | |
| Study design | 0.001 | |||||||
| Cohort | 5 | 7,414 | 2,454,058 | 1.05 (1.00–1.10) | 0.065 | 0.146 | 41.3 | |
| Case–control | 3 | 2,255 | 5,787 | 1.51 (0.99–2.29) | 0.054 | <0.001 | 90.5 | |
| Source of patients | 0.387 | |||||||
| Population-based | 6 | 8,579 | 2,427,022 | 1.29 (1.07–1.55) | 0.007 | <0.001 | 86.3 | |
| Industry-based | 2 | 1,090 | 32,823 | 0.96 (0.74–1.25) | 0.768 | 0.277 | 15.5 | |
| Number of cases | <0.001 | |||||||
| >1,000 | 4 | 8,461 | 22,351 | 1.05 (1.00–1.10) | 0.049 | 0.606 | 0.0 | |
| ≤1,000 | 4 | 1,208 | 2,437,494 | 1.98 (1.07–3.65) | 0.030 | <0.001 | 85.5 | |
| Exposure assessment | 0.436 | |||||||
| Questionnaire | 3 | 5,765 | 321,501 | 1.18 (0.96–1.46) | 0.116 | 0.079 | 60.6 | |
| Interview | 3 | 2,814 | 2,105,521 | 1.42 (0.96–2.12) | 0.080 | <0.001 | 93.4 | |
| Database | 2 | 1,090 | 32,823 | 0.96 (0.74–1.25) | 0.768 | 0.277 | 15.5 | |
| Publication year | 0.096 | |||||||
| >2007 | 5 | 7,559 | 341,275 | 1.32 (0.97–1.80) | 0.079 | <0.001 | 86.1 | |
| ≤2007 | 3 | 2,110 | 2,118,570 | 1.16 (0.93–1.43) | 0.182 | 0.031 | 71.2 | |
| Control factors | 0.018 | |||||||
| >6 | 4 | 6,500 | 322,504 | 1.59 (1.06–2.37) | 0.024 | <0.001 | 89.7 | |
| ≤6 | 4 | 3,169 | 2,137,341 | 1.05 (1.00–1.10) | 0.062 | 0.295 | 19.0 | |
| Control family history | 0.207 | |||||||
| Yes | 4 | 6,860 | 323,984 | 1.13 (1.03–1.24) | 0.007 | 0.166 | 41.0 | |
| No | 4 | 2,809 | 2,135,861 | 1.40 (0.88–2.21) | 0.155 | <0.001 | 90.5 | |
| Control smoking | 0.057 | |||||||
| Yes | 6 | 7,590 | 355,327 | 1.41 (1.03–1.92) | 0.030 | <0.001 | 84.7 | |
| No | 2 | 2,079 | 2,104,518 | 1.05 (1.00–1.11) | 0.050 | 0.149 | 51.9 | |
| Control alcohol | <0.001 | |||||||
| Yes | 3 | 448 | 19,959 | 2.71 (1.98–3.70) | ,0.001 | 0.755 | 0.0 | |
| No | 5 | 9,221 | 2,439,886 | 1.06 (1.01–1.10) | 0.016 | 0.443 | 0.0 | |
| Control BMI | 0.018 | |||||||
| Yes | 4 | 5,422 | 325,016 | 1.93 (0.97–3.83) | 0.061 | <0.001 | 89.7 | |
| No | 4 | 4,247 | 2,134,829 | 1.05 (1.00–1.10) | 0.035 | 0.308 | 16.7 |
Notes: P-value for significance test of effect size;
P-value for homogeneity between strata;
P-value for homogeneity in each strata.
Abbreviations: RR, relative risk; BMI, body mass index; CI, confidence interval.
Evaluation of heterogeneity
In this meta-analysis, the Q-test and the I2 index were used to evaluate the heterogeneity across studies. As shown in Figure 2, there was statistically significant heterogeneity among studies (P<0.001, I2=81.8%). Through the Galbraith plot, Parent et al’s study16 was identified as the major source of heterogeneity (Figure S1). After removal of this study, the combined RR (95% CI) was 1.06 (1.01–1.11), without significant heterogeneity (P=0.154, I2 =36.0%).
Dose–response meta-analysis
Three studies15,16,33 provided at least three levels of the duration of shift work and were included in dose–response meta-analysis. As shown in Figure 3, an increase in night-shift work of 5 years was statistically significantly associated with a 2.8% (95% CI: 0.3, 5.4%, P=0.030) increase in the risk of prostate cancer.
