Abstract
Both active and passive immunotherapy protocols decrease insoluble amyloid-ß42 (Aß42) peptide in animal models, suggesting potential therapeutic applications against the main pathological trigger in Alzheimer's disease (AD). However, recent clinical trials have reported no significant benefits from humanized anti-Aß42 antibodies. Engineered single-chain variable fragment antibodies (scFv) are much smaller and can easily penetrate the brain, but identifying the most effective scFvs in murine AD models is slow and costly. We show here that scFvs against the N- and C-terminus of Aß42 (scFv9 and scFV42.2, respectively) that decrease insoluble Aß42 in CRND mice are neuroprotective in Drosophila models of Aß42 and amyloid precursor protein neurotoxicity. Both scFv9 and scFv42.2 suppress eye toxicity, reduce cell death in brain neurons, protect the structural integrity of dendritic terminals in brain neurons and delay locomotor dysfunction. Additionally, we show for the first time that co-expression of both anti-Aß scFvs display synergistic neuroprotective activities, suggesting that combined therapies targeting distinct Aß42 epitopes can be more effective than targeting a single epitope. Overall, we demonstrate the feasibility of using Drosophila as a first step for characterizing neuroprotective anti-Aß scFvs in vivo and identifying scFv combinations with synergistic neuroprotective activities.
Introduction
Alzheimer's disease (AD) is the most prevalent neurodegenerative disorder and is characterized by the accumulation of the amyloid-ß1-42 (Aß42) peptide in plaques, hyperphosphorylated tau in neurofibrillary tangles and prominent neuronal loss in hippocampus and cortex (1). As posited by the amyloid cascade hypothesis, genetic evidence points to the accumulation of Aß42 as the triggering event in AD (2). The Aß42 peptide is generated following the sequential cleavage of the amyloid precursor protein (APP) by ß-secretase (BACE1) in the extracellular side and the γ-secretase complex inside the membrane. Familial forms of AD are linked to point mutations in APP and Presenilin1 and 2, key γ-secretase components, all of which favor the production of Aß42 peptide, thus supporting the triggering role of Aß42 in AD. Originally, the amyloid cascade hypothesis proposed that insoluble Aß42 present in highly organized amyloid fibers was the neurotoxic agent in AD, but this hypothesis was modified to include pre-amyloid assemblies such as oligomers and protofibrils, as these have shown to be more toxic in some experimental settings (3,4). However, both soluble and insoluble Aß42 assemblies may contribute to disease in the complex environment of the human brain; thus, neutralizing the toxicity of these different structures remains an unmet challenge in the field.
Despite tremendous advances in our understanding of AD pathogenesis, this knowledge has not yet led to the development of disease-modifying therapies. Over the last 15 years, different immunotherapy protocols have demonstrated reduction of insoluble Aß in the brain of animal models of AD (5,6). The promise of these strategies led to both active and passive immunotherapy trials in human patients. Active immunotherapy with aggregated Aß42 and adjuvant was halted after 6% of the patients developed meningoencephalitis (7,8). Efficacy data are not yet available on several ongoing active vaccination trials with novel Aβ vaccines designed to minimize harmful T-cell response (9). An alternative to prevent the uncontrolled immune response to Aß is the direct administration of humanized anti-Aß antibodies (passive immunotherapy). Antibody treatments reduced Aß deposition in the brain but in some cases have led to side effects including vasogenic edema (ARIA-E) and small hemorrhage (ARIA-H) (10,11). Despite superb preclinical data supporting efficacy in animal models, the humanized anti-Aß antibodies bapineuzumab (N-terminal) and solanezumab (central domain) did not show significant efficacy in large phase III trials (12,13). However, many researchers still believe that these or other anti-Aß antibodies may show clinical utility if administered in preclinical stages of AD. Looking ahead, the lack of rapid assays to compare the efficacy of anti-Aß antibodies and the relatively low brain penetration of peripherally administered antibodies present challenges for the development and testing of optimized Aß immunotherapies (9).
Antibody engineering is an alternative approach to increase brain penetration while limiting the deleterious effects of an uncontrolled immune response (14). Single-chain variable fragment (scFv) antibodies consist of the variable regions of the heavy and light chains fused into a small gene by a short linker. These antibodies can be purified and injected peripherally or introduced in viral vectors for persistent expression, providing increased flexibility for their administration compared with full antibodies. Owing to their small size (∼30 kDa), scFvs penetrate the brain more efficiently while preserving the epitope specificity of the parent antibodies. Many anti-Aß scFvs have been derived from full monoclonal antibodies or from phage and synthetic scFv libraries (15–18). Intracellular antibody capture technology provides a physiological environment for recognizing scFvs that bind oligomeric Aß (19). Despite the success in the isolation of scFvs that prevent Aß42 aggregation and toxicity in vitro, their effectiveness in animal models is critical to evaluate their therapeutic potential. We and others have previously shown efficacy of several anti-Aß scFv directly expressed in the brain of APP transgenic mice (18,20,21) and in the triple transgenic AD mouse (22,23). Other recent developments include less invasive alternatives for chronic therapeutics in humans, such as direct intranasal administration of scFvs and intramuscular injection of adeno-associated virus (AAV) (17,24–26), all of which support the potential of scFv as therapeutic agents against AD.
The main limitations of the current approach for optimizing passive anti-Aß immunotherapies in mice are the time and cost of testing in APP mouse models, and the lack of consistent assays enabling comparative efficacy studies. To explore alternative systems, we have utilized Aß42 transgenic Drosophila as a platform for in vivo selection of neuroprotective anti-Aß scFvs in a phenotypic model of AD. We combined transgenic flies expressing secreted human Aß42 (27) or APP carrying the Swedish mutation (APPswe) together with the previously described scFv9 (anti-Aß1-16) and scFv42.2 (anti-Aßx-42) (18), all under the control of UAS regulatory sequence. Both anti-Aß scFvs rescued partially the eye phenotype, reduced cell death, protected the architecture of the dendritic terminals in brain neurons and delayed the dysfunction of locomotor neurons. Moreover, the combination of both scFvs demonstrated synergistic protective activity, suggesting a new therapeutic use of anti-Aß antibodies. Interestingly, the scFvs exerted their protective activity without affecting the level of total Aß42. These observations suggest that binding of the anti-Aß scFvs to Aß42 was sufficient to reduce neurotoxicity, perhaps by masking its neurotoxic epitopes. Overall, the neuroprotective activity of anti-Aß scFvs in Drosophila supports the use of fruit flies for efficient in vivo screening of new recombinant anti-Aß antibodies with improved neuroprotective activity.
Results
Two anti-Aß scFvs independently and synergistically suppress Aß42 neurotoxicity in the eye
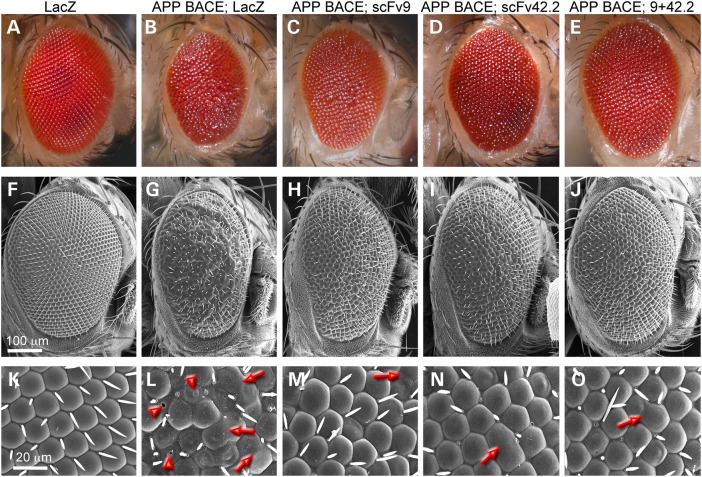
To examine the ability of anti-Aß scFvs to suppress the neurotoxicity of human Aß42, we introduced two previously characterized scFvs in a flexible, phenotypic model of Aß42 neurotoxicity: Drosophila. For this, we subcloned scFv9 and scFv42.2 (18) into the Drosophila expression vector pUASTv2 and generated transgenic flies to examine their ability to suppress Aß42 neurotoxicity in several assays. Flies co-expressing Aß42 and the reporter LacZ display small, glassy, depigmented eyes compared with flies only expressing LacZ (Fig. 1A and B). At higher magnification, the eye lattice is highly disorganized, ommatidia are fused, and the lenses show holes owing to late cell death (Fig. 1G and H). Co-expression of Aß42 with scFv9 or scFv42.2 partially rescues the Aß42 phenotype, with larger eyes and improved pigmentation (Fig. 1C and D). The eyes of these flies are better organized, with fewer fused ommatidia, and better differentiation of lenses with fewer broken lenses (Fig. 1I and J). As controls for the specificity of these scFvs, we generated flies expressing scFv40, an antibody that specifically recognizes Aß40, but not Aß42. Co-expression of Aß42 and scFv40 results in disorganized eyes with necrotic spots similar to the eyes of control flies co-expressing LacZ (Supplementary Material, Fig. S1A–C). As expected, the anti-Aß scFvs alone had no effect on eye formation (data not shown).
