Abstract
Immunotherapy, both active and passive, is increasingly recognized as a powerful approach to a wide range of diseases, including Alzheimer's and Parkinson's. Huntington's disease (HD), an autosomal dominant disorder triggered by misfolding of huntingtin (HTT) protein with an expanded polyglutamine tract, could also benefit from this approach. Individuals can be identified genetically at the earliest stages of disease, and there may be particular benefits to a therapy that can target peripheral tissues in addition to brain. In this active vaccination study, we first examined safety and immunogenicity for a broad series of peptide, protein and DNA plasmid immunization protocols, using fragment (R6/1), and knock-in (zQ175) models. No safety issues were found. The strongest and most uniform immune response was to a combination of three non-overlapping HTT Exon1 coded peptides, conjugated to KLH, delivered with alum adjuvant. An N586-82Q plasmid, delivered via gene gun, also showed ELISA responses, mainly in the zQ175 strain, but with more variability, and less robust responses in HD compared with wild-type controls. Transcriptome profiling of spleens from the triple peptide-immunized cohort showed substantial HD-specific differences including differential activation of genes associated with innate immune responses, absence of negative feedback control of gene expression by regulators, a temporal dysregulation of innate immune responses and transcriptional repression of genes associated with memory T cell responses. These studies highlight critical issues for immunotherapy and HD disease management in general.
Introduction
The immune system is increasingly recognized as a player in the pathogenic cascade of neurodegenerative diseases triggered by misfolding proteins, as well as a potential source of valuable targeted immunotherapies. Applying immunotherapeutic approaches to Alzheimer's and Parkinson's disease has shown significant promise in animal models, although most reported human trials to date have not reached efficacy criteria, possibly due to a combination of a lack of early enough biomarkers to identify cases, and heterogeneity of the underlying disease mechanisms within the broad neurological diagnoses (1,2). Huntington's disease (HD) is an autosomal dominant disorder that circumvents most of the above problems, since genetic diagnosis can be done at a relatively early pre-manifest stage, and the underlying primary gene defect is more uniform than for the neurodegenerations tested to date. The HD mutation is a trinucleotide repeat expansion leading to an increase in the length of a polyglutamine (polyQ) tract at the N terminus of the huntingtin (HTT) protein (3).The gene is expressed ubiquitously, although neurons appear to be the major affected cell type, and symptoms manifest at ages that are roughly inversely related to the unstable expanded polyQ (4). Mouse models using CAG repeat numbers that would characterize very early onset of HD in humans have been used to follow effects of the mutant gene in brain and peripherally, as well as for preliminary drug testing (5).
We have previously shown that intrastriatal delivery of an anti-HTT single-chain Fv antibody gene fragment directly into neurons can counteract the HD phenotype in cultures, drosophila and mouse models (6–10). Our pilot study of an active immunization protocol reported that vaccination with a mutant HTT Exon1 DNA fragment could ameliorate a glucose intolerance phenotype in a R6/2 mouse model (11). These results suggest that immunotherapies have significant potential for treating CNS and systemic phenotypes in HD. Here, we first comprehensively examine the immunogenicity and safety of multiple protein, peptide and plasmid vaccination protocols in the R6/1 fragment (12), and the zQ175 knock-in (13), mouse models of HD.
Systems biology approaches are being used to identify molecular networks that control immunity in response to vaccination (14,15). While numerous studies have reported on gene expression in human HD and HD models (16,17), little is known about the peripheral transcriptional signatures underlying the induction of antibody responses in HD. This study reports on a transcriptome analysis in the spleen of two HD mouse models after immunization with three non-overlapping peptides from HTT exon 1. The transcriptome analysis revealed HD-specific differences in the inflammatory/immune pathways that will further inform efforts to utilize active vaccination as a clinical therapeutic for HD.
Results
Safety of vaccine immunogens
Safety and immunogenicity of potential peptide, protein and/or DNA vaccines were evaluated. C57Bl/6J (B6) congenic males from both strains were crossed to wild-type CBA/J females. Wild-type F1 littermate mice served as controls. Genetic backgrounds were chosen to test two different H-2 regions (http://jaxmice.jax.org/jaxnotes/archive/433c.html). The choice of these two HD mouse models allowed us to examine immune response parameters in subjects that are very close to manifest, with potential onset of HD-related phenotypes during the study (R6/1) (12,18,19); and during the presymptomatic period of a later-onset full-length model (zQ175) (13,20).
As the mouse equivalent of a Phase 1a clinical trial, safety is a critical aspect of the analysis. All mice were weighed weekly, and none of the animals in any of the 11 experimental groups showed vaccine-related weight loss prior to the endpoints. There was also no evidence of muscle wasting or sickness behavior. In addition, for the mutant mice, it was possible that the immunizations would result in acceleration of the onset of neurological symptoms that become apparent from their overall behavior in the cages. This was never observed, either by the investigators, or by the animal care staff, who make notes on reduced function during routine weekly cage changes.
A combination of three non-overlapping HTT Exon 1 peptides is highly immunogenic in HD mutant mice
Immunogenicity was tested by both ELISA and Western blot (WB). While the two methods gave roughly comparable results, the epitopes available for binding from serum antibodies may differ. The study started with individual peptides. Fragments of antibodies to all three of these peptides have been shown to exhibit anti-HTT activity. AA1-17 is the peptide target of scFvC4 (10), AA49-60 was used by the Patterson lab to select intrabodies Happ1 and Happ3 (21), and AA74-88 is the antigen for EM48 (22). Of the three individual peptides, peptide AA1-17 elicited higher antibody titers than either AA49-60 or AA74-88; however, ELISA data for the R6/1 mice appeared consistently on the lower end for our assays. We therefore switched to testing the peptides delivered in combination, and did a full set of assays. Of all peptide/protein antigens tested in this entire series of experiments, a combination of three non-overlapping HTT Exon1 derived peptides (AA1-17, AA49-60, AA74-88; all KLH-conjugated with alum adjuvant) was most strongly immunogenic in HD mutant mice and control mice (Table 1, Fig. 1) across both assays. Critically, antibody titers to the triple peptide immunogen were robust in both HD mutant and control mice, although the R6/1 values were lower by ELISA. For protein immunization, the HTT Exon1-46Q protein fragment was linked to maltose binding protein (MBP) to retain solubility, with cleavage of the fusion just prior to injection. Although this protein contains the three non-overlapping peptides, plus an expanded polyQ, the protocol induced significantly lower antibody titers in both mutant and wild-type mouse strains (Table 1; WB not tested after consistently low ELISAs). The 46Q protein had a strong tendency to begin aggregating almost immediately upon cleavage from the MBP, which had the advantage of testing a different set of available epitopes, but also made the solution very non-homogeneous, and challenging to work with. We also used a series of WBs from lysates of cells transfected with HTTExon1-72Q to probe for the presence of aggregated forms of HTT, but never saw evidence of anti-aggregate antibodies in any of our sera (data not shown).
Table 1.