Figure 3.
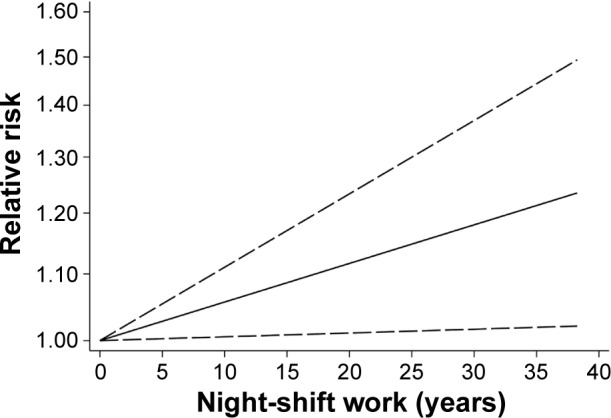
Dose–response analysis of the association between night-shift work and prostate cancer risk.
Note: Solid line represents the estimated odds ratios and the dotted lines represent the 95% confidence intervals.
Sensitivity analysis and cumulative meta-analysis
Sensitivity analyses were performed after sequential removal of each included study. The results indicated that the significance of the combined estimate was not influenced by any single study (Table S1). Cumulative meta-analysis was conducted via the assortment of studies by publication year. As shown in Figure S2, the effect of night-shift work tended to be significant, and the 95% CIs became increasingly narrower over time, indicating that the precision of the estimates was gradually boosted by the accumulation of more studies.
Publication bias
There was no evidence of study publication bias either with Begg’s test (P=0.174) or with Egger’s test (P=0.728). The trim-and-fill analysis imputed one missing study (Figure 4), which would not have altered the pooled result (RR =1.21, 95% CI: 1.03–1.42).
Figure 4.
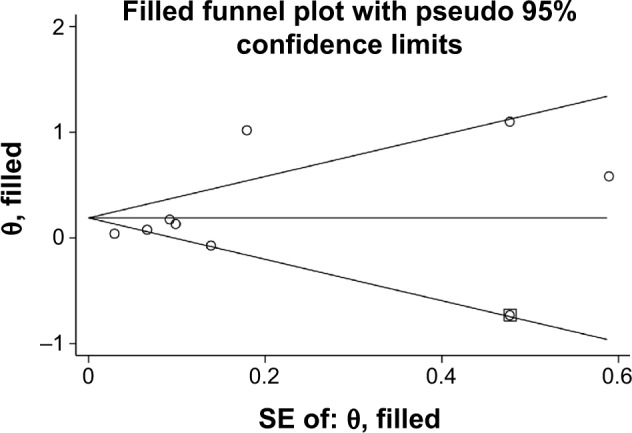
Trim-and-fill analysis identified one imputed study, which is represented by a hollow square.
Abbreviation: SE, standard error.
Discussion
The present meta-analysis involved 2,459,845 participants and 9,669 patients with prostate cancer from five cohort and three case–control studies. To the best of our knowledge, this is the first systematic review and meta-analysis to provide a comprehensive assessment of the association between night-shift work and prostate cancer. Overall, the pooled analysis of all included studies supports the idea that night-shift work is associated with an increased risk of prostate cancer (RR =1.24, 95% CI: 1.05–1.46, P=0.011).
Our study has some notable strengths. First, with the accumulated evidence and enlarged sample size, we have enhanced statistical power to derive a more precise and reliable estimation of the relationship between shift work and prostate cancer risk. Second, although marked between-study heterogeneity was observed, the combined estimate of the seven eligible studies was still significant after removal of Parent et al’s study,16 which contributed to heterogeneity. Third, robust results were obtained from cumulative meta-analysis and sensitivity analysis. Fourth, the results of Begg’s test, Egger’s test, and trim-and-fill analysis indicated no evidence of study publication bias. Fifth, subgroup analysis by study design suggested that the pooled RR was 1.05 (95% CI: 1.00–1.10) for cohort studies without significant heterogeneity (P=0.146). Cohort studies were less affected by recall bias and selection bias when compared with case–control studies.