Figure 1.
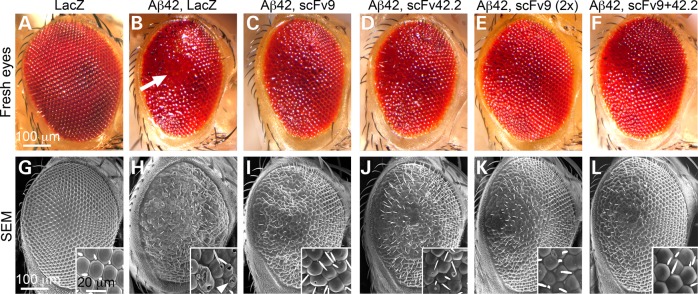
Anti-Aß4 scFvs suppress Aß42 neurotoxicity in the eye. (A–F) Fresh eyes and (G–L) scanning electron micrographs (SEM) of flies expressing LacZ or Aß42 constructs under the control of gmr-Gal4. Control flies expressing LacZ in all eye cells display highly organized eyes with hexagonal lenses (A and G). Flies co-expressing Aß42 and LacZ present small, depigmented and disorganized eyes with fused ommatidia and necrotic spots (B and H, arrow). Flies co-expressing Aß42 and scFv9 show larger and better-organized eyes, but fusions and disorganization are still evident (C and I). Flies co-expressing Aß42 and scFv42.2 show larger and slightly disorganized eyes, with fewer ommatidia fusions and holes (D and J). Flies co-expressing Aß42 with two copies of scFv9 show improved eye organization (E and K). Flies co-expressing Aß42 together with scFv9 and scFv42.2 display large eyes with improved organization and few ommatidia defects (F and L).
The preliminary observations in the eye suggest that scFv42.2 is more protective than scFv9, whereas scFv40 is not protective. We next evaluated the relative expression of the three scFvs in fly homogenates by western blot with an antibody against a common myc epitope in the C-terminal. We found that scFv9 was weaker than scFv40 and scFv42.2 (Supplementary Material, Fig. S1D). As scFv9 rescues the eye phenotype with weaker expression than scFv40, this result demonstrates the substrate specificity of the antibodies. To compare the protective activity of scFv9 and scFv42.2 at comparable expression levels, we generated flies expressing two copies of scFv9. These flies robustly suppressed Aß42 toxicity, resulting in larger and better-organized eyes with no necrotic spots and fewer ruptured lenses (Fig. 1E and K). This robust protection is consistent with previous findings that the N-terminal antibodies are more effective than antibodies against the central and C-terminal domains (14,18).
As scFv9 and scFv42.2 bind different Aß42 epitopes, it is reasonable to assume that the combination of both antibodies will exert a stronger protective activity than each antibody individually. To test this idea, we combined the two anti-Aß scFvs and co-expressed them with Aß42 in the eye. These flies exhibit larger eyes with improved organization and pigmentation (Fig. 1F and L). This result suggests that these small antibodies do not compete for binding to Aß42 and that they can work together to neutralize Aß42 neurotoxicity. Thus, the combination of anti-Aß scFvs demonstrated a synergistic protective activity against Aß42.
Anti-Aß scFvs suppress APP neurotoxicity in the eye
We next examined the ability of scFv9 or scFv42.2 to suppress neurotoxicity in a more physiological Drosophila model of Aß42 production and deposition. Flies expressing human APP and BACE1 can produce Aß42 by utilizing the endogenous γ-secretase activity of Drosophila (28). To determine the protective activity of anti-Aß scFvs against Aß42 derived from human APP, we co-expressed human APP carrying the Swedish mutation (APPswe) and BACE1 in the eye, which results in small and disorganized eyes compared with control LacZ eyes (Fig. 2A and B). At higher magnification, these eyes contain fused ommatidia, abnormally differentiated lenses and hairs, and broken lenses (Fig. 2F, G, K and L), phenotypes similar to those induced by Aß42 misexpression, albeit weaker (see Fig. 1). Flies co-expressing APPswe BACE1 and scFv9 exhibit larger and better-organized eyes (Fig. 2C and H) with fewer ommatidia fusions and no broken lenses (Fig. 2M). Co-expression of APPswe BACE1 and scFv42.2 further rescues eye disorganization (Fig. 2D and I), with better differentiation and organization of ommatidia (Fig. 2N), but the eyes are still small compared with LacZ alone. Finally, the combination of both anti-Aß scFvs with APPswe BACE1 achieve the best suppression, rescuing the size and the organization of the eye as well as the differentiation of the lenses with no necrotic spots (Fig. 2E, J and O). In all, these results support the protective activity of scFv9 and scFv42.2 and their synergistic action against APP neurotoxicity in the Drosophila eye, indicating that the anti-Aß scFvs can properly recognize the Aß42 epitopes form two different sources, secreted Aß42 or physiologically cleaved APP.
Figure 2.
Anti-Aß scFvs suppress APPswe toxicity in the eye. (A–E) Fresh eyes and (F–O) SEM of flies expressing LacZ or APPswe BACE1 constructs under the control of gmr-Gal4. Control flies expressing LacZ in all eye cells display highly organized eyes with hexagonal ommatidia (A, F and K). Flies co-expressing APPswe BACE1 and LacZ present small, depigmented and disorganized eyes (B, G and L). Flies co-expressing APPswe BACE1 and scFv9 or scFv42.2 exhibit small, but better-organized eyes (C, D, H, I, M and N). Flies co-expressing APPswe BACE1 together with scFv9 and scFv42.2 show larger eyes with improved organization of ommatidia and fewer broken lenses (E, J and O). Arrows indicate fused ommatidia and arrowheads show broken lens surface.
Anti-Aß scFvs are secreted in Drosophila
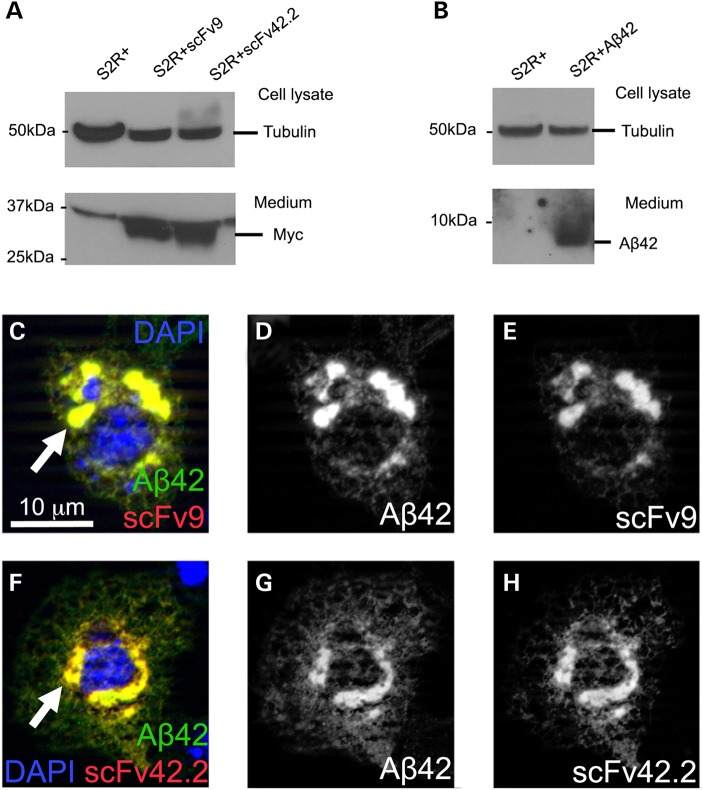
To verify that the scFvs are properly processed and secreted in Drosophila, we transfected S2R+ cells with vectors containing scFv9 or scFv42.2 under the control of UAS together with Actin-Gal4 to induce their expression. Then, we determined the presence of the scFvs in the medium by western blot. Both scFvs are properly processed because they accumulate robustly in the medium (Fig. 3A). Using the same conditions, we verified that S2 cells efficiently secrete Aß42, which we detected in the culture medium (Fig. 3B). The loading control on top shows the expression of tubulin in the cell lysates from the same cultures (Fig. 3A and B).
Figure 3.