A combination of three non-overlapping HTT exon1 peptides is highly immunogenic in HD mutant and control mice
| Immunogen | Mouse ID | zQ175 |
R6/1 | WT control |
|||
|---|---|---|---|---|---|---|---|
| ELISA | WB | ELISA | WB | ELISA | WB | ||
| AA1-17 peptide | 1 | >2000 | 2000 | 600 | 2000 | ND | ND |
| 2 | >2000 | 5000 | 600 | 2000 | ND | ND | |
| 3 | >2000 | 2000 | 200 | 2000 | ND | ND | |
| 4 | >2000 | 5000 | 225 | 2000 | ND | ND | |
| 5 | 900 | 2000 | 225 | 2000 | ND | ND | |
| AA49-60 peptide | 1 | >2000 | <500 | 375 | <200 | ND | ND |
| 2 | ND | ND | 75 | <200 | ND | ND | |
| 3 | 1000 | <500 | <50 | <200 | ND | ND | |
| 4 | 100 | <200 | 75 | <200 | ND | ND | |
| 5 | <50 | <200 | <50 | <200 | ND | ND | |
| AA74-88 peptide | 1 | >2000 | 2000 | 900 | <200 | ND | ND |
| 2 | 1200 | <200 | ND | <200 | ND | ND | |
| 3 | 350 | 200 | 150 | <200 | ND | ND | |
| 4 | <50 | 200 | <50 | <200 | ND | ND | |
| 5 | <50 | <200 | 75 | <200 | ND | ND | |
| Combined triple Peptidesa | 1 | >5000 | 2000 | 1200 | 2000 | 750 | 2000 |
| 2 | 5000 | 2000 | 2000 | 2000 | 5000 | 2000 | |
| 3 | 5000 | 2000 | 1000 | 2000 | 2000 | 2000 | |
| 4 | >1000 | 2000 | 250 | 2000 | 5000 | 2000 | |
| 5 | 1000 | 2000 | ND | ND | 500 | 2000 | |
| HTTexon1-Q46 protein | 1 | 270 | ND | 250 | ND | 250 | ND |
| 2 | 250 | ND | 350 | ND | 500 | ND | |
| 3 | 500 | ND | 90 | ND | 500 | ND | |
| 4 | 500 | ND | 220 | ND | ND | ND | |
| 5 | 250 | ND | 500 | ND | ND | ND | |
Anti-Htt antibodies in sera of immunized mice were measured by ELISA or Western blotting. Antibody titers >1000 are used to indicate relative responses, and are shaded in gray. Serum antibody titer is defined as the reciprocal of the dilution whose absorbance is 3× that of negative controls.
ND, Not determined.
aCombination of the three peptides above.
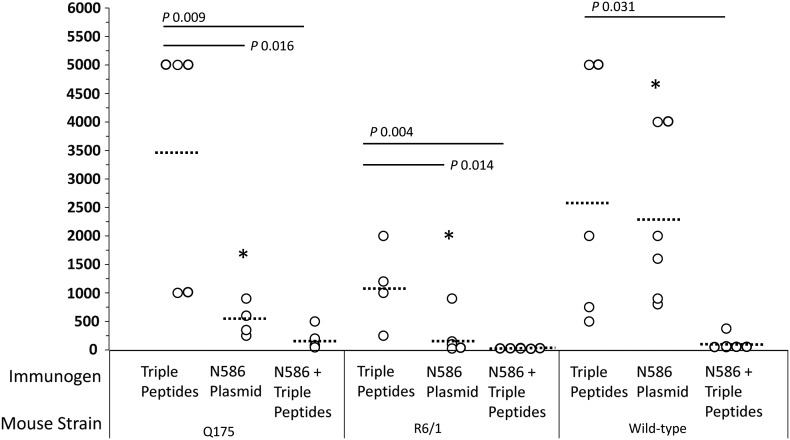
Figure 1.
A vaccine immunogen consisting of three non-overlapping HTT exon 1 peptides induces similar antibody titers in immunized HD mutant and wild-type mice. Serum antibody titers (reciprocal of the serum dilution whose absorbance is 3× that of negative controls) were determined by ELISA after immunization of zQ175, R6/1, or wild-type mice with a triple peptide combination, the N586 plasmid DNA or a prime/boost regimen (N586 prime, triple peptide boost). One-way ANOVA was used to identify differences among the three vaccine immunogens or the three mouse strains. Dotted lines = means. Tukey's HSD post-hoc test was then used to identify pairwise differences. P-values are indicated for the vaccine immunogens. The asterisks indicate a P-value of <0.05 for mice immunized with the N586 plasmid (wild-type and R6/1; wild-type and zQ175).
The HTT N586-Q82 plasmid DNA induced lower antibody titers in HD mutant mice than in WT controls
Of the two plasmid DNAs, HTT N586-Q82 was more immunogenic than HTT Exon1-Q97 (Fig. 1 and Table 2). Two strategies were used to augment the immunogenicity of the HTT plasmid DNAs. In one approach, a cytokine adjuvant (an Il4 plasmid DNA, mouse origin) (23) was co-administered with the HTT plasmid DNA. The Il4 plasmid did not have an adjuvant effect in wild-type (WT) mice immunized with either of the two HTT plasmids, or in the HD mouse model after immunization with HTT exon1-Q97, Surprisingly, co-administration of the Il4 plasmid had an inhibitory effect on antibody titers in the two HD mouse models after immunization with the HTT N586-Q82 plasmid.
Table 2.
The HTT N586-Q82 plasmid is more immunogenic than the HTT exon1-Q97 plasmid in HD mutant and control mice
| Immunogen | Mouse ID | zQ175 |
R6/1 | WT control |
|||
|---|---|---|---|---|---|---|---|
| ELISA | WB | ELISA | WB | ELISA | WB | ||
| HTTexon1-Q97 plasmid | 1 | 40 | <200 | 50 | <200 | <25 | <200 |
| 2 | 35 | <200 | <25 | <200 | <25 | <200 | |
| 3 | 25 | <200 | 40 | <200 | 70 | <200 | |
| 4 | 40 | <200 | 45 | <200 | 30 | <200 | |
| 5 | 45 | <200 | <25 | <200 | 25 | <200 | |
| HTTexon1-Q97 + IL-4 plasmids | 1 | 100 | <200 | <25 | <200 | 25 | <200 |
| 2 | 100 | <200 | <25 | <200 | 300 | <200 | |
| 3 | 40 | <200 | <25 | <200 | 90 | <200 | |
| 4 | <25 | <200 | 25 | <200 | 45 | <200 | |
| 5 | 175 | ND | 25 | <200 | 125 | <200 | |
| HTT N586-Q82 plasmid | 1 | 900 | 2000 | 100 | 2000 | 4000 | 5000 |
| 2 | 600 | <200 | 900 | 2000 | 1600 | 5000 | |
| 3 | 250 | <200 | 100 | 2000 | 4000 | 5000 | |
| 4 | ND | ND | <25 | 2000 | 800 | 5000 | |
| 5 | 350 | 2000 | <25 | <200 | 900 | 5000 | |
| 6 | 2000 | 5000 | |||||
| HTT N586-Q82 + IL-4 plasmids | 1 | 25 | <200 | 150 | 200 | 350 | 200 |
| 2 | 80 | <200 | <25 | <200 | >500 | 5000 | |
| 3 | 400 | <200 | 1900 | 200 | >500 | 5000 | |
| 4 | 60 | <200 | 100 | <200 | >500 | 5000 | |
| 5 | 25 | <200 | 150 | <200 | >500 | 5000 | |
| 6 | <25 | <200 | |||||
| 7 | >500 | 5000 | |||||
| HTTexon1-Q97 plasmid prime + triple peptide boost | 1 | 35 | ND | 90 | ND | 100 | ND |
| 2 | 240 | ND | 35 | ND | <25 | ND | |
| 3 | <25 | ND | <25 | ND | 450 | ND | |
| 4 | 45 | ND | <25 | ND | 50 | ND | |
| 5 | <25 | ND | <25 | ND | 175 | ND | |
| HTT N586-Q82 plasmid prime + triple peptide boost | 1 | 500 | ND | 35 | ND | 65 | ND |
| 2 | 200 | ND | <25 | ND | 50 | ND | |
| 3 | 80 | ND | <25 | ND | 50 | ND | |
| 4 | 45 | ND | <25 | ND | 60 | ND | |
| 5 | ND | ND | <25 | ND | 375 | ND | |
Anti-Htt antibodies in sera of immunized mice were measured by ELISA or Western blotting, as in Table 1. Antibody titers >500 are shaded in gray, since overall readings were lower.