Nonetheless, some important limitations should be discussed. First, the number of included studies was still relatively small. Therefore, considering the low statistical power, we can only cautiously conclude whether heterogeneity between studies or publication bias exists. In this study, significant between-study heterogeneity was detected. The included studies had significant variability in study design, risk estimates, ethnicity, sample size, method of exposure assessment, and adjustment for potential confounders. Heterogeneity may distort the meta-analysis and limit the generalizability of our findings to particular populations. However, given the low statistical power, we were not able to find an explanation from a meta-regression. Second, our studies showed varying levels of bias, especially recall and misclassification bias from exposure assessments. It is well known that the quality of exposure assessment is rather compromising in previous studies, mostly “ever” comparing to “never”. In several population-based studies, exposure to night-shift work was estimated by means of job-exposure matrix. In our meta-analysis, the included studies employed very different methods to define the exposure, and no two studies defined their primary exposure variables in exactly the same way, which may have resulted in some misclas-sification on exposure status and consequently may have caused dilution of the pooled effects when performing data synthesis.10 Third, not all studies collected or adjusted for common covariates. A meta-analysis cannot solve problems related to confounding factors that could be inherent in the included studies.35 Residual or unknown confounding can compromise the strength of the exposure–outcome association. Fourth, in subgroup analyses, a considerable portion of the results were not statistically significant, and the 95% CIs of the combined risk estimate tended to be broad due to the limited included studies, so the finding from our meta-analysis must be updated and confirmed when more evidence becomes available in future. Fifth, Gapstur et al’s study18 used prostate cancer death as the outcome of interest, which was different from the other studies. However, as shown in sensitivity analysis, the omission of this study did not change the significance of the combined estimate.
A relationship between night-shift work and the risk of prostate cancer has some biological plausibility. First, the melatonin pathway, which is closely related to circadian rhythms, is most frequently implicated in the observed elevated risk of cancer among night-shift workers. Melatonin appears to prevent cancer development through several pathways, including antioxidation, antimitosis, antiangiogenesis, and the regulation of the immune system.36 In terms of prostate cancer, previous studies showed that melatonin could directly inhibit the proliferation of prostate cancer cells in vitro and in vivo.37,38 Additionally, decreased excretion of melatonin may induce the continuous production of testosterone, which may influence the risk of prostate cancer as the growth and differentiation of the prostate is under androgen control.39 Second, decreased exposure to sunlight among night-shift workers reduces the production of a biologically active form of vitamin D that is able to suppress prostate cancer cell proliferation, which was also suspected to be involved,40 although the results of epidemiological studies between vitamin D and the risk of prostate cancer are still controversial.41 Third, a recent study reported a strong positive association between shift work and elevated prostate-specific antigen levels.13 There is evidence that prostate-specific antigen levels in serum are a marker of the future risk of prostate cancer development.42
There continues to be intense interest in the possibility that night-shift work is associated with an elevated risk of cancer. A massive amount of experimental evidence with acceptable quality supported the carcinogenic effects of shift work, and the International Agency for Research on Cancer reported that there was “sufficient evidence” for carcinogenicity in animal models.7 In humans, the findings to date from numerous epidemiologic studies and meta-analyses support such an association between night-shift work and the risk of breast cancer.8–10 However, regarding prostate cancer, the evidence was still limited and inconsistent. Therefore, we performed this meta-analysis, the results of which suggested that night-shift work was positively associated with prostate cancer. A major limitation of our study is the lack of a consistent definition of night-shift work in included studies as discussed above. Not long ago, a “molecular-timetable method” to detect body time using blood samples was developed in animal tests.43 In the future, if such methods are applied in human beings, the quality of exposure assessments would markedly improve.
In conclusion, our findings support a positive association between night-shift work and prostate cancer risk. Caution is needed in interpreting the results because of the limited number of eligible studies, the poor quality of exposure data, substantial between-study heterogeneity, different effects by study design, and methodological limitations of the existing studies. More future studies involving large, diverse occupational and geographic populations in this area are warranted, given the important public health and policy issues surrounding this topic and the increasing number of night-shift workers.
Supplementary materials
Galbraith plot analysis was used to evaluate heterogeneity.
Note: It indicated that Parent et al’s study16 was the potential source of heterogeneity.
Results from cumulative meta-analysis of the association between night-shift work and prostate cancer risk.
Abbreviations: CI, confidence interval; ES, estimate summary.
Table S1.