Anti-Aß scFvs are secreted and co-localize with Aß42 in S2 cells. (A and B) ScFvs and Aß42 are secreted in cultured S2 cells. In S2 cells transfected with scFv or Aß42 constructs, both scFv9 and scFv42.2 (A) and Aß42 (B) are detected in the culture medium by western blot. Equivalent amounts of cell lysates were used to detect Tubulin as loading control from each culture. (C–H) Subcellular distribution of Aß42 and anti-Aß scFvs in cultured S2 cells. Co-transfection of Aß42 and scFv9 (C–E) or scFv42.2 (F and G) shows perinuclear co-localization corresponding to ER and vesicles of the secretory pathway (arrows).
We took advantage of the large size of S2 cells compared with the Drosophila brain neurons to document the intracellular distribution of both scFvs and Aß42. Both scFv9 (Fig. 3C–E) and scFv42.2 (Fig. 3F–H) accumulate at high levels in the ER and secretory vesicles, where they co-localize with Aß42. Thus, the distribution of the three proteins is consistent with working signal peptides in each construct and with the efficient processing and secretion of the mature proteins.
Anti-Aß scFvs co-localize with Aß42 in Drosophila brain neurons
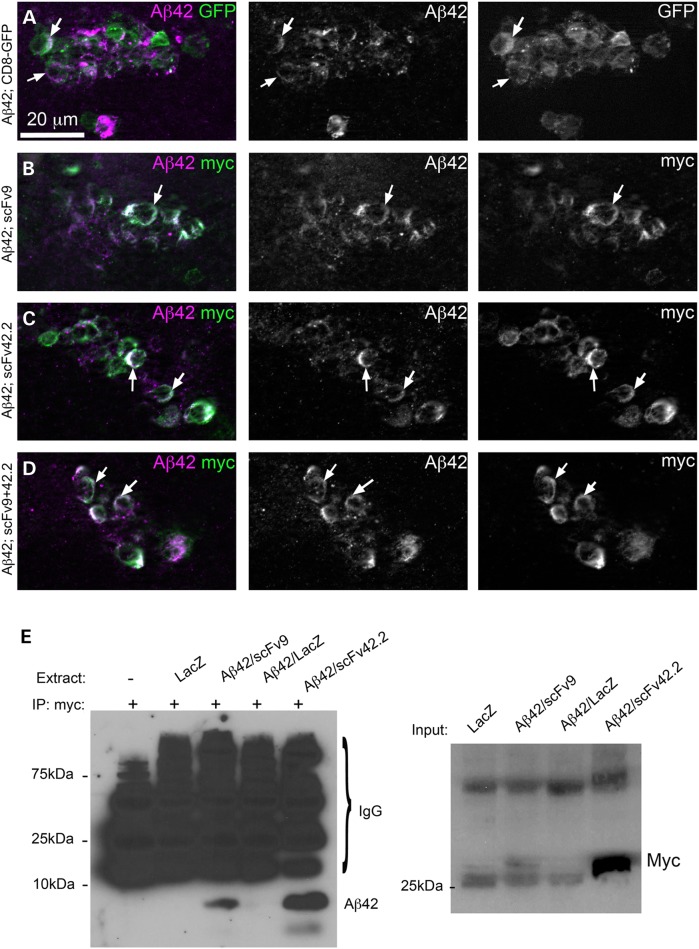
We next examined the distribution of the anti-Aß scFvs and Aß42 by immunofluorescence by detecting the myc tag of the scFvs. For these experiments, we expressed Aß42 and the scFvs on a small cluster of local interneurons of the antennal lobe under the control of the OK107-Gal4 line. Co-expression of Aß42 and CD8-GFP allows detection of Aß42 in the membrane of the brain interneurons (Fig. 4A). Co-expression of Aß42 and either scFv9 or scFv42.2 demonstrates the co-localization of Aß42 with both antibodies (Fig. 4B and C). However, co-expression of the anti-Aß scFvs has no overt effect on Aß42 accumulation and distribution (Fig. 4B and C). Co-expression of both anti-Aß scFvs together also demonstrates robust co-localization with no effect on Aß42 distribution (Fig. 4D). This distribution is consistent with the efficient secretion of both scFvs and Aß42 described in Figure 3.
Figure 4.
Anti-Aß scFvs co-localize with Aß42 in brain neurons. (A–D) Subcellular distribution of Aß42 and anti-Aß scFvs in brain neurons. The transgenes are expressed under the control of OK107-Gal4 and localized in a small cluster of local interneurons of the antennal lobe. (A) Co-expression of Aß42 (magenta) and CD8-GFP (green) shows co-distribution in the membrane (arrows) and punctate accumulation of Aß42 in the Golgi. (B) Co-expression of Aß42 and scFv9 (myc epitope, green) results in co-localization in the membrane without changes in Aß42 distribution. (C) Co-expression of Aß42 and scFv42.2 (myc epitope, green) shows co-localization in the membrane and no changes in Aß42 distribution. (D) Co-expression of Aß42 together with scFv9 and scFv42.2 results in no evident changes in Aß42 distribution. (E) Aß42 and scFvs co-immunoprecipitate in Drosophila brain neurons. Using beads coated with the anti-myc antibody to capture the scFvs, we detected Aß42 immunoreactivity in samples co-expressing Aß42 and scFv9 or scFv42.2. Flies co-expressing Aß42 and scFv42.2 produced higher Aß42 immunoreactivity, consistent with its higher expression levels. Three negative controls produced non-specific signal from antibody fragments released from the column. On the right, we show the input for the co-IP immunoblotted against anti-myc. As expected, the levels of scFv42.2 are several times higher than those of scFv9.
Both anti-Aß scFvs bind Aß42 in mammalian systems (18). To verify that the antibodies bind Aß42 in fly brains, we performed co-immunoprecipitation from Drosophila head homogenates. A control lane lacking Drosophila extract, flies expressing LacZ or flies expressing Aß42 and LacZ showed only non-specific bands following capture with anti-myc antibody (Fig. 4E). Only the extracts from flies co-expressing Aß42 with scFv9 or scFv42.2 produced a specific band following capture with anti-myc at around 4 kDa corresponding to Aß42 (Fig. 4E). The scFv42.2 captured higher amounts of Aß42 because it is expressed at higher levels than scFv9, as confirmed by the input (Fig. 4E). We also show that the reverse immunoprecipitation produces the same results (Supplementary material, Fig. S2). When the 4G8 anti-Aß42 antibody was bound to beads, control flies expressing LacZ alone or Aß42 with LacZ showed only non-specific bands. However, flies co-expressing Aß42 with scFv9 or scFv42.2 produced immunoreactivity at around 28 kDa. Again, flies expressing scFv42.2 showed the stronger signal owing to the higher expression of this scFv. These studies demonstrate the effective target engagement of the anti-Aß scFvs in Drosophila.
Anti-Aß scFvs protect neuronal architecture against Aß42 neurotoxicity
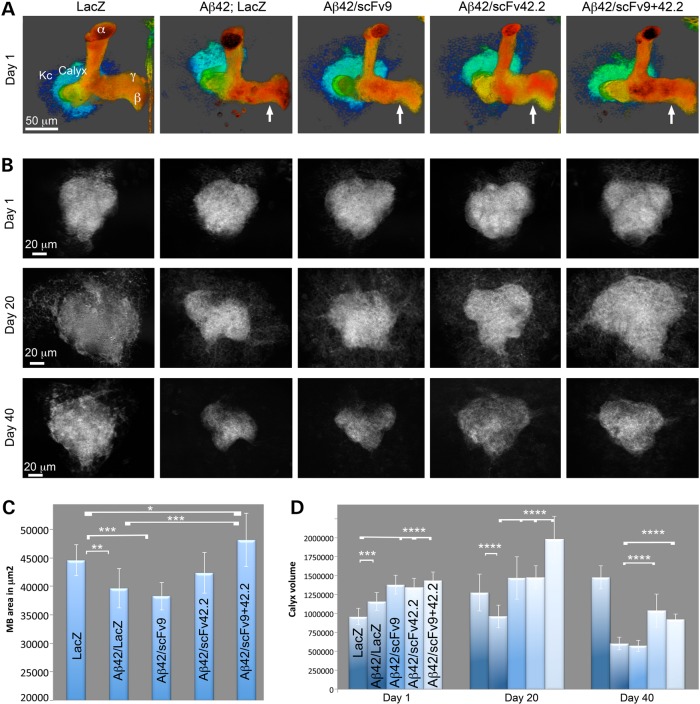
We next assessed the possible protective activity of the anti-Aß scFvs in brain neurons. For this, we took advantage of the robust anatomy of the mushroom bodies, the memory and learning center of Drosophila (Fig. 4A) (29). The Kenyon cells, the small mushroom body neurons in the posterior brain, form two tight clusters of 2500 neurons each that project their dendrites below the cortex into dense synaptic areas—the calyces. Their axons project through the calyces toward the anterior brain, where they branch into dorsal (α, α’) and medial (β, β’, γ) lobes (Fig. 4A) (30). Co-expression of LacZ and CD8-GFP had no effect on the development of the mushroom bodies, resulting in robust calyces and axonal lobes (Fig. 4A and C). However, co-expression of Aß42 and LacZ resulted in thinner axonal projections (Fig. 4A and C). Co-expression of Aß42 and scFv9 also produced thinner axonal projections, similar to the control Aß42/LacZ (Fig. 4A and C). Co-expression of Aß42 and scFv42.2 partially rescued the size of the mushroom body lobes, which were not significantly different from either LacZ or Aß42/LacZ (Fig. 4A and C). Finally, co-expression of Aß42 and both anti-Aß scFvs produced dorsal and medial lobes that were slightly larger than in control flies (Fig. 4A and C). As expected, the expression of the anti-Aß scFvs alone did not cause obvious problems during mushroom body differentiation (not shown). As the expression of Aß42 perturbs the development of the mushroom body axons, we did not study the ability of anti-Aß scFvs to prevent the progressive degeneration of these structures.