ND, Not determined.
The other approach to increase the immunogenicity of HTT plasmid DNA relied on including a non-plasmid immunogen as a vaccine boost. The vaccine boost chosen for this portion of the work was the combination of three non-overlapping HTT exon 1 peptides which is the most immunogenic of all regimens tested in this series (Fig. 1). The heterologous vaccines consisting of a priming dose with the HTT plasmid DNA followed by two boosts with the triple peptides did not augment antibody responses (Table 2) suggesting that different epitopes are being recognized in the priming and boosting immunogens.
Genome-wide RNA expression in the spleen of immunized HD mutant mice analyzed by GSEA
Of the eleven immunization strategies, the one that yielded the highest antibody titers utilized a combination of three non-overlapping HTT Exon 1 derived peptides as the immunogen (Table 1). Furthermore, the triple peptide immunogen elicited similar antibody titers in both HD mutant and WT mice (Fig. 1). Immune responses were probed further by assessing genome-wide RNA expression in the spleens of immunized mice using microarrays. Microarray data were analyzed using Gene Set Enrichment Analysis (GSEA) (24). The goal of GSEA is to determine whether members of a curated gene set (S) tend to occur toward the top or bottom of a ranked list (L) of differentially expressed genes. If members of S are found toward the top or bottom of L, then the gene set is correlated with the experimental phenotype.
Gene sets in a Molecular Signatures Database (MSigDB) from Kegg, Reactome, Immunologic signatures and GeneOntology (GO) were analyzed to identify those that correlated with strain differences in unimmunized mice or immunization differences in mutant HD and WT mice. Of the 26 high-ranking gene sets in our analysis, 11 correlated with strain differences in unimmunized mice, none of which is involved in innate immunity (manuscript in preparation), while 15 correlated with immunization differences in mutant and WT mice (Table 3). Detailed information on the 15 gene sets is shown in Supplementary Material, Table S1. Of the 15 gene sets, 11 showed positive enrichment scores (Supplementary Material, Table S2), and four showed negative enrichment scores (Supplementary Material, Table S3). An example of a GSEA result (Biocarta IL22BP) is shown in Supplementary Figure 1.
Table 3.
Fifteen gene sets in the MSigDB (Kegg, Reactome, Immunologic signatures and Gene Ontology) correlated with immunization differences in HD mutant and wild-type mice
| Comparison | Gene set | Number of probes in leading-edge subset/total number of probes | Immune-related genes in leading-edge subset |
|---|---|---|---|
| Immunized versus UI mutant | |||
| Imm R61 > R61 | Biocarta IL2RB | 3/39 | Socs3 |
| Imm R61 > R61 | Biocarta IL6 | 1/21 | Il6 |
| Imm R61 > R61 | Biocarta IL10 | 2/17 | Il6, Tnf |
| Imm R61 > R61 | Biocarta IL22BP | 4/16 | Socs3, Il22ra2, Il22 |
| Imm R61 < R61 | Reactome Ag processing |
72/188 | |
| Imm zQ175 > zQ175 | GeneOntology GSE13306Up |
33/192 | Nfkbia, H2-Q10, Tnfaip2, Traf4, Trat1 |
| Imm zQ175 > zQ175 | GeneOntology GSE13306Dn |
74/229 | Faslg |
| Immunized mutant versus immunized WT | |||
| Imm R61 > ImmWT | Biocarta CDMAC | 4/15 | Tnf, Nfkbia |
| Imm R61 < ImmWT | Biocarta MET | 16/37 | |
| Imm R61 < ImmWT | GeneOntology GSE8868DN2 |
74/180 | Aif1, Nfatc2, Fyb, Fcgr1, Lair1 |
| Imm zQ175 > Imm WT | Biocarta IL1R | 6/33 | Il6, Tnf, Il1b, Nfkbia, Il1rn, Il1rap |
| Imm zQ175 > ImmWT | Biocarta NTHI | 4/23 | Tnf, Il1b, Nfkbia, Tlr2 |
| Imm zQ175 > ImmWT | Biocarta PPARA | 8/53 | Tnf, Nfkbia |
| Imm zQ175 > ImmWT | GeneOntology GSE15733Up |
46/195 | Arg2, Cd177, Ccrl2, Cish, Tnfaip3, Cxcl14, Ifng |
| Imm zQ175 < ImmWT | GeneOntology GSE8868Dn |
79/180 | Il4r, Icam1, Nfatc2, Aif1, Fcgr1, H2-Q1 |
Gene sets that correlate with immunization differences in mutant HD mice function in pro-inflammatory pathways
Information for individual genes in the gene sets listed in Table 3 is provided in Supplementary Material, Table S4. The 15 gene sets (Tables 3 and 4) include biologically informative sets for nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) activation, antigen processing, cytokine signaling and macrophage activation (Table 5). Of these biologically informative sets, NFkB activation, cytokine signaling and pro-inflammatory macrophage responses were positively correlated with immunized mutant mice while antigen processing and anti-inflammatory macrophage responses were negatively correlated with immunized mutant mice. Immunized zQ175 mice showed up-regulation of NFkB and pro-inflammatory cytokine gene sets while immunized R61 mice showed up-regulation of pro-inflammatory macrophage gene sets when compared with immunized wild-type mice. Up-regulation of pro-inflammatory gene sets in immunized mutant HD mice was accompanied by down-regulation of anti-inflammatory macrophage gene sets when compared with immunized wild- type mice (Table 6).
Table 4.
Gene sets that correlate with immunization differences in mutant HD mice function in pro-inflammatory pathways
| Comparison | NFkB | Ag process | Cytokine signaling |
Macrophages |
||
|---|---|---|---|---|---|---|
| Pro-inflam | Anti-inflam | Pro-inflam | Anti-inflam | |||
| Imm R61 > R61 | ||||||
| Imm R61 < R61 | ||||||
| Imm zQ175 > zQ175 | ||||||
| Imm zQ175 > ImmWT | ||||||
| Imm R61 > Imm WT | ||||||
| Imm zQ175 < ImmWT | ||||||
| Imm R61 < Imm WT | ||||||
The fifteen gene sets (in Table 3) were organized into biologically functional groupings. Four functional groupings were identified. Shaded boxes represent affected pathways.
Table 5.
Individual immune-related genes are differentially expressed after immunization of mutant HD and wild-type mice
| Comparison | Innate immune responses |
T/B cell responses | |
|---|---|---|---|
| Pro-inflammatory | Anti-inflammatory | ||
| Imm R61 > Imm WT | Tnf (cytokine) | Nfkbia (negative regulator NFkB) | |
| Imm zQ175 > Imm WT |
Tnf, Il1b, Il1rap, Il6, Ifng, Cxcl14, Ccrl2 (cytokine/chemokine/receptor) Tlr2 (Toll-like receptor) Arg2 (ROS production) Cd177 (neutrophil activation) |
Nfkbia (negative regulator NFkB), Tnfaip3 (negative regulator TNF) I11rn (negative regulator IL1B) Cish (negative regulator JAK/STAT) |
|
| Imm R61 < Imm WT |
Aif1 (macrophages) Nfatc2 (homeostasis) |
Fcgr1 (phagocytic macrophages) Aif1 (CD4 T cells, Th2 cells) Nfatc2 (memory CD4, Th2 cells) Fyb (memory T cells) Lair1 (negative regulator T cell effector functions) |
|
| Imm zQ175 < Imm Wt |
Aif1 (macrophages) Nfatc2 (homeostasis) Icam1 (extravasation) |
Fcgr1 (phagocytic macrophages) Aif1 (CD4 T cells, Th2 cells) Nfatc2 (memory CD4, Th2 cells) Icam1 (memory CD8 T cells) Il4r (Th2 cells) H2-Q1 (Ag presentation/CD8 T cells) |
|
Genes in the leading-edge subset from eight gene sets (in Table 3) for immunized mutant versus immunized wild-type comparisons were sorted based on function and assigned to two categories, innate immune responses or T/B cell responses.