Results of the sensitivity analyses
| Excluded studies | RR (95% CI) | P-value | Model | I2 (P-value) |
|---|---|---|---|---|
| Kubo et al14 | 1.20 (1.03–1.41) | 0.023 | Random | 82.2 (P<0.001) |
| Conlon et al15 | 1.26 (1.04–1.53) | 0.019 | Random | 83.8 (P<0.001) |
| Schwartzbaum et al17 | 1.33 (1.06–1.69) | 0.016 | Random | 81.7 (P<0.001) |
| Kubo et al34 | 1.23 (1.04–1.45) | 0.015 | Random | 84.1 (P<0.001) |
| Parent et al16 | 1.06 (1.01–1.11) | 0.011 | Fixed | 36.0 (P=0.154) |
| Gapstur et al18 | 1.31 (1.05–1.63) | 0.016 | Random | 84.4 (P<0.001) |
| Yong et al19 | 1.30 (1.08–1.56) | 0.005 | Random | 83.9 (P<0.001) |
| Papantoniou et al33 | 1.27 (1.05–1.54) | 0.015 | Random | 84.2 (P<0.001) |
Abbreviations: RR, relative risk; CI, confidence interval.
Acknowledgments
This study was supported by grants from the National Key Clinical Specialty Construction Project of China, Key Medical Disciplines of Zhejiang Province, Health Sector Scientific Research Special Project (201002010), Combination of Traditional Chinese and Western Medicine Key Disciplines of Zhejiang Province (2012-XK-A23), Zhejiang Province Key Project of Science and Technology (2014C04008-2), National Natural Science Foundation of China (81472375, 81372773, 81201767), and Scientific Research Foundation of the Ministry of Public Health of China (WKJ2012-2-009).
Footnotes
Disclosure
The authors report that there are no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.van Die MD, Bone KM, Williams SG, Pirotta MV. Soy and soy isoflavones in prostate cancer: a systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014;113(5b):E119–E130. doi: 10.1111/bju.12435. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Cheng Y, Li S, et al. Dietary carrot consumption and the risk of prostate cancer. Eur J Nutr. 2014;53(8):1615–1623. doi: 10.1007/s00394-014-0667-2. [DOI] [PubMed] [Google Scholar]
- 5.Pukkala E, Martinsen JI, Weiderpass E, et al. Cancer incidence among firefighters: 45 years of follow-up in five Nordic countries. Occup Environ Med. 2014;71(6):398–404. doi: 10.1136/oemed-2013-101803. [DOI] [PubMed] [Google Scholar]
- 6.LeMasters GK, Genaidy AM, Succop P, et al. Cancer risk among firefighters: a review and meta-analysis of 32 studies. J Occup Environ Med. 2006;48(11):1189–1202. doi: 10.1097/01.jom.0000246229.68697.90. [DOI] [PubMed] [Google Scholar]
- 7.Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 8.Grundy A, Richardson H, Burstyn I, et al. Increased risk of breast cancer associated with long-term shift work in Canada. Occup Environ Med. 2013;70(12):831–838. doi: 10.1136/oemed-2013-101482. [DOI] [PubMed] [Google Scholar]
- 9.Hansen J, Lassen CF. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. 2012;69(8):551–556. doi: 10.1136/oemed-2011-100240. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Yeung KL, Chan WC, et al. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann Oncol. 2013;24(11):2724–2732. doi: 10.1093/annonc/mdt283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, Oh J. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;138(1):291–301. doi: 10.1007/s10549-013-2433-1. [DOI] [PubMed] [Google Scholar]
- 12.Ijaz S, Verbeek J, Seidler A, et al. Night-shift work and breast cancer – a systematic review and meta-analysis. Scand J Work Environ Health. 2013;39(5):431–447. doi: 10.5271/sjweh.3371. [DOI] [PubMed] [Google Scholar]
- 13.Flynn-Evans EE, Mucci L, Stevens RG, Lockley SW. Shiftwork and prostate-specific antigen in the national health and nutrition examination survey. J Natl Cancer Inst. 2013;105(17):1292–1297. doi: 10.1093/jnci/djt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo T, Ozasa K, Mikami K, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164(6):549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 15.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18(1):182–183. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 16.Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176(9):751–759. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 17.Schwartzbaum J, Ahlbom A, Feychting M. Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health. 2007;33(5):336–343. doi: 10.5271/sjweh.1150. [DOI] [PubMed] [Google Scholar]