In contrast to the axonal terminals, flies co-expressing Aß42 with either LacZ or anti-Aß scFvs exhibited slightly larger calycal volumes than control flies at Day 1 (Fig. 4B and D). Thus, we selected these compact dendritic structures to evaluate the progressive Aß42-dependent degeneration of brain neurons. In flies expressing only LacZ, the calycal volume expanded over time, 33% by Day 20 and 53% by Day 40 (Fig. 4B and D). In contrast, co-expression of Aß42 and LacZ resulted in 25% loss of calycal volume by Day 20 and 60% by Day 40 compared with control flies (Fig. 4B and D). As these dendritic fields appeared to be highly sensitive to the progressive toxicity of Aß42, we examined the ability of the anti-Aß scFvs to rescue the degeneration of the calyces. Co-expression of Aß42 and scFv9 resulted in 15% larger calyces at Day 1 and 35% larger calyces at Day 20 compared with Aß42/LacZ (Fig. 4B and D), indicating its protective activity in younger flies. However, in flies aged for 40 days, scFv9 demonstrated no protective activity against Aß42-dependent loss of calycal dendrites (Fig. 4B and D). Interestingly, co-expression of Aß42 and scFv42.2 resulted in larger calyces at all stages than Aß42/LacZ, with a 15% larger volume at Day 1, 35% larger by Day 20 and 42% larger volume at Day 40 (Fig. 4B and D). Thus, scFv42.2 demonstrated a more robust protective activity than scFv9 in brain neurons, consistent with its higher expression level. Finally, co-expression of Aß42 with both anti-Aß scFvs resulted in 19% larger calycal volume at Day 1, 52% larger volume at Day 20 and 35% larger volume at Day 40 compared with Aß42/LacZ (Fig. 4B and D). Although the combination of anti-Aß scFvs boosted the protection of the calyces up to Day 20, the protection by Day 40 was comparable with that of scFv42.2 alone, suggesting that high expression is critical for continued protection in older flies.
Anti-Aß scFvs delay the progressive locomotor dysfunction induced by Aß42
To determine the functional benefits of the anti-Aß scFvs, we analyzed the locomotor performance of adult flies as a surrogate assay for Aß42 neurotoxicity. For this, we expressed LacZ alone or Aß42 together with LacZ or the anti-Aß scFvs pan-neurally using the Elav-Gal4 driver and examined the ability of flies to walk vertically. A blinded assessment of all the genotypes at Day 12 revealed one group with reduced climbing ability that was subsequently identified as the positive control Aß42/LacZ (Fig. 5A). The other groups, including the negative control LacZ and all the combinations of Aß42 with anti-Aß scFvs, appeared to climb better than the Aß42 controls (Fig. 5A). The longitudinal analysis of the locomotor activity of these flies confirmed this initial observation, with the Aß42/LacZ flies reaching 50% climbing index by Day 5 and total loss of climbing by Day 15 (Fig. 5B, red lines). Then, flies co-expressing Aß42 and scFv9 (yellow lines) or co-expressing both anti-Aß scFvs (blue lines) reached 50% climbing index by Day 12, same as LacZ control flies (green lines) (Fig. 5B). Interestingly, flies co-expressing Aß42 and scFv42.2 (black lines) continued to climb for several more days, reaching 50% climbing by Day 16 (Fig. 5B). Overall, these observations support the physiological benefits of the anti-Aß scFvs in flies expressing Aß42.
Figure 5.
Anti-Aß scFvs protect neuronal architecture in the brain. (A and B) Expression of transgenes in the mushroom bodies with OK107-Gal4; UAS-CD8-GFP followed by GFP imaging. (A) Three-dimensional architecture of the mushroom body complex at Day 1 in flies of the indicated genotypes. Compared with LacZ, expression of Aß42 reduces the size of the axonal terminals of the mushroom bodies (arrow). Co-expression of scFv9 does not rescue Aß42 (arrow), but co-expression of scFv42.2 rescues partially (arrow). The combination of both anti-Aß scFvs completely rescues the size of the mushroom body lobes at Day 1 (arrow). (B) Single-plane images of calyces from flies of the indicated genotypes at Days 1, 20, 30 and 40. Day 1: compared with control LacZ, co-expression of Aß42 and LacZ results in slightly larger calyces. Co-expression of scFvs also induces slightly larger calycal sizes at Day 1. Day 20: flies co-expressing Aß42 and LacZ exhibit smaller calyces than LacZ controls. Co-expression of Aß42 and either scFv9 or scFv42.2 preserves the size of the calyces. Co-expression of both anti-Aß scFvs results in larger calyces. Day 40: co-expression of Aß42 and LacZ results in very small calyces that are not rescued by scFv9. Co-expression of Aß42 and scFv42.2 results in partial improvement in the volume of the calyces, similar to co-expression of both scFvs. (C) Quantification of the axonal surface at Day 1. (D) Quantification of calycal volume at three time points. Values are shown as averages and standard deviation of the media. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Anti-Aβ scFvs suppress neuronal loss in the brain but do not reduce Aß42 accumulation
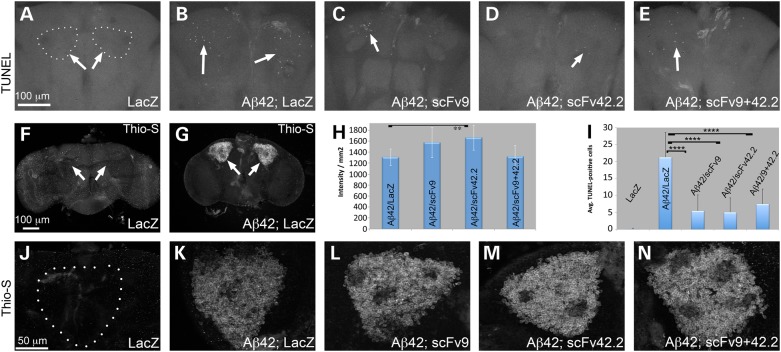
As both anti-Aß scFvs preserved the dendritic projections of the mushroom bodies for at least 20 days, we examined the ability of the scFvs to prevent Aß42-induced cell death in brain neurons. We expressed Aß42 with and without anti-Aß scFvs in mushroom body neurons, aged them for 20 days, and performed TUNEL to detect dying neurons. Control flies expressing LacZ alone showed no TUNEL-positive neurons in the Kenyon cell clusters, supporting the robustness of this key brain complex (Fig. 6A and I). In contrast, flies co-expressing Aß42 and LacZ accumulated >20 apoptotic cells per Kenyon cell cluster (Fig. 6B and I), demonstrating that Aß42 induces prominent neuronal cell death. Flies co-expressing Aß42 and either scFv9 or scFv42.2 accumulated an average of five apoptotic cells per Kenyon cell cluster (Fig. 6C, D and I), supporting their protective activity. In flies co-expressing Aß42 and both scFv9 and scFv42.2, the number of apoptotic cells per cluster was similar to the expression of individual anti-Aß scFvs (Fig. 6E and I). Overall, these results further support the neuroprotective activity of these anti-Aß scFvs, which could not be tested in the original experiments with AD mice because of the lack of significant cell death in relevant brain regions.
Figure 6.