Table 6.
Identification of eight additional gene sets for a comparison between immunized zQ175 and immunized wild-type mice (Imm zQ175 > ImmWT)
| Entrez gene Id | Gene symbol | 1 | 2a | 3a | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Gene description |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3553 | IL1B | X | X | X | X | X | X | X | X | X | Interleukin 1, beta | |
| 3569 | IL6 | X | X | X | X | X | X | X | X | X | Interleukin 6 | |
| 4792 | NFKBIA | X | X | X | X | X | X | X | Nuclear factor of kappa-light polypeptide gene enhancer in B cells inhibitor, alpha | |||
| 3557 | IL1RN | X | X | X | X | X | Interleukin 1 receptor antagonist | |||||
| 7128 | TNFAIP3 | X | X | X | X | X | X | Tumor necrosis factor, alpha-induced protein 3 | ||||
| 9034 | CCRL2 | X | X | X | Chemokine (C-C motif) receptor-like 2 | |||||||
| 384 | ARG2 | X | X | Arginase, type II | ||||||||
| 7097 | TLR2 | X | X | X | X | Toll-like receptor 2 | ||||||
| 7124 | TNF | X | X | X | X | X | X | X | Tumor necrosis factor | |||
| 3556 | IL1RAP | X | X | Interleukin 1 receptor accessory protein | ||||||||
| 3458 | IFNG | X | X | X | X | Interferon, gamma | ||||||
| 1154 | CISH | X | X | Cytokine inducible SH2-containing protein | ||||||||
| 9547 | CXCL14 | X | Chemokine (C-X-C motif) ligand 14 | |||||||||
| 57126 | CD177 | X | CD177 molecule |
Immune-related genes from the four leading-edge subsets (Table 3) were grouped and used to probe the entire MSigDB (v5.0). 1: Altemeier response to LPS with mechanical ventilation; 2: Biocarta IL1R; 3: GSE15733 BM versus spleen memory CD4 T cell up; 4: Hallmark TNFA signaling via NFKB; 5: HINATA NFKB targets keratinocyte up; 6: PID IL23; 7: Reactome immune system; 8: MARKS HDAC targets down; 9: KEGG NOD-like receptor signaling; 10: Lindstedt dendritic cell maturation. Asterisk denotes previously identified gene set.
adenotes previously identified gene set.
To explore additional pathways, we identified individual genes (details in Supplementary Material, Table S4) that function in immune responses in the leading-edge subset of each gene set (Table 3) for probing the entire MSigDB (v 5.0). Since multiple gene sets were identified in the analysis of immunized zQ175 and WT mice, we grouped immune-related genes from the four leading-edge subsets for this analysis. The analysis allowed us to identify eight additional gene sets that correlate with differential immune responses in immunized zQ175 and WT mice. Seven of the gene sets are inflammatory/immune pathways (Table 6). Of the seven gene sets, five represent early inflammatory pathways: inflammatory responses to lipopolysaccharides (LPS), TNFα signaling via NFκB targets, NOD-like receptor signaling which drives activation of NFκB and MAPK, and dendritic cell maturation in response to inflammatory stimuli. Two gene sets highlight additional immune responses; IL23 signaling which is involved in the development of Th17 responses and diverse immune responses in Reactome immune system.
Differential expression of individual genes highlights up-regulation of innate immune responses and down-regulation of memory T cell responses in immunized mutant HD mice
In GSEA, the leading-edge subset can be interpreted as the core of a gene set that accounts for the enrichment signal (24). Examination of genes in the leading-edge subset often reveals additional biologically informative sets. We undertook an analysis of immune-related genes in the leading-edge subsets from gene sets that correlated with immunization of mutant mice to identify additional biologically informative sets. The leading-edge subsets from eight gene sets for immunized mutant versus immunized wild-type comparisons were collapsed into four groupings; Imm R61 > Imm WT, Imm zQ175 > Imm WT, Imm R61 < Imm WT and Imm zQ175 < Imm WT. Individual genes were sorted based on function and assigned to pro-inflammatory responses, anti-inflammatory responses or T/B cell responses (Table 5).
In summary, immunized zQ175 mice showed up-regulation of genes that function in both pro-inflammatory and anti-inflammatory responses (Table 5). Genes associated with pro-inflammatory responses included those encoding cytokines, chemokines, related receptors and a Toll-like receptor. Genes associated with anti-inflammatory responses encoded negative regulators of inflammation. Immunized mutant mice also exhibited down-regulation of genes that function in Th2 responses and memory T cell responses.
Discussion
This ‘Phase 1’ safety and immunogenicity study was designed to initiate immunizations before the onset of major neurological phenotype. Given that 11 different immunization protocols were sampled with N = 5/ cohort, and that we collected postmortem spleen samples at what would be very early to mid-stage neurological disease, this study does not include efficacy endpoints. However, the study was able to test the possibility that immunizations to induce antibodies or innate responses that were specific for HTT could be toxic, and lead to an accelerated manifestation of disease phenotype. None of the protocols showed the weight loss or gross neurological behavioral changes that characterize the full manifestation of the HD phenotype in these mouse strains. This would have been especially obvious in the R6/1 mice, which display earlier deficits than zQ175. The next phase of this study should be efficacy, done in both male and female mutant mice, testing both triple peptides and a plasmid protocol. Long-term correction of behavioral abnormalities, survival and transcriptome correction would be the most efficient parameters to measure, since we are still not certain which conformation of HTT is the most toxic.
The CBAB6F1 genetic background was chosen to allow responses driven by two diverse H-2 haplotypes, while preserving uniform genetic backgrounds. Within responsive cohorts of N = 5 for each protocol, there was considerable variability of responses, suggesting that there are non-uniform parameters superimposed on the identical genetic backgrounds and age-matching. This variability is as prominent in wild-type as in mutant mice. The study as a whole did reveal classes of more uniform non-responsive immunization protocols that can be ruled out. It is clear that plasmid Il4, which has been used as an adjuvant in infectious disease plasmid immunization studies (23), does not improve responses in these trials. It is also clear that the prime-boost methods that are being tested in other DNA vaccine trials did not yield significant responses in any of the mice in our trials. The most likely explanation for the latter result is that the two different antigens/protocols are not presenting the same epitopes to the immune system; therefore, we are not actually priming. This may become a critical issue when considering which antibodies show the highest level of protection, even if their absolute levels are lower.
In our previous studies, we have shown that the single-chain variable region antibody, C4scFv, delivered as a gene, can modulate phenotypic effects of the mutant HTT Exon1 fragment in cells, drosophila and mouse striatum. However, the effects are short-term, and over time the misfolded protein accumulates, even in the continuous presence of an antibody fragment that binds to HTT AA1-17 (25). Kinetically, the mutant protein can misfold during brief windows of antibody dissociation, and will eventually form aggregates that cannot reversibly re-fold. We have had longer-term success by creating bifunctional fusion constructs that re-target the antigen-antibody complex to the proteasome, which more aggressively clears the mHTT Exon1 fragment prior to misfolding and aggregation (7). However, the mutant HD gene is expressed ubiquitously, and the necessity to counteract long-term accumulation may be less critical in the peripheral and non-neuronal cells that can be targeted with an active vaccination strategy. Active vaccination was able to modulate the peripheral pancreatic insufficiency phenotype in the severe R6/2 mouse model, with plasmid correcting more completely than AA1-17 peptide, despite a lower level of antibody as measured by WB (11).