- 18.Gapstur SM, Diver WR, Stevens VL, Carter BD, Teras LR, Jacobs EJ. Work schedule, sleep duration, insomnia, and risk of fatal prostate cancer. Am J Prev Med. 2014;46(3 Suppl 1):S26–S33. doi: 10.1016/j.amepre.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Yong M, Blettner M, Emrich K, Nasterlack M, Oberlinner C, Hammer GP. A retrospective cohort study of shift work and risk of incident cancer among German male chemical workers. Scand J Work Environ Health. 2014;40(5):502–510. doi: 10.5271/sjweh.3438. [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 22.Walker AM. Observation and Inference: An Introduction to the Methods of Epidemiology. Newton Lower Falls, MA: Epidemiology Resources; 1991. [Google Scholar]
- 23.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19(4):251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 26.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37(2):569–586. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, Moons KG. More than numbers: the power of graphs in meta-analysis. Am J Epidemiol. 2009;169(2):249–255. doi: 10.1093/aje/kwn340. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 32.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 33.Papantoniou K, Castano-Vinyals G, Espinosa A, et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer. 2015;137(5):1147–1157. doi: 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- 34.Kubo T, Oyama I, Nakamura T, et al. Industry-based retrospective cohort study of the risk of prostate cancer among rotating-shift workers. Int J Urol. 2011;18(3):206–211. doi: 10.1111/j.1442-2042.2010.02714.x. [DOI] [PubMed] [Google Scholar]
- 35.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367(9507):320–326. doi: 10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- 36.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13(4):257–264. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Moretti RM, Marelli MM, Maggi R, Dondi D, Motta M, Limonta P. Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol Rep. 2000;7(2):347–351. [PubMed] [Google Scholar]
- 38.Philo R, Berkowitz AS. Inhibition of Dunning tumor growth by melatonin. J Urol. 1988;139(5):1099–1102. doi: 10.1016/s0022-5347(17)42795-9. [DOI] [PubMed] [Google Scholar]
- 39.Dearnaley DP. Cancer of the prostate. BMJ. 1994;308(6931):780–784. doi: 10.1136/bmj.308.6931.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart LV, Weigel NL. Vitamin D and prostate cancer. Exp Biol Med (Maywood) 2004;229(4):277–284. doi: 10.1177/153537020422900401. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert R, Martin RM, Beynon R, et al. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control. 2011;22(3):319–340. doi: 10.1007/s10552-010-9706-3. [DOI] [PubMed] [Google Scholar]
- 42.Orsted DD, Nordestgaard BG, Jensen GB, Schnohr P, Bojesen SE. Prostate-specific antigen and long-term prediction of prostate cancer incidence and mortality in the general population. Eur Urol. 2012;61(5):865–874. doi: 10.1016/j.eururo.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Kasukawa T, Sugimoto M, Hida A, et al. Human blood metabolite timetable indicates internal body time. Proc Natl Acad Sci U S A. 2012;109(37):15036–15041. doi: 10.1073/pnas.1207768109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Galbraith plot analysis was used to evaluate heterogeneity.
Note: It indicated that Parent et al’s study16 was the potential source of heterogeneity.
Results from cumulative meta-analysis of the association between night-shift work and prostate cancer risk.
Abbreviations: CI, confidence interval; ES, estimate summary.
Table S1.
Results of the sensitivity analyses
| Excluded studies | RR (95% CI) | P-value | Model | I2 (P-value) |
|---|---|---|---|---|
| Kubo et al14 | 1.20 (1.03–1.41) | 0.023 | Random | 82.2 (P<0.001) |
| Conlon et al15 | 1.26 (1.04–1.53) | 0.019 | Random | 83.8 (P<0.001) |
| Schwartzbaum et al17 | 1.33 (1.06–1.69) | 0.016 | Random | 81.7 (P<0.001) |
| Kubo et al34 | 1.23 (1.04–1.45) | 0.015 | Random | 84.1 (P<0.001) |
| Parent et al16 | 1.06 (1.01–1.11) | 0.011 | Fixed | 36.0 (P=0.154) |
| Gapstur et al18 | 1.31 (1.05–1.63) | 0.016 | Random | 84.4 (P<0.001) |
| Yong et al19 | 1.30 (1.08–1.56) | 0.005 | Random | 83.9 (P<0.001) |
| Papantoniou et al33 | 1.27 (1.05–1.54) | 0.015 | Random | 84.2 (P<0.001) |
Abbreviations: RR, relative risk; CI, confidence interval.