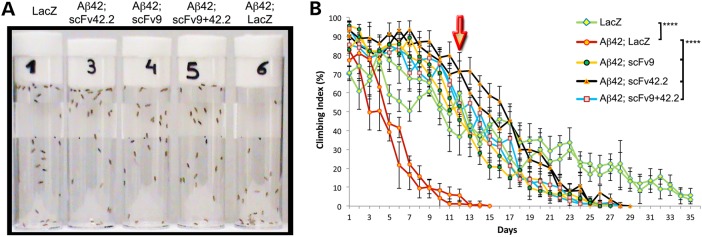
Anti-Aß scFvs prevent the progressive dysfunction of motor neurons. (A and B) Locomotor activity of flies expressing LacZ or Aß42 constructs under the control of elav-Gal4. (A) Snapshot of the locomotor assay at Day 12 showing all the genotypes covered. Only one vial (#6, Aß42; LacZ) contains flies with impaired climbing ability. Flies co-expressing anti-Aß scFvs show similar locomotor activity (#3, #4, #5) as negative control flies (#1, LacZ). (B) Longitudinal study of locomotor performance, each genotype is shown in duplicate. Flies expressing LacZ alone (green lines) reach 50% climbing index by Day 12 (arrow), whereas flies expressing Aß42 and LacZ (red) reach 50% climbing by Day 5. Flies co-expressing Aß42 and scFv9 (yellow) reach 50% climbing by Day 12. Flies co-expressing Aß42 and scFv42.2 (black) reach 50% climbing by Day 16. Flies co-expressing Aß42 and scFv9+42.2 (blue) reach 50% climbing by Day 13. ****P < 0.0001.
As the anti-Aß scFvs interact physically with Aß42 in mice and reduce Aß42 neurotoxicity in Drosophila, we asked whether the anti-Aß scFvs reduced Aß42 aggregation. To test this, we first stained Drosophila brains with thioflavin-S, a compound that intercalates into amyloid fibers and emits green fluorescence. Control flies expressing LacZ in the mushroom bodies revealed no localized fluorescence in Kenyon cells (Fig. 6F and J). In contrast, flies expressing Aß42 and LacZ accumulated intense fluorescence in the Kenyon cell clusters (Fig. 6G, K and H). Co-expression of Aß42 with scFv9 resulted in slightly increased fluorescent signal, but the change was not significant compared with control Aß42/LacZ (Fig. 6L and H). However, co-expression of Aß42 with scFv42.2 resulted in a significant increase in thioflavin-S signal (Fig. 6M and H). Finally, the combination of both anti-Aß scFvs had no effect on thioflavin-S intensity, reversing the effect of scFv42.2 (Fig. 6N and H). Although thioflavin-S is useful for qualitative studies and may not be sensitive to subtle changes in fibril formation, these results indicate that the direct binding of anti-Aß scFvs to Aß42 does not reduce Aß42 fibrillogenesis as described in rodent models of AD.
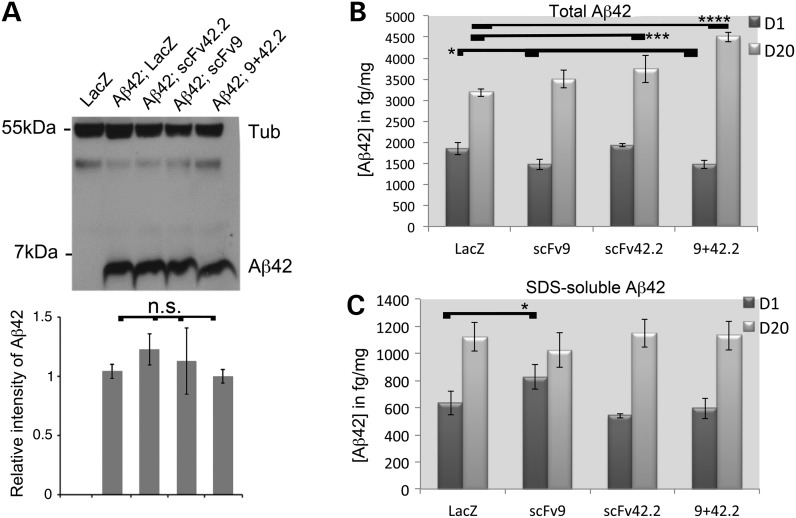
Anti-Aß scFvs do not reduce steady-state Aß42 levels
The expression of scFv9 and scFv42.2 in CRND8 mice reduced the levels of total Aß42 and insoluble Aß42. To determine the mechanism mediating the neuroprotection of anti-Aß scFvs in flies, we generated flies expressing Aß42 in the mushroom body neurons and detected Aß42 in western blot. In flies co-expressing Aß42 and scFv9 or both anti-Aß scFvs, we detected no significant changes in Aß42 levels. But we found a small increase in the level of Aß42 in flies co-expressing scFv42.2, consistent with the higher thioflavin-S intensity, that was not significant (P = 0.08) (Fig. 7A). ELISA is a more sensitive and quantitative method for protein analysis. We used the same flies to generate homogenates for detection of total Aß42 by ELISA at Days 1 and 20. In total Aß42 extracts at Day 1, we found small but significant reductions Aß42 in flies co-expressing scFv9 and scFv9+scFv42.2, but no differences in flies expressing scFv42.2 (Fig. 7B). In flies aged 20 days, all the anti-Aß scFv combinations increased the levels of total Aß42, but only scFv42.2 and scFv9+42.2 were significant.
Figure 7.
Anti-Aß scFvs are neuroprotective without affecting Aβ42 aggregation. (A–E) Accumulation of apoptotic cells in the Kenyon cell clusters at Day 20 in flies expressing the indicated constructs under the control of OK107-Gal4. Control flies expressing LacZ accumulate no TUNEL-positive cells (arrows) (A). Flies expressing Aß42 and LacZ accumulate ∼20 TUNEL-positive cells per Kenyon cell cluster (B). Flies co-expressing scFv9, scFv42.2 or both show significantly fewer apoptotic cells per Kenyon cell cluster (C–E). (I) Graphic representation of the average of apoptotic cells per cluster. Values are expressed as average ± standard error. (F–H and J–N) Accumulation of thioflavin-S in the Drosophila brain. Control flies expressing LacZ in the Kenyon cells (arrows) show no fluorescent signal (F and J). Flies co-expressing Aß42 and LacZ show strong and specific fluorescence (G and K). Co-expression of Aß42 and scFv9, scFv42.2 or both shows no effect on the thioflavin-S signal (L-N). (H) Quantification of the fluorescent signal from thioflavin-S shows significant increase in intensity in flies co-expressing Aß42 and scFv42.2. **P < 0.01; ****P < 0.0001.
To further determine whether the anti-Aß scFvs cause a shift in Aß42 aggregation, we also examined the SDS-soluble fraction of Aß42, which is proposed by some to contain the most toxic Aß42 aggregates. We only found a weak but significant increase in Aß42 in flies co-expressing scFv9 (Fig. 7C). No other significant changes were detected at either Day 1 or 20 (Fig. 7C). Hence, the most relevant findings of these analyses of Aß42 are that anti-Aß scFvs demonstrate protective activity without promoting Aß42 clearance, suggesting that the direct binding of anti-Aß scFvs is sufficient to block Aß42 neurotoxicity. These results are relevant because in murine models of AD, both full and engineered antibodies decrease the levels of Aß42 (5,18). But in Drosophila, the anti-Aß scFvs exert their neuroprotective activity without inducing a significant reduction of Aß42.
Discussion
ScFvs are a class of engineered antibodies with promising applications for AD immunotherapy. Many anti-Aß scFvs prevent Aß42 aggregation and reduce its neurotoxicity in vitro (14). Some anti-Aß scFvs also show benefits in mouse models of AD either by direct administration in the brain, nose or muscle (14) or by AAV-mediated expression in brain or muscle (18,22). These approaches report reduction of Aß deposition in the brain, supporting the amyloid attenuating effects observed with complete antibodies. Although anti-Aß scFvs have shown promising results in mouse models of AD, these are expensive and time-consuming experiments that mainly focus on Aß burden one scFv at a time. Furthermore, the physiological benefits of the anti-Aß scFvs are unclear because most AD mouse models overexpressing wild-type or mutant APP show no overt neurodegeneration. Thus, a new platform enabling efficient in vivo preliminary screening of the neuroprotective activity of new scFvs may be needed. Here, we demonstrate the feasibility of testing the neuroprotective activity of anti-Aß scFvs in Drosophila. As proof-of-principle, we used two scFvs known to reduce Aß42 burden in CRND8 mice (18) and show compelling evidence of their neuroprotective activity in several assays. ScFvs were expressed in flies previously, although modified for intracellular retention against Huntingtin (intrabodies) (31). A camelid heavy-chain antibody against oligomeric Aß was also tested recently in flies with the surprising result that it promoted the toxicity of Aß40 (32). We report here the first time that scFvs are expressed in flies with their original signal peptide, allowing them to interact with secreted Aß42. ScFv9 and scFv42.2 are properly secreted and folded in Drosophila based on their ability to suppress Aß42 and APP neurotoxicity. Overall, the results presented here support the use of Drosophila as a platform for screening multiple anti-Aß scFvs in the same experimental conditions to find the most effective in neurotoxicity assays.