The differential response of the two different HD mutant mouse strains to peptide versus. plasmid-encoded antigens suggests that, similar to the brain, the mouse model in which the pathogenic fragment of HTT is expressed directly is more severely impacted. It is unclear whether this is due to a more rapid overall progression of disease that affects both neuronal and non-neuronal cells in the R6/1 strain, or whether the presence of the fragment in each cell acts more aggressively. There is evidence for progressive presence of inflammatory cytokines in peripheral blood of HD individuals, starting relatively early in the timecourse (26). We can speculate that immunogenicity of peptides is affected by differences in a variety of cellular processes including uptake of peptides, processing and presentation of peptides by major histocompatibility complex (MHC) class II molecules in antigen presenting cells to T helper cells. On the other hand, uptake of plasmid immunogens results in intracellular expression of peptides which traffic through the MHC class I pathway. Again, these processes may differ in the two mutant mouse strains. Furthermore, defective internal cellular transport processes in T cells in the two mutant mouse strains may also affect the immunogenicity of plasmid immunogens because of data that dynein-mediated axonal transport is defective in HD (27), and similar proteins appear critical for the T cell synapse (28).
This vaccination study allowed us, for the first time, to comprehensively and specifically examine transcriptomes associated with HTT-specific antibody responses in HD versus WT spleen. Transcriptional changes in HD mice in response to active immunization are striking, and extend well beyond the pathways previously described. Previous studies of individuals with HD have shown increases in plasma levels of inflammatory cytokines and chemokines (26,29), as well as hyper-reactivity of isolated monocytes to lipopolysaccharides (LPS) stimulation (30). Mutant HTT appears to act autonomously to produce microglial activation via myeloid pathways (31). Hypersensitivities of the immune/inflammatory pathways have also been demonstrated in a range of assays using multiple fragment and full-length mouse models, with major emphasis on cytokines, and the NFκB and kynurenine pathways (32,33).
In the current profiling study, we show that immunization of mutant HD mice results in both positive and negative dysregulation. Of the eleven immunogens, the triple peptide combination was the most immunogenic, inducing similar serum antibody titers in HD and wild-type mice; therefore, this cohort was used for the more detailed and labor-intensive profiling. There is transcriptional activation of genes associated with innate immune responses, absence of negative feedback control of gene expression by regulators, a temporal dysregulation of innate immune responses and transcriptional repression of genes associated with memory T cell responses. Transcriptional up-regulation of genes associated with innate immune responses was more pronounced after immunization of zQ175 mice than R6/1 mice. In zQ175 mice, gene expression data highlight five signaling pathways, NFκB, TNF, IL1B, JAK/STAT and TLR2. For example, Il1b, Tnf, Nfkbia and Tnfaip3 are target genes for the transcription factor, NFκB1, with IL1B and TNF serving as positive regulators of gene expression while NFKBIA and TNFAIP3 are negative regulators of gene expression (KEGG database; (34,35). Similarly, target genes for TNF include Il1b, ll6 and Tnf (positive regulators) along with NFKBIA and TNFAIP3 (negative regulators) (34,35). Of note is that genes encoding both positive and negative regulators in the five signaling pathways are detected simultaneously, implying a failure in negative feedback mechanisms to inhibit expression of pro-inflammatory genes. Furthermore, the sustained expression of genes associated with innate immunity, 10 weeks after the initial immunization, suggests a failure in the temporal control of innate immune responses. These responses were not unexpected, although the comparison of the two mutant strains is informative.
However, in addition to transcriptional activation of innate immune markers, immunized HD mice exhibit transcriptional repression of markers associated with T and B cell responses. Three genes, Aif1, Nfatc2 and Fcgr1 are common to both R6/1 and zQ175 mice. Both AIF1 and NFATC2 function in innate and adaptive immune responses. AIF1 is expressed in macrophages and is a key regulator of inflammation, influencing cytokine production, inflammatory mediators and expression of adhesion molecules (36). AIF1 is also present in activated T cells and up-regulates IL4 production. Both Aif1 and Il4r are down-modulated in immunized zQ175 versus immunized wild-type mice. NFATc2 is a member of the NFAT family of transcription factors. NFAT signaling modulates the function of cells involved in innate immune responses such as macrophages, dendritic cells, mast cells, neutrophils and natural killer cells (37). In addition, NFAT influences lymphocyte development, activation and T cell differentiation and is expressed at higher levels in memory T cells (38). FCGR1 is expressed on macrophages and binds IgG with high affinity resulting in activation of phagocytic activity (39). Two additional genes, Fyb and Lair1, are down-regulated in immunized R6/1 versus immunized wild-type mice. FYB is upregulated in memory T cells (40) while LAIR1 is a negative regulator of T cell effector function (41). Finally, H2-Q1 (Qa1) and Icam1 are down-modulated in immunized zQ175 versus immunized wild-type mice. Qa1 functions in regulating adaptive immunity to both self and foreign antigens (42–44), while ICAM1 expression by mature dendritic cells determines the survival of activated CD8 T cells and the establishment of effective memory T cell responses (45). The genome-wide expression study therefore highlights an actively responsive transcriptional dysregulation of genes, affecting multiple cell types involved in innate and adaptive immune responses, after immunization of HD mice in vivo.
The question of whether transcriptional dysregulation of immune-related genes is observed after immunization of HD patients remains to be resolved. It would be valuable to examine the memory T cell population in humans at different stages of disease, using cell sorting. If there is an HD population that is receiving yearly influenza vaccinations, this might be an appropriate group in which to examine HD human immune responses with minimal additional stress. Should HD patients show a similar transcriptional dysregulation after immunization, our data suggest that active vaccination immunotherapeutic strategies for HD would have to include treatments aimed at restoring immune controls, although possibly this will be less critical if started very early. Possible immuno-modulatory approaches include the use of anti-inflammatory agents, cytokines, antibodies to specific cytokines or antibodies to specific cytokine receptors. Passive immunization delivered systemically is also currently being used for cancer and other diseases, and might be effective for broad coverage of peripheral cells here.
One critical issue for clinical development (of active and especially passive immunotherapy) is the epitope specificity of a protective antibody. In our previous active vaccination study (11), the HTT AA1-17 peptide yielded much higher titers of antibody than plasmid immunization, as measured by WB. However, the peptide gave only a partial protection from pancreatic insufficiency, while the plasmid was able to prevent or reverse the insulin deficiency in R6/2 mice. Since our prime-boost protocol strongly suggests that epitopes for the peptide versus the plasmid immunogens differ, one hypothesis would be that the previous plasmid induced a more ‘protective’ antibody response. It will be particularly interesting to determine whether the high antibody titers achieved with the new triple peptide protocol provide functional protection. This can then be compared with protection from the lower, but still measurable, titers produced with plasmid vaccination. Individual peptides can also be tested, although our preliminary data suggested that immunizing with a combination of the three peptides gave a much more robust response that single peptides alone.
Based on our current data, it is also possible that a very strong immune response will show mixed functional protection results, since there is also an increase in the inflammatory response. Modulation of the inflammatory process in the context of an active vaccination might improve both titer and efficacy of an active vaccine. This approach has recently been used to counteract the pathogenesis of misfolding alpha-synuclein (α-syn), by vaccinating with peptides that are below the threshold for antigen-specific T cell responses, and further engineered as functional mimotopes (46,47). It is of particular interest that a recent study using small anti- α-syn peptides to immunize a mouse model of Multiple Systems Atrophy (mice expressing human α-syn under the control of the Myelin Basic Protein promoter in oligodendrocytes) showed modulation of inflammatory cytokines that are increased in the untreated mouse model (46).
The results of this study in young adult HTT Exon1 transgenic R6/1, and full-length zQ175 knock-in, HD mouse models demonstrate that responses to potential therapeutic immunization protocols differ based on the antigens used. The immune reactions in the presence of the mutant HTT also differ from those of wild-type, both at the level of antibody response (plasmid only), and dramatically at the gene expression level (triple peptide tested). Several important new findings from these studies can be used to enhance future active vaccination directions, including critical follow-up efficacy studies of active vaccination in mouse models.