In the original report, expression of scFv9 and scFv42.2 in CRND8 mice by somatic brain transgenesis showed significant reduction of insoluble Aß42 in brain homogenates and fewer amyloid plaques by histological analysis (18). But, the neuroprotective activity of the anti-Aß scFvs could not be determined at the time because APP mice do not show overt neurodegeneration. We demonstrate here that both scFv9 and scFv42.2 prevent neuronal loss, protect the structural integrity of the eye and the dendritic fields of mushroom body neurons and increase the activity of motor neurons. Although the protective activity of scFv42.2 was stronger than scFv9 in both the mushroom bodies and the locomotor assay, this difference may be attributed to its higher expression level. In fact, two copies of scFv9 showed robust rescue of the eye phenotype, supporting the superior performance of N-terminal antibodies described in other models (14,18). Future efforts will take advantage of a common docking site (attP) (33) to ensure similar expression levels, enabling the direct comparison of the relative effectiveness of anti-Aß scFv.
The flexibility of Drosophila genetics allowed us to combine both anti-Aß scFvs and determine that they exert synergistic neuroprotective activities. The combined anti-Aß scFvs drastically improve eye organization and protect axonal and dendritic terminals in the mushroom body neurons. This is the first time that two scFvs have been used in combination in vivo, revealing a novel application for full antibodies or antibody fragments. N-terminal antibodies (e.g. scFv9) are known to bind soluble and insoluble forms of Aß42 because this domain is always exposed (14). Central and C-terminal antibodies (e.g. scFv42.2) can mostly bind monomeric Aß42, but their mechanism of action is not well understood. It is possible that N- and C-terminal anti-Aß antibodies act by different mechanisms, thus explaining the advantages of combining them. These observations suggest that treatments based on combinations of antibodies against different epitopes may provide significant benefits by exploiting their advantages and minimizing their limitations. As the challenges for delivering AD immunotherapy and reducing negative events have been known for the last 15 years, introducing two antibodies represents a minor modification to the current procedures with potential advantages.
The benefits of anti-Aß antibodies are explained by at least three non-exclusive hypotheses. The ‘peripheral sink’ hypothesis poses that antibodies sequester circulating Aß42 in the serum, thus increasing efflux from the brain and reducing Aß42 load in the brain, but subsequent studies have largely excluded this as a primarily mechanism of action (34). A second hypothesis suggests that Aß42 clearance is mediated by the activation of the immune response, particularly activated microglia and Fc-mediated phagocytosis. The third hypothesis proposes that anti-Aß scFvs directly prevent Aß42 aggregation and/or promote Aß42 disaggregation (5). Our experiments in Drosophila demonstrate the neuroprotective activity of two anti-Aß scFvs in animals lacking an adaptive immune response. This simplified experimental set-up indicates that anti-Aß scFvs are neuroprotective without implicating the first two mechanisms. Therefore, the direct binding of anti-Aß scFvs to Aß42 is the only mechanism that can explain their protective activity in Drosophila. According to some authors, optimal anti-Aß antibodies should remove soluble and insoluble Aß42 without increasing the levels of highly toxic, oligomeric Aß42 (35). But our results suggest that anti-Aß scFvs can be protective without clearing or disaggregating Aß42. Here, we propose an alternative mechanism, ‘Aß42 masking’, similar to the action of chaperones on misfolded substrates: binding of anti-Aß scFvs to Aß42 masks neurotoxic epitopes and prevents the interaction of Aß42 with cellular substrates, including neuronal membranes and synaptic proteins. According to this mechanism, the most effective anti-Aß antibodies would be those that stably bind Aß42 monomers, oligomers and other assemblies and mask their neurotoxicity.
Although we cannot discount the beneficial effect of the immune system on Aß42 clearance in rodent models and humans, there seems to be serious risks associated with the stimulation of the cellular response (5). Hence, engineered antibodies that avoid the immune response such as scFvs seem promising alternatives to full antibodies. New anti-Aß scFvs against linear or conformational epitopes can be efficiently selected in flies based on their neuroprotective activity and, then, modified to increase their brain penetration in mice or humans by adding targeting sequences (6). More complex approaches can include the dual targeting of Aß and tau, the two triggers of neurotoxicity in AD, following the same principle of binding and masking. Thus, changing the way we think about the role of aggregates in the toxicity of amyloids may contribute to the development of more efficient therapies for AD and other incurable proteinopathies.
Materials and Methods
Generation of constructs
The cDNAs encoding the IgK secretory signal fused to scFv9, scFv42.2 and scFv40 carrying c-myc and 6xHis tags (18) were released from pSecTag2B (Invitrogen) with PmeI and NheI. Then, these inserts were subcloned into a modified pUASTv2 vector (36) containing PmeI and NheI sites in the polylinker between NotI and EcoRI. The resulting clones were sequenced to confirm integrity of the ORFs and identified as pUASTv2-scFv9-myc-his, pUASTv2-scFv42.2-myc-his and pUASTv2-scFv40-myc-his.
Transgenic flies and Drosophila genetics
The constructs pUASv2-scFv9-myc-his, pUASTv2-scFv42.2-myc-his and pUASTv2-scFv40-myc-his were injected into yw embryos at Rainbow Transgenics following standard procedures (37) to generate multiple independent transgenic lines. The flies expressing two independent copies of Aß42 under the control of UAS were described previously (27). The flies carrying human APPswe BACE1 under the control of UAS were obtained from Vitruvian, Inc. and kindly provided by Mathew Mahoney. The driver strains OK107-Gal4 (mushroom bodies), gmr-Gal4 (all eye cells) and Elav-Gal4 (pan-neural), and the reporters UAS-LacZ and UAS-CD8-GFP were obtained from the Bloomington Drosophila Stock Center. Flies were maintained on standard Drosophila medium at 25°C. For experiments, homozygous females for the Gal4 strains were crossed with UAS males to generate progeny expressing Aβ42 in the desired tissue. All crosses were initially placed at 25°C for 2 days, progeny was raised at specified temperature depending on the assay and the adults were aged at the same temperature. All assays were performed using females.
External eye structure
We crossed gmr-Gal4: Aß42 females with LacZ, scFv9, scFv42.2 and scFv9+scFv42.2 (scFv9+42.2) males at 28°C and collected 1- to 2-day-old females. To image fresh eyes, we froze the flies at −20°C and collected eye images as Z-stacks with a Leica Z16 APO using a 2× Plan-Apo objective. Flattened in-focus images were produced with the Montage Multifocus module of the Leica Application Software. For scanning electron microscopy (SEM), flies were serially dehydrated in ethanol, air-dried in hexamethyldisilazane (Electron Microscope Sciences) and metal-coated for observation in a Jeol 1500 SEM.
Drosophila S2R+ cell culture, secreted proteins and immunofluorescence
Drosophila S2R+ cells were maintained in Schneider media at 26°C as described (38). To assess the expression and distribution of scFv and Aß42, pUASv2-scFv9-myc-his, pUASTv2-scFv42.2-myc-his and pUAS-Aß42 were co-transfected with a vector expressing Gal4 under control of the Drosophila Actin 5C promoter. Briefly, 4 × 105 cells were co-transfected in duplicate with 200 ng of pAC-Gal4 (Addgene) and scFv or Aß42 vectors in 24-well plates using the Qiagen Effectene Kit (Qiagen). Forty-eight hours later, we collected and precipitated the culture media (supernatant) and the cells (cell lysate) for western blot analysis following the procedures described later. We performed immunostaining using mab 3.1.1. anti-Aß42 (1:500) and chicken anti-myc (1: 250) antibodies.
Brain immunofluorescence
For co-localization studies, we co-expressed Aß42 with scFv9 and scFv42.2 in adult mushroom bodies with OK107-Gal4, fixed brains at Day 1 and incubated with chicken anti-myc (1:250, Sigma) and anti-Aß42 4G8 (1:150, Covance) antibodies. Whole-mount immunofluorescence of adult brain interneurons (63× objective) and larval eye discs (20× and 40× objectives) was conducted by fixing in 4% formaldehyde, washing with PBT and blocking with 3% bovine serum albumin. The secondary antibodies anti-mouse-Cy3 (Molecular Probes) and anti-chicken-FITC (Sigma) were used at 1:600. Final mounting of tissues was done on Vectashield antifade (Vector).
TUNEL staining
To document cell death, we used the TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay with fluorescein (Roche). We generated flies expressing LacZ alone or Aß42 together with LacZ, scFv9, scFv42.2 or scFv9+42.2 under the control of OK107-Gal4 at 27°C and aged them for 20 days. Once we fixed the whole brains, we followed the manufacturer's recommendations with a few changes to optimize penetration and signal in whole-mount samples. We permeabilized in 100 mm sodium citrate for 45 min at 65°C, washed, pre-incubated in 45 µl with 50% label solution for 30 min at 37°C, added 5 µl of enzyme, incubated for 3 h at 37°C, washed thoroughly and mounted in Vectashield. The Kenyon cell clusters were imaged as z-stacks with the 40× objective, and TUNEL-positive cells were manually counted from flattened stacks and entered in Excel to generate the graphs. Means were analyzed in GraphPad Prism using a one-way ANOVA with Tukey's multiple comparison.
Thioflavin-S staining
We generated flies expressing LacZ alone or Aß42 together with LacZ, scFv9, scFv42.2 or scFv9+42.2 under the control of OK107-Gal4 at 25°C. We fixed 1-day-old brains and incubated them with a 0.03% solution of freshly prepared and filtered thioflavin-S (Sigma) in 50% ethanol/PBS for 10 min, washed, mounted in Vectashield and imaged as a z-stack with the 40× objective. Thioflavin-S intensity was calculated from single-plane images containing the maximum area of Kenyon cells, the areas were outlined manually, the total intensity and area were calculated in Photoshop and the average intensity per square micrometer was calculated from those parameters. At least 10 images were analyzed per genotype, and the differences between the positive control (Aß42; LacZ) and the scFv constructs were determined by t-test.
Mushroom body degeneration
We crossed OK107-Gal4; CD8-GFP flies with LacZ alone (negative control) or with Aß42 together with LacZ, scFv9, scFv42.2 or scFv9+42.2 at 27°C. Adult flies were collected at Day 1 post-eclosion followed by aging for up to 40 days. We then imaged GFP-labeled mushroom bodies at Days 1, 20, 30 and 40 by dissecting, fixing and mounting the brains as described earlier. To quantify the surface of mushroom body axonal lobes, we imaged them as z-stacks with the 40× objective, flattened the Z-stacks and manually selected the maximum area from at least 10 images. To quantify the volume of the calyces, we collected Z-stacks with a 0.7-µm step using the 40× objective for 12 or more mushroom bodies. Then, the calycal area in each plane was traced manually in ImageJ with the free Measure Stack plug-in, which rendered the total volume for each stack. The average volume for each condition and time point was entered in Excel to create the graphs. Finally, data series were analyzed for statistical significance in GraphPad Prism using a one-way ANOVA with Tukey's multiple comparison.
Microscopy and image processing
We collected fluorescent images with AxioVision (Zeiss) in an Axio-Observer Z1 microscope (Zeiss) by optical sectioning using ApoTome (structured light microscopy) with 20× NA: 0.7 (air), 40× NA: 1.3 (air) and 63× NA: 1.4 (oil) objectives. Representative single-plane images of antennal lobe local interneurons and mushroom body calyces were extracted from Z-stacks (Figs 3 and 4). Three-dimensional images were created from Z-stacks using AxioVision rendered in the transparency mode, textured method and color-coded for depth (Fig. 4). Final images were combined using Adobe Photoshop and processing included brightness/contrast adjustment to whole images for optimal viewing and printing.
Locomotor assay
Flies carrying LacZ alone or Aß42 combined with LacZ, scFv9, scFv42.2 or both anti-Aß scFvs were crossed with the pan-neural driver elav-Gal4 at 25°C, and the progeny was tested daily for their ability to climb with the genotypes blinded to the experimenter as previously described (39). Briefly, 25–30 newborn adult females were placed in empty vials in duplicates. Every day, flies were forced to the bottom by firmly tapping against the surface. After 8 s, we recorded the number of flies that walked above 5 cm. This was repeated eight times to obtain the average climbing index each day (flies above line/total flies × 100). At the end of the experiment, the climbing index was plotted as a function of age in Excel. Data points are presented as mean values ± standard deviation. As we found no significant differences between replicates by t-test, we averaged them to analyze the different genotypes by a two-way ANOVA with Tukey's posthoc analysis. Significance was established with P-values of ≤0.01. Statistical analyses were performed with GraphPad Prism (Fig. 8).
Figure 8.
Anti-Aß scFvs suppresses Aβ42 neurotoxicity without reducing Aß42 levels. (A) Western blot in 1-day-old flies and quantification. Flies co-expressing Aß42 and the anti-Aß scFvs show similar levels of Aß42 as those co-expressing LacZ. Only flies co-expressing scFv42.2 showed slightly higher, but not significant levels of Aß42. A representative blot is shown, but the Aß42 quantification was done from three independent replicates. (B and C) Quantification of total Aβ42 and the SDS fraction at Days 1 (D1) and 20 (D20) by ELISA. (B) In young flies, co-expression of scFv9 and scFv9+42.2 led to a slight but significant reduction of Aß42 (n = 5). In 20-day-old flies, all the anti-Aß scFv combinations increase the levels of Aß42, but only scFv42.2 and scFv9+42.2 are significant. (C) The levels of Aß42 in the SDS fraction are mostly unchanged by the expression of anti-Aß scFvs, except for a modest increase in scFv9 at Day 1.
Tissue homogenates and western blot
Fly heads to detect Aß42 or whole flies to detect scFv9 and scFv42.2 were homogenized essentially as described (27). Tricine–SDS loading buffer was added to each sample, followed by boiling at 95°C for 5 min. Protein extracts were fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (SDS-PAGE) in 4–12% Bis–Tris gels (Invitrogen) under reducing conditions and electroblotted into nitrocellulose membranes. Membranes were blocked in Tris-buffered saline-T containing 5% non-fat milk and probed against Aß (1:1000, 6E10, Signet), myc (1:5000, 9E10, Abcam) and ß-Tubulin (1:2 500 000, Sigma) antibodies, followed by HRP-conjugated secondary antibodies at 1:2500. To optimize reactivity of the 6E10 antibody, membranes were boiled for 5 min in PBS. Immunoreactive bands were visualized by enhanced chemiluminescence (ECL, Amersham). Bands were quantified from three (Aß42) or four (scFv) independent experiments, normalized to the tubulin band, and reported as average ± standard deviation. To analyze the statistical significance of the band intensity, we used GraphPad to carry out a t-test.
Co-immunoprecipitation
We generated flies expressing Aβ42 together with LacZ, ScFv9 or scFv42.2 under the control of OK107-Gal4 at 25°C. A total of 40 heads of each genotype or control LacZ alone were homogenized in 30 µl of extraction buffer [1× IP buffer (Invitrogen), 0.15 m NaCl, 0.02 m MgCl2, 1× complete Mini (Roche) and 0.1 mm DTT]. The co-immunoprecipitation assay was conducted using the Dynabead co-immunoprecipitation kit (Invitrogen) according to the manufacturer's protocol with minor modifications. Briefly, 1.5 mg of Dynabeads was incubated with 7 µg of Myc or 4G8 antibodies for 14 h at 37°C with rotation. The antibody-coated beads were washed and incubated with extraction buffer containing 0.3% of BSA for 40 min at RT. After washing two additional times, the dynabead-Ab complex was incubated with 330 µl of extraction buffer and 25 µl of protein extract at 4°C for 14 h with gentle rotation. Upon washing, immunoprecipitated proteins were eluted by boiling in NuPAGE LDS sample buffer (Invitrogen) and detected by western blot in a 12% NuPAGE Bis–Tris gel using either the 6E10 anti-Aß42 or the anti-myc antibodies.
ELISA of total Aß42 and Aß42 fractions
To assess total Aß42 levels by ELISA, five groups of fly heads each were homogenized in 50 µl GnHCl extraction buffer [5 m guanidinium HCl, 50 mm Hepes pH 7.3, protease inhibitor cocktail (Thermo) and 5 mm EDTA] and centrifuged at 21 000g for 5 min at 4°C to remove cell debris. The resulting supernatant was analyzed as the total fly Aß42 sample. For preparation of the SDS-soluble fractions, five groups of fly heads each were sequentially extracted with 10 µl of Tris-buffered saline and 10 µl of 2% SDS containing protease inhibitor cocktail. Total Aß42 and 2% SDS fractions were diluted 1:20 and 1:400, respectively, and used for sandwich ELISA as described (40). Aß42 was captured with mAb 213 (human Aß35-42 specific) and detected by HRP-conjugated mAb Ab9 (40).
Supplementary Material
Funding
This work was supported in part by the National Institutes of Health grants DP2 OD002721-01 to P.F.-F. and AG18454 to T.E.G., a McKnight Brain Institute Research Development Award (UF Project 00112640) and start-up funds from the UF Department of Neurology to P.F.-F. and D.E.R.-L.
Supplementary Material
Acknowledgements
We thank Pedro Cruz for generating the constructs, Krishanu Mathur for assistance generating the transgenic flies, Amit Singh for sharing the optimized TUNEL protocol, the Bloomington Drosophila Stock Center for fly strains and the Developmental Studies Hybridoma Bank for antibodies.
Conflict of Interest statement. None declared.