Materials and Methods
Mice
For this phase 1 pilot project, all studies were done using female mice on a F1 hybrid CBA x B6 genetic background; N = 5 of each variant within each protocol (Future studies will use both sexes.). Wild-type CBA females were crossed to B6 congenic HD Exon1 fragment model R6/1 (12) and zQ175 knock-in mice (13). R6/1 mice were bred, genotyped and maintained in our colonies, with bimonthly verification of the repeat number (Q = 120–130). zQ175 mice were from the CHDI colony at the Jackson Laboratory (stock #027410), where they were bred, genotyped, maintained and outcrossed to CBA/J. F1 mice were shipped to Wadsworth at approximately 4 weeks of age (Q = 184–202). Non-transgenic littermates were used as wild-type controls. All procedures were approved by the Wadsworth Center Institutional Animal Care and Use Committee.
Immunization with HTT peptides
Use of a KLH-linked HTT AA1-17 peptide is similar to the KLH approaches taken in many human vaccines (http://clinicaltrials.gov/ct2/results?term=KLH), including the Affitope AD02 for Alzheimer's. We also knew that this peptide would induce a robust antibody response, since we had previously used it to produce a monoclonal anti-HTT AA1-17 antibody (48). Additional HTT Exon1 epitopes also linked to KLH, were 49PQLPQPPPQAQP60 [used by the Patterson lab to select Happ1 and Happ3 (21)]; and 74PPPGPAVAEEPLHRP88, the putative EM48 epitope. All peptides were synthesized and KLH-conjugated commercially (GenScript, Piscataway, NJ, USA). The latter is human-specific, and Wang et al., 2008, showed suppression of neuropil aggregates and neurological symptoms when a scFv intracellular antibody form of this monoclonal was very highly expressed from an adenovirus in striatum (22). Mice were immunized sub-cutaneously (sc) with 200 ul total volume (100ug of each peptide). Alum (Pierce/Life Technologies, Grand Island, NY, USA), which induces B cell responses and CD4 T cell responses but not CD8 T cell responses, was used as an adjuvant (49). Mice were primed at 6–12 weeks (R6/1) or 15–24 weeks (zQ175), and boosted twice at monthly/bimonthly intervals, with tissue collection 10 days after the second boost.
Immunization with HTT protein
The HTT Exon1-46Q protein linked to MBP was provided by CHDI, and cleaved and MBP removed just prior to immunizations with a Thrombin CleanCleave™ Kit (Sigma, St. Louis, MO, USA). Sc injections of 50ug of protein in Alum were done within 1 h of completing the cleavage/clean-up reaction.
Immunization with plasmid DNA
Two plasmids, HTT Exon1-97Q and N586-82Q, were tested ±plasmid Il4. Il4 was tested as an adjuvant because it has been reported to drive the development of TH2 responses and ultimately B cell response (23). HTT Exon1-97Q carries the full HTT Exon1 and has been used extensively, while N586-82Q covers significant additional HTT sequence, and has been used in mice and in cell cultures (50). Delivery used a particle-mediated epidermal delivery (PMED) device, developed by Dr Deborah Fuller, for the efficient epidermal delivery of the plasmid DNA (51). The Fuller lab (University of Washington) generously produced the delivery cartridges from plasmids supplied by the Messer lab. A total of 4 ug of either HTT Exon1-97Q or N586-82Q were delivered for each immunization (2ug at each at separate sites in the shaved abdomen). Il4 was co-administered (0.4ug) having been mixed with either of the two plasmids prior to PMED cartridge preparation.
Heterologous prime-boost immunization
This protocol was tested due to success in a range of vaccine studies (52). It combines the presentation of a presumably wider range of epitopes elicited when the protein is synthesized intracellularly with the stronger antibody responses that characterize protein/peptide immunogens. Plasmid was used for primary and the first boost, and protein (or peptide) for the second boost. Immunizations were conducted as described above for either sc. or PMED delivery.
ELISA
Anti-HTT antibodies in sera of immunized mice were measured by ELISA (53). Briefly, ELISA Maxisorp (Nunc) 96-well plates were coated with 25 ug/ml of MBP-Exon 1 in PBS and incubated at 4°C overnight. Plates were washed three times with PBS, 0.25% BSA and 0.05% Tween 20 and blocked with PBS, 0.25% BSA, 0.05% Tween and 1 mM EDTA for 1 h at room temperature. Serial-fold dilutions of sera in blocking buffer were added to the wells. Samples added to wells without bound antigen served as negative controls. After 2 h at room temperature, plates were washed three times and blocked with blocking buffer and 10% goat serum for 1 h. Plates were washed three times and alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma) was added to the wells. Plates were incubated for 2 h at room temperature and then washed three times. A p-nitrophenyl phosphate substrate solution (Sigma) was added to each well and optical densities were measured using an ELISA plate reader (Sunrise, Tecan) at 405 nm. Serum antibody titer is defined as the reciprocal of the dilution whose absorbance is three times that of negative controls.
Western blotting
ST14A cells were transiently transfected with a plasmid expressing Exon 1 of HTT fused to GFP, pcDNA3.1-HTTExon1-46Q-GFP, using the jetPEI™ DNA transfection reagent (VWR Inc., Bridgeport, NJ, USA), as previously described (7). Cells transfected with a GFP plasmid, pcDNA3.1-GFP, served as a control. Cells were harvested two days later, lysed in 10 mm Tris–HCl, 150 mm NaCl and 2% SDS containing cOmplete™ protease inhibitor cocktail (Roche, Indianapolis, IN, USA), and stored at −80°C. Protein concentrations were determined using the BCA protein assay kit (BioRad Laboratories, Hercules, CA, USA). A total of 20 ug of protein were denatured for 5 min at 95°C and electrophoresed in a 4–20% precision protein gel (Tris-HEPES SDS precast polyacrylamide Mini gel—Thermo Scientific). Electrophoretic transfer of proteins to a PVDF membrane was accomplished using the semi-dry transfer method and a buffer consisting of 25 mM Tris–HCl, 192 mM glycine and 20% methanol. The membrane was subsequently blocked in 5% non-fat dry milk in Tris buffered saline (TBS)-Tween 20 for 1 h at room temperature and incubated with appropriate dilutions of serum overnight at 4°C. Bound anti-HTT antibody was detected after a 1 h incubation with horseradish peroxidase conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by chemiluminescent detection.
Microarray gene expression in splenocytes
Spleens were dissociated by mechanical disruption and red blood cells were removed by hypotonic lysis in 0.15 M ammonium chloride. Splenocytes were lysed in RLT buffer (Qiagen, Valencia, CA, USA) and cell lysates were homogenized using a QIAshredder homogenizer (Qiagen). Cellular RNA was purified using RNeasy columns (Qiagen) and residual genomic DNA was digested with RQ1 DNase (Promega, Madison, WI, USA). RNA was precipitated and re-suspended in sterile water. RNA integrity was verified by agarose gel electrophoresis. RNA quality and concentration were measured using an Agilent 2100 Bioanalyzer (Life Technologies). cRNA was prepared according to the Affymetrix standard protocol as described (54) and hybridized to Affymetrix Mouse Gene 2.0 ST by SUNYMAC (Syracuse, NY, USA).