References
- 1.Overk C.R., Masliah E. (2014) Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochem. Pharmacol., 88, 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy J.A., Higgins G.A. (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science, 256, 184–185. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J. (2009) The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J. Neurochem., 110, 1129–1134. [DOI] [PubMed] [Google Scholar]
- 4.Benilova I., Karran E., De Strooper B. (2012) The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat. Neurosci., 15, 349–357. [DOI] [PubMed] [Google Scholar]
- 5.Wisniewski T., Goni F. (2014) Immunotherapy for Alzheimer's disease. Biochem. Pharmacol., 88, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer B., Masliah E. (2014) Immunotherapy for Alzheimer's disease: past, present and future. Front. Aging Neurosci., 6, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilman S., Koller M., Black R.S., Jenkins L., Griffith S.G., Fox N.C., Eisner L., Kirby L., Rovira M.B., Forette F. et al. (2005) Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology, 64, 1553–1562. [DOI] [PubMed] [Google Scholar]
- 8.Orgogozo J.M., Gilman S., Dartigues J.F., Laurent B., Puel M., Kirby L.C., Jouanny P., Dubois B., Eisner L., Flitman S. et al. (2003) Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology, 61, 46–54. [DOI] [PubMed] [Google Scholar]
- 9.Golde T.E. (2014) Open questions for Alzheimer's disease immunotherapy. Alzheimers Res. Ther., 6, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barakos J., Sperling R., Salloway S., Jack C., Gass A., Fiebach J.B., Tampieri D., Melancon D., Miaux Y., Rippon G. et al. (2013) MR imaging features of amyloid-related imaging abnormalities. AJNR, 34, 1958–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling R., Salloway S., Brooks D.J., Tampieri D., Barakos J., Fox N.C., Raskind M., Sabbagh M., Honig L.S., Porsteinsson A.P. et al. (2012) Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet. Neurol., 11, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M., Sabbagh M., Honig L.S., Porsteinsson A.P., Ferris S. et al. (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N. Engl. J. Med., 370, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., Raman R., Sun X., Aisen P.S. et al. (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N. Engl. J. Med., 370, 311–321. [DOI] [PubMed] [Google Scholar]
- 14.Robert R., Wark K.L. (2012) Engineered antibody approaches for Alzheimer's disease immunotherapy. Arch. Biochem. Biophys., 526, 132–138. [DOI] [PubMed] [Google Scholar]
- 15.Frenkel D., Solomon B., Benhar I. (2000) Modulation of Alzheimer's beta-amyloid neurotoxicity by site-directed single-chain antibody. J. Neuroimmunol., 106, 23–31. [DOI] [PubMed] [Google Scholar]
- 16.Liu R., Yuan B., Emadi S., Zameer A., Schulz P., McAllister C., Lyubchenko Y., Goud G., Sierks M.R. (2004) Single chain variable fragments against beta-amyloid (Abeta) can inhibit Abeta aggregation and prevent abeta-induced neurotoxicity. Biochemistry, 43, 6959–6967. [DOI] [PubMed] [Google Scholar]
- 17.Wang X.P., Zhang J.H., Wang Y.J., Feng Y., Zhang X., Sun X.X., Li J.L., Du X.T., Lambert M.P., Yang S.G. et al. (2009) Conformation-dependent single-chain variable fragment antibodies specifically recognize beta-amyloid oligomers. FEBS Lett., 583, 579–584. [DOI] [PubMed] [Google Scholar]
- 18.Levites Y., Jansen K., Smithson L.A., Dakin R., Holloway V.M., Das P., Golde T.E. (2006) Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J. Neurosci., 26, 11923–11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meli G., Visintin M., Cannistraci I., Cattaneo A. (2009) Direct in vivo intracellular selection of conformation-sensitive antibody domains targeting Alzheimer's amyloid-beta oligomers. J. Mol. Biol., 387, 584–606. [DOI] [PubMed] [Google Scholar]
- 20.Fukuchi K., Accavitti-Loper M.A., Kim H.D., Tahara K., Cao Y., Lewis T.L., Caughey R.C., Kim H., Lalonde R. (2006) Amelioration of amyloid load by anti-Abeta single-chain antibody in Alzheimer mouse model. Biochem. Biophys. Res. Commun., 344, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuchi K., Tahara K., Kim H.D., Maxwell J.A., Lewis T.L., Accavitti-Loper M.A., Kim H., Ponnazhagan S., Lalonde R. (2006) Anti-Abeta single-chain antibody delivery via adeno-associated virus for treatment of Alzheimer's disease. Neurobiol. Dis., 23, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan D.A., Mastrangelo M.A., Narrow W.C., Sullivan M.A., Federoff H.J., Bowers W.J. (2010) Abeta-directed single-chain antibody delivery via a serotype-1 AAV vector improves learning behavior and pathology in Alzheimer's disease mice. Mol. Ther., 18, 1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gimenez-Llort L., Rivera-Hernandez G., Marin-Argany M., Sanchez-Quesada J.L., Villegas S. (2013) Early intervention in the 3xTg-AD mice with an amyloid beta-antibody fragment ameliorates first hallmarks of Alzheimer disease. mAbs, 5, 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frenkel D., Solomon B. (2002) Filamentous phage as vector-mediated antibody delivery to the brain. Proc. Natl Acad. Sci. USA, 99, 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J., Pattanayak A., Song M., Kou J., Taguchi H., Paul S., Ponnazhagan S., Lalonde R., Fukuchi K. (2013) Muscle-directed anti-Abeta single-chain antibody delivery via AAV1 reduces cerebral Abeta load in an Alzheimer's disease mouse model. J. Mol. Neurosci., 49, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cattepoel S., Hanenberg M., Kulic L., Nitsch R.M. (2011) Chronic intranasal treatment with an anti-Abeta(30–42) scFv antibody ameliorates amyloid pathology in a transgenic mouse model of Alzheimer's disease. PLoS One, 6, e18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casas-Tinto S., Zhang Y., Sanchez-Garcia J., Gomez-Velazquez M., Rincon-Limas D.E., Fernandez-Funez P. (2011) The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum. Mol. Genet., 20, 2144–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greeve I., Kretzschmar D., Tschape J.A., Beyn A., Brellinger C., Schweizer M., Nitsch R.M., Reifegerste R. (2004) Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J. Neurosci., 24, 3899–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis R.L. (2005) Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci., 28, 275–302. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka N.K., Tanimoto H., Ito K. (2008) Neuronal assemblies of the Drosophila mushroom body. J. Comp. Neurol., 508, 711–755. [DOI] [PubMed] [Google Scholar]
- 31.Wolfgang W.J., Miller T.W., Webster J.M., Huston J.S., Thompson L.M., Marsh J.L., Messer A. (2005) Suppression of Huntington's disease pathology in Drosophila by human single-chain Fv antibodies. Proc. Natl Acad. Sci. USA, 102, 11563–11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wacker J., Ronicke R., Westermann M., Wulff M., Reymann K.G., Dobson C.M., Horn U., Crowther D.C., Luheshi L.M., Fandrich M. (2014) Oligomer-targeting with a conformational antibody fragment promotes toxicity in Abeta-expressing flies. Acta Neuropathol. Commun., 2, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groth A.C., Fish M., Nusse R., Calos M.P. (2004) Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics, 166, 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levites Y., Smithson L.A., Price R.W., Dakin R.S., Yuan B., Sierks M.R., Kim J., McGowan E., Reed D.K., Rosenberry T.L. et al. (2006) Insights into the mechanisms of action of anti-Abeta antibodies in Alzheimer's disease mouse models. FASEB J., 20, 2576–2578. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y.H., Giunta B., Zhou H.D., Tan J., Wang Y.J. (2012) Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat. Rev. Neurol., 8, 465–469. [DOI] [PubMed] [Google Scholar]
- 36.Brand A.H., Perrimon N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- 37.Rubin G.M., Spradling A.C. (1982) Genetic transformation of Drosophila with transposable element vectors. Science, 218, 348–353. [DOI] [PubMed] [Google Scholar]
- 38.Rincon-Limas D.E., Lu C.H., Canal I., Botas J. (2000) The level of DLDB/CHIP controls the activity of the LIM homeodomain protein apterous: evidence for a functional tetramer complex in vivo. Embo J., 19, 2602–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Funez P., Casas-Tinto S., Zhang Y., Gomez-Velazquez M., Morales-Garza M.A., Cepeda-Nieto A.C., Castilla J., Soto C., Rincon-Limas D.E. (2009) In vivo generation of neurotoxic prion protein: role for hsp70 in accumulation of misfolded isoforms. PLoS Genet., 5, e1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J., Miller V.M., Levites Y., West K.J., Zwizinski C.W., Moore B.D., Troendle F.J., Bann M., Verbeeck C., Price R.W. et al. (2008) BRI2 (ITM2b) inhibits Abeta deposition in vivo. J. Neurosci., 28, 6030–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.