Bioinformatics and statistical analyses
Traditional strategies for gene expression analysis focus on identifying individual genes whose expression differ between experimental groups. A common approach is to focus on the handful of genes at the top and bottom of a ranked list. Expression of a small subset of these genes is then verified by RT-PCR. Some of the limitations of single-gene analysis include (a) the generation of a long list of statistically significant genes without any unifying biological theme; (b) the potential to miss important effects on pathways and (c) the likelihood that different groups studying the same biological system will generate lists of statistically significant genes with little overlap (24). An approach that has been developed to overcome these limitations of single-gene analysis is GSEA. The method derives its power by focusing on gene sets or groups of genes that share common biological function. The GSEA method has been extensively tested against single-gene analysis and has provided insights into biological pathways for several cancer-related data sets when single-gene analysis showed little similarity between independent studies (24).
In our study, Affymetrix CEL files were subjected to GSEA (24) using Molecular Signatures Database v5.0 (MSigDB). Twenty-one pairwise sample comparisons (Supplementary Material, Table S5) were tested on multiple curated gene sets including those from BioCarta, Gene Ontology, Kegg and Reactome. Of the 41,344 mouse probe set IDs (Mouse Gene 2.0 ST array), 17,774 (43%) mapped to HUGO (Human Genome Organization) genes. For this study, we limited our analysis to comparisons between immunized (Imm) versus unimmunized (UnImm) mutant mice, immunized mutant versus immunized WT, unimmunized mutant versus WT, and unimmunized R6/1 versus unimmunized zQ175. We identified the top scoring 26 gene sets for six sample comparisons: imm R6/1 versus UnImm R6/1; Imm zQ175 versus UnImm zQ175; Imm R6/1 versus Imm WT; Imm zQ175 versus Imm WT; UnImm R6/1 versus UnImm zQ175 and UnImm R6/1 versus UnImm WT. Since the present study reports on immunization differences in mutant and wild-type mice, we focused on gene sets for four sample comparisons: imm R6/1 versus UnImm R6/1; Imm zQ175 versus UnImm zQ175; Imm R6/1 versus Imm WT; Imm zQ175 versus Imm WT. Fifteen gene sets correlated with immunization differences in mutant and wild-type mice (present report; values in Supplementary Material, Table S1) while eleven gene sets (manuscript in preparation) correlated with strain differences in unimmunized mice.
Supplemental Material
Funding
This work was supported under a research contract from CHDI Foundation, Inc., which also supplied the HTT Exon1-MBP protein, and the founder Q175 mice from a dedicated colony at the Jackson Laboratory; the B6.HDR6/1 founder colony was supported by National Institutes of Health grant NS053912 to A.M.
Supplementary Material
Acknowledgements
We thank Dr Deborah H. Fuller (Department of Microbiology, University of Washington, Seattle, WA, USA) for discussions of the immunization protocols, particularly for the plasmids. Her laboratory also generously facilitated the gene gun experiments and production of the cartridges. We thank Christy Stagnar for excellent technical assistance with the gene gun and the ELISA assays. Dr Abigail Snyder-Keller provided valuable assistance with the mouse colony and dissections.
Conflict of Interest statement. None declared.
References
- 1.Lemere C.A. (2013) Immunotherapy for Alzheimer's disease: hoops and hurdles. Mol. Neurodegener., 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer B., Masliah E. (2014) Immunotherapy for Alzheimer's disease: past, present and future. Front. Aging Neurosci., 6, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Huntington's Disease Collaborative Research Group. (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell, 72, 971–983. [DOI] [PubMed] [Google Scholar]
- 4.Gusella J.F., MacDonald M.E., Ambrose C.M., Duyao M.P. (1993) Molecular genetics of Huntington's disease. Arch. Neurol., 50, 1157–1163. [DOI] [PubMed] [Google Scholar]
- 5.Crook Z.R., Housman D. (2011) Huntington's disease: can mice lead the way to treatment? Neuron, 69, 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messer A., Joshi S.N. (2013) Intrabodies as neuroprotective therapeutics. Neurotherapeutics, 10, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler D.C., Messer A. (2011) Bifunctional anti-huntingtin proteasome-directed intrabodies mediate efficient degradation of mutant huntingtin exon 1 protein fragments. PLoS One, 6, e29199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder-Keller A., McLear J.A., Hathorn T., Messer A. (2010) Early or late-stage anti-N-terminal Huntingtin intrabody gene therapy reduces pathological features in B6.HDR6/1 mice. J. Neuropathol. Exp. Neurol., 69, 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfgang W.J., Miller T.W., Webster J.M., Huston J.S., Thompson L.M., Marsh J.L., Messer A. (2005) Suppression of Huntington's disease pathology in Drosophila by human single-chain Fv antibodies. Proc. Natl. Acad. Sci. U S A, 102, 11563–11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lecerf J.M., Shirley T.L., Zhu Q., Kazantsev A., Amersdorfer P., Housman D.E., Messer A., Huston J.S. (2001) Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington's disease. Proc. Natl. Acad. Sci. U S A, 98, 4764–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller T.W., Shirley T.L., Wolfgang W.J., Kang X., Messer A. (2003) DNA vaccination against mutant huntingtin ameliorates the HDR6/2 diabetic phenotype. Mol. Ther., 7, 572–579. [DOI] [PubMed] [Google Scholar]
- 12.Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S.W. et al. (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell, 87, 493–506. [DOI] [PubMed] [Google Scholar]
- 13.Menalled L.B., Kudwa A.E., Miller S., Fitzpatrick J., Watson-Johnson J., Keating N., Ruiz M., Mushlin R., Alosio W., McConnell K. et al. (2012) Comprehensive behavioral and molecular characterization of a new knock-in mouse model of Huntington's disease: zQ175. PLoS One, 7, e49838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakaya H.I., Wrammert J., Lee E.K., Racioppi L., Marie-Kunze S., Haining W.N., Means A.R., Kasturi S.P., Khan N., Li G.M. et al. (2011) Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol., 12, 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Querec T.D., Akondy R.S., Lee E.K., Cao W., Nakaya H.I., Teuwen D., Pirani A., Gernert K., Deng J., Marzolf B. et al. (2009) Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol., 10, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valor L.M. (2015) Transcription, epigenetics and ameliorative strategies in Huntington's Disease: a genome-wide perspective. Mol. Neurobiol., 51, 406–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seredenina T., Luthi-Carter R. (2012) What have we learned from gene expression profiles in Huntington's disease? Neurobiol . Dis., 45, 83–98. [DOI] [PubMed] [Google Scholar]
- 18.Davies S.W., Turmaine M., Cozens B.A., DiFiglia M., Sharp A.H., Ross C.A., Scherzinger E., Wanker E.E., Mangiarini L., Bates G.P. (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell, 90, 537–548. [DOI] [PubMed] [Google Scholar]
- 19.Sathasivam K., Hobbs C., Mangiarini L., Mahal A., Turmaine M., Doherty P., Davies S.W., Bates G.P. (1999) Transgenic models of Huntington's disease. Philos. Trans. R. Soc. Lond B Biol Sci, 354, 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heikkinen T., Lehtimaki K., Vartiainen N., Puolivali J., Hendricks S.J., Glaser J.R., Bradaia A., Wadel K., Touller C., Kontkanen O. et al. (2012) Characterization of neurophysiological and behavioral changes, MRI brain volumetry and 1H MRS in zQ175 knock-in mouse model of Huntington's disease. PLoS One, 7, e50717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Southwell A.L., Ko J., Patterson P.H. (2009) Intrabody gene therapy ameliorates motor, cognitive, and neuropathological symptoms in multiple mouse models of Huntington's disease. J. Neurosci., 29, 13589–13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C.E., Zhou H., McGuire J.R., Cerullo V., Lee B., Li S.H., Li X.J. (2008) Suppression of neuropil aggregates and neurological symptoms by an intracellular antibody implicates the cytoplasmic toxicity of mutant huntingtin. J. Cell Biol., 181, 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arrington J., Braun R.P., Dong L., Fuller D.H., Macklin M.D., Umlauf S.W., Wagner S.J., Wu M.S., Payne L.G., Haynes J.R. (2002) Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvants for DNA vaccines. J. Virol., 76, 4536–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S. et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U S A, 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler D.C., McLear J.A., Messer A. (2012) Engineered antibody therapies to counteract mutant huntingtin and related toxic intracellular proteins. Prog. Neurobiol., 97, 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjorkqvist M., Wild E.J., Thiele J., Silvestroni A., Andre R., Lahiri N., Raibon E., Lee R.V., Benn C.L., Soulet D. et al. (2008) A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J. Exp. Med., 205, 1869–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith G.A., Rocha E.M., McLean J.R., Hayes M.A., Izen S.C., Isacson O., Hallett P.J. (2014) Progressive axonal transport and synaptic protein changes correlate with behavioral and neuropathological abnormalities in the heterozygous Q175 KI mouse model of Huntington's disease. Hum. Mol. Genet., 23, 4510–4527. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Cofreces N.B., Robles-Valero J., Cabrero J.R., Mittelbrunn M., Gordon-Alonso M., Sung C.H., Alarcon B., Vazquez J., Sanchez-Madrid F. (2008) MTOC translocation modulates IS formation and controls sustained T cell signaling. J. Cell Biol., 182, 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wild E., Magnusson A., Lahiri N., Krus U., Orth M., Tabrizi S.J., Bjorkqvist M. (2011) Abnormal peripheral chemokine profile in Huntington's disease. PLoS Curr., 3, RRN1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trager U., Andre R., Magnusson-Lind A., Miller J.R., Connolly C., Weiss A., Grueninger S., Silajdzic E., Smith D.L., Leavitt B.R. et al. (2014) Characterisation of immune cell function in fragment and full-length Huntington's disease mouse models. Neurobiol. Dis., 73C, 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotti A., Benner C., Kerman B.E., Gosselin D., Lagier-Tourenne C., Zuccato C., Cattaneo E., Gage F.H., Cleveland D.W., Glass C.K. (2014) Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat. Neurosci., 17, 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwan W., Trager U., Davalos D., Chou A., Bouchard J., Andre R., Miller A., Weiss A., Giorgini F., Cheah C. et al. (2012) Mutant huntingtin impairs immune cell migration in Huntington disease. J. Clin. Invest., 122, 4737–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trager U., Andre R., Lahiri N., Magnusson-Lind A., Weiss A., Grueninger S., McKinnon C., Sirinathsinghji E., Kahlon S., Pfister E.L. et al. (2014) HTT-lowering reverses Huntington's disease immune dysfunction caused by NFkappaB pathway dysregulation. Brain, 137, 819–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanehisa M., Goto S., Sato Y., Kawashima M., Furumichi M., Tanabe M. (2014) Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res., 42, D199–D205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanehisa M., Goto S. (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res., 28, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y.Y., Yan D.J., Chen Z.W. (2013) Role of AIF-1 in the regulation of inflammatory activation and diverse disease processes. Cell Immunol., 284, 75–83. [DOI] [PubMed] [Google Scholar]
- 37.Fric J., Zelante T., Wong A.Y., Mertes A., Yu H.B., Ricciardi-Castagnoli P. (2012) NFAT control of innate immunity. Blood, 120, 1380–1389. [DOI] [PubMed] [Google Scholar]
- 38.Dienz O., Eaton S.M., Krahl T.J., Diehl S., Charland C., Dodge J., Swain S.L., Budd R.C., Haynes L., Rincon M. (2007) Accumulation of NFAT mediates IL-2 expression in memory, but not naive, CD4+ T cells. Proc. Natl. Acad. Sci. U S A, 104, 7175–7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gautier E.L., Shay T., Miller J., Greter M., Jakubzick C., Ivanov S., Helft J., Chow A., Elpek K.G., Gordonov S. et al. (2012) Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol., 13, 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dluzniewska J., Zou L., Harmon I.R., Ellingson M.T., Peterson E.J. (2007) Immature hematopoietic cells display selective requirements for adhesion- and degranulation-promoting adaptor protein in development and homeostasis. Eur. J. Immunol., 37, 3208–3219. [DOI] [PubMed] [Google Scholar]
- 41.Saverino D., Fabbi M., Merlo A., Ravera G., Grossi C.E., Ciccone E. (2002) Surface density expression of the leukocyte-associated Ig-like receptor-1 is directly related to inhibition of human T-cell functions. Hum. Immunol., 63, 534–546. [DOI] [PubMed] [Google Scholar]
- 42.Flaherty L., Elliott E., Tine J.A., Walsh A.C., Waters J.B. (1990) Immunogenetics of the Q and TL regions of the mouse. Crit. Rev. Immunol., 10, 131–175. [PubMed] [Google Scholar]
- 43.Jensen P.E., Sullivan B.A., Reed-Loisel L.M., Weber D.A. (2004) Qa-1, a nonclassical class I histocompatibility molecule with roles in innate and adaptive immunity. Immunol. Res., 29, 81–92. [DOI] [PubMed] [Google Scholar]
- 44.Jiang H. (2005) The Qa-1 dependent CD8+ T cell mediated regulatory pathway. Cell. Mol. Immunol., 2, 161–167. [PubMed] [Google Scholar]
- 45.Scholer A., Hugues S., Boissonnas A., Fetler L., Amigorena S. (2008) Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity, 28, 258–270. [DOI] [PubMed] [Google Scholar]
- 46.Mandler M., Valera E., Rockenstein E., Mante M., Weninger H., Patrick C., Adame A., Schmidhuber S., Santic R., Schneeberger A. et al. (2015) Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol. Neurodegener., 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandler M., Valera E., Rockenstein E., Weninger H., Patrick C., Adame A., Santic R., Meindl S., Vigl B., Smrzka O. et al. (2014) Next-generation active immunization approach for synucleinopathies: implications for Parkinson's disease clinical trials. Acta Neuropathol., 127, 861–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller T.W., Zhou C., Gines S., MacDonald M.E., Mazarakis N.D., Bates G.P., Huston J.S., Messer A. (2005) A human single-chain Fv intrabody preferentially targets amino-terminal Huntingtin's fragments in striatal models of Huntington's disease. Neurobiol. Dis., 19, 47–56. [DOI] [PubMed] [Google Scholar]
- 49.Lofthouse S.A., Andrews A.E., Nash A.D., Bowles V.M. (1995) Humoral and cellular responses induced by intradermally administered cytokine and conventional adjuvants. Vaccine, 13, 1131–1137. [DOI] [PubMed] [Google Scholar]
- 50.Tebbenkamp A.T., Green C., Xu G., Denovan-Wright E.M., Rising A.C., Fromholt S.E., Brown H.H., Swing D., Mandel R.J., Tessarollo L. et al. (2011) Transgenic mice expressing caspase-6-derived N-terminal fragments of mutant huntingtin develop neurologic abnormalities with predominant cytoplasmic inclusion pathology composed largely of a smaller proteolytic derivative. Hum. Mol. Genet., 20, 2770–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuller D.H., Loudon P., Schmaljohn C. (2006) Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods, 40, 86–97. [DOI] [PubMed] [Google Scholar]
- 52.Ranasinghe C., Ramshaw I.A. (2009) Genetic heterologous prime-boost vaccination strategies for improved systemic and mucosal immunity. Expert Rev. Vaccines, 8, 1171–1181. [DOI] [PubMed] [Google Scholar]
- 53.Gu R., Stagnar C., Zaichenko L., Ramsingh A.I. (2012) Induction of mucosal HIV-specific B and T cell responses after oral immunization with live coxsackievirus B4 recombinants. Vaccine, 30, 3666–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostrowski S.E., Reilly A.A., Collins D.N., Ramsingh A.I. (2004) Progression or resolution of coxsackievirus B4-induced pancreatitis: a genomic analysis. J. Virol., 78, 8229–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.